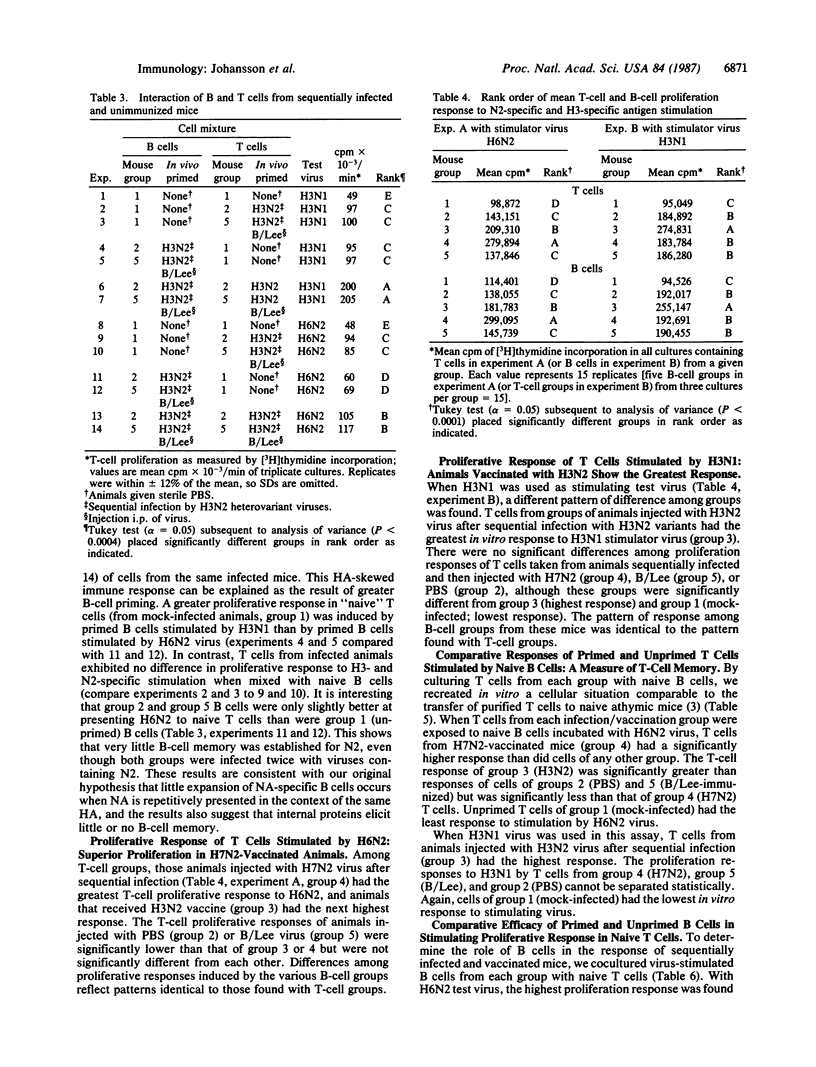
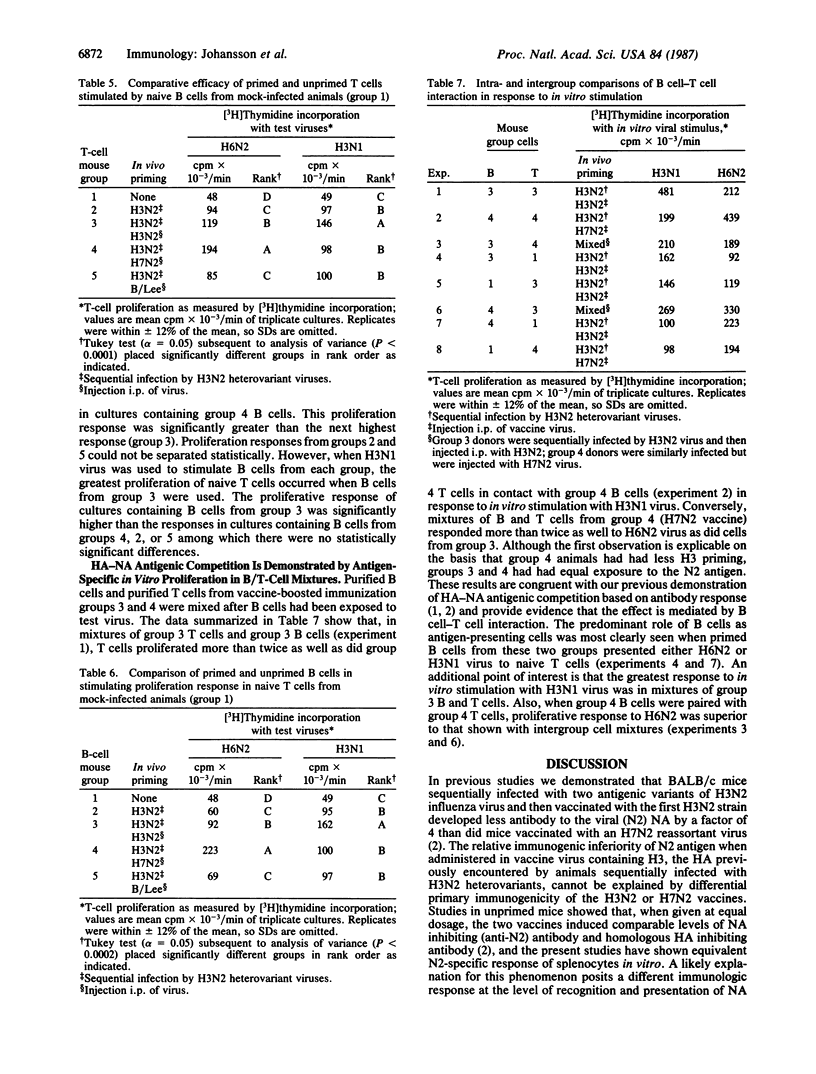
Abstract
Parenteral vaccination of BALB/c mice primed by infection with H3N2 variants of influenza A virus results in a reduced production of N2 antibody in response to homologous (H3N2) vaccine compared with the response to an H7N2 vaccine equal in N2 immunologenicity. We now have studied the interaction in vitro of purified splenic B and T lymphocytes from variably immunized mice to ascertain the cellular basis of the hemagglutinin (HA)-influenced antibody response to neuraminidase (NA). Assay of the proliferative response of T cells in B/T-cell mixtures stimulated by H3N1 (HA-specific) and H6N2 (NA-specific) reassortant (recombinant) viruses in vitro has enabled us to differentiate cellular responses to HA and NA antigens. Using a factorial design in analysis of B/T-cell mixtures, we have shown that: (i) intravirionic HA is dominant over NA in both B- and T-cell priming; (ii) an increase in H3-specific B cells occurs in mice administered boosters of H3N2 vaccine, and an increase in N2-specific B cells occurs in those given a booster of H7N2 vaccine; and (iii) memory B cells function as antigen-presenting cells and interact with memory helper T cells in the mediation of intravirionic HA-NA antigenic competition in favor of HA. The damping of response to the NA antigen in favor of HA with reinfection prohibits balanced immunologic response to the two antigens. The present studies define further the complex immunology of influenza virus infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chesnut R. W., Colon S. M., Grey H. M. Antigen presentation by normal B cells, B cell tumors, and macrophages: functional and biochemical comparison. J Immunol. 1982 Apr;128(4):1764–1768. [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Couch R. B., Kasel J. A., Gerin J. L., Schulman J. L., Kilbourne E. D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J Infect Dis. 1974 Apr;129(4):411–420. doi: 10.1093/infdis/129.4.411. [DOI] [PubMed] [Google Scholar]
- FRANCIS T., Jr, DAVENPORT F. M., HENNESSY A. V. A serological recapitulation of human infection with different strains of influenza virus. Trans Assoc Am Physicians. 1953;66:231–239. [PubMed] [Google Scholar]
- Hutchings P., Rayner D. C., Champion B. R., Marshall-Clarke S., Macatonia S., Roitt I., Cooke A. High efficiency antigen presentation by thyroglobulin-primed murine splenic B cells. Eur J Immunol. 1987 Mar;17(3):393–398. doi: 10.1002/eji.1830170314. [DOI] [PubMed] [Google Scholar]
- Kakiuchi T., Chesnut R. W., Grey H. M. B cells as antigen-presenting cells: the requirement for B cell activation. J Immunol. 1983 Jul;131(1):109–114. [PubMed] [Google Scholar]
- Kilbourne E. D., Cerini C. P., Khan M. W., Mitchell J. W., Jr, Ogra P. L. Immunologic response to the influenza virus neuraminidase is influenced by prior experience with the associated viral hemagglutinin. I. Studies in human vaccinees. J Immunol. 1987 May 1;138(9):3010–3013. [PubMed] [Google Scholar]
- Kilbourne E. D. Comparative efficacy of neuraminidase-specific and conventional influenza virus vaccines in induction of antibody to neuraminidase in humans. J Infect Dis. 1976 Oct;134(4):384–394. doi: 10.1093/infdis/134.4.384. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986 Oct 1;164(4):1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig M. J., Mozes E., Shearer G. M., Sela M. Antigenic competition and genetic control of the immune response. A hypothesis for intramolecular competition. Cell Immunol. 1973 Aug;8(2):299–310. doi: 10.1016/0008-8749(73)90119-6. [DOI] [PubMed] [Google Scholar]
- Taussig M. J., Mozes E., Shearer G. M., Sela M. Studies on the mechanism of antigenic competition: analysis of competition between synthetic polypeptide antigens. Eur J Immunol. 1972 Oct;2(5):448–452. doi: 10.1002/eji.1830020513. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Kilbourne E. D. Reactions of antibodies with surface antigens of influenza virus. J Gen Virol. 1968 Dec;3(3):315–326. doi: 10.1099/0022-1317-3-3-315. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]



