Abstract
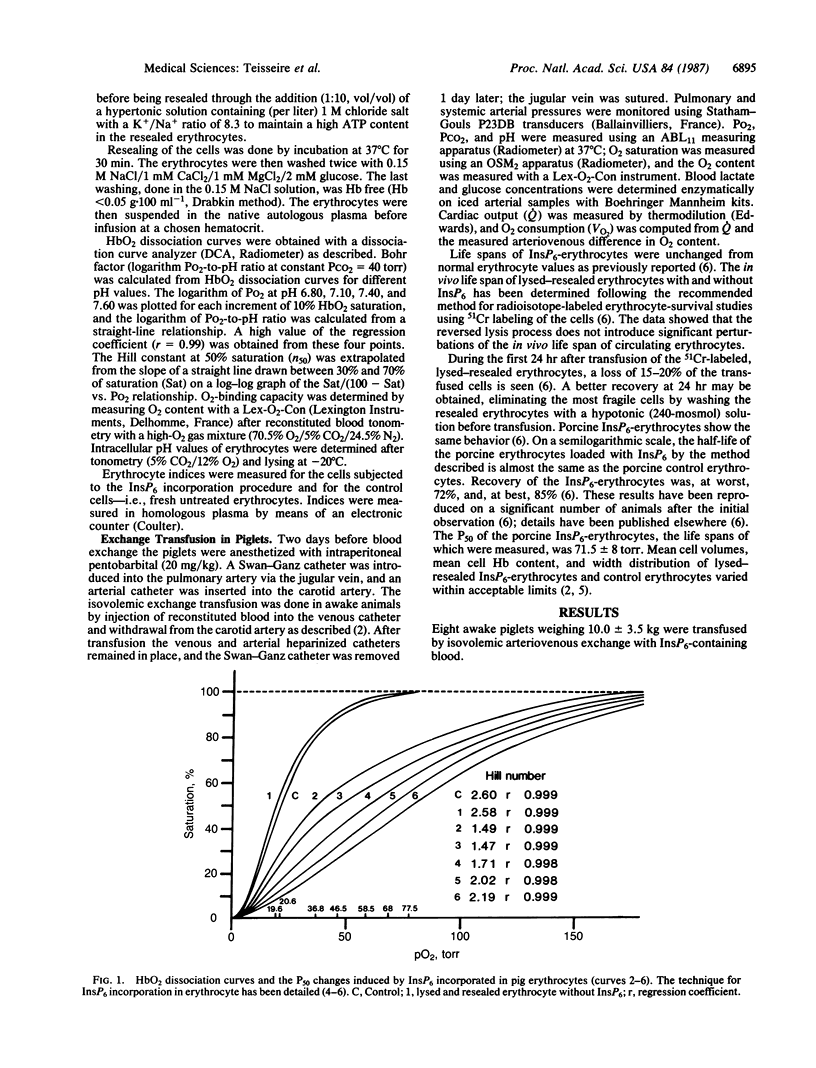
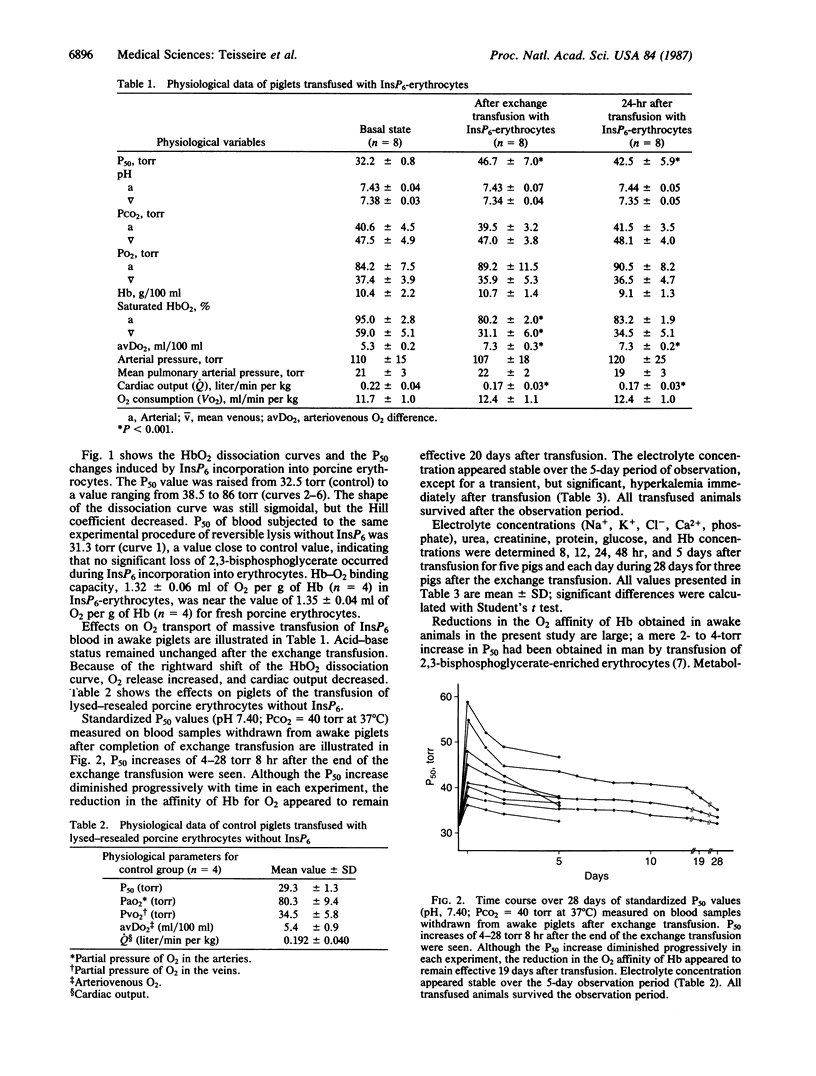
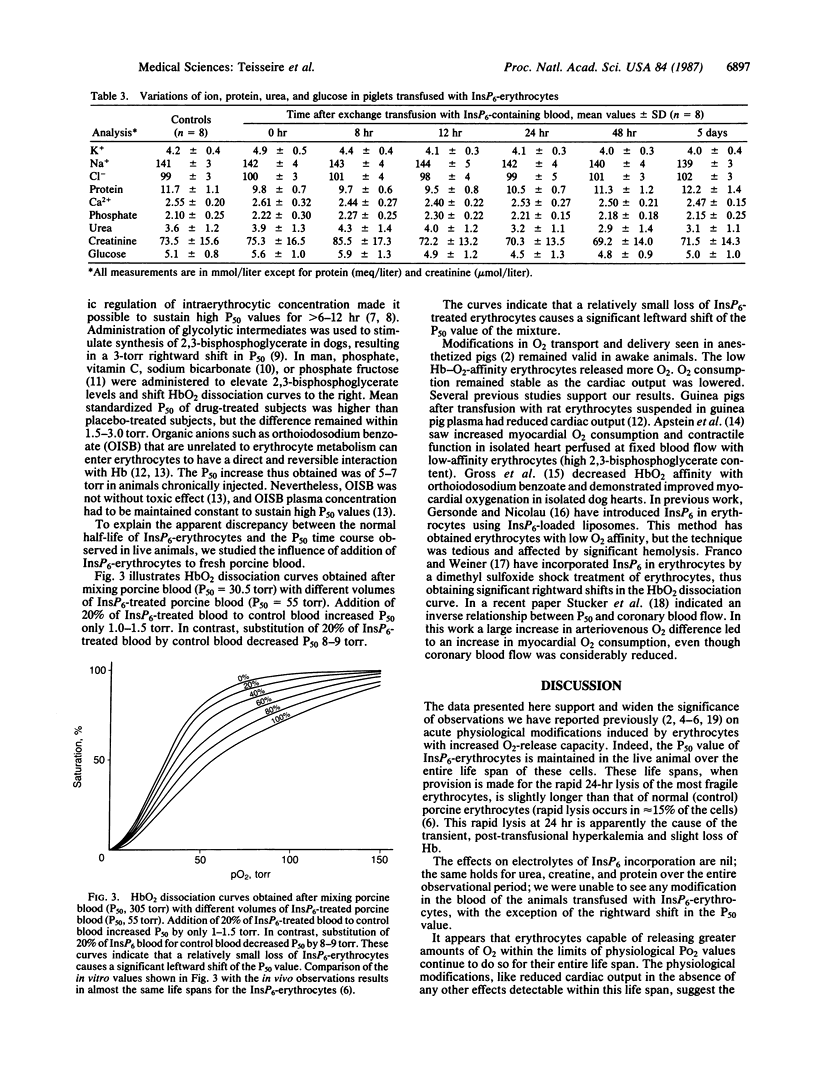
A continuous lysing and resealing procedure with erythrocytes permitted incorporation in these cells of inositol hexaphosphate (InsP6), a strong allosteric effector of Hb. This leads to significant rightward shifts of the HbO2 dissociation curves with in vitro P50 (partial pressure of O2 at 50% Hb saturation), values increasing from 32.2 +/- 1.8 torr for control erythrocytes to 86 +/- 60 torr (pH 7.40; PCO2 40 torr at 37 degrees C; 1 torr = 1.333 X 10(2) Pa). The shape of the dissociation curve was still sigmoidal, although the Hill coefficient was decreased. The life span of InsP6-loaded erythrocytes equaled that of control erythrocytes. The long-term physiological effects of the InsP6-loaded erythrocytes on piglets were increased O2 release and reduced cardiac output. The reduced O2 affinity of the InsP6-loaded erythrocytes was still effective 20 days after transfusion in awake piglets. The electrolyte concentration appeared stable over the 5-day observation period except for a transient, but significant, hyperkalemia immediately after transfusion. The reductions in the O2 affinity of Hb reported here are large compared with previously reported values. Introduction of InsP6 into viable erythrocytes improves tissue oxygenation when, for any reason, normal blood flow is impaired.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apstein C. S., Dennis R. C., Briggs L., Vogel W. M., Frazer J., Valeri C. R. Effect of erythrocyte storage and oxyhemoglobin affinity changes on cardiac function. Am J Physiol. 1985 Apr;248(4 Pt 2):H508–H515. doi: 10.1152/ajpheart.1985.248.4.H508. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Edalji R., Benesch R. Reciprocal interaction of hemoglobin with oxygen and protons. The influence of allosteric polyanions. Biochemistry. 1977 Jun 14;16(12):2594–2597. doi: 10.1021/bi00631a003. [DOI] [PubMed] [Google Scholar]
- Bristow J. D., Metcalfe J., Krall M. A., Welch J. E., Black J. A., Dhindsa D. S. Reduction of blood oxygen affinity in dogs by infusion of glycolytic intermediates. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jul;43(1):102–106. doi: 10.1152/jappl.1977.43.1.102. [DOI] [PubMed] [Google Scholar]
- Cudd A., Nicolau C. Intracellular fate of liposome-encapsulated DNA in mouse liver. Analysis using electron microscope autoradiography and subcellular fractionation. Biochim Biophys Acta. 1985 Jun 30;845(3):477–491. doi: 10.1016/0167-4889(85)90214-9. [DOI] [PubMed] [Google Scholar]
- Dennis R. C., Vito L., Weisel R. D., Valeri C. R., Berger R. L., Hechtman H. B. Improved myocardial performance following high 2-3 diphosphoglycerate red cell transfusions. Surgery. 1975 Jun;77(6):741–747. [PubMed] [Google Scholar]
- Farber M. O., Sullivan T. Y., Fineberg N., Carlone S., Manfredi F. Effect of decreased O2 affinity of hemoglobin on work performance during exercise in healthy humans. J Lab Clin Med. 1984 Aug;104(2):166–175. [PubMed] [Google Scholar]
- Franco R. S., Barker R., Novick S., Weiner M., Martelo O. J. Effect of inositol hexaphosphate on the transient behavior of red cells following a DMSO-induced osmotic pulse. J Cell Physiol. 1986 Nov;129(2):221–229. doi: 10.1002/jcp.1041290214. [DOI] [PubMed] [Google Scholar]
- Gersonde K., Nicolau C. Improvement of the red blood cell O2 release capacity by lipid vesicle-mediated incorporation of inositol hexaphosphate. Blut. 1979 Jul;39(1):1–7. doi: 10.1007/BF01008069. [DOI] [PubMed] [Google Scholar]
- Gross G. J., Warltier D. C., Hardman H. F. Effect of ortho-iodo sodium benzoate on hemoglobin-oxygen affinity in normal and ischemic myocardium. J Pharmacol Exp Ther. 1977 Oct;203(1):72–81. [PubMed] [Google Scholar]
- Laver M. B., Jackson E., Scherperel M., Tung C., Tung W., Radford E. P. Hemoglobin-O2 affinity regulation: DPG, monovalent anions, and hemoglobin concentration. J Appl Physiol Respir Environ Exerc Physiol. 1977 Oct;43(4):632–642. doi: 10.1152/jappl.1977.43.4.632. [DOI] [PubMed] [Google Scholar]
- Lenfant C., Torrance J., English E., Finch C. A., Reynafarje C., Ramos J., Faura J. Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J Clin Invest. 1968 Dec;47(12):2652–2656. doi: 10.1172/JCI105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. G., Brewer G. J. Beneficial effect of rightward hemoglobin-oxygen dissociation curve shift for short-term high-altitude adaptation. J Lab Clin Med. 1981 Jul;98(1):145–154. [PubMed] [Google Scholar]
- Stücker O., Vicaut E., Villereal M. C., Ropars C., Teisseire B. P., Duvelleroy M. A. Coronary response to large decreases of hemoglobin-O2 affinity in isolated rat heart. Am J Physiol. 1985 Dec;249(6 Pt 2):H1224–H1227. doi: 10.1152/ajpheart.1985.249.6.H1224. [DOI] [PubMed] [Google Scholar]
- Teisseire B. P., Ropars C., Vallez M. O., Herigault R. A., Nicolau C. Physiological effects of high-P50 erythrocyte transfusion on piglets. J Appl Physiol (1985) 1985 Jun;58(6):1810–1817. doi: 10.1152/jappl.1985.58.6.1810. [DOI] [PubMed] [Google Scholar]
- Teisseire B. P., Soulard C. D., Hérigault R. A., Leclerc L. F., Laver M. B. Effects of chronic changes in hemoglobin-O2 affinity in rats. J Appl Physiol Respir Environ Exerc Physiol. 1979 Apr;46(4):816–822. doi: 10.1152/jappl.1979.46.4.816. [DOI] [PubMed] [Google Scholar]



