Abstract
Mutations in CLDN14, encoding tight junction protein claudin 14, cause profound deafness in mice and humans. We identified a Pakistani family, in which the affected individuals were homozygous for a known pathogenic mutation c.254 T>A resulting in p.V85D substitution in CLDN14; however, in contrast to the previously reported families with mutations in CLDN14, most of the affected individuals in this family exhibit only a severe hearing loss. In order to identify the contribution of CLDN14 to less than profound deafness, we screened for mutations of CLDN14 in 30 multiplex and 57 sporadic cases with moderately severe to severe hearing loss from Pakistan. We identified one other affected individual homozygous for p.V85D substitution. Comparison of audiometric data from all patients indicates that mutations in CLND14 cause varying degrees of hearing loss, which may be enhanced at high frequencies. This suggests that a modifier can reduce the severity of hearing loss associated with mutations of CLDN14. Our data indicates that mutations in CLDN14 should be explored when considering the etiology of less severe hearing loss.
Keywords: CLDN14, Claudin, Tight junctions, DFNB29, Hearing loss, Pakistan
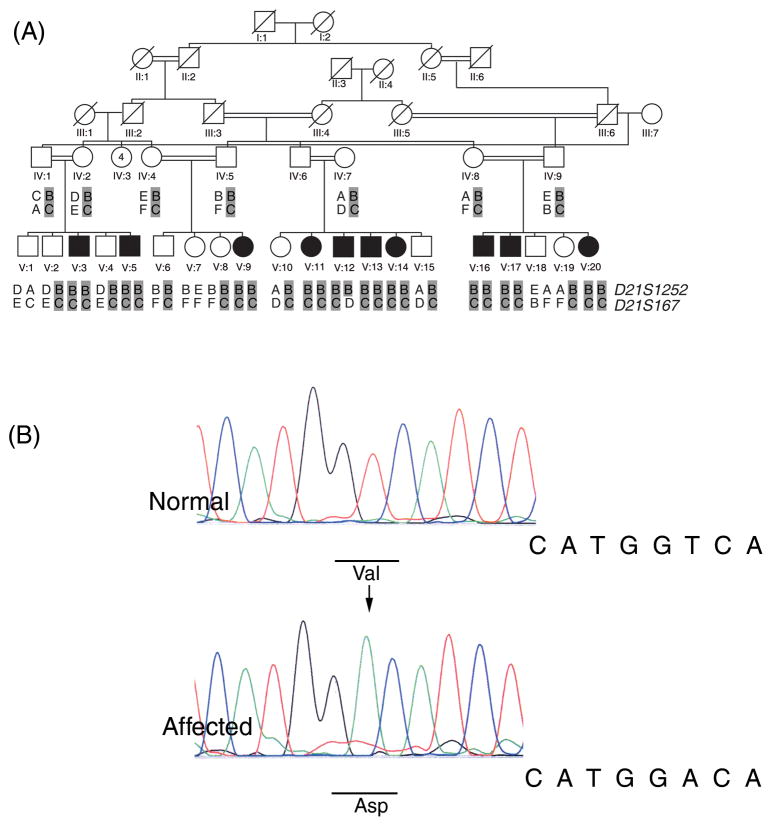
CLDN14 encodes a protein which participates in the formation of tight junctions in different epithelial cells including those of the cochlear sensory epithelia. Patients with mutations in CLDN14 and Cldn14 knockout mice are profoundly deaf.1, 2 In order to determine the etiology of less severe hearing loss in Pakistan, we recruited thirty consanguineous families with multiple affected individuals and fifty seven sporadic cases with moderate to severe hearing loss (50–90dB HL) with help of audiologists and schools for special children. After Institutional Review Board approval and written informed consent was acquired, DNA samples were obtained from blood samples of all participants. Linkage analyses for 45 of the known loci of recessively inherited hearing loss were performed for family HLRB5 by genotyping fluorescently labeled microsatellite markers including D21S1252 and D21S167 for DFNB29. All other families and 57 sporadic cases with hearing loss were similarly checked for linkage or homozygosity for markers flanking CLDN14. Only HLRB5 was consistent with linkage to CLDN14 (Figure 1a) while the single affected individual SA18 was homozygous for the genotyped markers. Sequencing revealed the same mutation c.254 T>A (p.V85D) in CLDN14 segregating in affected individuals in HLRB5 and SA18 (Figure 1b). Affected individuals in family HLRB5 and individual SA18 were homozygous for the same allele linked to the deafness phenotype for marker D21S1252 and also shared Single Nucleotide Polymorphisms (SNPs) in CLDN14 (Table 1), which indicates that this mutation probably arose on a common ancestral chromosome. However, carriers for this mutation are not frequent in the population, since we did not identify this variant in 100 control DNA samples from Sheikhupura (200 chromosomes).
Figure 1.
a) Pedigree with haplotype data for family HLRB5. Solid symbols denote affected individuals. Results of genotyping are shown for two microsatellite markers. The deafness associated haplotype is shaded in gray. Alleles for each marker are denoted by letters. Allele sizes in base pairs are: D21S1252; A, 249; B, 247; C, 245; D, 239; E, 237 D21S167; A, 162; B, 154; C, 152; D,146; E, 138; F, 132.
b) Sequence trace files for the c.254 T>A mutation observed in family HLRB5 and SA18 from a normal and affected sample. The mutation is indicated by an arrow in the trace from the affected individual. The normal and mutated codons are underlined in the respective traces.
Table 1.
Genotypic data for DFNB29 linked haplotypes
Genotypic data for short tandem repeat markers and single nucleotide polymorphisms in families HLRB5 and SA18 for DFNB29-linked haplotypes with c.254 T>A mutation in CLDN14.
| Position1 | Marker | Variation | Allele HLRB5 | Allele SA18 |
|---|---|---|---|---|
| 37826859 | D21S1252 | (AC)n | 247 | 247 |
| 37826859 | c.254 T>A | A | A | |
| 37834641 | rs219778 | T>C | T | T |
| 37834704 | rs2068750 | G>A | G | G |
| 37834721 | rs219777 | C>T | C | C |
| 37834835 | rs219776 | G>A | A | A |
| 37834914 | rs2835363 | T>A | A | A |
| 37834944 | rs219775 | C>T | T | T |
| 37835043 | rs219774 | G>A | G | G |
| 37835164 | rs60419768 | C>T | C | C |
| 37835185 | rs34627708 | InsC | - | - |
| 37835241 | rs72477120 | InsAA | - | - |
| 37835333 | rs219773 | C>T | C | C |
| 37835347 | rs219772 | T>A | T | T |
| 37835501 | rs219771 | G>A | G | G |
| 37835648 | rs219770 | T>C | T | T |
| 37835675 | rs219769 | G>T | G | G |
| 38195766 | D21S167 | (GT)n | 152 | 158 |
Nucleotide positions on chromosome 21 from UCSC February 2009, NCBI 37/hg19 assembly.
“Ins”=Insertion “-” = Deletion
Recently, inheritance of a synonymous SNP in CLDN14 was reported to be associated with kidney stones and low bone mineral density.3 Therefore, we examined three members of family HLRB5 for kidney stones. However, Renal ultrasounds failed to detect kidney stones in two affected individuals V:9, V:11 who were homozygous for the mutation and one normal individual V:7, heterozygous for the c.254 T>A mutation.
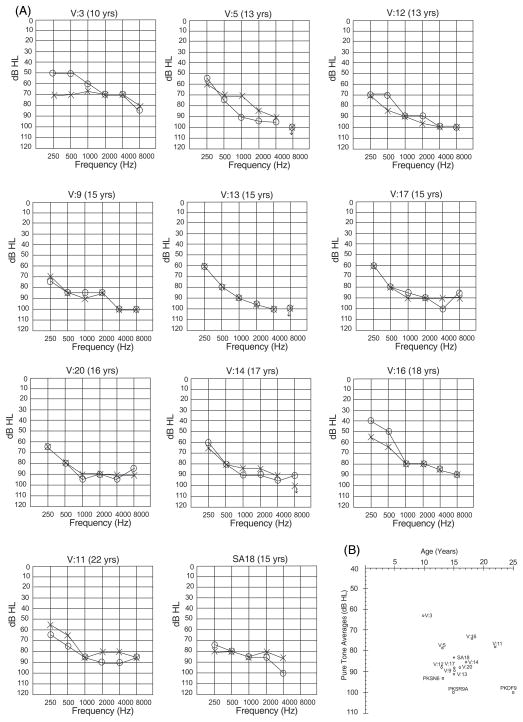
Pure-tone air conduction averages (PTA) for hearing thresholds at 500, 1000, 2000 and 4000 Hz were calculated to compare the severity of deafness of all affected individuals. Hearing loss thresholds were classified as normal 0–25dB HL, mild 26–40dB HL, moderate 41–55dB HL, moderately severe 56–70dB HL, severe 71–90dB HL and profound >90dB HL.4 Ten individuals (ages 10–22yrs) in family HLRB5 are hearing impaired. According to parents and self reports, hearing loss was not progressive. The audiometric data for all affected individuals in family HLRB5 gathered two years apart suggests that there is no deterioration of hearing. However, hearing loss may progress slowly and additional time is needed to conclusively establish stability of hearing thresholds.
The best hearing among the affected individuals in family HLRB5 is of a 10 year old child, V:3, PTA 63dB HL (moderately severe hearing loss), while the worst is of a 15 year old child, V:13, PTA 91dB HL (profound deafness) (Figure 2). All other affected individuals in family HLRB5 and SA18 have a severe hearing loss, PTA 74–88dB HL (Figure 2). The previously reported families with p.V85D mutation have profound deafness (PTA 93–100). Thus the hearing loss associated with mutations in CLDN14 can range from moderately severe to profound deafness.
Figure 2.
a) Audiograms for all affected family members of family HLRB5 and SA18. Age at time of first audiometry is indicated on top of each audiogram. “o” indicates air conduction for right ear, while “x” indicates air conduction for left ear
b) Average thresholds at pure tones (500, 1000, 2000 and 4000 Hz) for the better hearing ears in family HLRB5 (circles), individual SA18 (triangle) and previously published families (squares) with mutations in CLDN14. The degree of hearing loss is different across and within families with mutations in CLDN14. All families have the same mutation, except for family PKSN6.
Variations can also be seen at different frequencies when comparing audiometric data from all families with CLDN14 mutations. At low frequencies (250 and 500 Hz) the hearing loss ranges from moderate to severe. At conversational frequencies (500, 1000, 2000 Hz) hearing loss ranges from moderately severe to profound, while at high frequencies it ranges from severe to profound. However, for family HLRB5, the loss at high frequencies is still less than that reported earlier, with 7 out of 10 individuals responding to these tones while none of the patients in the previously published reports responded to the 8000 Hz frequency.5
This study shows that individuals with mutations of CLDN14 may have different degrees of hearing loss and the loss is greater at high frequencies. Some preservation of low frequency hearing with a greater degree of high frequency hearing loss may indicate involvement of CLDN14 in deafness, a finding similar to the hearing loss associated with mutant alleles of GJB2.6 Although CLDN14 is not a contributor to profound deafness in Turkey, Tunis and Spain,7–9 it is possible that some cases of less severe hearing loss in these and other countries could be attributed to CLDN14 mutations.
p.V85D is probably a null mutation as functional assays show that its ectopic expression in fibroblast cell lines results in mislocalization of this mutant CLDN14 and its absence from plasma membranes in contrast to the wild type protein.10 These data suggest that in patients with p.V85D mutation, the dysfunction is due to loss of CLDN14 from tight junctions. It is known that CLDN14 is absent from tight junctions in Cldn14 knockout mice leading to progressive loss of hair cells.2 Mutations in CLDN14 resulting in hearing loss may similarly cause hair cell loss in humans. However, the different degree of hearing loss in humans with CLDN14 mutations indicates that the extent of hair cell loss may vary. In Cldn14−/− mice, variation in hair cell loss is seen with the base of the cochlea affected earlier than the apex. This may also be the case in humans. Since the apex of the cochlea responds to low frequency sounds, it may account for the residual hearing at the 4000 and 8000 Hz observed in the individuals reported in this study. Alternately, variations resulting in regulation of another inner ear expressed claudin may partially compensate for the loss of CLDN14.
Our research indicates that the same pathogenic mutations may not result in identical phenotypic severity and genetic or epigenetic factors may modify the clinical course of this genetic disorder. The search for modifier genes is difficult in humans11 though a few modifiers for deafness have been mapped or cloned using linkage analyses or candidate gene approaches12. In family HLRB5, one individual is profoundly deaf and other individuals exhibit moderately severe to severe hearing losses. A clear demarcation of phenotype is absent, making linkage analyses for mapping a modifier locus difficult. However, mice are more amenable to search for modifiers of different phenotypes and many genes which modify effects of deafness causing genes have been identified12. Therefore transferring Cldn14 null allele to different genetic backgrounds and isolating the most and least affected strains may help in identification of modifying variants in one or more causative genes.
Acknowledgments
We thank the families for participating in the study. We are grateful to Mr. Khalil Hashmi for facilitating sample collection from patients. We thank Prof. M. Akram Dogar and Prof. Adul Lateef Mughal for sample collection from unaffected control samples from Sheikhupura and Dr. Andrew Griffith for his expert opinion. We are indebted to Dr. Thomas B. Friedman for his valuable comments on the manuscript. This research was supported by Grant Number R01TW007608 from the Fogarty International Center and National Institute of Deafness and other Communication Disorders, National Institutes of Health, USA.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest
References
- 1.Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–172. doi: 10.1016/s0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- 3.Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Gene. 2009;41:926–930. doi: 10.1038/ng.404. [DOI] [PubMed] [Google Scholar]
- 4.Katz J. Handbook of Clinical Audiology. Williams & Wilkins; 1994. [Google Scholar]
- 5.Ahmed ZM, Riazuddin S, Friedman TB, Riazuddin S, Wilcox ER, Griffith AJ. Clinical manifestations of DFNB29 deafness. Adv Otorhinolaryngol. 2002;61:156–160. doi: 10.1159/000066828. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox SA, Saunders K, Osborn AH, Arnold A, Wunderlich J, Kelly T, et al. High frequency hearing loss correlated with mutations in the GJB2 gene. Hum Genet. 2000;106:399–405. doi: 10.1007/s004390000273. [DOI] [PubMed] [Google Scholar]
- 7.Uyguner O, Emiroglu M, Uzumcu A, Hafiz G, Ghanbari A, Baserer N, et al. Frequencies of gap- and tight-junction mutations in Turkish families with autosomal-recessive non-syndromic hearing loss. Clin Genet. 2003;64:65–69. doi: 10.1034/j.1399-0004.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 8.Arican ST, Incesulu A, Inceoglu B, Tekin M. Alterations in the GJB3 and CLDN14 genes in families with nonsyndromic sensorineural hearing loss. Genet Couns. 2005;16:309–311. [PubMed] [Google Scholar]
- 9.Belguith H, Tlili A, Dhouib H, Ben Rebeh I, Lahmar I, Charfeddine I, et al. Mutation in gap and tight junctions in patients with non-syndromic hearing loss. Biochem Biophys Res Commun. 2009;385:1–5. doi: 10.1016/j.bbrc.2009.02.125. [DOI] [PubMed] [Google Scholar]
- 10.Wattenhofer M, Reymond A, Falciola V, Charollais A, Caille D, Borel C, et al. Different mechanisms preclude mutant CLDN14 proteins from forming tight junctions in vitro. Hum Mutat. 2005;25:543–549. doi: 10.1002/humu.20172. [DOI] [PubMed] [Google Scholar]
- 11.Génin E, Feingold J, Clerget-Darpoux F. Identifying modifier genes of monogenic disease: strategies and difficulties. Hum Genet. 2008;124:357–368. doi: 10.1007/s00439-008-0560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson KR, Zheng QY, Noben-Trauth K. Strain background effects and genetic modifiers of hearing in mice. Brain Res. 2006;1091:79–88. doi: 10.1016/j.brainres.2006.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]