Abstract
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease that can cause significant kidney disease. Our goal was to assess the relative mortality risk associated with SLE in pediatric and adult populations with end-stage renal disease (ESRD) maintained on hemodialysis (HD). We performed Kaplan–Meier survival analysis from data collected by the United States Renal Data System (USRDS) in strata of pediatric and adult patients. This file includes data on all Medicare-reimbursed renal replacement patients. Cox proportional hazard models were used to assess mortality after adjusting for race and gender. Subjects were censored at transplantation or at end of follow-up. Pediatric patients with ESRD secondary to SLE had a 2-fold increased risk of death compared with other pediatric patients with ESRD (hazard ratio [HR]: 2.4, 95% confidence interval [CI]: 1.5–3.7). Adult patients with ESRD secondary to SLE were also at increased risk of death compared with other adult patients (HR: 1.7, 95% CI: 1.2–2.7). The most common causes of death in both pediatric and adult patients with SLE were cardiovascular disease and cardiac arrest. Our study demonstrates that there is a significant increase in mortality secondary to cardiovascular disease in pediatric and adult patients with ESRD secondary to SLE. Patients with ESRD secondary to SLE may need aggressive monitoring for traditional risk factors for atherosclerosis and the diagnosis of SLE alone may be an independent risk factor for death in patients with ESRD.
Keywords: Systemic lupus erythematosus, Pediatrics, Dialysis, Mortality
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with varied clinical presentations. SLE accounts for up to 10% of patients with pediatric rheumatic diseases with an estimated prevalence of between 5,000 and 10,000 children in the United States [1]. In adults, the prevalence of SLE is estimated at 20 to 150 cases per 100,000, with the prevalence in African-American women 2.5- to 3-fold higher than in Caucasian women [2, 3].
Up to 60% of adults and 80% of pediatric patients with SLE will have kidney involvement at some point in their disease course [1, 4]. Clinically, kidney involvement in SLE may vary from mild hematuria or proteinuria to acute or chronic kidney disease. Additionally, a number of patients will progress to end-stage renal disease (ESRD) requiring renal replacement therapy [5–7]. The renal pathology in these cases can have a broad range of activity, from minimal mesangial involvement (International Society of Nephrology and Renal Pathology Society [ISNRPS] class I) to diffuse proliferative glomerulonephritis (ISNRPS class IV) and advanced sclerosing lupus nephritis (ISNRPS class VI) [8].
In order to evaluate outcomes in patients with ESRD secondary to SLE, we utilized a large national database to define hazard ratios for mortality in individuals with ESRD due to SLE compared with other causes of ESRD. Our goal was to compare differences in mortality among three groups:
Pediatric patients with ESRD secondary to SLE vs pediatric patients with other causes of ESRD
Adult patients with ESRD secondary to SLE vs adults with ESRD secondary to other causes
Pediatric vs adult patients with ESRD secondary to SLE
Materials and methods
We performed a retrospective longitudinal analysis using the 2006 dataset of patients from the USRDS standard analytic file obtained from the USRDS.
United States Renal Data System
The United States Renal Data System (USRDS), operated by the National Institute of Diabetes and Digestive and Kidney Diseases and the Health Care Financing Administration, collects data from patients in the United States who have received maintenance renal replacement. The database includes information on mortality and kidney transplantation in all ESRD patients in the United States. To be included in the database, patients must be receiving chronic dialysis therapy or have undergone renal transplantation. Patients are excluded if they receive dialysis for acute kidney injury only, die of kidney failure before receiving dialysis or renal transplantation, or do not accept renal replacement therapy [9].
Data analysis
Causes of ESRD were dichotomized into systemic lupus erythematosus (ICD 9 code: 710.0) vs all other diagnoses. Demographic and clinical characteristics were explored among adults and children in the USRDS dataset. According to the USRDS definition, pediatric patients were defined as age ≤ 18 years at first ESRD service.
Kaplan–Meier survival analyses were performed. The time at risk was calculated from 1 January 1990 through 31 December 2004, the last date of the USRDS data collection period in this dataset. Patients were censored at renal transplantation or at the end of follow-up.
Cox proportional hazard models were used to assess whether SLE as a cause of ESRD was associated with an increased risk of death. For the adjusted analysis, in order to have the most parsimonious model, only the significant predictors from the unadjusted analysis and clinically relevant variables were used in the analysis. The proportional hazards assumption was not violated for any variable over time (p values >0.05 by means of global test of Schoenfeld residuals). P values less than 0.05 were considered significant. Data were analyzed using STATA, version 9 (Stata Corporation, College Station, TX, USA).
Results
A total of 98,483 patients identified were maintained on hemodialysis (HD) between 1 January 1990 and 31 December 2004. There were 1,513 patients with ESRD secondary to SLE, 171 of whom were pediatric patients with an age at first ESRD dialysis service ≤ 18 years. Demographic characteristics are presented in Table 1. Pediatric patients with SLE were older than other pediatric patients with ESRD maintained on HD; in contrast, adult patients with SLE were younger than other adult patients with ESRD (p<0.01). There was an increased percentage of female gender and black race in patients with SLE, both pediatric and adult populations, compared with other patients (p<0.01).
Table 1.
Demographic characteristics of the cohort
| SLE causing ESRD | Other causes of ESRD | |||
|---|---|---|---|---|
| Pediatric patients, n=171 |
Adult patients, n=1,342 |
Pediatric patients, n=3,276 |
Adult patients, n=93, 694 |
|
| Mean age at initiation of HD, years (SD) | 15.2 (2.1) | 39 (13.7) | 11.6 (5.2) | 58.5 (15.5) |
| Percentage black race | 66 | 55 | 35 | 38 |
| Percentage female | 79 | 83 | 44 | 47 |
| Years receiving HD, mean (SD) | 4.9 (5.6) | 8.6 (4.8) | 4.7 (6.1) | 7.7 (4.1) |
SLE, systemic lupus erythematosus; ESRD, end-stage renal disease; HD, hemodialysis; SD, standard deviation
Pediatric patients with ESRD secondary to SLE were older with an increased female and black race predominance compared with other pediatric patients (p<0.01)
Adults with ESRD secondary to SLE were younger with an increased female and black race percentage compared with other adults (p<0.01)
There were also significantly more black race patients in the pediatric SLE population than in adults with SLE (p<0.01)
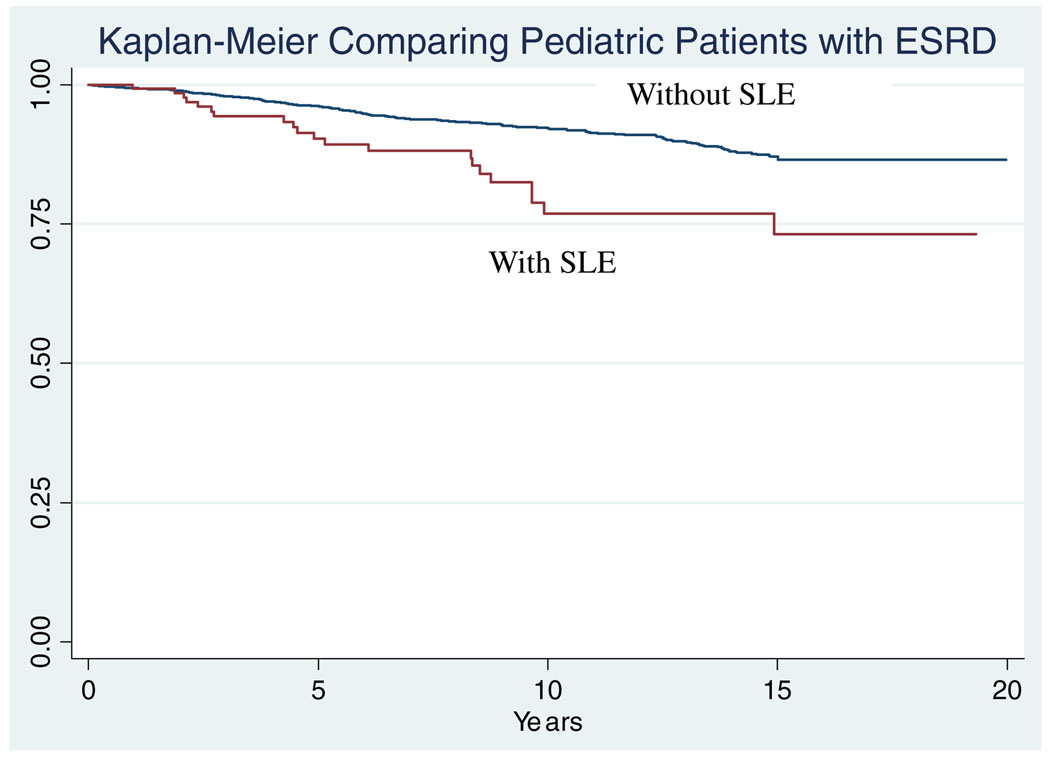
There were a total of 29 deaths in the pediatric patients with SLE and 316 deaths in pediatric patients with other causes of ESRD. Pediatric patients with SLE were older at death compared with others (24.3 years in SLE vs 21.6 years in others, p<0.001). The Kaplan–Meier curve is shown in Fig. 1. Pediatric patients with SLE had a 2-fold increased risk of death compared with other pediatric patients (hazard ratio [HR]: 2.4, 95% confidence interval [CI]: 1.5–3.7). Even after adjusting for race, gender, and age at death, pediatric patients with SLE were at increased risk of death (HR: 3.1, 95% CI: 1.8–5.1) compared with other patients their age.
Fig. 1.
Years at risk are noted on the x-axis. Pediatric patients with end-stage renal disease (ESRD) secondary to systemic lupus erythematosus (SLE) died earlier than pediatric patients with ESRD from other causes
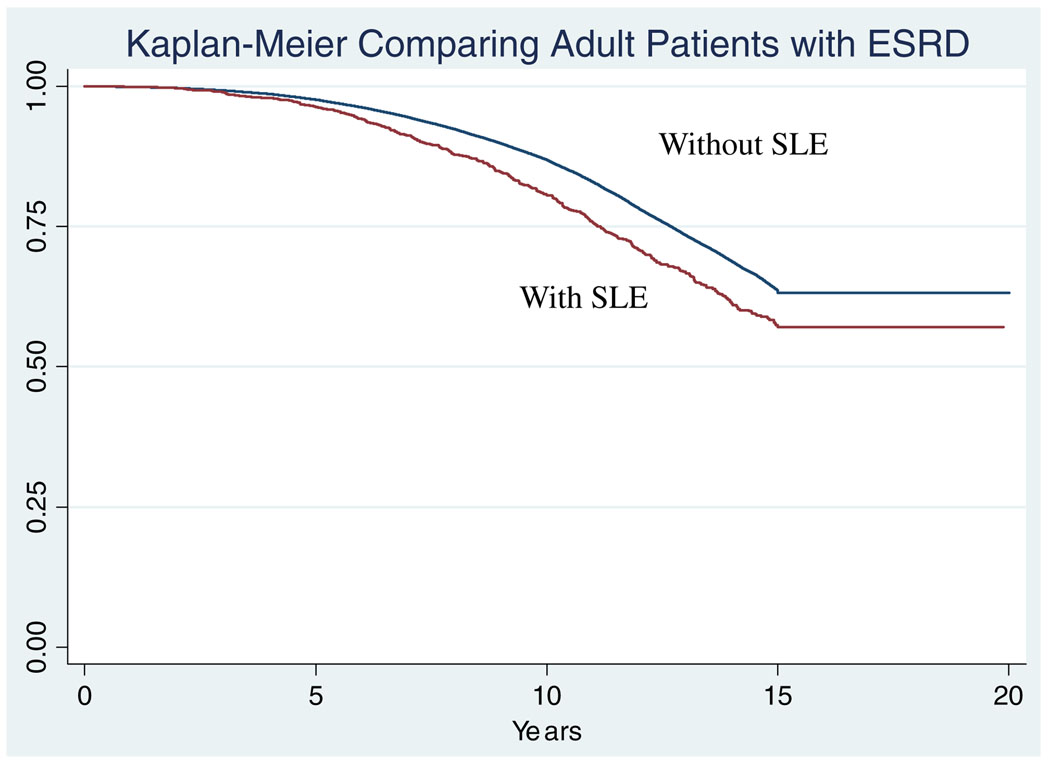
There were a total of 559 deaths in adult patients with ESRD secondary to SLE, and 58,336 deaths in adults with ESRD secondary to other causes. Adult patients with SLE were significantly younger at death than other adults maintained on HD (49.4 years in SLE patients vs 66.1 years in others, p<0.001). The Kaplan–Meier curve comparing these populations is shown in Fig. 2. Adult patients with SLE were at increased risk of death compared with other adult patients in the USRDS database (HR: 1.7, 95% CI: 1.2–2.7). After adjusting for age at death and gender, adult patients with SLE maintained an increased risk of death (HR 1.7, 95% CI: 1.1–2.6)
Fig. 2.
More adult patients with SLE died in follow-up than other adult patients with ESRD
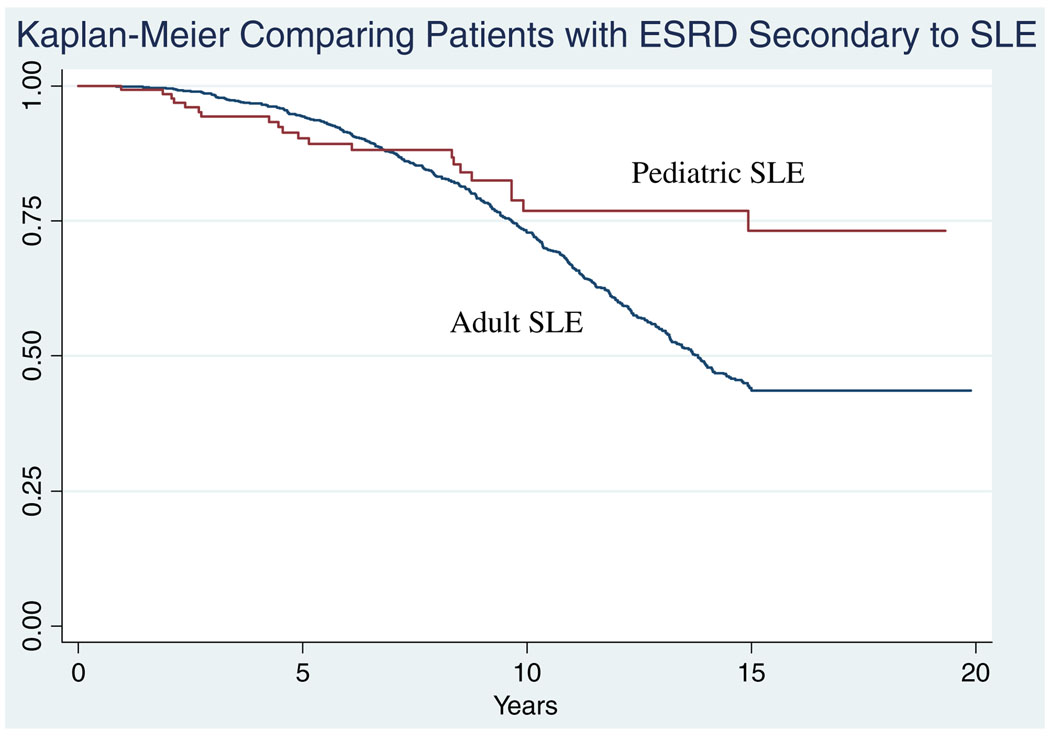
The Kaplan–Meier curve comparing pediatric and adult patients with ESRD secondary to SLE is shown in Fig. 3. There was no significant difference in the hazard ratio between these groups (HR: 1.09 95% CI: 0.62–1.94).
Fig. 3.
There was no difference between the Kaplan–Meier curves of adult and pediatric patients with ESRD secondary to SLE
For this analysis, we censored patients at the time of renal transplantation. This was done because previous study has noted that patients with SLE are less likely to receive renal transplantation than other patients with ESRD, perhaps leading to disparate groups for comparison [10]. In a separate analysis of those patients who did receive renal transplantation, the trend of the hazard ratios remained the same, with increased mortality in the pediatric patients with SLE (HR: 2.11, 95% CI: 1.07–4.16), as well as for adults with SLE (HR: 1.73, 95% CI: 0.59–5.11). Even when comparing these potentially uneven groups, the trend toward increased mortality in the patients with SLE remains.
Only the top five reasons for death were noted in the USRDS dataset. The most common causes of death in pediatric patients with ESRD secondary to SLE were cardiovascular disease and cardiac arrest (75%), followed by septicemia/infections (25%). In pediatric patients with other causes of ESRD, the five most common causes of death were cardiovascular disease (26%), infections (12%), other (10%), diabetes mellitus (7%), and disorders of the nervous system (5%). In adult patients with ESRD secondary to SLE the leading causes of death were cardiovascular disease (30%), infections (20%), of unknown etiology (15%), diabetes mellitus (12%), and lung disease (7%). In adults with other causes of ESRD, the most common reasons for death were cardiovascular disease (25%), other etiologies (12%), infections (8%), malignant neoplasms (7%), and coagulation disorders (6%).
Discussion
In this study, we found that pediatric patients who initiated HD when ≤ 18 years of age with ESRD secondary to SLE are at an almost 2-fold higher risk of death compared with pediatric patients with ESRD secondary to other causes. Strikingly, there was no significant difference in the risk of death between pediatric and adult patients with SLE, indicating that the pediatric population with SLE is at significant risk. In both pediatric and adult patients with SLE, the vast majority of the deaths were secondary to cardiovascular disease.
In adults with chronic inflammatory rheumatic conditions such as SLE and rheumatoid arthritis, numerous reports have shown an increased incidence of cardiovascular events compared with age-matched normal controls [11, 12]. In women with SLE aged 35–55 years, the incidence of myocardial infarction was 50 times higher than for normal controls. This increased risk of cardiovascular events is not explained by traditional risk factors such as hypertension, diabetes, or smoking [13].
In a series of 31 children with SLE, 16% of them had abnormalities in cardiac perfusion on thallium perfusion scans [14]. In a cohort of 221 pediatric patients with SLE, proteinuria was associated with increased carotid wall intima media thickness [15]. Some studies have shown increased markers for subclinical atherosclerosis in young adults with SLE and it is hypothesized that the atherosclerotic process begins earlier in children with chronic inflammatory diseases [16].
Inflammation may play a pathological role in the development of cardiovascular disease. Serum markers of inflammation such as C-reactive protein and tumor necrosis factor-α have been shown to mediate the development of atherosclerosis [17–20]. The amount of inflammation in adult patients with SLE has been shown to correlate with increased carotid intima-media thickness, a surrogate of subclinical atherosclerosis [16, 21, 22]. In addition, increased disease activity and damage scores in adults with SLE correlate with atherosclerosis [23]. In the USRDS dataset, there is no information on disease activity indices or inflammatory markers; thus, we are not able to correlate the degree of inflammation with cardiovascular disease. However, an increased inflammatory state leading to atherosclerosis may account for the much younger age at death for the adult patients with ESRD secondary to SLE compared with other patients.
As regards more traditional risk factors, elevated triglyceride and very-low-density lipoprotein (VLDL) have been found in both pediatric and adult patients with SLE [24–26]. Additionally, increased low-density lipoprotein (LDL) oxidation has been found in pediatric patients with SLE [25]. In patients with ESRD secondary to other causes, dyslipidemia may also occur [27, 28]. Markedly increased triglyceride levels and small dense LDL particles have been reported in patients receiving hemodialysis. However, there is less of an association between LDL levels and the presence of cardiovascular disease in patients receiving long-term hemodialysis compared with the general population. Medications used to treat SLE, such as glucocorticoids, may also contribute to lipid dysregulation [24]. Hypertension, another traditional risk factor for atherosclerosis, has been seen in up to 60% of adults with SLE who are 10 years from their initial diagnosis [29].
Guidelines have been developed to help diagnosis and treat these traditional risk factors in patients with SLE [30]. These include counseling on regular exercise and diet to maintain a BMI <25 kg/m2, treating dyslipidemia with a goal LDL of < 100 mg/dl, checking blood pressure at each visit and between visits for those on corticosteroid therapy, tobacco cessation, and monitoring yearly fasting glucose. It is unclear if checking C-reactive protein as a marker for inflammatory cardiovascular risk in patients with SLE is helpful as it can be elevated in patients with active disease.
For patients with renal disease secondary to SLE, nephrotic range proteinuria has been associated with increased carotid intimal wall thickness in pediatric patients with SLE. Nephritis can also be associated with hypertension, which may be a contributor to atherosclerosis in some of these patients [4, 31].
This study is limited by the retrospective nature of the data. Detection of incident cases of ESRD in the USRDS is not complete because data from patients who die of ESRD before receiving renal replacement therapy is not included. This may dilute the hazard ratio as the sickest patients would not be included in the analysis. Pediatric patients with SLE were older than patients with other causes of ESRD at first entry into the USRDS database. This may be because SLE presents later in adolescence, usually after puberty, whereas other causes of ESRD in the pediatric population, such as urological disease, present in early childhood. This age difference may be why pediatric patients with SLE were older at death. Interestingly, even after adjusting for this difference in age, mortality was higher in pediatric patients with SLE suggesting that the disease itself, either through inflammation or mechanisms not yet known, may increase mortality.
Our study demonstrates that there is a significant increase in mortality, particularly secondary to cardiovascular disease, in pediatric and adult patients with ESRD secondary to SLE compared with other patients with ESRD. This is especially true for pediatric patients with SLE who began HD on or before age 18 years, where the risk of death was 2-fold higher than their peers. This suggests that pediatric and adult patients with ESRD secondary to SLE need careful monitoring and aggressive treatment for traditional risk factors for atherosclerosis such as hypertension and obesity. Additionally, they should also be evaluated for more non-traditional risk factors, which could result in an inflammatory state and contribute to cardiovascular disease. However, based on this study, it seems possible that the diagnosis of SLE alone may be an independent risk factor for death in patients with ESRD.
Contributor Information
Sangeeta Sule, Email: ssule@jhmi.edu, Johns Hopkins University, 200 North Wolfe Street, Suite 2126, Baltimore, MD 21205, USA.
Barbara Fivush, Johns Hopkins University, 200 North Wolfe Street, Suite 2126, Baltimore, MD 21205, USA.
Alicia Neu, Johns Hopkins University, 200 North Wolfe Street, Suite 2126, Baltimore, MD 21205, USA.
Susan Furth, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
References
- 1.Stichweh D, Arce E, Pascual V. Update on pediatric systemic lupus erythematosus. Curr Opin Rheumatol. 2004;16:577–587. doi: 10.1097/01.bor.0000137852.42270.0f. [DOI] [PubMed] [Google Scholar]
- 2.Jimenez S, Cervera R, Font J, Ingelmo M. The epidemiology of systemic lupus erythematosus. Clin Rev Allergy Immunol. 2003;25:3–12. doi: 10.1385/CRIAI:25:1:3. [DOI] [PubMed] [Google Scholar]
- 3.Workgroup National Arthritis Data. Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, Pillemer SR, Reveille JD, Stone JH. Workgroup National Arthritis Data (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the united states. I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 4.Bogdanovic R, Nikolic V, Pasic S, Dimitrijevic J, Lipkovska-Markovic J, Eric-Marinkovic J, Ognjanovic M, Minic A, Stajic N. Lupus nephritis in childhood: a review of 53 patients followed at a single center. Pediatr Nephrol. 2004;19:36–44. doi: 10.1007/s00467-003-1278-y. [DOI] [PubMed] [Google Scholar]
- 5.Elliot V, Cairns T, q, Cook HT. Evolution of lesions over 10 years in a patient with SLE: flowchart approach to the new International Society of Nephrology (ISN)/Renal Pathology Society (RPS) classification of lupus nephritis. Am J Kidney Dis. 2006;47:184–190. doi: 10.1053/j.ajkd.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 6.Hagelberg S, Lee Y, Bargman J, Mah G, Schneider R, Laskin C, Eddy A, Gladman D, Urowitz M, Hebert D, Silverman E. Longterm followup of childhood lupus nephritis. J Rheumatol. 2002;29:2635–2642. [PubMed] [Google Scholar]
- 7.Rood MJ, ten Cate R, van Suijlekom-Smit LW, den Ouden EJ, Ouwerkerk FE, Breedveld FC, Huizinga TW. Childhood-onset systemic lupus erythematosus: clinical presentation and prognosis in 31 patients. Scand J Rheumatol. 1999;28:222–226. doi: 10.1080/03009749950155580. [DOI] [PubMed] [Google Scholar]
- 8.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M International Society of Nephrology Working Group on the Classification of Lupus Nephritis, Renal Pathology Society Working Group on the Classification of Lupus Nephritis. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 9.US Renal Data System. USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2004. [Google Scholar]
- 10.Bartosh SM, Fine RN, Sullivan EK. Outcome after transplantation of young patients with systemic lupus erythematosus: a report of the North American Pediatric Renal Transplant Cooperative Study. Transplantation. 2001;72:973–978. doi: 10.1097/00007890-200109150-00047. [DOI] [PubMed] [Google Scholar]
- 11.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, D'Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 12.Van Doornum S, McColl G, Wicks IP. Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum. 2002;46:862–873. doi: 10.1002/art.10089. [DOI] [PubMed] [Google Scholar]
- 13.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Cote R, Grover SA, Fortin PR, Clarke AE, Senecal JL. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Gazarian M, Feldman BM, Benson LN, Gilday DL, Laxer RM, Silverman ED. Assessment of myocardial perfusion and function in childhood systemic lupus erythematosus. J Pediatr. 1998;132:109–116. doi: 10.1016/s0022-3476(98)70494-9. [DOI] [PubMed] [Google Scholar]
- 15.Schanberg LE, Sandborg C, Barnhart HX, Ardoin SP, Yow E, Evans GW, Mieszkalski KL, Ilowite NT, Eberhard A, Levy DM, Kimura Y, von Scheven E, Silverman E, Bowyer SL, Punaro L, Singer NG, Sherry DD, McCurdy D, Klein-Gitelman M, Wallace C, Silver R, Wagner-Weiner L, Higgins GC, Brunner HI, Jung L, Soep JB, Reed A Atherosclerosis Prevention in Pediatric Lupus Erythematosus Investigators. Premature atherosclerosis in pediatric systemic lupus erythematosus: risk factors for increased carotid intima-media thickness in the Atherosclerosis Prevention in Pediatric Lupus Erythematosus Cohort. Arthritis Rheum. 2009;60:1496–1507. doi: 10.1002/art.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manzi S, Selzer F, Sutton-Tyrrell K, Fitzgerald SG, Rairie JE, Tracy RP, Kuller LH. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 18.Wang TJ, Nam BH, Wilson PW, Wolf PA, Levy D, Polak JF, D'Agostino RB, O'Donnell CJ. Association of C-reactive protein with carotid atherosclerosis in men and women: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2002;22:1662–1667. doi: 10.1161/01.atv.0000034543.78801.69. [DOI] [PubMed] [Google Scholar]
- 19.Mangge H, Hubmann H, Pilz S, Schauenstein K, Renner W, Marz W. Beyond cholesterol-inflammatory cytokines, the key mediators in atherosclerosis. Clin Chem Lab Med. 2004;42:467–474. doi: 10.1515/CCLM.2004.081. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 21.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, Crow MK, Schwartz JE, Paget SA, Devereux RB, Salmon JE. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 22.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, Linton MF, Raggi P, Stein CM. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 23.Petri M, Perez-Gutthann S, Spence D, Hochberg MC. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med. 1992;93:513–519. doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 24.Ilowite NT, Samuel P, Ginzler E, Jacobson MS. Dyslipo-proteinemia in pediatric systemic lupus erythematosus. Arthritis Rheum. 1988;31:859–863. doi: 10.1002/art.1780310706. [DOI] [PubMed] [Google Scholar]
- 25.Frostegard J. Autoimmunity, oxidized LDL and cardiovascular disease. Autoimmun Rev. 2002;1:233–237. doi: 10.1016/s1568-9972(02)00059-9. [DOI] [PubMed] [Google Scholar]
- 26.Posadas-Romero C, Torres-Tamayo M, Zamora-Gonzalez J, Aguilar-Herrera BE, Posadas-Sanchez R, Cardoso-Saldana G, Ladron de Guevara G, Solis-Vallejo E, El Hafidi M. High insulin levels and increased low-density lipoprotein oxidizability in pediatric patients with systemic lupus erythematosus. Arthritis Rheum. 2004;50:160–165. doi: 10.1002/art.11472. [DOI] [PubMed] [Google Scholar]
- 27.Parekh RS, Zhang L, Fivush BA, Klag MJ. Incidence of atherosclerosis by race in the dialysis morbidity and mortality study: a sample of the US ESRD population. J Am Soc Nephrol. 2005;16:1420–1426. doi: 10.1681/ASN.2004080661. [DOI] [PubMed] [Google Scholar]
- 28.Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, Powe NR, Coresh J, Klag MJ. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int. 2008;74:1335–1342. doi: 10.1038/ki.2008.449. [DOI] [PubMed] [Google Scholar]
- 29.Fong KY, Thumboo J, Koh ET, Chng HH, Leong KH, Koh WH, Howe HS, Leong KP, Lim B, Koh DR, Ng SC, Feng PH, Boey ML. Systemic lupus erythematosus: initial manifestations and clinical features after 10 years of disease. Ann Acad Med Singapore. 1997;26:278–281. [PubMed] [Google Scholar]
- 30.Elliott JR, Manzi S. Cardiovascular risk assessment and treatment in systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2009;23:481–494. doi: 10.1016/j.berh.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Falaschi F, Ravelli A, Martignoni A, Migliavacca D, Sartori M, Pistorio A, Perani G, Martini A. Nephrotic-range proteinuria, the major risk factor for early atherosclerosis in juvenile-onset systemic lupus erythematosus. Arthritis Rheum. 2000;43:1405–1409. doi: 10.1002/1529-0131(200006)43:6<1405::AID-ANR26>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]