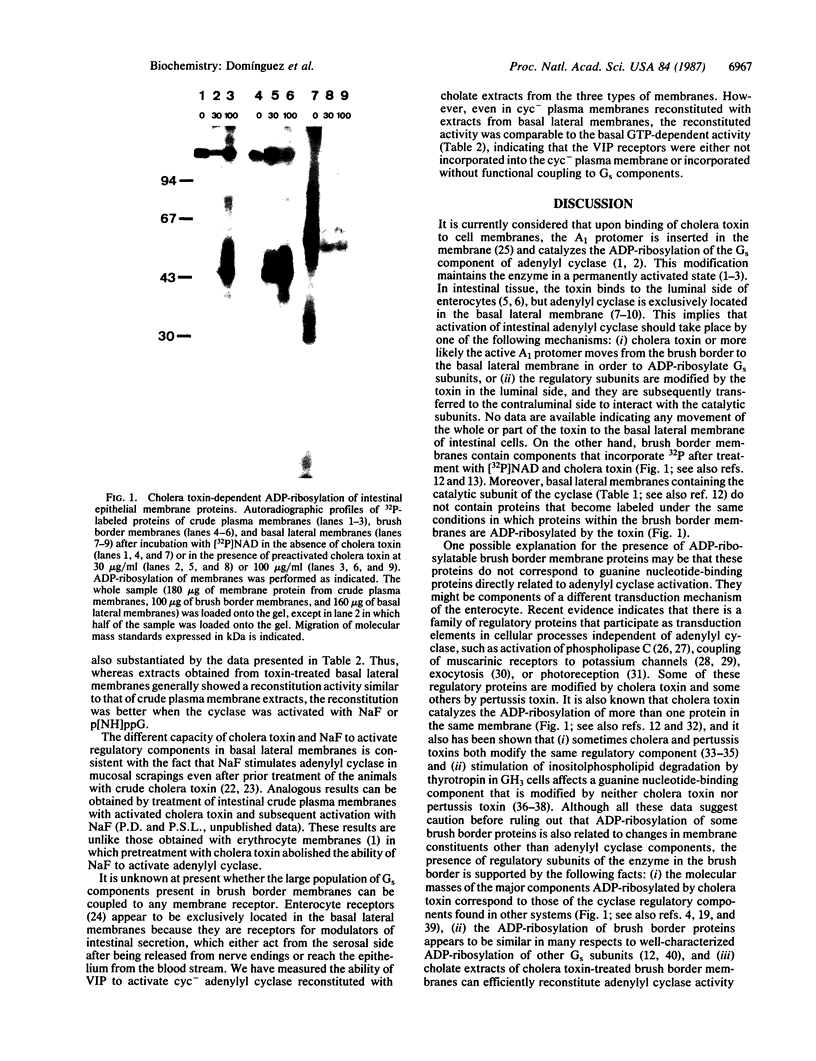
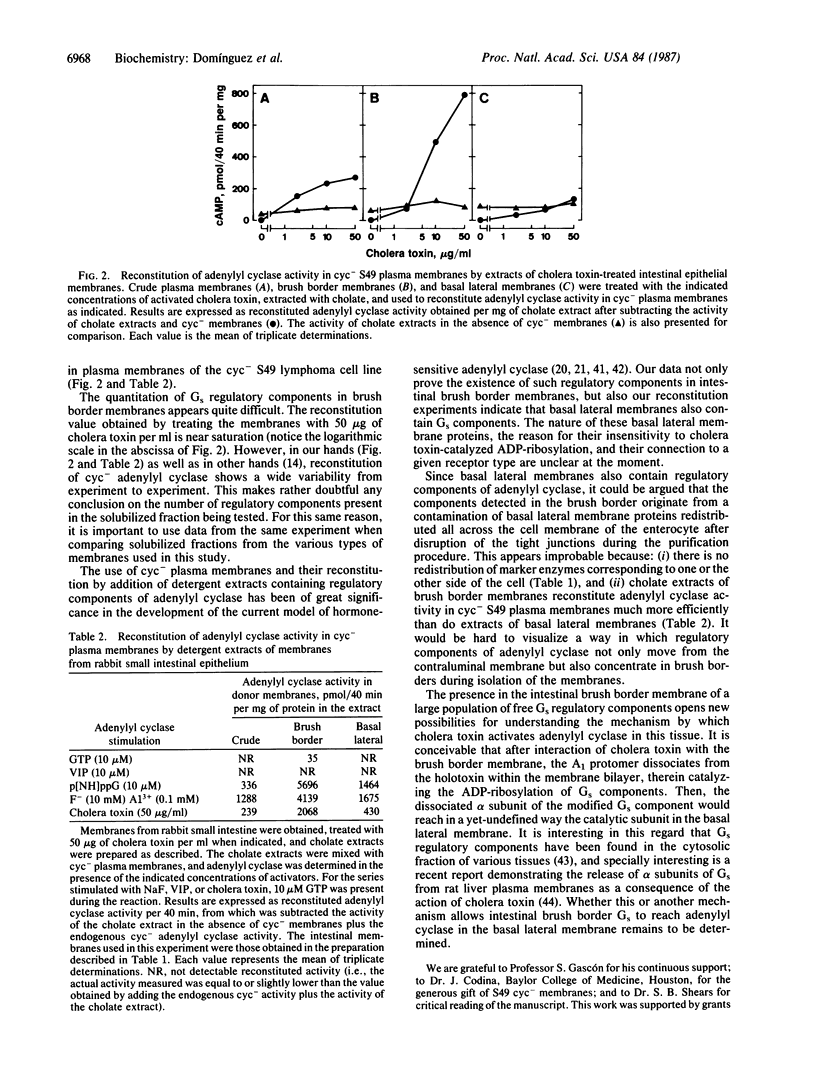
Abstract
Cholera toxin alters intestinal function by stimulation of adenylyl cyclase [ATP pyrophosphate-lyase (cyclizing) or adenylate cyclase, EC 4.6.1.1]. The mechanism of this activation is unknown and particularly puzzling because adenylyl cyclase is confined to the basal lateral membrane of enterocytes, whereas it is the brush border membrane that binds the toxin and contains proteins that undergo cholera toxin-catalyzed ADP ribosylation. It is shown that cholate extracts from cholera toxin-treated brush border membranes can efficiently reconstitute adenylyl cyclase activity in the guanine nucleotide-binding regulatory component (Gs)-deficient cyc- variant of the S49 mouse lymphoma cell line (cyc- cells lack the alpha subunit of Gs needed to activate the catalytic subunit of adenylyl cyclase). Moreover, NaF (in the presence of Al3+) and guanyl-5'-yl imidodiphosphate mediate strong activation of cyc- adenylyl cyclase provided the cholate extracts of brush border membranes are also present. Therefore, it appears that brush border membranes contain high levels of regulatory subunits of adenylyl cyclase in the absence of catalytic subunits. This represents a previously unrecognized feature of this transduction system that presumably plays an important role in the derangement of intestinal cell function by cholera toxin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abood M. E., Hurley J. B., Pappone M. C., Bourne H. R., Stryer L. Functional homology between signal-coupling proteins. Cholera toxin inactivates the GTPase activity of transducin. J Biol Chem. 1982 Sep 25;257(18):10540–10543. [PubMed] [Google Scholar]
- Aub D. L., Frey E. A., Sekura R. D., Cote T. E. Coupling of the thyrotropin-releasing hormone receptor to phospholipase C by a GTP-binding protein distinct from the inhibitory or stimulatory GTP-binding protein. J Biol Chem. 1986 Jul 15;261(20):9333–9340. [PubMed] [Google Scholar]
- Barros F., Domínguez P., Velasco G., Lazo P. S. Na+/H+ exchange is present in basolateral membranes from rabbit small intestine. Biochem Biophys Res Commun. 1986 Jan 29;134(2):827–834. doi: 10.1016/s0006-291x(86)80495-8. [DOI] [PubMed] [Google Scholar]
- Bennett N., Dupont Y. The G-protein of retinal rod outer segments (transducin). Mechanism of interaction with rhodopsin and nucleotides. J Biol Chem. 1985 Apr 10;260(7):4156–4168. [PubMed] [Google Scholar]
- Bhat M. K., Iyengar R., Abramowitz J., Bordelon-Riser M. E., Birnbaumer L. Naturally soluble component(s) that confer(s) guanine nucleotide and fluoride sensitivity to adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3836–3840. doi: 10.1073/pnas.77.7.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder H. J., Lemp G. F., Gardner J. D. Receptors for vasoactive intestinal peptide and secretin on small intestinal epithelial cells. Am J Physiol. 1980 Mar;238(3):G190–G196. doi: 10.1152/ajpgi.1980.238.3.G190. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Coffino P., Tomkins G. M. Selection of a variant lymphoma cell deficient in adenylate cyclase. Science. 1975 Feb 28;187(4178):750–752. doi: 10.1126/science.163487. [DOI] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Codina J., Hildebrandt J. D., Sekura R. D., Birnbaumer M., Bryan J., Manclark C. R., Iyengar R., Birnbaumer L. Ns and Ni, the stimulatory and inhibitory regulatory components of adenylyl cyclases. Purification of the human erythrocyte proteins without the use of activating regulatory ligands. J Biol Chem. 1984 May 10;259(9):5871–5886. [PubMed] [Google Scholar]
- Critchley D. R., Magnani J. L., Fishman P. H. Interaction of cholera toxin with rat intestinal brush border membranes. Relative roles of gangliosides and galactoproteins as toxin receptors. J Biol Chem. 1981 Aug 25;256(16):8724–8731. [PubMed] [Google Scholar]
- De Jonge H. R. The response of small intestinal villous and crypt epithelium to choleratoxin in rat and guinea pig. Evidence against a specific role of the crypt cells in choleragen-induced secretion. Biochim Biophys Acta. 1975 Jan 13;381(1):128–143. doi: 10.1016/0304-4165(75)90195-6. [DOI] [PubMed] [Google Scholar]
- Domínguez P., Barros F., Lazo P. S. The activation of adenylate cyclase from small intestinal epithelium by cholera toxin. Eur J Biochem. 1985 Feb 1;146(3):533–538. doi: 10.1111/j.1432-1033.1985.tb08684.x. [DOI] [PubMed] [Google Scholar]
- Enomoto K., Gill D. M. Cholera toxin activation of adenylate cyclase. Roles of nucleoside triphosphates and a macromolecular factor in the ADP ribosylation of the GTP-dependent regulatory component. J Biol Chem. 1980 Feb 25;255(4):1252–1258. [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazo P. S., Barros F., Domínguez P., Rivaya A., Velasco G. Adenylate cyclase from rabbit small intestine: activation by cholera toxin and interaction with calcium. Arch Biochem Biophys. 1985 Jun;239(2):587–594. doi: 10.1016/0003-9861(85)90728-3. [DOI] [PubMed] [Google Scholar]
- Litosch I., Wallis C., Fain J. N. 5-Hydroxytryptamine stimulates inositol phosphate production in a cell-free system from blowfly salivary glands. Evidence for a role of GTP in coupling receptor activation to phosphoinositide breakdown. J Biol Chem. 1985 May 10;260(9):5464–5471. [PubMed] [Google Scholar]
- Lynch C. J., Morbach L., Blackmore P. F., Exton J. H. Alpha-subunits of Ns are released from the plasma membrane following cholera toxin activation. FEBS Lett. 1986 May 12;200(2):333–336. doi: 10.1016/0014-5793(86)81163-2. [DOI] [PubMed] [Google Scholar]
- Martin T. F., Bajjalieh S. M., Lucas D. O., Kowalchyk J. A. Thyrotropin-releasing hormone stimulation of polyphosphoinositide hydrolysis in GH3 cell membranes is GTP dependent but insensitive to cholera or pertussis toxin. J Biol Chem. 1986 Aug 5;261(22):10141–10149. [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Lin M. C. NAD glycohydrolase and ADP-ribosyltransferase activities are intrinsic to the A1 peptide of choleragen. J Biol Chem. 1979 Dec 10;254(23):11993–11999. [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Murakami T., Yasuda H. Rat heart cell membranes contain three substrates for cholera toxin-catalyzed ADP-ribosylation and a single substrate for pertussis toxin-catalyzed ADP-ribosylation. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1355–1361. doi: 10.1016/s0006-291x(86)80432-6. [DOI] [PubMed] [Google Scholar]
- Murer H., Ammann E., Biber J., Hopfer U. The surface membrane of the small intestinal epithelial cell. I. Localization of adenyl cyclase. Biochim Biophys Acta. 1976 May 21;433(3):509–519. doi: 10.1016/0005-2736(76)90277-7. [DOI] [PubMed] [Google Scholar]
- Parkinson D. K., Ebel H., DiBona D. R., Sharp G. W. Localization of the action of cholera toxin on adenyl cyclase in mucosal epithelial cells of rabbit intestine. J Clin Invest. 1972 Sep;51(9):2292–2298. doi: 10.1172/JCI107039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Quill H., Weiser M. M. Adenylate and guanylate cyclase activities and cellular differentiation in rat small intestine. Gastroenterology. 1975 Aug;69(2):470–478. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Resolution of some components of adenylate cyclase necessary for catalytic activity. J Biol Chem. 1977 Oct 25;252(20):6966–6969. [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase. Reconstitution of the uncoupled variant of the S40 lymphoma cell. J Biol Chem. 1979 May 10;254(9):3333–3340. [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]
- Van Dop C., Yamanaka G., Steinberg F., Sekura R. D., Manclark C. R., Stryer L., Bourne H. R. ADP-ribosylation of transducin by pertussis toxin blocks the light-stimulated hydrolysis of GTP and cGMP in retinal photoreceptors. J Biol Chem. 1984 Jan 10;259(1):23–26. [PubMed] [Google Scholar]
- Verghese M., Uhing R. J., Snyderman R. A pertussis/choleratoxin-sensitive N protein may mediate chemoattractant receptor signal transduction. Biochem Biophys Res Commun. 1986 Jul 31;138(2):887–894. doi: 10.1016/s0006-291x(86)80579-4. [DOI] [PubMed] [Google Scholar]
- Walker W. A., Field M., Isselbacher K. J. Specific binding of cholera toxin to isolated intestinal microvillous membranes. Proc Natl Acad Sci U S A. 1974 Feb;71(2):320–324. doi: 10.1073/pnas.71.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling M. W., Mircheff A. K., Van Os C. H., Wright E. M. Subcellular distribution of nucleotide cyclases in rat intestinal epithelium. Am J Physiol. 1978 Nov;235(5):E539–E545. doi: 10.1152/ajpendo.1978.235.5.E539. [DOI] [PubMed] [Google Scholar]
- Wisnieski B. J., Bramhall J. S. Photolabelling of cholera toxin subunits during membrane penetration. Nature. 1981 Jan 22;289(5795):319–321. doi: 10.1038/289319a0. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R. J., Kent P. A., Fain J. N. Evidence that thyrotropin-releasing hormone-induced increases in GTPase activity and phosphoinositide metabolism in GH3 cells are mediated by a guanine nucleotide-binding protein other than Gs or Gi. Biochem Biophys Res Commun. 1986 Aug 14;138(3):1383–1389. doi: 10.1016/s0006-291x(86)80436-3. [DOI] [PubMed] [Google Scholar]