Abstract
Here we report that ALDH1L1 (FDH, a folate enzyme with tumor suppressor-like properties) inhibits cell motility. The underlying mechanism involves F-actin stabilization, re-distribution of cytoplasmic actin towards strong preponderance of filamentous actin, and formation of actin stress fibers. A549 cells expressing FDH demonstrated a much slower recovery of GFP-actin fluorescence in a FRAP assay, as well as an increase in G-actin polymerization and a decrease in F-actin depolymerization rates in pyren-actin fluorescence assays indicating the inhibition of actin dynamics. These effects were associated with robust dephosphorylation of the actin depolymerizing factor cofilin by PP1 and PP2A serine/threonine protein phosphatases but not the cofilin-specific phosphatases slingshot and chronophin. In fact, the PP1/PP2A inhibitor calyculin prevented cofilin dephosphorylation and restored motility. Inhibition of FDH-induced apoptosis by the JNK inhibitor SP600125 or the pan-caspase inhibitor zVAD-fmk did not restore motility or levels of phospho-cofilin, indicating that the observed effects are independent from FDH function in apoptosis. Interestingly, cofilin siRNA or expression of phosphorylation-deficient S3A cofilin mutant resulted in a decrease of G-actin and the actin stress fiber formation, the effects seen upon FDH expression. In contrast, the expression of S3D mutant, mimicking constitutive phosphorylation, prevented these effects further supporting the cofilin-dependent mechanism. Dephosphorylation of cofilin and inhibition of motility in response to FDH can be also prevented by the increased folate in media. Furthermore, folate depletion itself, in the absence of FDH, resulted in cofilin dephosphorylation and inhibition of motility in several cell lines. Our experiments showed that these effects were folate-specific and not a general response to nutrient starvation. Overall, this study demonstrates the presence of distinct intracellular signaling pathways regulating motility in response to folate status and points toward mechanisms involving folates in promoting a malignant phenotype.
Keywords: ALDH1L1, cofilin, phosphatases, actin, folate
Introduction
Enhanced motility of transformed cells causes tumor spreading and represents a hallmark of the malignant phenotype in advanced tumors (Hanahan and Weinberg, 2000). Transition to the migratory phenotype requires reorganization of the cytoskeleton, a complex network of actin microfilaments, microtubules and intermediate filaments (Ananthakrishnan and Ehrlicher, 2007). The leading role in this process is played by actin due to its unique dynamic properties, associated with two interchangeable protein forms, globular (G-actin) and filamentous (F-actin) (dos Remedios et al., 2003). Actin filaments are polar in their nature, with two distinct ends, pointed and barbed (Pantaloni et al., 2001). Polymerization of actin is essentially unidirectional taking place at the barbed ends (Winder and Ayscough, 2005). Rapid elongation of actin filaments, required for actin-based motility, strictly depends on the availability of globular actin monomers. To enable actin polymerization, the content of G-actin should exceed a critical concentration, that can be achieved by depolymerization of F-actin at the pointed end (Pantaloni et al., 2001). Association and dissociation of actin filaments are regulated by multiple actin-binding proteins (dos Remedios et al., 2003; Winder and Ayscough, 2005). One of these proteins, actin-depolymerizing factor cofilin, is the major calcium-independent regulator of actin dynamics (Wiggan et al., 2005). Cofilin is a small, 19 kDa, ubiquitous protein capable of binding both filamentous and globular actin, facilitating the turnover between the two forms (Yamaguchi and Condeelis, 2007). Its function is tightly regulated in the cell by several mechanisms (DesMarais et al., 2005; Oser and Condeelis, 2009; van Rheenen et al., 2009). Phosphorylation of cofilin at a single serine residue, Ser3, by LIMK/TESK kinases prevents its binding to actin and inhibits, while dephosphorylation restores, the actin-related activity (DesMarais et al., 2005; Wiggan et al., 2005; Yamaguchi and Condeelis, 2007).
Cell motility is regulated by a complex network of extracellular signals including essential nutrients (Jin et al., 2007; Merlot and Firtel, 2003). One of such nutrients is folate, a vitamin serving as a coenzyme for the fundamental metabolic reactions of one-carbon transfer (Wagner, 1995). Folate abundance is beneficial for proliferation of cancer cells, which require efficient folate pathways to support de novo nucleotide biosynthesis and methylation processes (Wagner, 1995). This is the basis for treatment of malignancies by antifolate drugs, which inhibit folate enzymes (Zhao and Goldman, 2003). Not much is known, however, about the role of folate in cell motility. An early study reported that in vegetative amoeba actin nucleation activity is stimulated by folate pointing toward an association between folate availability and motility (Hall et al., 1989). In higher organisms, disruption of the actin cytoskeleton reversibly increases the proportion of folate receptors on the cell surface and the rate of 5-methyltetrahydrofolate delivery (Lewis et al., 1998). Low extracellular folate status is also associated with altered expression of genes involved in cell adhesion, migration and invasion indicating a potential role for folate in these processes (Crott et al., 2004; Crott et al., 2008; Jhaveri et al., 2001). Furthermore, a study of cardiac development revealed dramatic alterations in components of the actin cytoskeleton network, including down-regulation of cofilin, in folate receptor knockout mice (Zhu et al., 2007). This later finding is in line with the proteomic study, which demonstrated down-regulation of cofilin in rats kept on folate-deficient diet (Chanson et al., 2005).
Intracellular folate status is defined by folate supplementation, its transport and enzymes of folate pathways. Impairment of any of these elements could result in cellular stress (Wagner, 1995; Zhao and Goldman, 2003; Zhao et al., 2009). In recent years we have demonstrated that one of the folate enzymes, ALDH1L1 (10-formyltetrahydrofolate dehydrogenase, also named FDH), possesses tumor suppressor-like properties: it is down regulated in malignancies and induces apoptosis upon expression in cancer cells (Krupenko and Oleinik, 2002; Oleinik and Krupenko, 2003). The enzyme converts 10-formyltetrahydrofolate to tetrahydrofolate and serves as an important regulator of intracellular folate pools (Oleinik et al., 2006). The mechanism of its suppressor effects is likely associated with the depletion of 10-formyltetrahydrofolate (Oleinik et al., 2005), which is an essential donor of formyl group in two reactions in the de novo purine pathway (Fox and Stover, 2008). Here we report that folate stress, induced by either FDH expression or folate depletion, inhibits migration and invasion of cancer cells by a mechanism associated with robust dephosphorylation of cofilin by two major cellular phosphatases, PP1 and PP2A, and alterations in actin cytoskeleton.
Results
FDH inhibits motile characteristics of the cell
We have examined effects of FDH on chemotactic migration and invasive potential in transwell migration and invasion assays, respectively. A549/Tet-On cells (Oleinik and Krupenko, 2003) capable of inducible FDH expression were tested in these experiments. This inducible system allows gradual expression of FDH (depending on the concentration of the inducer, doxycycline) which mimics physiologically relevant protein levels. After induction of FDH, cell migration across the fibronectin-coated membrane and invasive potential were both decreased by as much as 66% (Fig. 1a). Doxycycline is a known inhibitor of matrix metalloproteases (Franco et al., 2006) and as such could exert anti-migratory effects. In our experiments the concentrations of doxycycline were much lower than those reported to inhibit metalloproteases (Tyagi et al., 1996) and did not affect control A549 cells not capable of FDH expression (Supplement Fig. S1).
Figure 1.
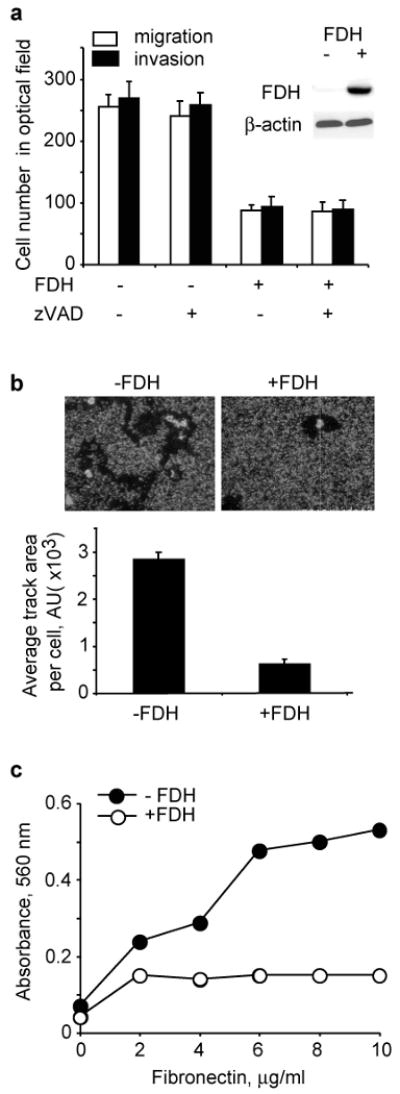
Changes of motile characteristics in A549 cells upon FDH expression. (a) Migration and invasion of FDH deficient and FDH-expressing cells (inset shows levels of FDH with actin as a loading control) in the absence or in the presence zVAD-fmk. (b) Migration track of a single cell in the absence (-) and in the presence (+) FDH (top panel); bottom panel shows average track length calculated with NIH Image Software. (c) Adhesion potential of FDH-expressing and FDH-deficient cells. Experiments were performed in triplicate; average ± SD is shown.
To confirm that the decrease in migration/invasion ability was not due to apoptosis, the experiments were performed in the presence of zVAD-fmk. We have previously shown that this caspase inhibitor protects cells from FDH-induced toxicity by inhibiting apoptosis (Oleinik and Krupenko, 2003). Our experiments demonstrated similar effects of FDH on cell motility in the presence and in the absence of zVAD-fmk (Fig. 1a), indicating that cell death does not account for the inhibition of migration caused by FDH expression. In a control experiment zVAD-fmk by itself did not affect migration/invasion (Supplement Fig. S1).
We also examined the influence of FDH on the ability of individual cells to form migration tracks through a field of fluorescent micro-spheres (Yujiri et al., 2000). As cells migrate, they engulf the spheres leaving a non-fluorescent track that can be quantified. These experiments demonstrated a clear difference in the track-forming ability between the cells expressing FDH and control A549 cells: the average migration area for an individual cell expressing FDH compared to FDH deficient A549 cells was reduced by about 80% (Fig. 1b).
Alteration of G/F-actin ratio and actin dynamics in response to FDH
G/F-actin ratio is an indicator of the extent of stabilization of actin fibers and focal adhesion of the cell (Turner et al., 2007). Cell adhesion assays have demonstrated that FDH weakens cellular ability to attach to fibronectin (Fig. 1c) indicating decreased adhesion potential. Confocal microscopy showed changes in actin cytoskeleton in FDH-expressing compared to FDH-deficient cells with the appearance of stress fibers and visible decrease in cytosolic G-actin content (Fig. 2a). To confirm this observation, we have separated actin into G and F fractions and evaluated their relative content (nucleus-associated actin was excluded from calculations). Examination of G-actin to F-actin ratio revealed a distinct shift towards prevalence of F-actin in FDH expressing cells compared with control FDH deficient cells (Fig. 2b). We also observed an overall decrease of cytoplasmic G-actin in FDH-expressing cells (Fig. 2b, inset). The levels of total actin were essentially the same in both types of cells (Fig. 2b, inset).
Figure 2.
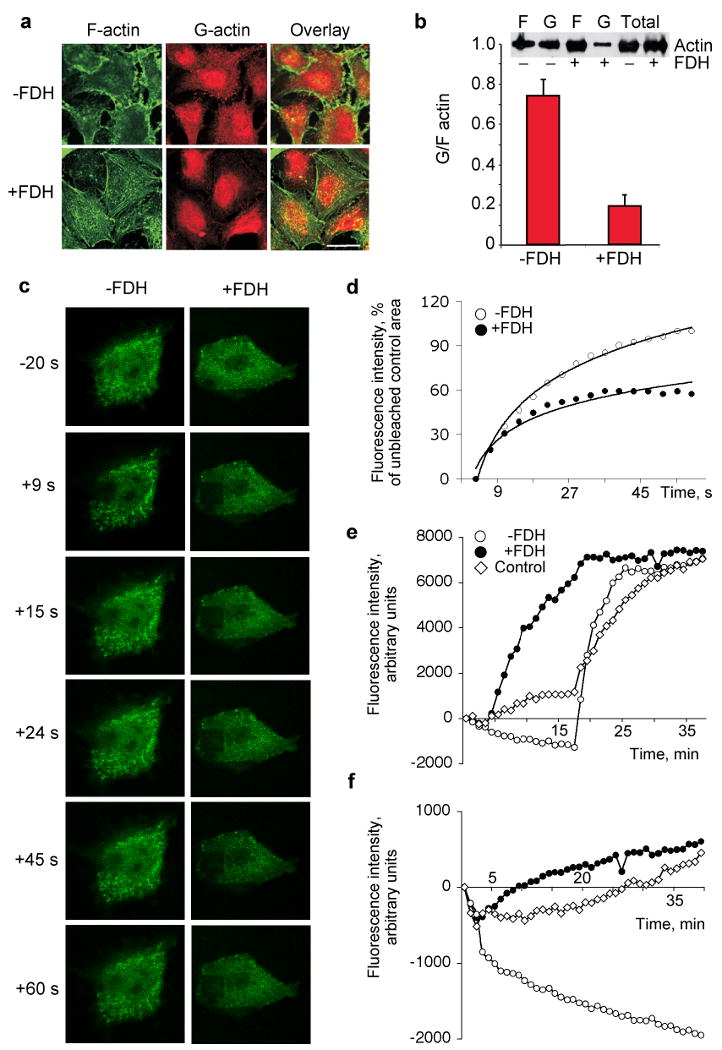
FDH induces shift in G/F actin ratio and inhibits actin dynamics. (a) Levels of F-actin (green) and G-actin (red) in FDH deficient (-) and FDH expressing (+) A549 cells imaged by confocal microscopy. Yellow indicates co-localization. Bar, 20 μm. (b) Bar graph, G/F actin ratio in cytosol of -FDH and +FDH cells (quantified from three Western blots as described in Materials and Methods). Inset shows a representative Western blot of F, G and total actin in FDH-expressing and FDH-deficient cells (c) FRAP analysis of actin treadmilling rate in A549 cells. Representative microphotographs show re-distribution of GFP-actin fusion after photobleaching in control FDH-deficient (-FDH) and FDH-expressing (+FDH) A549 cells. Time (seconds) after photobleaching is indicated. The first panel (-20 s) shows cells before photobleaching. (d) Quantification of FRAP data from (c) for FDH-deficient cells (-FDH, closed circles) and FDH-expressing cells (+FDH, open circles). The rate of fluorescence recovery was analyzed using Leica Confocal Software; average of nine experiments was calculated for each time-point. (e) Actin polymerization rate by A549 cell lysates with (closed circles) or without (open circles) FDH expression evaluated by the increase of fluorescence intensity of pyrene conjugated to G-actin. Control experiment (open diamonds): pyrene G-actin was incubated with lysis buffer. (f) Actin depolymerization rate by the same lysates as in (e) evaluated by the decrease of fluorescence intensity of the pre-formed pyrene F-actin.
Fluorescence Recovery After Photobleaching (FRAP) assays have demonstrated much faster recovery of the fluorescence in GFP-actin transfected cells in the absence of FDH (Fig. 2, c and d). Since this parameter is an indicator of actin treadmilling rate, our experiments show that actin dynamics is significantly inhibited in FDH-expressing cells compared to control FDH-deficient cells. The fluorescent pyren-actin polymerization assay has further revealed an increase in actin polymerization rates upon FDH expression (Fig. 2e). A similar type of assay demonstrated a significantly decreased ability of these cells to depolymerize F-actin (Fig. 2f). Based on these experiments we have concluded that FDH inhibits actin dynamics through suppression of filamentous actin depolymerization.
FDH induces robust dephosphorylation of cofilin
In eukaryotes, actin dynamics is regulated by actin-depolymerization factor cofilin (dos Remedios et al., 2003; Winder and Ayscough, 2005). This process depends on phosphorylation status of the protein (DesMarais et al., 2005). To address the potential role of cofilin in the effects of FDH on cell motility, we have examined the levels of total and phosphorylated cofilin in the presence and in the absence of FDH using immunoblot assays with specific antibodies. We observed that the total levels of cofilin were similar in FDH proficient and deficient cells (Fig. 3a). In contrast, levels of phosphorylated cofilin were dramatically decreased in FDH-proficient cells as compared to the FDH-deficient cells (Fig. 3a). The magnitude of this effect depended on FDH levels and was enhanced over time of FDH induction (Fig. 3a). Confocal microscopy has confirmed overall dephosphorylation of cofilin in response to FDH expression (Fig. 3b).
Figure 3.
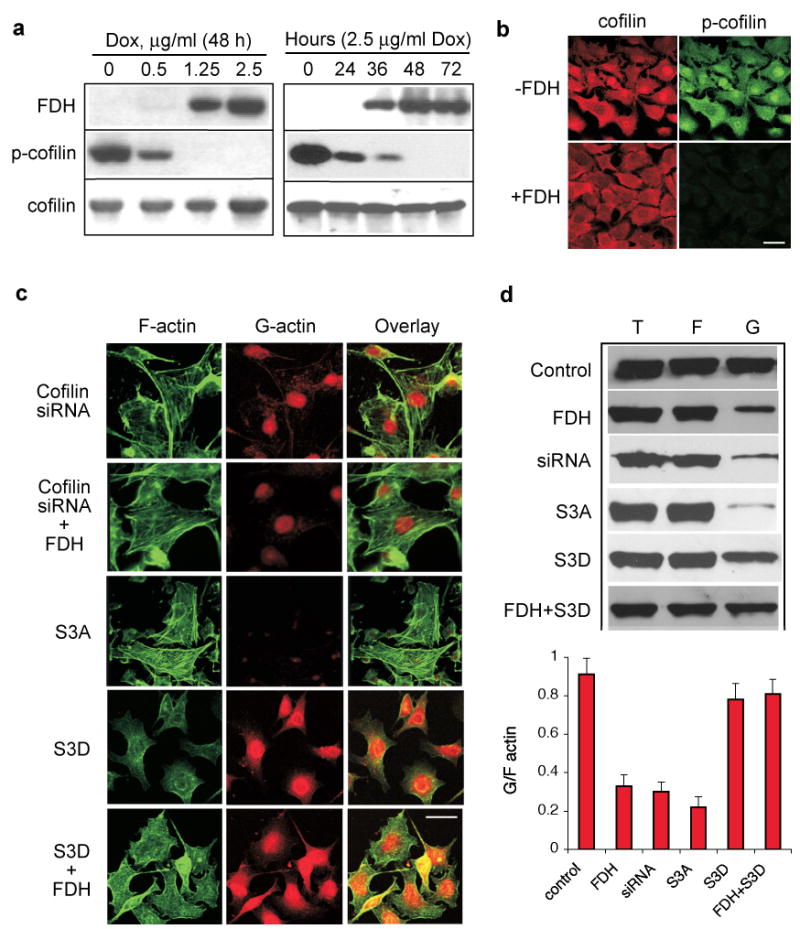
Decrease of phosphorylated cofilin upon FDH expression and effects of cofilin mutants/siRNA on G/F-actin and the FDH-induced phenotype. (a) Western blot analysis of cofilin and its phosphorylated form in A549/Tet-On cells induced for FDH expression with increasing concentrations of Dox (left panel) and at different time points after induction with 2.5 μg/ml of Dox (right panel). (b) Levels of cofilin (red) and its phosphorylated form (green) assessed by confocal microscopy in FDH deficient (-) and FDH expressing cells (+). Bar, 20 μm. (c) Confocal imaging of F-actin (green) and G-actin (red) in FDH-deficient or FDH-expressing (+FDH) A549/Tet-On cells with different cofilin status: S3A and S3D, cells transfected with corresponding mutant; siRNA, cells with knocked down cofilin. Yellow indicates co-localization. Bar, 20 μm. (d) Western blot analysis (top panel) of G-, F-, and the total actin in cells treated as indicated for panel (c); bar graph, ratio of G/F actin quantified from the the western blots using Quantity One software (Bio-Rad). Average (±SD) of two independent experiments is shown. Cells were analyzed 48 h or 72 h (siRNA) post-transfection. FDH induction was initiated 6 h after transfection.
Alterations in cofilin status result in the loss of cytosolic G-actin and stress fibers formation
To investigate whether FDH-independent alterations in the cofilin status have an effect on cellular phenotype with regard to actin, we have knocked down cofilin in A549 cells using a siRNA approach. A strong down-regulation of the protein was observed in these experiments between 24 and 120 h post-transfection of the cofilin-targeting siRNA duplex (Supplement Fig. S2). Simultaneously with the decrease in cofilin levels the decrease in G-actin content and formation of actin stress fibers were revealed (Fig. 4c and 4d). Similar changes were seen in the cells transiently expressing phosphorylation-deficient cofilin mutant, S3A (Fig. 4c and 4d; expression of the mutant is shown in Supplement Fig. S2). These experiments strongly indicate that the direct disruption of cofilin levels/phosphorylation produces effects on actin seen upon FDH induction. Not surprisingly, the effects produced by the combination of either cofilin knock down or the mutant expression with FDH induction were the same as effects seen upon a single insult (Fig. 4c and 4d).
Figure 4.
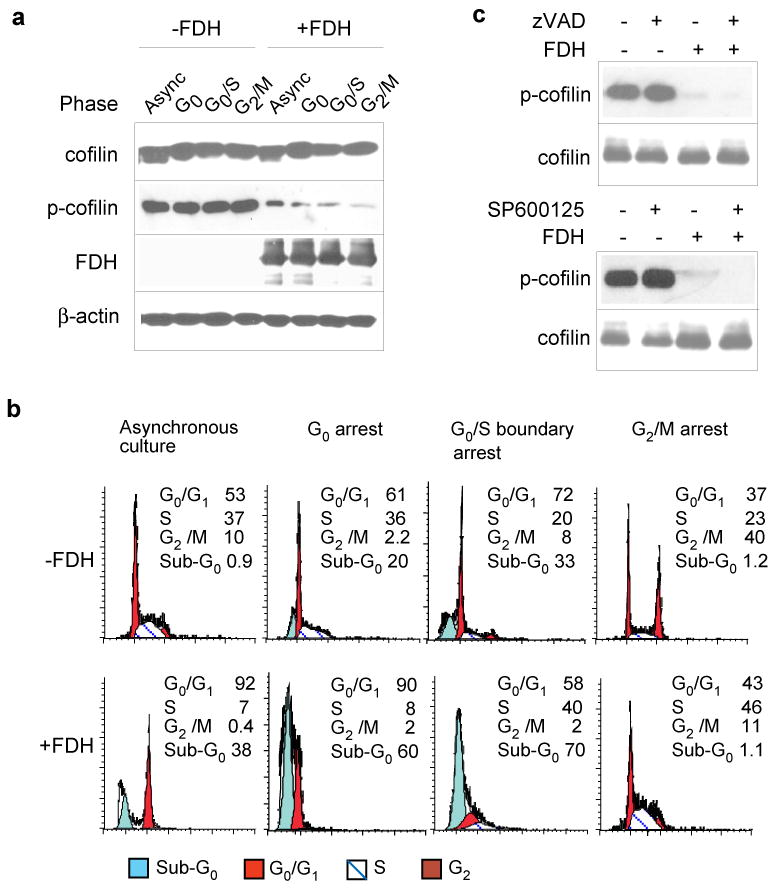
FDH-induced cofilin dephosphorylation is not a part of antiproliferative response. (a) Levels of cofilin and p-cofilin (Western blot) in A549/Tet-On cells synchronized at different phases of the cell cycle, in the absence or in the presence of FDH. FDH was induced by the addition of 2.5 μg/ml doxycycline 48 h prior to the cell collection. Actin is shown as a loading control. (b) FACS analysis of the above cells. Apoptotic cells (sub-G0) were excluded from calculation of the percentage of cells for different phases, shown in the histogram. Synchronization was achieved as described in the Supplemental Materials. (c) Western blot analysis of cofilin and p-cofilin in the cells induced for FDH expression (2.5 μg/ml of Dox for 48 h) and treated with either 50 μM of zVAD (top panel) or 10 μM of SP600125 (bottom panel).
The cofilin mutant mimicking constitutive phosphorylation reverses effects of FDH on actin
We have suggested that, if cofilin dephosphorylation is a mechanism by which FDH affects actin dynamics, the S3D cofilin mutant mimicking constitutive phosphorylation should reverse the phenotype seen in FDH-expressing cells. This was based on the assumption that the balance between phosphorylated and non-phosphorylated cofilin is a key to support simultaneously actin polymerization and depolymerization (Oser and Condeelis, 2009; van Rheenen et al., 2009). Transient transfection of A549 cells with corresponding plasmid resulted in an effective expression of the S3D mutant (Supplement Fig. S2). We observed that cells expressing this mutant did not form actin stress fibers upon FDH induction and did not lose G-actin (as can be judged by confocal microscopy and Western blot, Fig. 4c and 4d). Furthermore, transwell migration assays showed that the presence of S3D mutant restored motility of FDH-expressing cells (Supplement Fig. S2). Overall, these results indicated that S3D cofilin mutant indeed reverses the FDH-induced phenotype.
FDH-induced cofilin dephosphorylation is not a part of the antiproliferative response
FDH-induced antiproliferative effects include G1 arrest and apoptosis (Oleinik and Krupenko, 2003). Therefore, alterations in cofilin status could potentially be a part of these responses. To determine whether cofilin dephosphorylation could be caused by cell cycle arrest, we have evaluated the total cofilin and its phosphorylated form in synchronized cells in different phases of the cell cycle. We observed that the levels of total cofilin and phospho-cofilin remained constant regardless of the cell cycle phase, while upon FDH expression the levels of phospho-cofilin were drastically diminished and became undetectable without noticeable change of the total cofilin (Fig. 4a). These effects were uniform throughout the cell cycle suggesting that the FDH-dependent drop in phospho-cofilin content was not cell cycle specific (Fig. 4, a and b) and thus was not a result of FDH-induced G1 arrest.
Cofilin dephosphorylation was also not associated with induction of apoptosis: the decrease in phospho-cofilin levels was essentially independent of the proportion of apoptotic cells (Fig. 4b, bottom panel). Strong dephosphorylation of cofilin was seen even in the absence of the apoptotic sub-G0 peak (Fig. 4a and 4b, bottom far right panel). Furthermore, zVAD-fmk treatment, which effectively protects cells from FDH-induced apoptosis (Oleinik and Krupenko, 2003), did not prevent cofilin dephosphorylation (Fig. 4c). Another compound that protects against FDH-induced apoptosis (Oleinik et al., 2007), JNK inhibitor SP600125, also had no effect on the levels of total cofilin/phospho-cofilin upon FDH expression (Fig. 4c). The FDH-induced apoptosis in A549 cells proceeds through the p53 signaling pathway (Oleinik et al., 2005). In contrast, FDH-induced inhibition of motile characteristics does not depend on p53 status: A549 cells lacking p53 also revealed strongly decreased migration potential upon FDH expression (data not shown).
Effect of folate supplementation on cell motility
We have previously shown that high folate supplementation can partially compensate for antiproliferative and apoptotic effects of FDH (Krupenko and Oleinik, 2002). In a similar manner, high folate supplementation (10 μM leucovorin in the media) restored the levels of phosphorylated cofilin in FDH-expressing cells (Fig. 5a). This supplementation has also shifted G/F actin ratio in these cells towards the ratio observed in FDH-deficient cells and restored motility as well as invasion and adhesion potential (Fig. 5a). We have also evaluated effects of folate depletion on the same parameters in FDH-deficient cells (Fig. 5b and Supplement Fig. S3). We observed that the lack of media folate resulted in a strong decrease in the levels of phosphorylated cofilin, decreased G/F-actin ratio, and decreased motility/adhesion, the effects similar to those produced by FDH (Fig. 5b). Restoration of folate supplementation returned these parameters to the levels found in cells kept on normal folate regimen (Fig. 5b).
Figure 5.
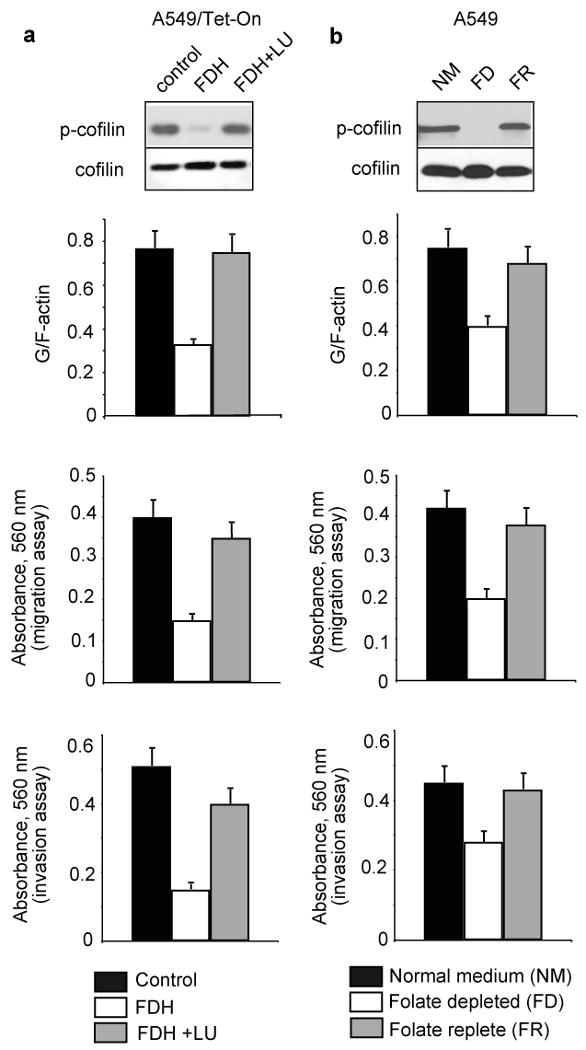
Folate supplementation controls cellular motility and related effects of FDH. (a) Reversal of FDH effects on motile characteristics by high folate supplementation (top panel, G/F actin ratio; middle panel, migration; bottom panel, invasion). Control, untreated cells/regular media; FDH, cells induced for FDH expression/regular media; FDH+LU, cells induced for FDH expression grown on regular media supplemented with 10 μM leucovorin. (b) G/F actin ratio (top panel), and migration (middle panel) and invasion (bottom panel) characteristics of A549 cells grown in normal folate (NM, control, 2.2 μM folic acid), folate-depleted (FD, folate free) and folate replete (FR, 2.2 μM folate added after folate depletion) medium. Insets show phosphorylated cofilin. In depletion experiments, prior to analysis cells were kept for 3 days in folate-free media supplemented with dialyzed FBS. In repletion experiments, cells were analyzed 24 h after the return to regular folate-containing media.
Cofilin is dephosphorylated by PP1 and PP2A in response to FDH
To study whether the decrease of phospho-cofilin is a result of activated dephosphorylation and not the lack of kinase activity, we have monitored phospho-cofilin levels in A549 cell lysates after mixing them with the lysates from FDH expressing cells. We observed rapid time-dependent dephosphorylation of cofilin upon addition of the FDH-containing lysate (with presumably activated cofilin phosphatases) (Fig. 6a). To identify the phosphatase responsible for cofilin dephosphorylation in response to FDH, we used pull down assays with a cofilin-specific antibody. Immunoblot analysis revealed PP1 and PP2A in the pull-down preparation, while slingshot or chronophin, two cofilin-specific phosphatases (Huang et al., 2006), were not detected (Fig. 6b). To confirm that PP1 and PP2A were responsible for cofilin dephosphorylation, we monitored phospho-cofilin dephosphorylation by the lysate from FDH-expressing cells in the presence of the specific PP1/PP2A inhibitor, calyculin, which does not inhibit slingshot or chronophin (Huang et al., 2006; Niwa et al., 2002). In agreement with the results of the pull-down experiments, calyculin blocked dephosphorylation of cofilin (Fig. 6a).
Figure 6.
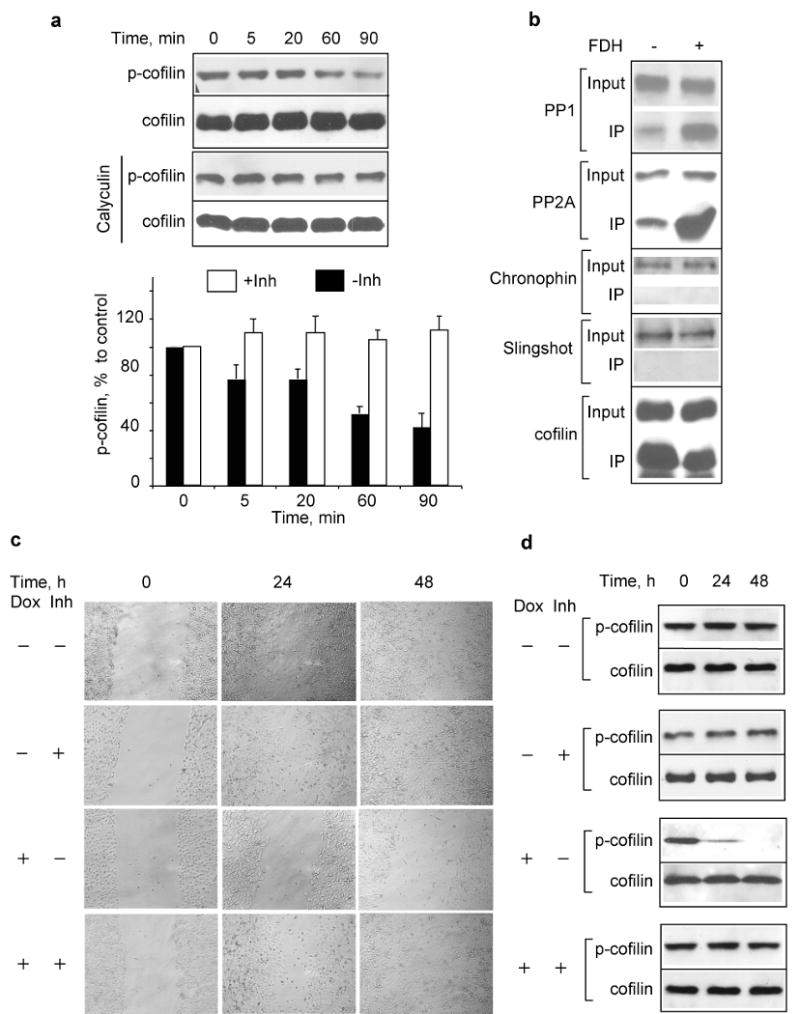
FDH activates dephosphorylation of cofilin by PP1 and PP2A. (a) Time dependent in vitro dephosphorylation of p-cofilin by lysate from FDH-expressing cells (top panel); the same assay in the presence of 1.0 μM of PP1/PP2A inhibitor calyculin (bottom panel); bar graph, levels of p-cofilin quantified from the panels (-Inh, in the absence of calyculin; +Inh, in the presence calyculin) using Quantity One software (Bio-Rad). Average (±SD) of three independent experiments is shown. (b) Western blot analysis of phosphatases pulled down with cofilin antibody from FDH expressing and FDH-deficient cells. (c) Motility of FDH-expressing A549 cells (wound healing assays) is restored by the addition of calyculin. (d) Calyculin prevents FDH-induced cofilin dephosphorylation in vivo. Time (h) after addition of calyculin to the cell culture is indicated. FDH expression was induced 24 h before addition of calyculin.
Inhibition of PP1 and PP2A prevents FDH-induced cofilin dephosphorylation and restores motility
We further studied whether inhibition of PP1 and PP2A is sufficient to rescue cells from the effects of FDH on motility. After FDH induction, A549 cells were subjected to wound healing or transwell migration assays in the presence or absence of calyculin. In these experiments, cells were grown in the presence of pan-caspase inhibitor zVAD-fmk to prevent FDH-associated apoptosis. The treatment with 2.5 nM calyculin protected cells from FDH and restored motility (Fig. 6c and Supplement Fig. S4). In agreement with the in vitro experiments, dephosphorylation of cofilin in response to FDH was also inhibited in cells upon calyculin treatment (Fig. 6d). The inhibitor itself did not produce any visible effects on the levels of phosphorylated cofilin or motility (Fig. 6, c and d, and Supplement Fig. S4).
Discussion
We have previously demonstrated that FDH produces strong antiproliferative effects in cancer cells, which can be reversed by supplementation with high concentrations of folate (Krupenko and Oleinik, 2002). It was not clear, however, whether the FDH suppressor mechanism is limited to cell proliferation or whether it affects other cellular functions, such as migration and adhesion potential. The present studies demonstrate that FDH inhibits cell motility through a specific, folate regulated pathway, that is independent of apoptotic signaling and imply that the anti-motile effects of FDH/folate stress are associated with the inhibition of actin turnover. The conclusion was based on the decreased ratio between G- and F-actin in FDH expressing cells with the strong shift towards preponderance of F-actin suggesting a decreased ability to depolymerize filamentous actin. This was further confirmed by direct measurements of the rates of actin polymeryzation/depolymerization. While actin dynamics is a complex process involving many components, actin depolymerizing factor cofilin is considered as one of the major players in this process (DesMarais et al., 2005). Of note, the anti-motile effects of FDH were not associated with the co-accumulation of cofilin and G-actin in nuclei (Supplement Fig. S5), a phenomenon observed under some stress conditions (Bamburg and Wiggan, 2002; Chhabra and dos Remedios, 2005).
Phosphorylation of cofilin at a single serine residue, Ser3, is a common mechanism regulating its activity (DesMarais et al., 2005; Wang et al., 2007). Therefore, robust dephosphorylation of cofilin upon FDH expression indicated that this is the likely upstream event involved in redistribution of F and G actin. In support of this view, expression of the phosphorylation-deficient S3A cofilin mutant in A549 cells produced the phenotype seen upon FDH expression that is characterized by decreased G-actin content in the cytosol and formation of actin stress fibers. Furthermore, the S3D mutant mimicking constitutive phosphorylation reverses the effects of FDH. Previously non-phosphorylated cofilin was commonly viewed as the active protein facilitating actin turnover in contrast to phosphorylated cofilin, which was considered non-active (DesMarais et al., 2005; Wang et al., 2007). Our findings appear controversial to this view. The complexity of cofilin-dependent processes, however, is not yet completely understood: numerous studies indicate that phosphorylation status of the protein does not directly reflect the rate of actin polymerization/depolymerization and associated motility (Song et al., 2006; Wang et al., 2007; Wang et al., 2006). Generally, the function of cofilin could be determined by many factors including the balance between phosphorylated/non-phosphorylated forms and the participation of other actin-regulating proteins, and perhaps is cell type specific (dos Remedios et al., 2003; Lai et al., 2008; Oser et al., 2009; Wang et al., 2007; Winder and Ayscough, 2005).
The recently introduced concept of a cofilin activity cycle consolidates the somewhat controversial observations regarding active/non-active cofilin (Oser and Condeelis, 2009; van Rheenen et al., 2009). It is based on the phenomenon of a constant turnover of cofilin between phosphorylated and non-phosphorylated forms as the protein moves between three cellular compartments: plasma membrane, cytosol and actin filaments. This model implies that actin dynamics can be inhibited even in the presence of significant levels of non-phosphorylated cofilin, a presumably activated protein, and that rather the local cofilin activity at a specific compartment defines the process (van Rheenen et al., 2009). Indeed, in unstimulated cells not undergoing actin skeleton rearrangement, the majority of cofilin can be either phosphorylated or non-phosphorylated depending on the cell type (Oser and Condeelis, 2009). The cofilin activity cycle hypothesis also states that the initial activation of cofilin requires different mechanisms depending on the starting point in the cycle. Furthermore, cofilin can be uncoupled from actin regulation in any of the three compartments thus disabling the cycle (van Rheenen et al., 2009). One of the mechanisms, for example, to inactivate non-phosphorylated cofilin is through binding PI(4,5)P2 at the plasma membrane (van Rheenen et al., 2007). It could be suggested, therefore, that if the cycle is stalled at any point, the actin turnover will be suppressed resulting in inhibited motility as the downstream effect. Thus, the idea that the cofilin turnover rather than the prevalence of one of the forms is crucial to support the actin dynamics provides a rational explanation for our findings.
Mechanistically, the overall levels of phosphorylated cofilin depend on the relative rate of its phosphorylation by LIM kinases compared to the rate of dephosphorylation, which in turn would depend on a specific phosphatase recruited for this process. The list of phosphatases capable of cofilin dephosphorylation includes so far PP1 and PP2A (Ambach et al., 2000), PP2B (Meberg et al., 1998), PP2C (Zhan et al., 2003), slingshot (Niwa et al., 2002) and chronophin (Gohla et al., 2005). Our experiments demonstrate that in FDH-stressed cells two phosphatases, PP1 and PP2A, were involved in cofilin dephosphorylation but not slingshot and chronophine, which are recognized as cofilin-specific phosphatases (Huang et al., 2006; Wiggan et al., 2005). Interestingly, inhibition of the phosphatases with calyculin prevented FDH-induced cofilin dephosphorylation and protected motility in a cell culture model. PP1 and PP2A are two major serine/threonine phosphatases, which dephosphorylate a large number of targets including components of cytoskeleton (Cohen, 2002; Eichhorn et al., 2009; Janssens et al., 2008). Their recruitment for cofilin dephosphorylation in response to changes in intracellular/extracellular folate status perhaps defines such a robust protein dephosphorylation. This in turn indicates the necessity for a rapid inhibition of actin dynamics and reflects the fact that the folate related stress is recognized as a strong insult, which prompts the cell to a fast and decisive response.
We have previously shown that antiproliferative effects of FDH are associated with activation of JNKs and p53 as downstream effectors (Ghose et al., 2009; Oleinik et al., 2007; Oleinik et al., 2005). These central players in controlling cellular proliferation are also implicated in regulation of migration (Roger et al., 2006; Williams et al., 2006; Xia and Karin, 2004). In the case of FDH-induced stress, however, mechanisms independent of either JNKs or p53 appear to be involved in the inhibition of cell motility. Indeed, inhibitors of JNKs, as well as the lack of p53, did not prevent effects of FDH on cofilin phosphorylation, actin remodeling and motility while effectively protected cells against apoptosis (Oleinik et al., 2007; Oleinik et al., 2005).
FDH effects on cell motility, F/G actin distribution and levels of phosphorylated cofilin are a part of a more general cellular response to the disturbance of folate metabolism and are similar to the effects produced by folate depletion. As such, these effects were reversed by the excess of extracellular folate. We propose that the mechanism underlying FDH effects includes the following steps: (i) recruitment of PP1 and PP2A; (ii) dephosphorylation of cofilin; and (iii) inhibition of actin depolymerization. On a broader scale, our study indicates that folate is required for the proper cell migration while folate depletion decreases motile potential of the cell. Furthermore, it appears that these effects are folate specific rather than a general response to nutrient starvation (Supplement Fig. S3). The mechanism of folate control of cell motility, revealed by this study, might be relevant to the function of folate in the prevention of neural tube defects. Indeed, cell motility is important for normal development (Montell, 2008) while a limited motility at conditions of insufficient folate supplementation is likely to result in the incomplete neural tube closure. On the other hand, inhibition of this regulatory mechanism by excessive folate supplementation could enhance the metastatic potential of transformed cells perhaps promoting invasive tumors.
Materials and Methods
Inducible FDH expression
A non-small cell lung carcinoma cell line A549 capable of inducible FDH expression (A549/Tet-On) reported previously (Oleinik and Krupenko, 2003) was used in this study. To induce FDH expression, cells growing in 6-well plates (0.5×106 cells per well) were kept on media containing doxycycline (final concentration 0-2.5 μg/ml).
Evaluation of cell motility
Transwell migration, invasion, migration track, wound healing and adhesion assays were used to characterize cell motility. Detailed descriptions of these assays are provided in supplemental materials.
Actin polymerization/depolymerization assays
The kinetics of actin polymerization/depolymerization was measured using a non-muscle Actin Polymerization Biochem kit (Cytoskeleton, Denver, CO) according to the manufacturer's manual. Fluorescence measurements were performed at 25 °C using a Perkin Elmer 1420 Multilabel Counter. The excitation wavelength was set at 355 nm and the emission wavelength was set at 460 nm.
FRAP (Fluorescence Recovery After Photobleaching) assays
Photobleaching experiments were performed using a Leica TCS SP2 AOBS confocal microscope; 488-nm line and 63× numerical aperture 1.2 water immersion objective were used for GFP imaging. A549 and A549/FDH cells were transiently transfected with a GFP/actin construct (kind gift from Dr. Imhof) (Ballestrem et al., 1998). Six hours later doxycycline was added to induce FDH expression. Assays were performed 48 h post-induction (corresponding to 54 h post-transfection). Two pre-bleach scans of an entire image were followed by 10 scan iterations of a rectangular region of interest at 100% intensity of 30 mW argon-ion 488 nm laser (transmission intensity). After bleaching the fluorescence recovery was measured automatically every 3 to 4 s. The intensity of fluorescence of the bleached area was normalized to neighboring non-bleached area to account for normal photobleaching during the monitoring period.
G-actin/F-actin assay
G- and F-actin were measured as described elsewhere (Turner et al., 2007). Cells were lysed in 50 mM PIPES buffer, pH 6.9 containing 50 mM NaCl 5 mM MgCl2, 5 mM EGTA, 5% glycerol, 0.1% Nonidet P40, 0.1% Triton X-100, 0.1% Tween 20, 1 mM ATP and protease inhibitor cocktail (Sigma). Cell lysates were centrifuged for 10 min at 500×g then for 30 min at 18,000×g and the supernatant (containing G-actin) was collected. The 18,000×g pellet containing F-actin was washed and resuspended in SDS-PAGE loading buffer. Levels of actin in each fraction were evaluated after Western blot with actin-specific antibody.
In vitro cofilin dephosphorylation assay
After rinsing with ice-cold PBS, cells were collected in ice-cold phosphatase extraction buffer (20 mM imidazole HCl, pH 7.2, 2 mM EDTA, 0.2% β-mercaptoethanol, 2 mg/ml rabbit liver glycogen, 1 mM benzamidine, 1 mM PMSF, 10 μg/ml of each aprotinin, leupeptin, antipain and pepstatin) and immediately sonicated. Lysate aliquots from FDH-expressing and FDH-free cells were mixed in pre-chilled tubes in the absence or in the presence of 1.0 μM of calyculin and incubated at 30 °C. The reaction was stopped by heating at 95 °C in SDS loading buffer for 3min. Levels of phosphorylated cofilin were assessed by SDS-PAGE/immunoblotting and quantified using Quantity One Software (BioRad).
Knock down of cofilin by siRNA
Stealth siRNA duplex targeting cofilin 1 (targeted sequence: GGG AUC AAG CAU GAA UUG CAA GCA A) and a negative control Stealth duplex were purchased from Invitrogen. Thirty pmol of oligonucleotides were used to transfect A549 cells (2×106) by electroporation with Amaxa nucleofector according to manufacturer's instructions. Cells were collected at 24-120 h post-transfection, lysed and analyzed by Western blot assays for the presence of cofilin 1 protein.
Transient expression of cofilin mutants
Vectors for expression of S3A and S3D cofilin mutants were generous gifts from Dr. Shieh (Hsu et al.). Cells (2×106) were transfected with 5.0 μg of pXJN-HA/cofilin vector DNA using Amaxa nucleofector according to the protocol optimized for A549 cells. Expression of the mutants was detected by Western blot assays with the cofilin-specific antibody (due to HA-tag the mutant cofilin appears as a band migrating slower than 19 kDa wild-type endogenous cofilin).
Phosphatase pull-down
A slurry of protein G conjugated beads (100 μl) was pre-incubated with cofilin antibody (10 μl) for 1 h at 4 °C with gentle mixing. Lysate of A549 FDH-deficient cells was added to the mixture and incubated 1 h. Beads were washed several times with the phosphatase extraction buffer, mixed with the lysate of ATG10.26 cells obtained 48 h FDH post-induction, and incubated at 30 °C for 30 min. Beads were washed several times with the above buffer, heated at 95 °C in SDS-PAGE loading buffer for 3min and analyzed by SDS-PAGE and immunoblotting with antibodies against PP2A (Upstate), PP1β, PP1γ (Santa Cruz Biotechnology), slingshot (Abcam) and chronophin (Cell Signaling).
Supplementary Material
Acknowledgments
The construct for expression of GFP/actin fusion was a kind gift from Dr. Imhof. Vectors for expression of S3A and S3D cofilin mutants were generous gifts from Dr. Shieh. The authors would like to thank Dr. Condeelis for the helpful discussion. This work was supported by National Institutes of Health Grants DK54388 and CA95030.
Footnotes
Additional description of Materials and Methods is included in supplemental materials.
Conflict of interest: The authors declare no conflict of interest.
References
- Ambach A, Saunus J, Konstandin M, Wesselborg S, Meuer SC, Samstag Y. The serine phosphatases PP1 and PP2A associate with and activate the actin-binding protein cofilin in human T lymphocytes. Eur J Immunol. 2000;30:3422–31. doi: 10.1002/1521-4141(2000012)30:12<3422::AID-IMMU3422>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ananthakrishnan R, Ehrlicher A. The forces behind cell movement. Int J Biol Sci. 2007;3:303–17. doi: 10.7150/ijbs.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestrem C, Wehrle-Haller B, Imhof BA. Actin dynamics in living mammalian cells. J Cell Sci. 1998;111(Pt 12):1649–58. doi: 10.1242/jcs.111.12.1649. [DOI] [PubMed] [Google Scholar]
- Bamburg JR, Wiggan OP. ADF/cofilin and actin dynamics in disease. Trends Cell Biol. 2002;12:598–605. doi: 10.1016/s0962-8924(02)02404-2. [DOI] [PubMed] [Google Scholar]
- Chanson A, Sayd T, Rock E, Chambon C, Sante-Lhoutellier V, Potier de Courcy G, et al. Proteomic analysis reveals changes in the liver protein pattern of rats exposed to dietary folate deficiency. J Nutr. 2005;135:2524–9. doi: 10.1093/jn/135.11.2524. [DOI] [PubMed] [Google Scholar]
- Chhabra D, dos Remedios CG. Cofilin, actin and their complex observed in vivo using fluorescence resonance energy transfer. Biophys J. 2005;89:1902–8. doi: 10.1529/biophysj.105.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PT. Protein phosphatase 1--targeted in many directions. J Cell Sci. 2002;115:241–56. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- Crott JW, Choi SW, Ordovas JM, Ditelberg JS, Mason JB. Effects of dietary folate and aging on gene expression in the colonic mucosa of rats: implications for carcinogenesis. Carcinogenesis. 2004;25:69–76. doi: 10.1093/carcin/bgg150. [DOI] [PubMed] [Google Scholar]
- Crott JW, Liu Z, Keyes MK, Choi SW, Jang H, Moyer MP, et al. Moderate folate depletion modulates the expression of selected genes involved in cell cycle, intracellular signaling and folate uptake in human colonic epithelial cell lines. J Nutr Biochem. 2008;19:328–35. doi: 10.1016/j.jnutbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesMarais V, Ghosh M, Eddy R, Condeelis J. Cofilin takes the lead. J Cell Sci. 2005;118:19–26. doi: 10.1242/jcs.01631. [DOI] [PubMed] [Google Scholar]
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, et al. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–73. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795:1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- Franco C, Ho B, Mulholland D, Hou G, Islam M, Donaldson K, et al. Doxycycline alters vascular smooth muscle cell adhesion, migration, and reorganization of fibrillar collagen matrices. Am J Pathol. 2006;168:1697–709. doi: 10.2353/ajpath.2006.050613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose S, Oleinik NV, Krupenko NI, Krupenko SA. 10-formyltetrahydrofolate dehydrogenase-induced c-Jun-NH2-kinase pathways diverge at the c-Jun-NH2-kinase substrate level in cells with different p53 status. Mol Cancer Res. 2009;7:99–107. doi: 10.1158/1541-7786.MCR-08-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohla A, Birkenfeld J, Bokoch GM. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat Cell Biol. 2005;7:21–9. doi: 10.1038/ncb1201. [DOI] [PubMed] [Google Scholar]
- Hall AL, Warren V, Condeelis J. Transduction of the chemotactic signal to the actin cytoskeleton of Dictyostelium discoideum. Dev Biol. 1989;136:517–25. doi: 10.1016/0012-1606(89)90277-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hsu FF, Lin TY, Chen JY, Shieh SY. p53-Mediated transactivation of LIMK2b links actin dynamics to cell cycle checkpoint control. Oncogene. 29:2864–76. doi: 10.1038/onc.2010.40. [DOI] [PubMed] [Google Scholar]
- Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol. 2006;18:26–31. doi: 10.1016/j.ceb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–21. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Jhaveri MS, Wagner C, Trepel JB. Impact of extracellular folate levels on global gene expression. Mol Pharmacol. 2001;60:1288–95. doi: 10.1124/mol.60.6.1288. [DOI] [PubMed] [Google Scholar]
- Jin S, DiPaola RS, Mathew R, White E. Metabolic catastrophe as a means to cancer cell death. J Cell Sci. 2007;120:379–83. doi: 10.1242/jcs.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupenko SA, Oleinik NV. 10-formyltetrahydrofolate dehydrogenase, one of the major folate enzymes, is down-regulated in tumor tissues and possesses suppressor effects on cancer cells. Cell Growth Differ. 2002;13:227–36. [PubMed] [Google Scholar]
- Lai FP, Szczodrak M, Block J, Faix J, Breitsprecher D, Mannherz HG, et al. Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 2008;27:982–92. doi: 10.1038/emboj.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Smith AK, Kamen BA. Receptor-mediated folate uptake is positively regulated by disruption of the actin cytoskeleton. Cancer Res. 1998;58:2952–6. [PubMed] [Google Scholar]
- Meberg PJ, Ono S, Minamide LS, Takahashi M, Bamburg JR. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil Cytoskeleton. 1998;39:172–90. doi: 10.1002/(SICI)1097-0169(1998)39:2<172::AID-CM8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Merlot S, Firtel RA. Leading the way: Directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J Cell Sci. 2003;116:3471–8. doi: 10.1242/jcs.00703. [DOI] [PubMed] [Google Scholar]
- Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–5. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–46. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- Oleinik NV, Krupenko NI, Krupenko SA. Cooperation between JNK1 and JNK2 in activation of p53 apoptotic pathway. Oncogene. 2007;26:7222–30. doi: 10.1038/sj.onc.1210526. [DOI] [PubMed] [Google Scholar]
- Oleinik NV, Krupenko NI, Priest DG, Krupenko SA. Cancer cells activate p53 in response to 10-formyltetrahydrofolate dehydrogenase expression. Biochem J. 2005;391:503–11. doi: 10.1042/BJ20050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleinik NV, Krupenko NI, Reuland SN, Krupenko SA. Leucovorin-induced resistance against FDH growth suppressor effects occurs through DHFR up-regulation. Biochem Pharmacol. 2006;72:256–66. doi: 10.1016/j.bcp.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Oleinik NV, Krupenko SA. Ectopic expression of 10-formyltetrahydrofolate dehydrogenase in a549 cells induces g(1) cell cycle arrest and apoptosis. Mol Cancer Res. 2003;1:577–88. [PubMed] [Google Scholar]
- Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. J Cell Biochem. 2009;108:1252–62. doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–87. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni D, Le Clainche C, Carlier MF. Mechanism of actin-based motility. Science. 2001;292:1502–6. doi: 10.1126/science.1059975. [DOI] [PubMed] [Google Scholar]
- Roger L, Gadea G, Roux P. Control of cell migration: a tumour suppressor function for p53? Biol Cell. 2006;98:141–52. doi: 10.1042/BC20050058. [DOI] [PubMed] [Google Scholar]
- Song X, Chen X, Yamaguchi H, Mouneimne G, Condeelis JS, Eddy RJ. Initiation of cofilin activity in response to EGF is uncoupled from cofilin phosphorylation and dephosphorylation in carcinoma cells. J Cell Sci. 2006;119:2871–81. doi: 10.1242/jcs.03017. [DOI] [PubMed] [Google Scholar]
- Turner DP, Moussa O, Sauane M, Fisher PB, Watson DK. Prostate-derived ETS factor is a mediator of metastatic potential through the inhibition of migration and invasion in breast cancer. Cancer Res. 2007;67:1618–25. doi: 10.1158/0008-5472.CAN-06-2913. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Kumar SG, Alla SR, Reddy HK, Voelker DJ, Janicki JS. Extracellular matrix regulation of metalloproteinase and antiproteinase in human heart fibroblast cells. J Cell Physiol. 1996;167:137–47. doi: 10.1002/(SICI)1097-4652(199604)167:1<137::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- van Rheenen J, Condeelis J, Glogauer M. A common cofilin activity cycle in invasive tumor cells and inflammatory cells. J Cell Sci. 2009;122:305–11. doi: 10.1242/jcs.031146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rheenen J, Song X, van Roosmalen W, Cammer M, Chen X, Desmarais V, et al. EGF-induced PIP2 hydrolysis releases and activates cofilin locally in carcinoma cells. J Cell Biol. 2007;179:1247–59. doi: 10.1083/jcb.200706206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C. Biochemical role of folate in cellular metabolism. In: Bailey LB, editor. Folate in Health and Disease. Marcel Dekker, Inc.; New York: 1995. pp. 23–42. [Google Scholar]
- Wang W, Eddy R, Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer. 2007;7:429–40. doi: 10.1038/nrc2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Mouneimne G, Sidani M, Wyckoff J, Chen X, Makris A, et al. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J Cell Biol. 2006;173:395–404. doi: 10.1083/jcb.200510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggan O, Bernstein BW, Bamburg JR. A phosphatase for cofilin to be HAD. Nat Cell Biol. 2005;7:8–9. doi: 10.1038/ncb0105-8. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Wiklund ML, Wikman S, Hultmark D. Rac1 signalling in the Drosophila larval cellular immune response. J Cell Sci. 2006;119:2015–24. doi: 10.1242/jcs.02920. [DOI] [PubMed] [Google Scholar]
- Winder SJ, Ayscough KR. Actin-binding proteins. J Cell Sci. 2005;118:651–4. doi: 10.1242/jcs.01670. [DOI] [PubMed] [Google Scholar]
- Xia Y, Karin M. The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol. 2004;14:94–101. doi: 10.1016/j.tcb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yujiri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, et al. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-kappa B activation. Proc Natl Acad Sci U S A. 2000;97:7272–7. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Q, Bamburg JR, Badwey JA. Products of phosphoinositide specific phospholipase C can trigger dephosphorylation of cofilin in chemoattractant stimulated neutrophils. Cell Motil Cytoskeleton. 2003;54:1–15. doi: 10.1002/cm.10079. [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22:7431–57. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med. 2009;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Cabrera RM, Wlodarczyk BJ, Bozinov D, Wang D, Schwartz RJ, et al. Differentially expressed genes in embryonic cardiac tissues of mice lacking Folr1 gene activity. BMC Dev Biol. 2007;7:128. doi: 10.1186/1471-213X-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


