Abstract
Nephrocalcinosis is the deposition of calcium salts in renal parenchyma and can be intratubular or interstitial. Animal model studies indicate that intratubular nephrocalcinosis is a result of increased urinary supersaturation. Urinary supersaturation with respect to calcium oxalate (CaOx) and calcium phosphate (CaP) are generally achieved at different locations in the renal tubules. As a result experimental induction of hyperoxaluria in animals with CaP deposits does not lead to growth of CaOx over CaP. Interstitial nephrocalcinosis has been seen in mice with lack of crystallization modulators Tamm–Horsfall protein and osteopontin. Sodium phosphate co-transporter or sodiumhydrogen exchanger regulator factor-1 null mice also produced interstitial nephrocalcinosis. Crystals plug the tubules by aggregating and attaching to the luminal cell surface. Structural features of the renal tubules also play a role in crystal retention. The crystals plugging the terminal collecting ducts when exposed to the metastable pelvic urine may promote the formation of stone.
Keywords: Nephrocalcinosis, Nephrolithiasis, Calcium oxalate, Napt2a null mice, Calcium phosphate
Introduction
Nephrocalcinosis describes the deposition of radiologically demonstrable calcium salts in renal parenchyma and is different from crystal deposition in the lumens of collecting systems, ureters, bladder, etc., which is called nephrolithiasis or urolithiasis. Nephrocalcinosis can be divided into two categories, intratubular or interstitial. Kidney stones develop in the renal calyces and pelvis [1]. Randall suggested that interstitial subepithelial deposits of carbon containing calcium phosphate arising from pathologic conditions of the renal papilla erode through to the papillary surface and form a Type 1 precalculus lesion [2]. He further suggested that excessive supersaturation associated with tubular epithelial cell necrosis leads to crystallization and crystal deposition in the terminal collecting ducts producing a Type 2 precalculus lesion. In both cases the lesion acts as a nidus for further deposition of salts resulting in the formation of calculi in the pelvis or papillary ducts. Randall’s plaque generally refers to Type 1 precalculus lesion.
Recent studies by Stoller and associates [3–5] and Evan and colleagues [1, 6–9] of kidneys of a variety of stone patients including, idiopathic, intestinal bypass surgery for obesity, primary hyperoxaluria, brushite, cystine and distal tubular acidosis have provided a detailed description of plaque and histopathological changes in kidneys of the stone formers and shed more light on the role of Randall’s plaque in stone formation. Randall’s plaques consist of poorly crystalline biological apatite and are suggested to originate in the renal interstitium close to the collagen fibers and the basement membrane of the Loops of Henle [10]. With the exception of kidneys of those who form stones after bariatric surgery for obesity, Randall’s plaques were seen in almost all the renal papillary biopsies including those from the non-stone forming papillae [1]. On the other hand intratubular apatite crystals were seen in renal collecting ducts of all stone formers except those of the ID stone formers. Kidneys of intestinal bypass surgery did not show any plaques or interstitial crystal deposits, only apatitic crystal deposits in some inner medullary collecting ducts [10]. In a rare case both CaOx and CaP crystals were observed in the collecting ducts. Luminal surface of the inner medullary collecting ducts also showed focal hyaluronan staining [11] which was generally absent in the ID stone formers’ kidneys. Our light and electron microscopic studies of a renal papilla and associated CaOx kidney stones showed calcium phosphate (CaP) deposition in the interstitium and CaOx in the ducts of Bellini [12]. Calcium oxalate crystals did not appear to develop on the interstitial deposits of CaP.
Animal models of renal crystal deposition
A variety of animals including mice, rabbits, rats and pigs have been used to study in vivo the pathogenesis of different types of stones. So far, most investigations have been limited to calcific stone disease using rats and mice and I will therefore focus on studies of CaOx and CaP crystal deposition in kidneys of these animals. With respect to renal morphology, kidneys of mice, rabbits and rats are unlike those of humans and are unipapillate while those of pigs are similar to those of humans in being multipapillate. Rats and mice are however, the two most commonly used experimental animals in medical research including stone disease.
Calcium oxalate deposition: rats
Hyperoxaluria, one of the most common risk factors of ID CaOx stone disease, can by itself induce CaOx deposition of in the kidneys by the administration of vitamin B6, glycolic acid, glyoxylate, sodium oxalate, ammonium oxalate (AOx), ethylene glycol (EG), or hydroxy-L-proline (HLP). Additional administration of vitamin D or calcium chloride promotes hypercalciuria and faster CaOx crystal deposition [13]. Consumption of refined sugars such as sucrose, glucose, fructose, xylitol or sorbitol by rats on ethylene glycol also results in CaOx crystal deposition in the rat kidneys [14]. Acute hyperoxaluria can be induced through intraperitoneal injection while chronic hyperoxaluria through food and/or water [15, 16]. Literature survey indicates that a significant majority of the studies utilize EG as the hyperoxaluria inducing agent.
A number of studies have been carried out to investigate aspects of calcium oxalate stone formation in male and female Sprague–Dawley rats. Acute hyperoxaluria leads to rapid deposition of CaOx crystals in the kidneys, starting from proximal tubules and moving to the renal papillae [17, 18]. Crystals appear in the renal tubules, moving with the urine eventually clearing the kidneys. Amount of deposits and their stay within the kidneys depends upon the quantity of lithogen administered. Higher amounts lead to significantly high number of deposits throughout the kidneys. Crystals are retained at the renal corticomedullary junctions, papillary tips and fornices. These are the sites with changes in luminal diameter of renal tubules and large crystal aggregates cannot move with the urine flow [19].
Hyperoxaluria has also been induced by sustained oxalate release through subcutaneous implantation of oxalate containing osmotic minipumps [20, 21]. Most of the oxalate, however, deposits around the pump opening or is taken up by the skin [21, 22]. Only a small portion ends up in the urine. As a result, renal CaOx crystal deposition is limited and occurs only when a large amount of oxalate is delivered. Otherwise hyperoxaluria leads only to CaOx crystalluria.
When hyperoxaluria is induced by administration of 0.75–1% EG, there is an increase in urinary excretion of oxalate within 2 days and hyperoxaluria is established within 3 days, CaOx crystalluria within 2 weeks and CaOx nephrolithiasis within 4–6 weeks [15]. Urinary pH and excretion of citrate are significantly decreased. Other urinary factors and renal creatinine clearance remain within normal range. Crystal deposition starts in the lumina of collecting ducts of the renal papilla and large stone-like deposits are formed associated with the renal papillae and/ or fornices. Higher concentrations of EG or induction of acute hyperoxaluria by intraperitoneal administration of oxalate leads to crystal deposition throughout the kidneys and in all sections of the renal tubules, from proximal tubules to the papillary collecting ducts. Administration of HLP also leads to CaOx nephrolithiasis in the male rats [23] following a similar course of events generating comparable urinary biochemistry. However, crystals are, in general, seen throughout the kidneys.
Kidneys of nephrolithic rats show deposits of CaOx crystals in the renal tubules. Epithelial cells lining the crystal-containing renal tubules are damaged, showing signs of degeneration followed by regeneration [23]. Crystals appear first in the tubular lumen [24], thereafter many of the crystals move into inter and intracellular locations and eventually into the interstitium. Movement into interstitium is associated with inflammation, attracting many inflammatory cells including leukocytes, monocytes and macrophages [25]. Eventually interstitial crystals disappear [26, 27]. CaOx crystals often block the terminal collecting ducts on papillary surface and grow into large deposits (Figs. 1, 2b). Crystals are often seen attached to the basement membrane (Fig. 2a, b) [18]. After the loss of surface epithelium, a result of loosening of tight junctions [23], these deposits become exposed to the pelvic urine, and continue to grow as large papillary stones.
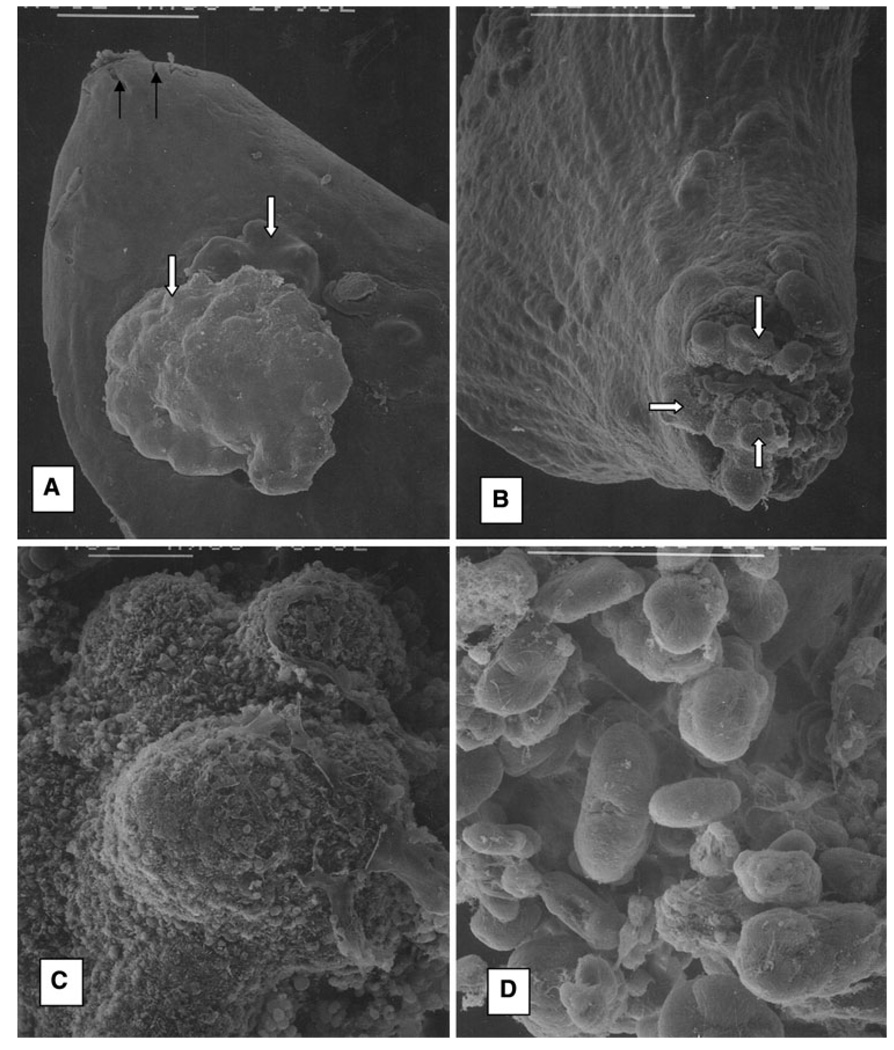
Fig. 1.
Scanning electron microscopic analysis of the calcium oxalate crystal deposition in rat kidneys. Rats received EG in water and calcium chloride mixed with chow for 8 weeks, a Renal papilla with surface protrusions (solid white arrows). Longitudinal section through similar renal papillae from the hyperoxaluric rats show collecting ducts filled with CaOx crystals (Fig. 2b). Solid black arrows point to the opening of the ducts of Bellini at the papillary tip. b Renal papilla. Aggregates of CaOx crystals plugged the ducts of Bellini and protruded through at the tip. c Higher magnification of the protruding crystal aggregates, d Close-up of some of the dumbbell shaped CaOx monohydrate crystals. Bar 500 µm, a, b; 50 µm c, d
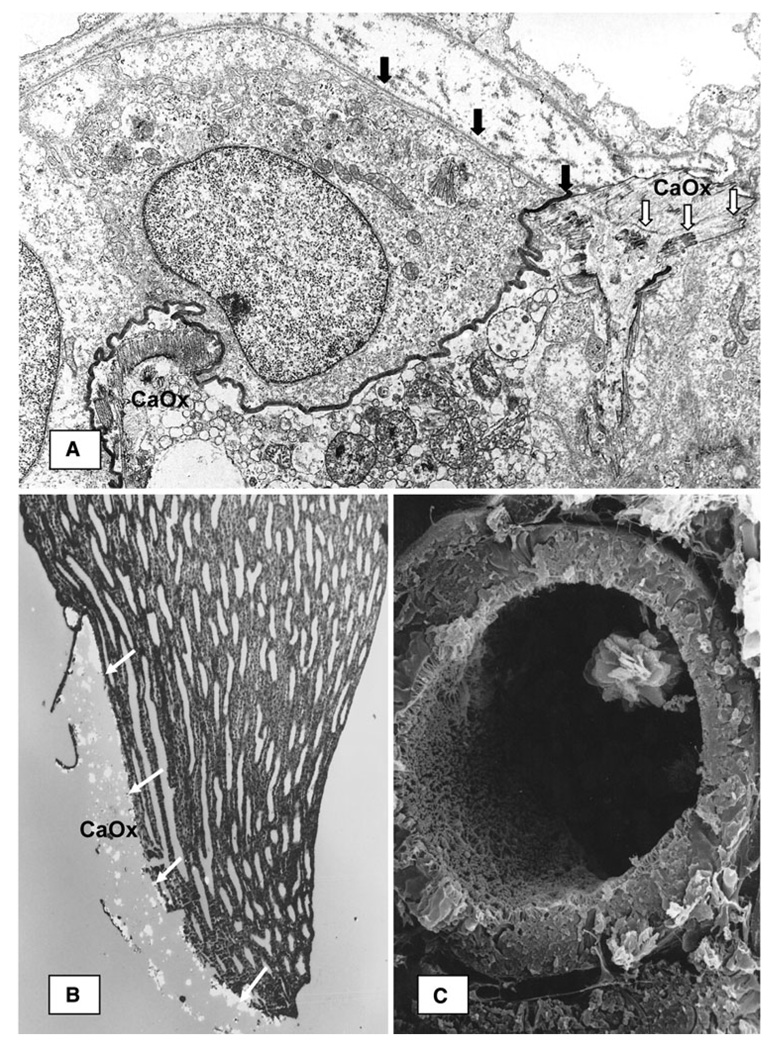
Fig. 2.
CaOx crystal deposition in renal tubules of the hyperoxaluria rats. a Transmission electron microscopic image of a renal tubule from kidney of rat made hyperoxaluric by intraperitoneal administration of sodium oxalate [18]. Luminal surface of the epithelial cell is stained dark. Basement membrane of the epithelial cell (dark arrows) continues in the organic matrix of the CaOx crystal (white arrows). Tubular lumen is filled with cellular degradation products and another CaOx crystal close to the luminal epithelial surface, b Light micrograph of longitudinal section through a large intratubular CaOx crystal deposit at papillary tip of a kidney from EG-fed male rat. Birefringent crystals are lined up against the basement membrane (white arrows). c Scanning electron micrograph of proximal tubule from kidney of a rat made hyperoxaluric by feeding EG
Hyperoxaluria leads [28] to a gradual increase in urinary levels of alkaline phosphatase (AP), gamma-glutamyl transpeptidase (GGTP), and N-acetyl-β-glucoseaminidase (NAG), enzymes often reflective of proximal tubular injury. Urinary excretion of NAG was most significantly increased and correlated highly with urinary excretion of Ox. Hyperoxaluric rats had increased urinary excretion of LDH, malodialdehyde, 8-isoprostane, and hydrogen peroxide, indicating the involvement of free radicals in oxalate and CaOx-associated renal toxicity [23, 29]. Administration of antioxidant such as vitamin E resulted in reduced levels of lipid peroxides in the kidneys and decreased urinary excretion of LDH and lipid peroxides. In one study vitamin E administration improved renal tissue antioxidant status and prevented CaOx crystal deposition [30]. After 8 weeks on a hyperoxaluric diet, male Sprague–Dawley rats excreted higher amounts of arachidonic acid and prostaglandin E2 than the normal controls. We investigated MCP-1 production by renal epithelial cells in response to an exposure to Ox and CaOx crystals [31]. Immunohisto-chemical investigation of the paraffin-embedded sections of rat kidneys using a rabbit anti-rat monocyte chemo-attractant protein-1 (MCP-1) antibody showed cells of crystal-containing tubules to satin positive for MCP-1.
Other studies have provided evidence for the activation of the rennin–angiotensin system during the development of hyperoxaluria-induced tubulointerstitial lesions of CaOx crystals [32, 33]. Reduction of angiotensin production by inhibiting the angiotensin converting enzyme as well as blocking the angiotensin receptor reduced crystal deposition and ameliorated the associated inflammatory response. CaOx crystal deposition in rat kidneys activates the rennin–angiotensin system and increases renin expression in the kidneys and serum [34].
CaOx crystal deposition in the kidneys also increases the expression of Tamm–Horsfall protein (THP), CD 44, osteopontin (OPN), bikunin and other inter-alpha-inhibitor (ITI) related macromolecules, prothrombin (PT), and heparan sulfate (HS) as determined by immunocytochemical localization using specific antibodies [35]. There was no increase in either the production or excretion of THP [36, 37], only increased retention in the kidneys where THP surrounded the retained crystals. Other studies have, however, shown either a decrease [38] or an increase [39] in THP expression and production. Production and urinary excretion of OPN [40], PT [41], various ITI-related proteins [42, 43] and HS [42] was substantially increased as determined by detection of their specific mRNAs. These molecules are involved in modulation of crystallization as well as inflammatory cascade.
In conclusion, results of rat model studies indicate that renal CaOx crystal deposition is mostly intratubular, nephrocalcinosis, and is associated with cellular injury, inflammation and cellular regeneration. Reactive oxygen species are produced during hyperoxaluria and nephrolithiasis and are most likely involved in various signaling events.
Calcium oxalate crystal deposition: pigs
Feeding HLP to the male or female pigs produces hyperoxaluria [44, 45], which reaches a maximum and then stays at that level in males and drops to the control levels in the females. Light microscopic examination of the renal papillae was performed in the male pigs and showed CaOx crystal deposition, papillary tip encrustation and tissue injury.
Calcium oxalate crystal deposition: mice
Even though most rats with experimentally induced hyperoxaluria develop CaOx renal deposits, mice with hyperoxaluria alone either do not produce any CaOx crystals deposits or only a few crystals in their kidneys [46, 47]. Daily intra-abdominal injections of glyoxylate have been shown to produce CaOx nephrolithiasis in mice. However, renal crystal deposition increased in the first week and then started to decrease, crystals disappearing from the kidneys after 2 weeks [48, 49].
Genetic manipulations of alanine glyoxylate aminotransferase (AGT) or anion transporter Slc26a6 have produced hyperoxaluric mice. AGT null mice develop severe hyperoxaluria but develop only a few CaOx crystal deposits in their kidneys [48]. Mice lacking anion transporter Slc26a6 also develop severe hyperoxaluria [15, 18], but only those with higher urinary calcium excretion develop CaOx crystal deposits [15] and bladder calculi. We have hypothesized that in mice, experimental induction of hyperoxaluria alone is not sufficient to produce CaOx nephrolithiasis. It requires an increase in the urinary excretion of both calcium and oxalate [50]. Male gender also appears to be a critical factor in crystal deposition. We were successful in producing CaOx crystals deposits in only male hypercalciuric Npt2a KO mice. In the case of AGT KO, when killed at age of 6 months, only the male mice (n = 62 of 118) developed CaOx bladder calculi while none of the female AGT KO mice (n = 212) had bladder calculi.
Calcium phosphate crystal deposition: rats
A number of rat models are available to study the role of hypercalciuria in the development of ID CaOx stone disease. A genetic rat model of hyercalciuria was developed by selective breeding of spontaneously hypercalciuric male and female Sprague–Dawley rats. Hypercalciuria in these genetic hypercalciuric (GH) rats is associated with increased intestinal vitamin D receptors, calcium absorption and normal serum vitamin D3 (1,25-dihydroxyvitamin D3) [51, 52]. The GH rats inbred for 29 generations, when put on a 1.2% calcium diet, developed deposits of poorly crystalline CaP, in the urinary space. The frequency of stone formation increased progressively over time. In one experiment, one in five GH rats had stones after 6 weeks, three of five after 12 weeks, and five of five after 18 weeks. When rats of the 45th inbred generation, who excrete 8–10 times more calcium as the controls were given ammonium chloride in their drinking water and a diet of 1.2% calcium, there was a decrease in their urinary supersaturation with respect to brushite and CaOx [53]. However, they still produced stones of poorly crystalline CaP. Induction of hyperoxaluria by the administration of 3% HLP to GH rats resulted in the deposition of CaOx in the urinary space [54].
Experimental induction of hypercalciuria in rats by administration of vitamin D results in the intratubular deposition of CaP [55]. Only the female weanling rats given AIN-76 diet become hypercalciuric and produce intratubular concretions of CaP [56] (Fig. 3). Diet with 30% lactose causes hypercalciuria and CaOx dihydrate crystalluria without any change in the urinary oxalate or crystal deposition in the kidneys [57].
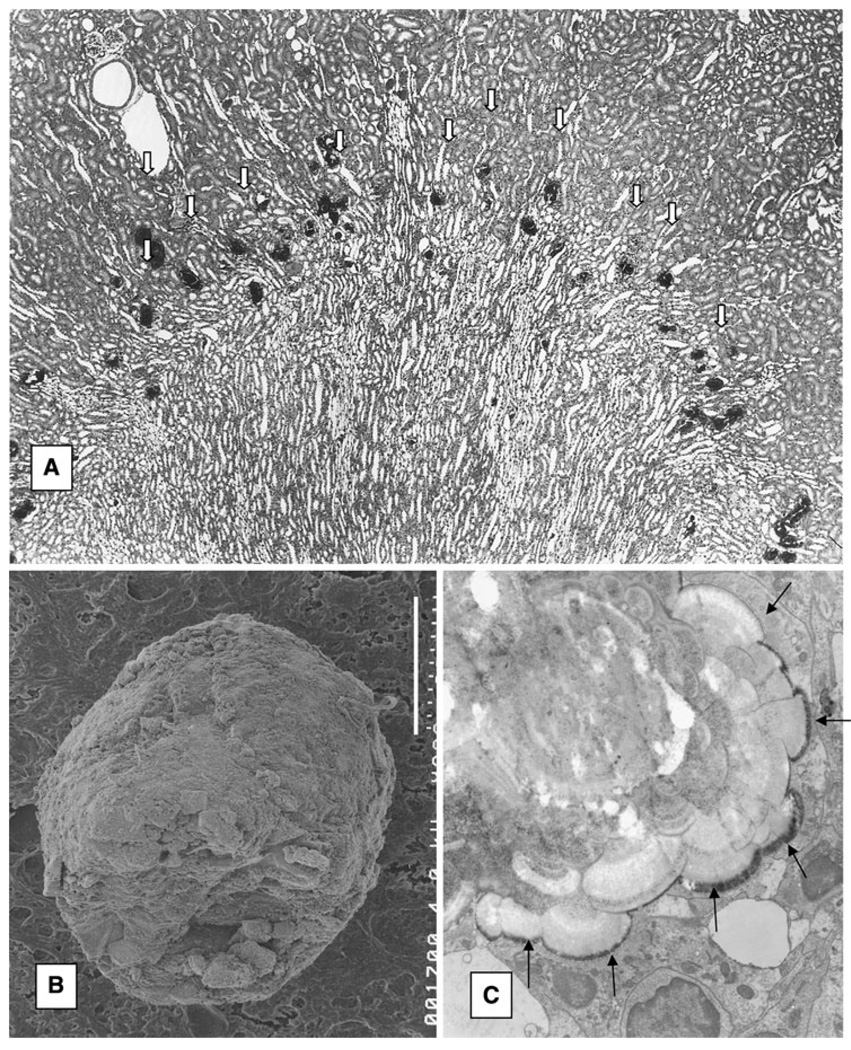
Fig. 3.
CaP crystal deposition in rat kidneys. Female weanling rats were given AIN 76 diet for 28 days, a Renal CaP deposits (arrows) present in the corticomedullary junction. b Scanning electron micrograph of a CaP crystal deposit. Bar 20 µm. c Transmission electron microscopic illustration of CaP deposit. Arrows point at the junction between the crystals and the tubular epithelium. Concentric laminations are evident. Surface of the large deposit is covered with needle-shaped crystals
Calcium phosphate crystal deposition, mice
Tamm–Horsfall and osteopontin null mice
THP, also known as uromodulin, is a kidney-specific protein, synthesized in cells of the thick ascending limbs of the loop of Henle. It coats the luminal side of the epithelium and is most abundant in a human urine (50–100 mg/day) [58]. It is consistently present in the stone matrix and has high affinity with calcium phosphate crystals [59]. Normal THP is a potent inhibitor of CaOx crystal aggregation [60] and reduced urinary excretion of THP by stone formers has been reported [61, 62]. In addition, stone formers are shown to produce THP with abnormal molecular structure which promotes crystal aggregation [63].
The first, direct evidence for THP’s involvement in stone formation was provided by ablating the murine THP gene [46, 64]. Two independent knockout mouse models were created that used the homologous recombination approach to ablate the THP gene in the mouse embryonic stem (ES) cells [64, 65]. Mice derived from the genetically engineered ES cells were completely viable, thrived, bred normally and exhibited no noticeable behavioral changes. Unlike the wild-type mice which strongly expressed THP, THP was completely absent in the kidney and urine of the THP KO mice, as evidenced by Northern blotting, RT-PCR, Western blotting and immunohistochemical staining. When stained with the von Kassa solutions, the kidneys of THP KO mice, but not the wild-type controls, exhibited micro-crystals mainly in the renal papillary region [64]. The great majority of the crystals, consisting exclusively of calcium phosphate, were located interstitially or in the basement membrane zone [66]. Overall, 14–17% of the KO mice developed papillary calcinosis from two different cohorts. Induction of hypercalciuria and hyperoxaluria by the administration of vitamin D3 and ethylene glycol, respectively, lead to copious crystal deposition in the kidneys of 76% of the THP knockout mice. There was no crystal deposition in kidneys of the wild-type mice, with or without the excessive intake of calcium and oxalate. These results indicate that defects in THP production may contribute to crystallization in the kidneys and promote stone formation.
We have recently determined that nephrocalcinosis increases with age and is present in 100% of the 15-month-old THP−/− mice. Interstitial crystals, consisting of poorly crystalline biological apatite, were mostly seen in widened basement membrane zone of the collecting tubules and thin limbs. TEM of the interstitial crystals showed onion-ring or tree-trunk appearance, with electron-dense materials interspersed by less-dense materials presumably consisting of proteinaceous substances. The crystals were not accompanied by inflammatory cell infiltration. Thus, the older THP−/− mice have renal interstitial deposits of poorly crystalline biological apatite with phenotypic characteristics of renal papillary interstitial deposits of ID stone formers.
Ten percent of the mice lacking osteopontin (OPN) spontaneously formed interstitial deposits of calcium phosphate within the renal papillae [46]. Lack of both OPN and THP proteins caused renal crystallization in 39.3% of the double-null mice. Urinalysis revealed elevated concentrations of urine phosphorus and calcium phosphate supersaturation in THP-null and OPN/THP-double null mice, suggesting that impaired phosphorus handling may be linked to interstitial papillary calcinosis in THP- but not in OPN-null mice.
Sodium phosphate co-transporter (Npt2a) null mice
Mice with disrupted Npt2a co-transporter gene (Npt2a−/−) were created by targeted mutagenesis [67]. Npt2a−/− mice are viable and fertile with normal gross appearance and behavior. They exhibit increased urinary excretion of Pi, ∼80% decrease in renal brush border membrane Na/Pi co-transporter, and hypophosphatemia, which leads to increased serum 1,25 (OH)2D levels, overexpression of intestinal calcium channels, increased intestinal calcium hyperabsorption and development of hypercalcemia and hypercalciuria [67, 68]. Npt2a−/− mice develop renal deposits of apatitic CaP [69] (Fig. 4a). Deposits are present in newborn, weanling as well as adult mice.
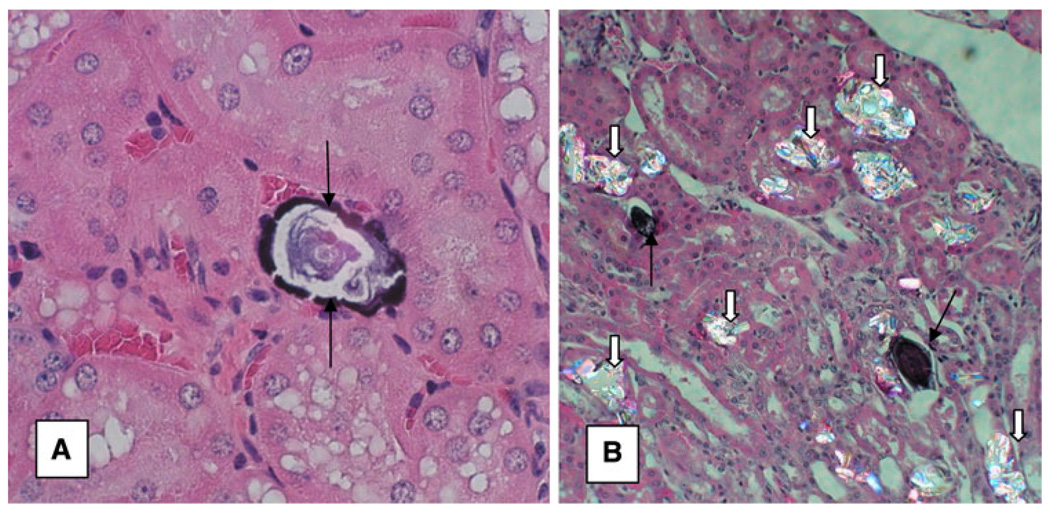
Fig. 4.
Crystal deposits in Npt2a−/− mice, a 11-month-old mice show renal interstitial CaP deposit (arrows). Original magnification ×40. b 4-month-old male mice were fed glyoxylate to induce hyperoxaluria leading to deposition of CaOx crystals (white arrows) in the kidneys. Black arrows point to CaP deposits. CaOx and CaP crystals are deposited at different locations in the renal kidneys. Original magnification ×20
We determined the sites and extent of CaP deposition in the kidneys of male and female KO mice of 5 days, 14 days, 2 months, 4 months, 6 months, and 11 months of age using various microscopic techniques [50]. The kidneys of mice of all ages investigated, from 5 days to 11 months of age, contained both tubular as well as interstitial crystal deposits. By 6 months most crystal deposits appeared located in the renal interstitium. Crystal deposits were von Kossa positive and often showed growth of ring-like internal substructure. Interestingly, many of the deposits were just beneath the papillary surface epithelium. Energy dispersive microanalyses of the deposit revealed the presence of calcium and phosphorus peaks and electron diffraction analysis identified them to be poorly crystalline biological apatite.
Sodium-hydrogen exchanger regulator factor-1 (NHERF-1) null mice
12-week-old NHERF-1 null mice have normal urinary volume and experience hypercalciuria, hyperphosphaturia, hypermagnesuria, and uric aciduria. At 6 months of age there is a correction in urinary phosphate/creatinine ratio. By 1 year these mice are still hypercalcuric with uric aciduria but become hypophosphatemic. These mice show a few interstitial CaP deposits in their renal papillae at 48–54 weeks of age. By 72 months of age there is a many-fold increase in the interstitial CaP deposits. These mice also have very low expression of Npt2. Npt2a links with PDZ containing NHERF-1 through COOH-terminal PDZ-binding domain.
Calcium oxalate/calcium phosphate association in animal models
Since CaP is a substrate for the development and growth of idiopathic (ID) CaOx kidney stones, role of CaP in renal deposition of CaOx has been investigated using both the rat and mice models. Hyperoxaluria was induced in hypercal-ciuric rats and mice, with the expectation that hypercalciuria will lead to CaP deposition and hyperoxaluria-induced CaOx crystals will deposit upon the preexisting CaP crystals. Under similar urinary CaOx or CaP supersaturations, male weanling rats were prone to form CaOx deposits while female weanling rats were susceptible to produce CaP deposits in their kidneys. Crystals in females rats were localized to the corticomedullary junction while in males to the renal papillae. The difference in location of CaOx and CaP crystals within the renal tubules may be the result of urine supersaturation for CaP being reached at levels above collecting duct while that of CaOx in collecting ducts and lower nephron sections. Only the male NPt2a–/– hyper-calciuric mice produced CaOx crystals which did not deposit on the preexisting CaP crystals (Fig. 4b). The results indicate that, deposition of intratubular CaP does not influence intratubular deposition of CaOx, and gender and supersaturation play an important role in the type and location of crystal deposition in the kidneys.
Concluding remarks
CaOx as well as CaP crystal deposition in animal models is induced by increasing urinary excretion of calcium and/or oxalate thereby increasing urinary supersaturation with respect to CaOx or CaP. All the animal models of genetically or environmentally induced hyperoxaluria, be they mice, rats, or pigs lead to CaOx crystalluria. Male rats and mice are susceptible to renal deposition of CaOx crystals under hyperoxaluric conditions while it is difficult to induce CaOx crystal deposition in kidneys of the female rats and mice. Obstruction of ducts located near the medullary surface or papillary tips results in erosion of the overlying ductal epithelium and the urothelium, forming a lesion comparable to Randall Type II precalculus lesion. Induction of hypercalciuria in rats leads to CaP crystals in their renal tubules, renal calyces and pelvis. Thus in rats both hyperoxaluria and hypercalciuria produce intratubular crystal deposits. On the other hand, hypercalciuric mice and mice with crystallization inhibitor deficiency can produce both intratubular as well as interstitial deposits of CaP.
Idiopathic kidney stones are defined as deposits of CaOx/CaP on a subepithelial plaque of poorly crystalline biological apatite present on the renal papillary surface. Thus ID stones have both luminal as well as interstitial components. None of the animal models have so far been able to mimic this condition in which CaP crystals originating in the renal medullary interstitium act as nidus for the development of a CaOx kidney stone in the renal pelvis. The interstitial component appears to be complex to model perhaps a result of a lack of insight into the interstitial environment and thus of capability to manipulate it.
As originally suggested by Randall, there appear to be two basic mechanisms for the formation of stones. In one, stone formation begins when subepithelial interstitial deposits of CaP at the papillary tip lose their epithelial covering and become exposed to the pelvic urine metastable with respect to CaOx. This leads to the deposition of CaOx crystals on the preformed CaP plaque and the formation of CaOx kidneys stone. In the other, high supersaturation leads to crystallization in the terminal collecting ducts. Almost any salt can precipitate in the renal tubules depending upon its supersaturation in the urine. Crystals plug the tubules by aggregating and attaching to the luminal cell surface. Structural features of the renal tubules at the papillary tip may also play a role in crystal retention. The crystal plug when exposed to the metastable pelvic urine promotes the formation of stone. Based on the currently available information therefore we can conclude the following: (1) interstitial nephrocalcinosis, the deposition of poorly crystalline CaP in the renal interstitium is particularly common in the human renal papillae; (2) intratubular nephrocalcinosis involves tubular deposition of a variety of crystals including CaP, CaOx, cystine or uric acid; (3) idiopathic calcific stone formers demonstrate both interstitial as well as intratubular nephrocalcinosis; (4) interstitial nephrocalcinosis may or may not lead to the formation of kidney stones.
Footnotes
Proceedings paper from the 3rd International Urolithiasis Research Symposium, Indianapolis, Indiana, USA, 3–4 December 2009.
References
- 1.Evan AP. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol. 2010;25(5):831–841. doi: 10.1007/s00467-009-1116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randall A. The origin and growth of renal calculi. Ann Surg. 1937;105:1009. doi: 10.1097/00000658-193706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Low RK, Stoller ML. Endoscopic mapping of renal papillae for Randall’s plaques in patients with urinary stone disease. J Urol. 1997;158:2062. doi: 10.1016/s0022-5347(01)68153-9. [DOI] [PubMed] [Google Scholar]
- 4.Low RK, Stoller ML, Schreiber CK. Metabolic and urinary risk factors associated with Randall’s papillary plaques. J Endourol. 2000;14:507. doi: 10.1089/end.2000.14.507. [DOI] [PubMed] [Google Scholar]
- 5.Stoller ML, Low RK, Shami GS, et al. High resolution radiography of cadaveric kidneys: unraveling the mystery of Randall’s plaque formation. J Urol. 1996;156:1263. doi: 10.1016/s0022-5347(01)65565-4. [DOI] [PubMed] [Google Scholar]
- 6.Evan AP, Coe FL, Lingeman JE, et al. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int. 2006;69:2227. doi: 10.1038/sj.ki.5000268. [DOI] [PubMed] [Google Scholar]
- 7.Evan AP, Lingeman J, Coe F, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int. 2007;71:795. doi: 10.1038/sj.ki.5002113. [DOI] [PubMed] [Google Scholar]
- 8.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 9.Evan AP, Lingeman JE, Coe FL, et al. Role of interstitial apatite plaque in the pathogenesis of the common calcium oxalate stone. Semin Nephrol. 2008;28:111. doi: 10.1016/j.semnephrol.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evan AP, Coe FL, Gillen D, et al. Renal intratubular crystals and hyaluronan staining occur in stone formers with bypass surgery but not with idiopathic calcium oxalate stones. Anat Rec (Hoboken) 2008;291:325. doi: 10.1002/ar.20656. [DOI] [PubMed] [Google Scholar]
- 12.Khan SR, Finlayson B, Hackett R. Renal papillary changes in patient with calcium oxalate lithiasis. Urology. 1984;23:194. doi: 10.1016/0090-4295(84)90021-9. [DOI] [PubMed] [Google Scholar]
- 13.Asplin JR, Mandel NS, Coe FL. Evidence of calcium phosphate supersaturation in the loop of Henle. Am J Physiol. 1996;270:F604. doi: 10.1152/ajprenal.1996.270.4.F604. [DOI] [PubMed] [Google Scholar]
- 14.Conyers RA, Bais R, Rofe AM. Oxalosis and the E-Ferol toxicity syndrome. JAMA. 1986;256:2677. doi: 10.1001/jama.1986.03380190047021. [DOI] [PubMed] [Google Scholar]
- 15.Khan SR. Animal models of kidney stone formation: an analysis. World J Urol. 1997;15:236. doi: 10.1007/BF01367661. [DOI] [PubMed] [Google Scholar]
- 16.McMartin K. Are calcium oxalate crystals involved in the mechanism of acute renal failure in ethylene glycol poisoning? Clin Toxicol (Phila) 2009;47:859. doi: 10.3109/15563650903344793. [DOI] [PubMed] [Google Scholar]
- 17.Khan SR, Finlayson B, Hackett RL. Histologic study of the early events in oxalate induced intranephronic calculosis. Invest Urol. 1979;17:199. [PubMed] [Google Scholar]
- 18.Khan SR, Finlayson B, Hackett RL. Experimental calcium oxalate nephrolithiasis in the rat. Role of the renal papilla. Am J Pathol. 1982;107:59. [PMC free article] [PubMed] [Google Scholar]
- 19.Khan SR, Hackett RL. Retention of calcium oxalate crystals in renal tubules. Scanning Microsc. 1991;5:707. [PubMed] [Google Scholar]
- 20.Khan SR, Finlayson B, Hackett RL. Experimental induction of crystalluria in rats using mini-osmotic pumps. Urol Res. 1983;11:199. doi: 10.1007/BF00272279. [DOI] [PubMed] [Google Scholar]
- 21.Marengo SR, Chen DH, Evan AP, et al. Continuous infusion of oxalate by minipumps induces calcium oxalate nephrocalcinosis. Urol Res. 2006;34:200. doi: 10.1007/s00240-006-0043-7. [DOI] [PubMed] [Google Scholar]
- 22.Marengo SR, Zhang A, Traverso EJ. Partitioning of 14C-oxalate excretion in rats during a persistent oxalate challenge. Urol Res. 2008;36:319. doi: 10.1007/s00240-008-0155-3. [DOI] [PubMed] [Google Scholar]
- 23.Khan SR, Glenton PA, Byer KJ. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int. 2006;70:914. doi: 10.1038/sj.ki.5001699. [DOI] [PubMed] [Google Scholar]
- 24.de Bruijn WC, Boeve ER, van Run PR, et al. Etiology of calcium oxalate nephrolithiasis in rats I. Can this be a model for human stone formation? Scanning Microsc. 1995;9:103. [PubMed] [Google Scholar]
- 25.de Water R, Noordermeer C, van der Kwast TH, et al. Calcium oxalate nephrolithiasis: effect of renal crystal deposition on the cellular composition of the renal interstitium. Am J Kidney Dis. 1999;33:761. doi: 10.1016/s0272-6386(99)70231-3. [DOI] [PubMed] [Google Scholar]
- 26.Khan SR, Shevock PN, Hackett RL. Acute hyperoxaluria, renal injury and calcium oxalate urolithiasis. J Urol. 1992;147:226. doi: 10.1016/s0022-5347(17)37202-6. [DOI] [PubMed] [Google Scholar]
- 27.Khan SR, Thamilselvan S. Nephrolithiasis: a consequence of renal epithelial cell exposure to oxalate and calcium oxalate crystals. Mol Urol. 2000;4:305. [PubMed] [Google Scholar]
- 28.Khan SR, Shevock PN, Hackett RL. Urinary enzymes and calcium oxalate urolithiasis. J Urol. 1989;142:846. doi: 10.1016/s0022-5347(17)38928-0. [DOI] [PubMed] [Google Scholar]
- 29.Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol. 1997;157:1059. [PubMed] [Google Scholar]
- 30.Thamilselvan S, Menon M. Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int. 2005;96:117. doi: 10.1111/j.1464-410X.2005.05579.x. [DOI] [PubMed] [Google Scholar]
- 31.Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol. 2004;8:75. doi: 10.1007/s10157-004-0292-0. [DOI] [PubMed] [Google Scholar]
- 32.Toblli JE, Ferder L, Stella I, et al. Enalapril prevents fatty liver in nephrotic rats. J Nephrol. 2002;15:358. [PubMed] [Google Scholar]
- 33.Toblli JE, Ferder L, Stella I, et al. Effects of angiotensin II subtype 1 receptor blockade by losartan on tubulointerstitial lesions caused by hyperoxaluria. J Urol. 2002;168:1550. doi: 10.1016/S0022-5347(05)64519-3. [DOI] [PubMed] [Google Scholar]
- 34.Umekawa T, Hatanaka Y, Kurita T, et al. Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in rat kidneys. J Am Soc Nephrol. 2004;15:635. doi: 10.1097/01.asn.0000113321.49771.2d. [DOI] [PubMed] [Google Scholar]
- 35.Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci. 2004;9:1450. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 36.Gokhale JA, Glenton PA, Khan SR. Localization of Tamm-Horsfall protein and osteopontin in a rat nephrolithiasis model. Nephron. 1996;73:456. doi: 10.1159/000189110. [DOI] [PubMed] [Google Scholar]
- 37.Gokhale JA, Glenton PA, Khan SR. Biochemical and quantitative analysis of Tamm-Horsfall protein in rats. Urol Res. 1997;25:347. doi: 10.1007/BF01294664. [DOI] [PubMed] [Google Scholar]
- 38.Marengo SR, Chen DH, Kaung HL, et al. Decreased renal expression of the putative calcium oxalate inhibitor Tamm-Horsfall protein in the ethylene glycol rat model of calcium oxalate urolithiasis. J Urol. 2002;167:2192. [PubMed] [Google Scholar]
- 39.Katsuma S, Shiojima S, Hirasawa A, et al. Global analysis of differentially expressed genes during progression of calcium oxalate nephrolithiasis. Biochem Biophys Res Commun. 2002;296:544. doi: 10.1016/s0006-291x(02)00840-9. [DOI] [PubMed] [Google Scholar]
- 40.Khan SR, Johnson JM, Peck AB, et al. Expression of osteopontin in rat kidneys: induction during ethylene glycol induced calcium oxalate nephrolithiasis. J Urol. 2002;168:1173. doi: 10.1016/S0022-5347(05)64621-6. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki K, Tanaka T, Miyazawa K, et al. Gene expression of prothrombin in human and rat kidneys: basic and clinical approach. J Am Soc Nephrol. 1999;10 Suppl 14:S408. [PubMed] [Google Scholar]
- 42.Iida S, Inoue M, Yoshii S, et al. Molecular detection of heparan sulfate proteoglycan mRNA in rat kidney during calcium oxalate nephrolithiasis. J Am Soc Nephrol. 1999;10 Suppl 14:S412. [PubMed] [Google Scholar]
- 43.Moriyama MT, Glenton PA, Khan SR. Expression of inter-alpha inhibitor related proteins in kidneys and urine of hyperoxaluric rats. J Urol. 2001;165:1687. [PubMed] [Google Scholar]
- 44.Mandel NS, Henderson JD, Jr, Hung LY, et al. A porcine model of calcium oxalate kidney stone disease. J Urol. 2004;171:1301. doi: 10.1097/01.ju.0000110101.41653.bb. [DOI] [PubMed] [Google Scholar]
- 45.Kaplon DM, Penniston KL, Darriet C, et al. Hydroxypro-line-induced hyperoxaluria using acidified and traditional diets in the porcine model. J Endourol. 2010;24(3):355–359. doi: 10.1089/end.2009.0202. [DOI] [PubMed] [Google Scholar]
- 46.Mo L, Liaw L, Evan AP, et al. Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Horsfall protein, or both. Am J Physiol Renal Physiol. 2007;293:F1935. doi: 10.1152/ajprenal.00383.2007. [DOI] [PubMed] [Google Scholar]
- 47.Wesson JA, Johnson RJ, Mazzali M, et al. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol. 2003;14:139. doi: 10.1097/01.asn.0000040593.93815.9d. [DOI] [PubMed] [Google Scholar]
- 48.Okada A, Nomura S, Higashibata Y, et al. Successful formation of calcium oxalate crystal deposition in mouse kidney by intraabdominal glyoxylate injection. Urol Res. 2007;35:89. doi: 10.1007/s00240-007-0082-8. [DOI] [PubMed] [Google Scholar]
- 49.Okada A, Yasui T, Hamamoto S, et al. Genome-wide analysis of genes related to kidney stone formation and elimination in the calcium oxalate nephrolithiasis model mouse: detection of stone-preventive factors and involvement of macrophage activity. J Bone Miner Res. 2009;24:908. doi: 10.1359/jbmr.081245. [DOI] [PubMed] [Google Scholar]
- 50.Khan SR, Glenton PA. Calcium oxalate crystal deposition in kidneys of hypercalciuric mice with disrupted type IIa sodium-phosphate cotransporter. Am J Physiol Renal Physiol. 2008;294:F1109. doi: 10.1152/ajprenal.00620.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bushinsky DA, Frick KK, Nehrke K. Genetic hypercalciuric stone-forming rats. Curr Opin Nephrol Hypertens. 2006;15:403. doi: 10.1097/01.mnh.0000232881.35469.a9. [DOI] [PubMed] [Google Scholar]
- 52.Bushinsky DA, Parker WR, Asplin JR. Calcium phosphate supersaturation regulates stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2000;57:550. doi: 10.1046/j.1523-1755.2000.00875.x. [DOI] [PubMed] [Google Scholar]
- 53.Bushinsky DA, Grynpas MD, Asplin JR. Effect of acidosis on urine supersaturation and stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2001;59:1415. doi: 10.1046/j.1523-1755.2001.0590041415.x. [DOI] [PubMed] [Google Scholar]
- 54.Bushinsky DA, Asplin JR, Grynpas MD, et al. Calcium oxalate stone formation in genetic hypercalciuric stone-forming rats. Kidney Int. 2002;61:975. doi: 10.1046/j.1523-1755.2002.00190.x. [DOI] [PubMed] [Google Scholar]
- 55.Okamoto N, Aruga S, Tomita K, et al. Chronic acid ingestion promotes renal stone formation in rats treated with vitamin D3. Int J Urol. 2007;14:60. doi: 10.1111/j.1442-2042.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 56.Khan SR, Glenton PA. Deposition of calcium phosphate and calcium oxalate crystals in the kidneys. J Urol. 1995;153:811. [PubMed] [Google Scholar]
- 57.Hennequin C, Tardivel S, Medetognon J, et al. A stable animal model of diet-induced calcium oxalate crystalluria. Urol Res. 1998;26:57. doi: 10.1007/s002400050024. [DOI] [PubMed] [Google Scholar]
- 58.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm- Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis. 2003;42:658. doi: 10.1016/s0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 59.Atmani F, Glenton PA, Khan SR. Identification of proteins extracted from calcium oxalate and calcium phosphate crystals induced in the urine of healthy and stone forming subjects. Urol Res. 1998;26:201. doi: 10.1007/s002400050047. [DOI] [PubMed] [Google Scholar]
- 60.Hess B, Nakagawa Y, Coe FL. Inhibition of calcium oxalate monohydrate crystal aggregation by urine proteins. Am J Physiol. 1989;257:F99. doi: 10.1152/ajprenal.1989.257.1.F99. [DOI] [PubMed] [Google Scholar]
- 61.Glauser A, Hochreiter W, Jaeger P, et al. Determinants of urinary excretion of Tamm-Horsfall protein in non-selected kidney stone formers and healthy subjects. Nephrol Dial Transplant. 2000;15:1580. doi: 10.1093/ndt/15.10.1580. [DOI] [PubMed] [Google Scholar]
- 62.Jaggi M, Nakagawa Y, Zipperle L, et al. Tamm-Horsfall protein in recurrent calcium kidney stone formers with positive family history: abnormalities in urinary excretion, molecular structure and function. Urol Res. 2007;35:55. doi: 10.1007/s00240-007-0083-7. [DOI] [PubMed] [Google Scholar]
- 63.Hess B, Nakagawa Y, Parks JH, et al. Molecular abnormality of Tamm-Horsfall glycoprotein in calcium oxalate nephrolithiasis. Am J Physiol. 1991;260:F569. doi: 10.1152/ajprenal.1991.260.4.F569. [DOI] [PubMed] [Google Scholar]
- 64.Mo L, Huang HY, Zhu XH, et al. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. 2004;66:1159. doi: 10.1111/j.1523-1755.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 65.Bates JM, Raffi HM, Prasadan K, et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 2004;65:791. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 66.Anderson JC, Williams JC, Jr, Evan AP, et al. Analysis of urinary calculi using an infrared microspectroscopic surface reflectance imaging technique. Urol Res. 2007;35:41. doi: 10.1007/s00240-006-0077-x. [DOI] [PubMed] [Google Scholar]
- 67.Beck L, Karaplis AC, Amizuka N, et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA. 1998;95:5372. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tenenhouse HS, Gauthier C, Martel J, et al. Na/P(i) cotransporter (Npt2) gene disruption increases duodenal calcium absorption and expression of epithelial calcium channels 1 and 2. Pflugers Arch. 2002;444:670. doi: 10.1007/s00424-002-0865-2. [DOI] [PubMed] [Google Scholar]
- 69.Chau H, El-Maadawy S, McKee MD, et al. Renal calcification in mice homozygous for the disrupted type IIa Na/Pi cotransporter gene Npt2. J Bone Miner Res. 2003;18:644. doi: 10.1359/jbmr.2003.18.4.644. [DOI] [PubMed] [Google Scholar]