Abstract
Rationale
Monocytes recruited to ischemic myocardium originate from a reservoir in the spleen, and the release from their splenic niche relies on angiotensin-II (Ang-II) signaling.
Objective
Since monocytes are centrally involved in tissue repair after ischemia, we here hypothesized that early ACE inhibitor therapy impacts healing after myocardial infarction partly via effects on monocyte traffic.
Methods and Results
In a mouse model of permanent coronary ligation, Enalapril arrested the release of monocytes from the splenic reservoir and consequently reduced their recruitment into the healing infarct by 45%, as quantified by flow cytometry of digested infarcts. Time-lapse intravital microscopy revealed that Enalapril reduces monocyte motility in the spleen. In vitro migration assays and Western blotting showed that this was caused by reduced signaling through the Ang-II receptor subtype 1. We then studied the long-term consequences of blocked splenic monocyte release in atherosclerotic apoE-/- mice, in which infarct healing is impaired due to excessive inflammation in the cardiac wound. Enalapril improved histological healing biomarkers and reduced inflammation in infarcts measured by fluorescence molecular tomography (FMT-CT) of proteolytic activity. ACE inhibition improved MRI-derived ejection fraction by 14% on day 21, despite initially comparable infarct size. In apoE-/- mice, ischemia reperfusion injury resulted in larger infarct size, enhanced monocyte recruitment and was reversible by Enalapril treatment. Splenectomy reproduced anti-inflammatory effects of Enalapril.
Conclusion
This study suggests that benefits of early ACE inhibition after MI can partially be attributed to its potent anti-inflammatory impact on the splenic monocyte reservoir.
Keywords: Monocyte, spleen, ACE inhibitor, myocardial infarction, heart failure, wound healing
ACE inhibitors, which lower systemic and tissue levels of angiotensin II (Ang-II) by reducing cleavage of the C-terminal dipeptide from Ang-1, were developed as antihypertensive agents. They have evolved into a mainstay of heart failure therapy; current guidelines recommend to start therapy early after MI 1, and are based on clinical trials showing that ACE inhibition decreases mortality 2. Initially, these effects were attributed to hemodynamic unloading of the left ventricle 3. Subsequently, it became clear that ACE inhibitors also decrease hypertrophy and fibrosis through direct effects on heart tissue 4. These well described effects reduce left ventricular remodeling following MI, and therapy is therefore continued indefinitely.
The effect of ACE inhibitors on the monocyte response during the first week after MI is less well understood. There is emerging evidence that Ang-II, which sharply increases in circulation after myocardial infarction, is a pro-inflammatory peptide that closely interacts with the immune system, including monocytes 4-7. Furthermore, Ang-II can modulate the regional cytokine milieu8-11. It has recently been shown that 40-70% of monocytes acutely recruited to the infarct originate from a splenic reservoir 6. In the steady state, monocytes reside in clusters of ~50 cells in the subcapsular red pulp of the spleen. Upon interaction of Ang-II with its cell surface receptor, Ang-II receptor subtype 1 (AT1R), splenic monocytes increase their motility and intravasate into nearby splenic veins. In the mouse, this “emergency reservoir” releases up to one million monocytes within 24 hours post MI 6, which are subsequently recruited into the infarct mainly via interaction of the chemokine MCP-1 with its cognate receptor CCR2 12. The cells then assume a central role in orchestrating the healing wound 13-16. They promote removal of cell debris and extracellular matrix through proteolysis and phagocytosis and regulate tissue repair by stimulating angiogenesis and fibroblasts. However, an excessive inflammatory response is deleterious. In atherosclerotic apoE-/- mice with coronary ligation, the co-existing systemic inflammation associated with hyperlipidemia increases levels of circulating monocytes and their recruitment to the infarct 17. Consequently, scar healing is compromised and development of heart failure accentuated 17, 18. In patients, increased levels of blood monocytes during MI also correlate with enhanced left ventricular dilation and adverse outcome 19-21.
Here we hypothesized that ACE inhibition reduces splenic release of monocytes, decreases their recruitment into the infarct, curbs excessive infarct inflammation, and promotes coordinated wound healing. We further propose that the effect of ACE inhibition on the innate immune system presents a new mechanism that at least partially explains the reduction of heart failure following MI.
Methods
A detailed method section is available online.
Animal models
Female C57BL/6J and apoE-/- mice were purchased from Jackson Labs. Cx3cr1gfp/+ mice were obtained by breeding Cx3cr1gfp/gfp mice with C57BL/6J mice. Cx3cr1gfp/+ mice have one Cx3cr1 allele replaced with cDNA encoding eGFP, and can be used to track monocytes 22. ApoE-/- mice had an average age of 45 weeks and were on a high-cholesterol diet. The institutional animal welfare committee approved the research reported.
MI, ischemia reperfusion injury and splenectomy were performed as described online 6, 23. Mice were treated with a dose of 100 mg/kg 24, 25 Enalapril daily for the acute studies (sacrifice one day after MI) or 20 mg/kg Enalapril daily 26, 27 for the chronic studies. Treatment was started two days before MI and continued for seven days thereafter. In additional cohorts, we initiated Enalapril treatment one hour or twenty-four hours after coronary ligation. Hydralazine was given at a dose of 15 mg/kg 28 daily. Treatment was done by gastric gavage.
Histology
Histology of spleens and hearts was assessed as described in the online methods.
Flow Cytometry
Mice were sacrificed on days 1, 2, 3 and 5 after MI and 1 day after ischemia reperfusion injury for flow cytometry (n = 3 – 5 mice per group and time point). Spleens, hearts and blood were prepared and analyzed for total organ monocyte numbers by multicolor flow cytometry 18.
ELISA for serum Ang-II levels
Ang-II concentration in blood was determined with an Ang-II ELISA.
Intravital microscopy
Intravital microscopy of splenic monocytes was done in Cx3cr1gfp/+ mice. During isoflurane anesthesia, the spleen was exteriorized and imaged with a prototypical intravital laser scanning microscope (IV100, Olympus Corporation) 6, 29.
In vitro migration
Migration experiments using Ang-II as a chemoattractant were performed using sorted monocytes from spleen. For the receptor blocking condition, cells were pretreated with the AT1R antagonist losartan.
Western for Ang-II Type 1 (AT1R) receptor on splenic monocytes
Splenic monocytes were isolated by FACS and dimerization of the AT1 receptor was assessed by Western blotting.
Quantitative PCR
Infarct tissue was examined for expression of markers of inflammatory monocytes (Ly-6C), differentiated macrophages (CD68; Mac3), TNF-α, TGF-β, myeloperoxidase, and appropriate controls (GAPDH). TGF-β mRNA was also measured in monocytes isolated from the heart.
FMT-CT
On day 1 after MI we performed FMT-CT imaging 17, 30, 31 to interrogate the magnitude of inflammation 24 hours after injection of 5 nmol of a pan-cathepsin protease sensor (Prosense-680, PerkinElmer) in apoE-/- mice with and without Enalapril treatment and apoE-/- mice that were splenectomized at the time of coronary ligation.
MRI
We performed in vivo MRI on day 21 after MI in apoE-/- mice with and without Enalapril treatment and in apoE-/- mice that were splenectomized at the time of coronary ligation 23.
Ischemia reperfusion injury
Ischemia reperfusion injury (IRI) was studied in wild type mice, apoE-/- with and without Enalapril treatment, and in apoE-/- that were splenectomized immediately prior to ischemia. The area at risk was assessed using fluorescent microspheres, and the resulting infarct size by triphenyltetrazolium chloride (TTC) staining.
Blood pressure measurements
Blood pressure was monitored using the non-invasive tail cuff method 32.
Statistics
Results are expressed as mean ± SEM. Statistical comparisons between two groups were evaluated by Student's t-test and corrected by ANOVA for multiple comparisons. A value of p < 0.05 was considered to indicate statistical significance.
Results
ACE inhibition prevents the release of splenic monocytes in acute MI
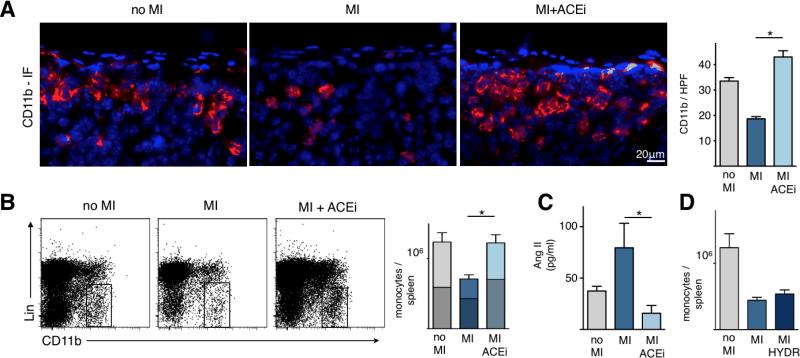
Myocardial injury or administration of Ang-II results in increased motility of monocytes in the spleen 6. We therefore investigated if lowering levels of Ang-II through ACE inhibition impacts splenic monocytes. First, we assessed monocyte numbers in the spleen on day 1 after permanent coronary ligation with and without Enalapril treatment. The subcapsular region is the site where monocytes are stored in the spleen 6. Immunoflurescence staining for CD11b showed significant reduction of CD11b+ cells in the subcapsular region after MI. Treatment with Enalapril completely abolished splenic loss of monocytes after MI (p<0.0001; Figure 1A).
Figure 1. ACE inhibition abolishes the release of splenic monocytes in acute MI.
A. Splenic sections stained with CD11b-specific antibodies (red) and 4′,6′-diamidino-2-phenylindole (DAPI) (blue) showing the subcapsular red pulp. Right panel shows enumeration of CD11b+ cells in the subcapsular red pulp.
B. Flow cytometric analysis of spleens. Rectangle indicates monocyte gate, Lin: lineage markers. Bars are divided to display contribution of monocyte subsets (upper part / lighter color encodes Ly-6Chigh monocytes, whereas the lower / darker part shows Ly-6Clow monocytes).
C. Serum levels of Angiotensin II measured by ELISA.
D. Total number of monocytes in the spleen in control mice (no MI) or 1 day after MI with and without Hydralazine (HYDR) treatment, a direct vasodilator. Mean ± SEM; * p < 0.05.
To quantify total monocyte numbers in the whole spleen, we used flow cytometry one day after coronary ligation. MI induced massive release of monocytes from the spleen. Monocyte numbers in spleens of mice with MI were reduced by 42% compared to spleens from naive mice (Figure 1B, p = 0.01). In contrast, infarcted mice treated with Enalapril showed practically no release of splenic monocytes (no MI, 1.24 × 106 monocytes per spleen; MI plus Enalapril, 1.22 × 106 monocytes per spleen; p = n.s.; Figure 1B). The effect was observed for both subsets, Ly6-Chigh as well as Ly6-Clow monocytes.
Next we investigated if the effect on splenic monocytes was related to vasodilatory properties of ACE inhibitors. We confirmed that Enalapril significantly reduced Ang-II serum levels in plasma (p < 0.05; Figure 1C). We then treated mice with the direct vasodilator Hydralazine and chose a dose of 15mg/kg/day, which had previously been shown to reduce systolic blood pressure to < 80 mmHg 28. Flow cytometric analysis of the spleen showed that Hydralazine did not reduce the release of monocytes after MI (p = n.s. versus untreated MI, Figure 1D). We thus concluded that monocyte retention in the spleen by Enalapril is likely not related to its vasodilatory properties, since lowering of blood pressure with a direct vasodilator did not reproduce its effect. Blood pressure data in various cohorts are shown in Online Table I.
ACE inhibition reduces the motility of splenic monocytes
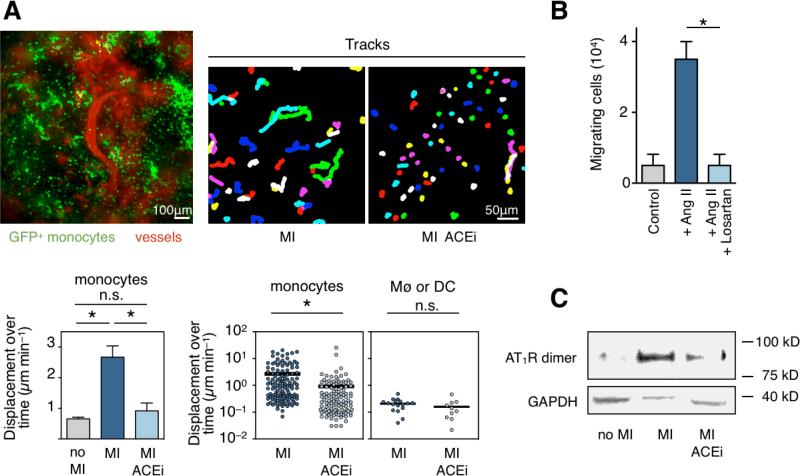
Next we aimed to investigate how ACE inhibition reduces monocyte release from the spleen. We employed time-lapse intravital microscopy, a techique that allows to track endogenous monocytes in the subcapsular red pulp of the spleen in live animals 6. We investigated the spleens of Cx3cr1gfp/+ mice, in which one Cx3cr1 allele is replaced with cDNA encoding eGFP. Thus, cells that express the fractalkine receptor are rendered GFP+ and can be followed with fluorescence imaging. Within the splenic capsule, 95% of the GFP+ cell population consists of monocytes, macrophages and dendritic cells 6. The present study confirmed that splenic monocytes increase motility after MI (4-fold increase, p < 0.01, Figure 2A, Online Movie I). Previously, it was shown that this increase in motility leads to intravasation into sinusoids and splenic veins and departure of the cells from the organ 6. When treated with Enalapril, monocytes did not increase motility after MI (Figure 2A, Online Movie I). Splenic macrophages or dendritic cells showed very low displacement and there was no difference between both groups, as expected (Figure 2A).
Figure 2. ACE inhibition curtails motility and release of splenic monocytes through reduced Ang-II/AT1R signalling.
A. Intravital microscopy of GFP+ cells in the spleen subcapsular red pulp of Cx3cr1gfp/+ mice. Example from a control mouse (no MI, upper left panel): cells expressing the fractalkine receptor are GFP+ (green), vessels are shown in red as highlighted by a fluorescent blood pool agent. Tracks are shown for monocytes in spleens of mice 1 day after coronary ligation (upper middle and right panel). Average displacement over time of all GFP+ splenic monocytes is shown on lower left (mean ± SEM, * p < 0.01). Displacement over time of single splenic monocytes is shown in lower middle panel and splenic macrophages or DCs on the right.
B. In vitro migration of splenic monocytes without the presence of Ang II (control), in response to Ang II and after treatment with AT1R blocker Losartan.
C. Western blot analysis of the AT1R on splenic monocytes in the steady state (no MI), and 1 day after MI with or without Enalapril. GAPDH was used as loading control.
In vitro cell migration experiments corroborated the importance of Ang-II signaling for monocyte motility. Ang-II induced rigorous migration of monocytes isolated from the spleen; however, co-treatment with the AT1R antagonist Losartan reduced this effect significantly (Figure 2B), suggesting that mobilization of splenic monocytes requires Ang-II/AT1R signaling.
Next, we investigated dimeric forms of the AT1R on monocytes sorted from spleens by Western blot. Ang-II/AT1R signaling requires dimerization and covalent cross-linking of the receptor, an event that can be quantified by Western blot using reduced gels 6, 33. We found that MI triggers AT1R dimerization in splenic monocytes. However, Enalapril reduced this effect (Figure 2C). These data describe the mechanism how ACE inhibition blocks splenic monocyte release: they indicate that ACE inhibition reduces Ang II/AT1R signaling in monocytes, which directly affects monocyte motility, and thus departure from the splenic reservoir.
Blocking of the splenic monocyte release diminishes recruitment to the infarct
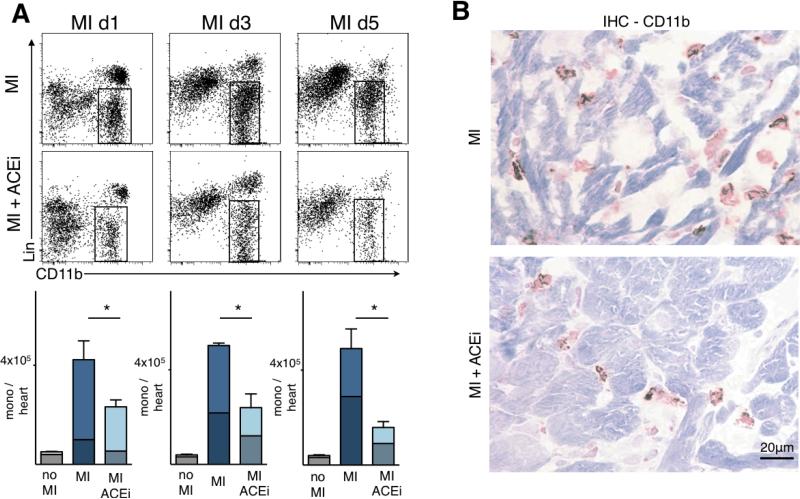
Reduced mobilization of splenic monocytes by Enalpril should decrease accumulation of these cells in ischemic myocardium. Flow cytometry confirmed a marked reduction in the number of monocytes accumulating in the infarct on day 1, 3 and 5 after permanent coronary ligation (Figure 3A). The inflammatory response on day 1 after MI is dominated by Ly-6Chigh monocytes 18. Accordingly, the impact of Enalapril was more profound on this subset (Figure 3A). However, Ly-6Clow monocyte numbers were also reduced in the heart, especially at later time points (Figure 3A). Immunoreactive staining for CD11b and MAC-3 confirmed that Enalapril reduced the number of myeloid cells in one-day old infarcts (Figure 3B). The number of CD11b+ cells per high power field was reduced from 52 ± 4 to 31 ± 4 (p < 0.01), and the area positive for MAC-3 from 5.8 ± 0.7 to 3.5 ± 0.4% (p < 0.01).
Figure 3. Reduction of infarct inflammation by Enalapril.
A. Flow cytometric analysis of hearts after MI: dot plots from untreated animals (top row) and Enalapril treated animals (lower row) and the according enumeration (bottom) on days 1, 3 and 5 after coronary ligation. Bars are divided to display contribution of monocyte subsets (upper part / lighter color encodes Ly-6Chigh monocytes, whereas the lower / darker part shows Ly-6Clow monocytes). Mean ± SEM; * p < 0.05.
B. Immunohistochemistry for CD11b+ cells in a one day old infarct.
Since some patients will not be on ACE inhibitor therapy prior to their first MI, we next tested the effects of Enalapril 1 hour or 24 hours after coronary ligation. This experiment models the situation of a first MI, when treatment is started early after ischemia. The impact of ACE inhibition on monocyte flux was preserved, but somewhat attenuated when started with a 24 hour delay, probably because there was a period of uninhibited monocyte release after MI (Online Figure I).
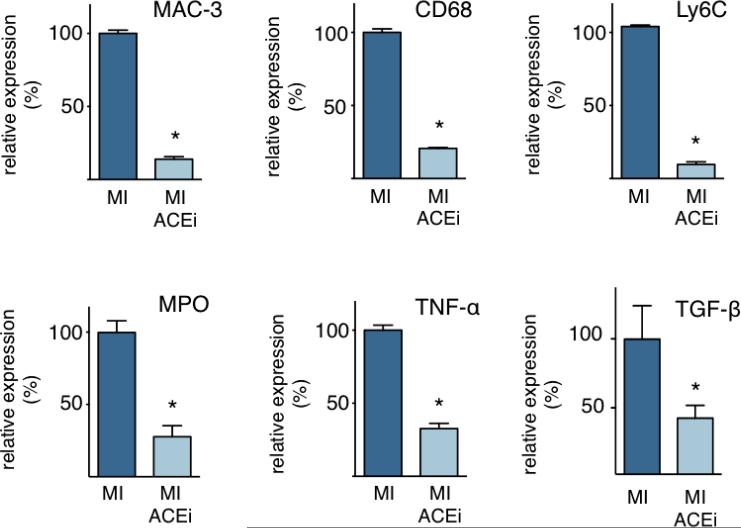
Quantitative PCR of infarct tissue showed that Enalapril profoundly reduced expression of TGF-β, myeloperoxidase, and TNF-α (Figure 4). MAC-3, CD68, and Ly-6C, which are genes primarily expressed by inflammatory monocytes and macrophages, were also reduced (Figure 4). Further, we assessed the mRNA level of TGF-β in monocytes that were isolated by flow activated cell sorting and found levels that were 4.3-fold higher than in infarct tissue (p < 0.01 versus infarct tissue). Thus, we concluded that monocytes may be a source of TGF-β after MI.
Figure 4. Enalapril reduces expression of inflammatory genes in the infarct.
Quantitative PCR from infarct tissue on day 1 after MI showing relative expression of MAC-3, CD68, Ly-6C, MPO, TNF-α and TGF-β. Mean ± SEM; * p < 0.05.
ACE inhibition reduces inflammation in infarcts of apoE-/- mice
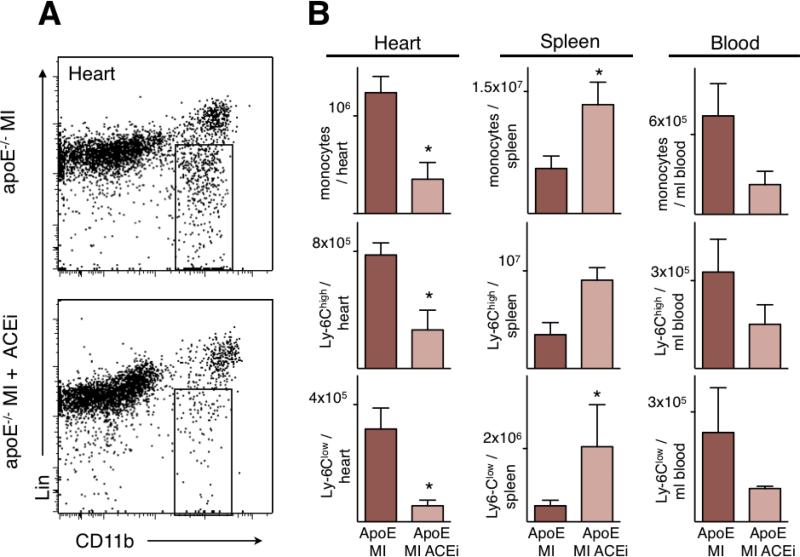
We next tested whether inhibiting the mobilization of the splenic monocyte reservoir affects cardiac wound healing. We chose to investigate infarct healing in apoE-/- mice, because their heightened systemic inflammation, specifically the increased availability of monocytes in circulation, may resemble patients with atherosclerosis better 17. This model is particularly helpful when innate immune responses to ischemia and tissue repair are studied 34. Flow cytometric analysis of apoE-/- mice on day 1 after permanent coronary ligation showed that Enalapril prevented the release of splenic monocytes and reduced blood monocytosis (Figure 5). Notably, monocyte numbers in the infarct were reduced to levels seen in wild type mice (wild type, 0.42 × 106 ± 0.1 × 106; apoE-/-, 1.23 × 106 ± 0.1 × 106; apoE-/- Enalapril, 0.35 × 106 ± 0.1 × 106). The treatment reduced the number of both, Ly-6Chigh and Ly-6Clow monocytes (Figure 5B).
Figure 5. Impact of Enalapril on monocyte flux in apoE-/- mice.
A. Representative flow cytometry dot plots of infarct tissue from apoE-/- mice 1 day after MI. The gate shows CD11b positive lineage negative monocytes and macrophages. Lin: Lineage markers.
B. Quantitation of monocytes and their subsets by flow cytometry in heart, spleen and blood of apoE-/- mice 1 day after MI. Mean ± SEM; * p < 0.05.
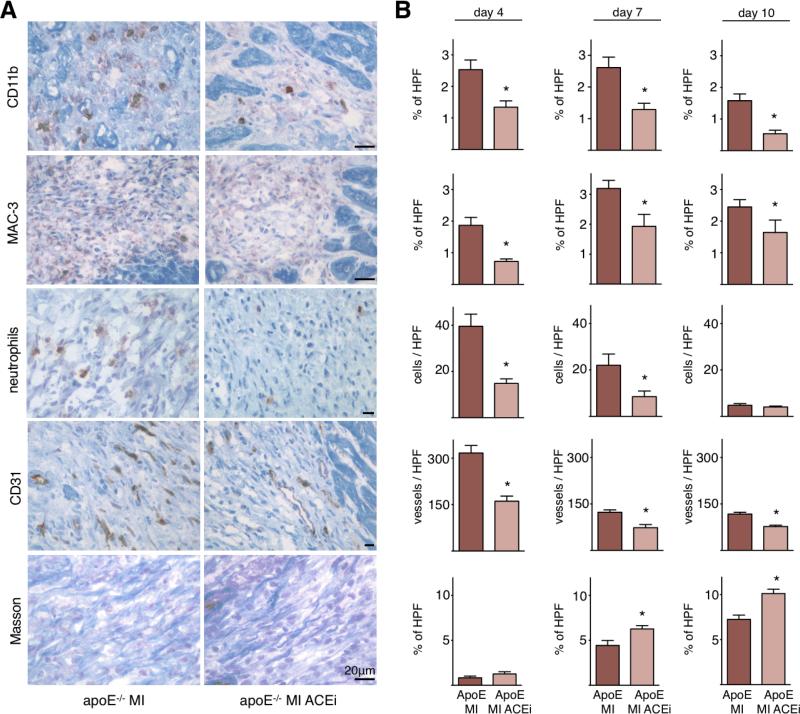
Wound healing was then assessed by histology on day 4, 7 and 10 after coronary ligation. Immunoreactive staining of the infarcted tissue from apoE-/- mice showed that Enalapril supported the resolution of inflammation, as numbers of inflammatory cells, including monocytes, neutrophils and macrophages were reduced (Figure 6). We had previously found that the infarcts of apoE-/- mice contain more microvessels and less collagen, likely due to increased delivery of pro-angiogenic factors and proteases by monocytes 18. This was reversed in the current study: Enalapril decreased the number of neovessels and augmented collagen content in the border zone (Figure 6). Thus, reduction of inflammation by Enalapril improved infarct healing in apoE-/- mice.
Figure 6. Impact of Enalapril on infarct healing in apoE-/- mice.
A. Representative microscopy images of respective stains on day 7.
B. Quantitation on day 4, 7 and 10 after permanent coronary ligation. ApoE: apoE-/- mice, HPF: high power field. Mean ± SEM; * p < 0.05.
Early ACE inhibitor treatment reduces protease activity and improves LV remodeling in apoE-/- mice with MI
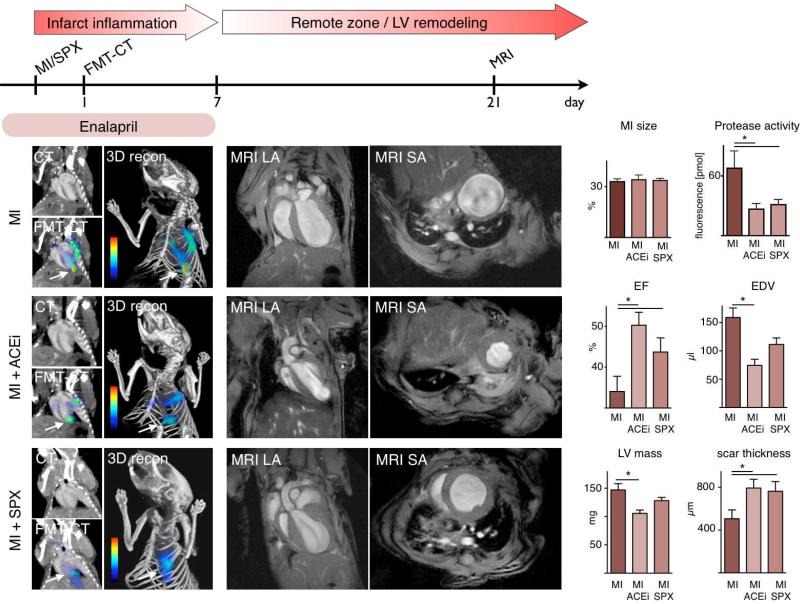
We next evaluated the effects of improved healing in Enalapril-treated mice on left ventricular remodeling. Infarct size and proteolytic activity were measured by FMT-CT 17 on day 1 after MI, followed by MRI on day 21 to evaluate left ventricular anatomy and function. A group of apoE-/- mice received 20 mg/kg Enalapril (see flow chart in Figure 7) and was compared to untreated apoE-/- mice. Importantly, treatment was terminated on day 7 to study ACE inhibitor effects during the early healing phase after MI. CT imaging found that infarct size 35 on day 1 was similar between study groups. To assess infarct inflammation in vivo, we quantitated proteolytic activity in infarcts using an activatable fluorescent protease sensor and FMT-CT 17, 30. Regions of interest were identified in the FMT data set based on anatomical information provided by fusion with CT images. In line with flow cytometric results showing lower monocyte numbers, Enalapril reduced proteolytic activity in the infarct (Figure 7, p < 0.05). Enalapril therapy was discontinued on day 7, when inflammation in murine infarcts subsides and actions of the ACE inhibitor on the remote myocardium would predominate. Mice were then followed up on day 21 after MI by MRI volumetry. Untreated mice showed substantial left ventricular remodeling and a reduced ejection fraction. However, MRI parameters improved in the Enalapril treated cohort. These mice had higher ejection fractions and smaller end-diastolic volumes (Figure 7, p < 0.05). Increased scar thickness (Figure 7, p < 0.05) indicated that the treatment had influenced infarct healing, as infarct expansion and thinning is a hallmark of impaired healing 15. Left ventricular mass was also reduced (Figure 7).
Figure 7. Enalapril reduces early protease activity and subsequently LV remodeling in apoE-/- mice with MI.
Top: Flow chart illustrating the set up of the study.
Left: FMT-CT 1 day after MI. Arrows denote apical infarct signal. Additional activation of the protease sensor is seen in the vicinity of the decending aorta and may be due to inflammatory atherosclerotic lesions. Infarct size was measured by contrast-enhanced CT.
Middle and right panels: Cardiac MRI, LA: long axis, SA: short axis view. ACEi: Enalapril treatment, SPX: splenectomy. Mean ± SEM; * p < 0.05.
When Enalapril treatment was started one hour after coronary ligation, the strong anti-inflammtory effects seen by FMT-CT were preserved (protease sensor activation: MI 67.6 ± 17.0 pmol, MI Enalapril 26.2 ± 8.2 pmol, p < 0.05). In the same mice, MRI derived ejection fraction on day 21 was higher when compared to untreated mice (control MI, 34±4%, MI Enalapril 43±2%, p < 0.05).
Early ACE inhibitor treatment has beneficial effects on left ventricular hemodynamics, and reduces local ACE activity in the heart. We wanted to explore to what extent the impact on the monocyte flux contributes to the overall benefits of Enalapril. We thus neutralized the splenic monocyte reservoir by surgical removal of the spleen at the time of coronary ligation. As in Enalapril treated mice, this results in a reduced availability of splenic monocytes, but leaves non-spleen targets of Enalapril untouched. Hence, this cohort models the isolated splenic effect of ACE inhibition. FMT-CT showed a similar reduction of protease activity in the infarct of apoE-/- mice by Enalapril and splenectomy (Figure 7, p < 0.05). This is in line with the notion that both procedures reduce the recruitment of monocytes into the infarct, cells that are the major source of proteases in MI 31. In apoE-/- mice, splenectomy had a beneficial effect on left ventricular remodeling, albeit to a lesser degree than treatment with Enalapril (Figure 7, p < 0.05). Compared to untreated apoE-/- mice, the EF was improved by 9% in splenectomized mice, and by 16% in mice treated with Enalapril. Similar trends were observed for the end-diastolic volume, left ventricular mass and scar thickness, all measured by MRI (Figure 7).
Ischemia reperfusion injury is increased in apoE-/- mice and ameliorated by Enalapril as well as splenectomy
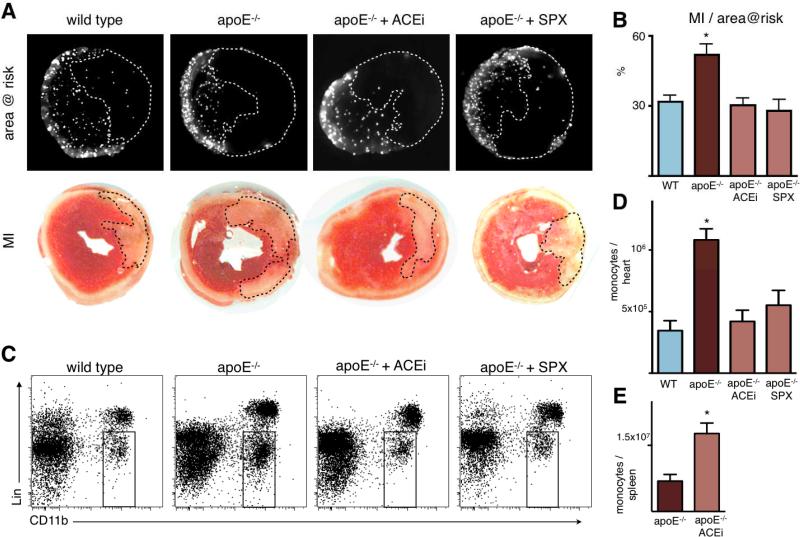
Most patients with acute MI reach the hospital in time for reperfusion therapy. We therefore investigated the role of monocytes in ischemia reperfusion injury. During thirty-five minutes of ischemia, mice were injected with fluorescent microspheres to delineate the area at risk. Twenty-four hours after reperfusion, infarct size was determined by TTC staining, and area at risk by fluorescence imaging of myocardial rings (Figure 8A). Compared to wild type mice, the infarct/area at risk ratio was profoundly increased in apoE-/- mice (Figure 8B), and this was paralleled by an increased number of monocytes recruited to the injured myocardium (Figure 8C, D). Treatment with Enalapril and surgical removal of the splenic monocyte reservoir reduced the infarct/area at risk ratio in a similar fashion. Likewise, the number of monocytes recruited to the heart was reduced in parallel by Enalapril treatment and splenectomy (Figure 8C, D). Analysis of spleens revealed that Enalapril inhibited the release of the splenic monocyte reservoir following ischemia reperfusion injury (Figure 8E).
Figure 8. Ischemia reperfusion injury is enhanced in apoE-/- mice with inflammatory atherosclerosis, and attenuated by Enalapril and splenectomy.
A: Fluorescence reflectance images display the area at risk, which is void of microspheres injected during ischemia. Lower panel shows TTC staining of the same myocardial short axis slice.
B: Quantification of ischemia reperfusion injury as a percentage of the area at risk. Mean ± SEM; * p < 0.05 versus all other groups.
C: Representative flow cytometry dot plots of heart tissue from apoE-/- mice 1 day after ischemia. The gate shows CD11b positive lineage negative monocytes and macrophages.
D: Quantification of CD11b positive lineage negative monocytes/macrophages. * p < 0.05 versus all other groups.
E: Flow cytometric quantification of splenic monocytes 24 hours after ischemia in apoE-/- mice with and without Enalapril treatment (ACEi). Mean ± SEM; * p < 0.05.
Discussion
In 2010, an estimated 785,000 Americans will have a new acute coronary syndrome, one every 25 seconds 36. Most of these patients will receive ACE inhibitor therapy within 24 hours. Here we identified a new mechanism that contributes to the beneficial effects of early ACE inhibitor therapy after myocardial infarction. In mice with coronary ligation, Enalapril decreased Ang-II/AT1R signaling on monocytes in the spleen, and thus inhibited their massive mobilization from the splenic reservoir. Preventing the release of splenic monocytes subsequently controlled their recruitment in infarcts, and short-term treatment during the wound healing phase after MI had a profound long-term impact on LV remodeling.
Coronary ligation in apoE-/- mice allows the study of MI in the context of pre-existing chronic inflammation 17. In these mice, hyperlipidemia induces a progressive increase of the monocyte pool 37. The cells are recruited into the vessel wall, a process that is now well understood to drive atherosclerotic lesion formation and progression 38, 39. We chose this model because it likely reproduces innate immune processes during infarct repair in man with higher fidelity than a model of coronary ligation in otherwise ‘healthy’ wild type mice 34, as patients experience infarcts due to thrombotic complication of an atherosclerotic plaque. The pre-existing heightened immune activity and the increased levels of blood monocytes at the time of MI result in enhanced recruitment of monocytes into the infarct 17. Blood monocytosis by itself (i.e., in the absence of atherosclerosis or hypercholesterolemia, but induced by LPS injections) causes excessive monocyte recruitment in infarcts, impaired infarct healing and accelerated left ventricular dilation 17. Of note, a parallel relationship of increased blood monocyte levels and left ventricular remodeling was recently described in patients after MI 21. In the current study, Enalapril substantially reduced the mobilization of monocytes from the spleen and lowered their numbers in blood and infarct of wild type as well as apoE-/- mice. Therefore, the anti-inflammatory properties of ACE inhibition may mitigate the accentuated immune activity in patients with acute coronary syndromes and thus partly contribute to improving their prognosis 19-21.
Histological assessment of Enalapril-treated apoE–/– mice after MI showed faster resolution of inflammation, and normalization of wound healing parameters such as number of neovessels and collagen content in the border zone. Direct action of Ang-II on fibroblasts promotes collagen synthesis and ACE inhibition reduces collagen content in the remote zone 40, 41 and scar 42. In the current study, we found increased collagen content in the scar of treated apoE-/- mice. Because Enalapril drastically reduced numbers of inflammatory monocytes and protease activity, it likely changed the balance of matrix breakdown and synthesis 43. Increased scar thickness measured by MRI indicates that improved healing reduced infarct expansion, which then resulted in attenuated left ventricular remodeling and higher ejection fraction in Enalapril treated mice. Anatomic and functional MR data measured 3 weeks after MI were improved although therapy was limited to the first week, suggesting that treatment during the early period of infarct healing garners long-term benefits. ACE inhibition has also been shown to modulate inflammatory cytokine expression, for instance TNF-α or TGF-β 8, which may influence left ventricular remodeling and heart failure. Since these regulators of innate immunity 14 are found in monocytes, it is likely that the effects of ACE inhibition on monocyte traffic, at least partially, contribute to a change in the regional cytokine milieu. This is supported by the 4.3-fold higher TGF-β mRNA levels in monocytes when compared to infarct tissue levels.
Similar as in previous reports which showed increased recruitment of monocytes to the infarct after permanent coronary artery occlusion in apoE-/- mice with atherosclerosis and blood monocytosis 17, we found that monocyte recruitment is more than doubled after ischemia reperfusion injury in these mice. The majority of recruited cells belonged to the inflammatory Ly-6Chigh subset, which carry high payloads of potentially harmful inflammatory mediators. When compared to wild type, the infarct size was increased in apoE-/- mice. Treatment with Enalapril as well as splenectomy reversed the number of recruited monocytes and the quantity of infarcted tissue to levels seen in wild type mice. The lower number of monocytes in heart tissue was due to inhibited splenic release following ACE inhibitor treatment. These findings position monocytes as therapeutic targets in ischemia reperfusion injury, and confirm that ACE inhibitors reduce infarct size 44-46 in a model that accounts for the heightened activity of the immune system in atherosclerosis 38.
ACE inhibitor therapy has many reported targets, including beneficial systemic hemodynamic effects and decrease of Ang-II tissue levels 4. To study the relative contribution of splenic effects, we removed the splenic monocyte reservoir surgically in apoE-/- mice. Infarct protease activity was reduced to the same extent by splenectomy and Enalapril, which suggests dominance of the splenic effects in the acute anti-inflammatory properties of Enalapril. Ejection fraction on day 21 post MI was improved partially when compared to Enalapril treatment (9 versus 16% improvement over untreated mice with MI). It is important to stress that these beneficial effects of splenectomy were observed in apoE-/- mice, which recruit too many monocytes to the healing infarct 17. In healthy individuals, the existence of a rapidly deployable reservoir of myeloid cells is likely an evolutionary advantage as it allows the immune system to respond to injury quickly. The spleen may have many favorable effects for wound healing, and health in general. This is highlighted by a study of veterans that lost their spleen due to a World War II injury and consequently had an increased cardiovascular mortality 47. Also, the wholesale removal of the organ may compromise other, not yet known protective functions in infarct healing.
Current guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA) recommend that oral ACE inhibitor therapy should be started within the first 24 hours of suspected acute myocardial infarction in patients without contraindications. If the observations of this study translate into humans, the treatment would also curb inflammation in patients with a raised level of immune activity due to co-existing atherosclerotic disease. The data presented here provide additional mechanistic insight into the mortality reduction by early ACE inhibitor treatment found in clinical studies (HEART 48, SMILE 49), and suggest that systemic or local infarct inflammation may be an important therapeutic target for guiding therapy in individual patients. The level of inflammation in the infarct could be monitored by monocyte/macrophage imaging. To this end, the phagocytic or inflammatory properties of myeloid cells could be harnessed for MR imaging 50, 51. Alternatively, as done in this study, a key monocyte function could serve as an imaging biomarker. We used fluorescence molecular tomography to quantify protease-dependent activation of an optical beacon in the myocardium, which can be accomplished with nuclear imaging in patients 52. While these technologies are not yet translated into clinical care, the level of circulating monocytes may offer an approximation for the number of cells in the infarct. However, this association needs to be confirmed in patients.
In conclusion, we show that ACE inhibitor treatment has a profound impact on the innate immune response after MI, and that this newly discovered mechanism contributes substantially to the benefits of early ACE inhibitor therapy after MI. The inhibition of monocyte mobilization from their splenic reservoir represents a powerful anti-inflammatory action which may have therapeutic implications beyond treatment of hypertension and heart failure.
Supplementary Material
Acknowledgements
We gratefully acknowledge the help of Elisabeth Zhang, MS; Rainer Kohler, PhD, Martin Etzrodt, MS, Brena Sena, MS.
Funding Sources
This work was funded in part by grants from NHLBI (R01HL095629 and R01HL096576) and American Heart Association (SDG0835623D) to MN, R24-CA92782 and UO1-HL08073 to RW, and Deutsche Herzstiftung e. V. to FL, and the Korea Research Foundation Grant (KRF-2009-013-E00027) to WWL.
Non-standard Abbreviations and Acronyms
- ACEi
Angiotensin Converting Enzyme Inhibitor
- Ang-II
angiotensin II
- AT1R
Ang-II type 1 receptor
- IVM
Intravital Microscopy
- IRI
Ischemia reperfusion injury
- FMT-CT
Fluorescence Molecular Tomography in conjunction with Xray Computed Tomography
- MRI
Magnetic Resonance Imaging
- SPX
Splenectomy
Footnotes
Disclosures
None.
References
- 1.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SCJ, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation. 2004;110:e82–292. [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr., Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 4.Dzau VJ, Bernstein K, Celermajer D, Cohen J, Dahlof B, Deanfield J, Diez J, Drexler H, Ferrari R, Van Gilst W, Hansson L, Hornig B, Husain A, Johnston C, Lazar H, Lonn E, Luscher T, Mancini J, Mimran A, Pepine C, Rabelink T, Remme W, Ruilope L, Ruzicka M, Schunkert H, Swedberg K, Unger T, Vaughan D, Weber M. Pathophysiologic and therapeutic importance of tissue ACE: a consensus report. Cardiovasc Drugs Ther. 2002;16:149–160. doi: 10.1023/a:1015709617405. [DOI] [PubMed] [Google Scholar]
- 5.Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, Phillips LK, Goldstein MJ, Bhat R, Raine CS, Sobel RA, Steinman L. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci U S A. 2009;106:14948–14953. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swirski F, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo J, Kohler R, Chudnovskiy A, Waterman P, Aikawa E, Mempel T, Libby P, Weissleder R, Pittet M. Identification of Splenic Reservoir Monocytes and Their Deployment to Inflammatory Sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsubakimoto Y, Yamada H, Yokoi H, Kishida S, Takata H, Kawahito H, Matsui A, Urao N, Nozawa Y, Hirai H, Imanishi J, Ashihara E, Maekawa T, Takahashi T, Okigaki M, Matsubara H. Bone marrow angiotensin AT1 receptor regulates differentiation of monocyte lineage progenitors from hematopoietic stem cells. Arterioscler Thromb Vasc Biol. 2009;29:1529–1536. doi: 10.1161/ATVBAHA.109.187732. [DOI] [PubMed] [Google Scholar]
- 8.Blais CJ, Lapointe N, Rouleau JL, Clement R, Bachvarov DR, Adam A. Effects of captopril and omapatrilat on early post-myocardial infarction survival and cardiac hemodynamics in rats: interaction with cardiac cytokine expression. Can J Physiol Pharmacol. 2002;80:48–58. doi: 10.1139/y01-096. [DOI] [PubMed] [Google Scholar]
- 9.Peng H, Carretero OA, Vuljaj N, Liao TD, Motivala A, Peterson EL, Rhaleb NE. Angiotensin-converting enzyme inhibitors: a new mechanism of action. Circulation. 2005;112:2436–2445. doi: 10.1161/CIRCULATIONAHA.104.528695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young JB. Angiotensin-converting enzyme inhibitors and cytokines in heart failure: dose and effect? J Am Coll Cardiol. 1999;34:2068–2071. doi: 10.1016/s0735-1097(99)00476-3. [DOI] [PubMed] [Google Scholar]
- 11.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 13.Cleutjens JP, Blankesteijn WM, Daemen MJ, Smits JF. The infarcted myocardium: simply dead tissue, or a lively target for therapeutic interventions. Cardiovasc Res. 1999;44:232–241. doi: 10.1016/s0008-6363(99)00212-6. [DOI] [PubMed] [Google Scholar]
- 14.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 15.Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res. 2009;81:474–481. doi: 10.1093/cvr/cvn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French BA, Kramer CM. Mechanisms of Post-Infarct Left Ventricular Remodeling. Drug Discov Today Dis Mech. 2007;4:185–196. doi: 10.1016/j.ddmec.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R, Nahrendorf M. Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol. 2010;55:1629–1638. doi: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, Mitamura H, Ogawa S. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol. 2002;39:241–246. doi: 10.1016/s0735-1097(01)01721-1. [DOI] [PubMed] [Google Scholar]
- 20.Mariani M, Fetiveau R, Rossetti E, Poli A, Poletti F, Vandoni P, D'Urbano M, Cafiero F, Mariani G, Klersy C, De Servi S. Significance of total and differential leucocyte count in patients with acute myocardial infarction treated with primary coronary angioplasty. Eur Heart J. 2006;27:2511–2515. doi: 10.1093/eurheartj/ehl191. [DOI] [PubMed] [Google Scholar]
- 21.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, Komukai K, Kataiwa H, Nakamura N, Hirata K, Tanaka A, Akasaka T. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54:130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nature Immunology. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 23.Nahrendorf M, Hu K, Frantz S, Jaffer FA, Tung CH, Hiller KH, Voll S, Nordbeck P, Sosnovik D, Gattenlöhner S, Novikov M, Dickneite G, Reed GL, Jakob P, Rosenzweig A, Bauer WR, Weissleder R, Ertl G. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation. 2006;113:1196–1202. doi: 10.1161/CIRCULATIONAHA.105.602094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gard PR, Mandy A, Sutcliffe MA. Evidence of a possible role of altered angiotensin function in the treatment, but not etiology, of depression. Biol Psychiatry. 1999;45:1030–1034. doi: 10.1016/s0006-3223(98)00101-2. [DOI] [PubMed] [Google Scholar]
- 25.Nascimben L, Friedrich J, Liao R, Pauletto P, Pessina AC, Ingwall JS. Enalapril treatment increases cardiac performance and energy reserve via the creatine kinase reaction in myocardium of Syrian myopathic hamsters with advanced heart failure. Circulation. 1995;91:1824–1833. doi: 10.1161/01.cir.91.6.1824. [DOI] [PubMed] [Google Scholar]
- 26.Liu YH, Xu J, Yang XP, Yang F, Shesely E, Carretero OA. Effect of ACE inhibitors and angiotensin II type 1 receptor antagonists on endothelial NO synthase knockout mice with heart failure. Hypertension. 2002;39:375–381. doi: 10.1161/hy02t2.102796. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Carretero OA, Liu YH, Shesely EG, Yang F, Kapke A, Yang XP. Role of AT2 receptors in the cardioprotective effect of AT1 antagonists in mice. Hypertension. 2002;40:244–250. doi: 10.1161/01.hyp.0000029095.23198.ad. [DOI] [PubMed] [Google Scholar]
- 28.Kanamori H, Takemura G, Li Y, Okada H, Maruyama R, Aoyama T, Miyata S, Esaki M, Ogino A, Nakagawa M, Ushikoshi H, Kawasaki M, Minatoguchi S, Fujiwara H. Inhibition of Fas-associated apoptosis in granulation tissue cells accompanies attenuation of postinfarction left ventricular remodeling by olmesartan. Am J Physiol Heart Circ Physiol. 2007;292:H2184–94. doi: 10.1152/ajpheart.01235.2006. [DOI] [PubMed] [Google Scholar]
- 29.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nahrendorf M, Waterman P, Thurber G, Groves K, Rajopadhye M, Panizzi P, Marinelli B, Aikawa E, Pittet MJ, Swirski FK, Weissleder R. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler Thromb Vasc Biol. 2009;29:1444–1451. doi: 10.1161/ATVBAHA.109.193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nahrendorf M, Sosnovik DE, Waterman P, Swirski FK, Pande AN, Aikawa E, Figueiredo JL, Pittet MJ, Weissleder R. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ Res. 2007;100:1218–1225. doi: 10.1161/01.RES.0000265064.46075.31. [DOI] [PubMed] [Google Scholar]
- 32.Daugherty A, Rateri D, Hong L, Balakrishnan A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J Vis Exp. 2009;15:1291. doi: 10.3791/1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AbdAlla S, Lother H, Langer A, el Faramawy Y, Quitterer U. Factor XIIIA transglutaminase crosslinks AT1 receptor dimers of monocytes at the onset of atherosclerosis. Cell. 2004;119:343–354. doi: 10.1016/j.cell.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nahrendorf M, Badea C, Hedlund LW, Figueiredo JL, Sosnovik DE, Johnson GA, Weissleder R. High-resolution imaging of murine myocardial infarction with delayed-enhancement cine micro-CT. Am J Physiol Heart Circ Physiol. 2007;292:H3172–8. doi: 10.1152/ajpheart.01307.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2010 Update. A Report From the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 37.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. The Journal of Clinical Investigation. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 39.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulder P, Devaux B, Richard V, Henry JP, Wimart MC, Thibout E, Mace B, Thuillez C. Early versus delayed angiotensin-converting enzyme inhibition in experimental chronic heart failure. Effects on survival, hemodynamics, and cardiovascular remodeling. Circulation. 1997;95:1314–1319. doi: 10.1161/01.cir.95.5.1314. [DOI] [PubMed] [Google Scholar]
- 41.Wollert KC, Studer R, Doerfer K, Schieffer E, Holubarsch C, Just H, Drexler H. Differential effects of kinins on cardiomyocyte hypertrophy and interstitial collagen matrix in the surviving myocardium after myocardial infarction in the rat. Circulation. 1997;95:1910–1917. doi: 10.1161/01.cir.95.7.1910. [DOI] [PubMed] [Google Scholar]
- 42.Jugdutt BI, Lucas A, Khan MI. Effect of angiotensin-converting enzyme inhibition on infarct collagen deposition and remodelling during healing after transmural canine myocardial infarction. Can J Cardiol. 1997;13:657–668. [PubMed] [Google Scholar]
- 43.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 44.Zughaib ME, Sun JZ, Bolli R. Effect of angiotensin-converting enzyme inhibitors on myocardial ischemia/reperfusion injury: an overview. Basic Res Cardiol. 1993;88(Suppl 1):155–167. doi: 10.1007/978-3-642-72497-8_11. [DOI] [PubMed] [Google Scholar]
- 45.Przyklenk K, Kloner RA. Relationships between structure and effects of ACE inhibitors: comparative effects in myocardial ischaemic/reperfusion injury. Br J Clin Pharmacol. 1989;28(Suppl 2):167S–175S. doi: 10.1111/j.1365-2125.1989.tb03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazar HL, Bao Y, Rivers S, Colton T, Bernard SA. High tissue affinity angiotensin-converting enzyme inhibitors improve endothelial function and reduce infarct size. Ann Thorac Surg. 2001;72:548–53. doi: 10.1016/s0003-4975(01)02779-5. [DOI] [PubMed] [Google Scholar]
- 47.Robinette CD, Fraumeni JFJ. Splenectomy and subsequent mortality in veterans of the 1939-45 war. Lancet. 1977;2:127–129. doi: 10.1016/s0140-6736(77)90132-5. [DOI] [PubMed] [Google Scholar]
- 48.Pfeffer MA, Greaves SC, Arnold JM, Glynn RJ, LaMotte FS, Lee RT, Menapace FJJ, Rapaport E, Ridker PM, Rouleau JL, Solomon SD, Hennekens CH. Early versus delayed angiotensin-converting enzyme inhibition therapy in acute myocardial infarction. The healing and early afterload reducing therapy trial. Circulation. 1997;95:2643–2651. doi: 10.1161/01.cir.95.12.2643. [DOI] [PubMed] [Google Scholar]
- 49.Ambrosioni E, Borghi C, Magnani B. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study Investigators. N Engl J Med. 1995;332:80–85. doi: 10.1056/NEJM199501123320203. [DOI] [PubMed] [Google Scholar]
- 50.Sosnovik DE, Nahrendorf M, Weissleder R. Molecular magnetic resonance imaging in cardiovascular medicine. Circulation. 2007;115:2076–2086. doi: 10.1161/CIRCULATIONAHA.106.658930. [DOI] [PubMed] [Google Scholar]
- 51.Swirski FK, Wildgruber M, Ueno T, Figueiredo JL, Panizzi P, Iwamoto Y, Zhang E, Stone JR, Rodriguez E, Chen JW, Pittet MJ, Weissleder R, Nahrendorf M. Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest. 2010;120:2627–34. doi: 10.1172/JCI42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su H, Spinale FG, Dobrucki LW, Song J, Hua J, Sweterlitsch S, Dione DP, Cavaliere P, Chow C, Bourke BN, Hu XY, Azure M, Yalamanchili P, Liu R, Cheesman EH, Robinson S, Edwards DS, Sinusas AJ. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation. 2005;112:3157–3167. doi: 10.1161/CIRCULATIONAHA.105.583021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.