Abstract
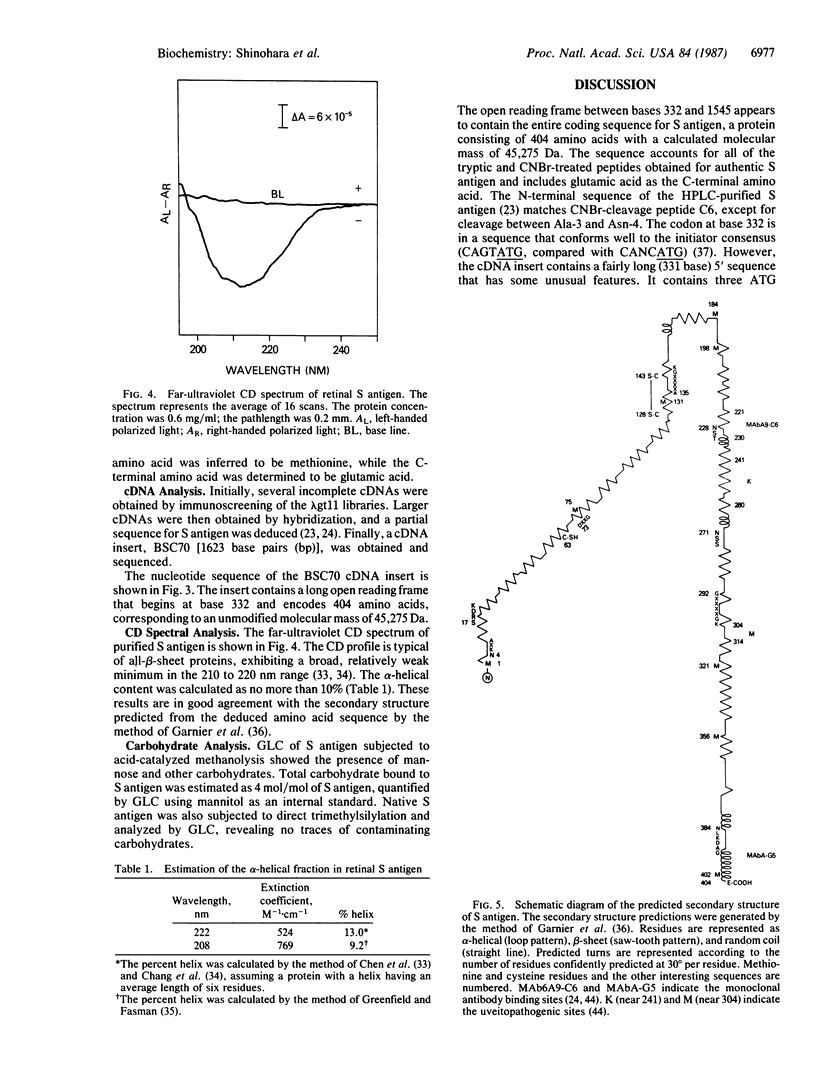
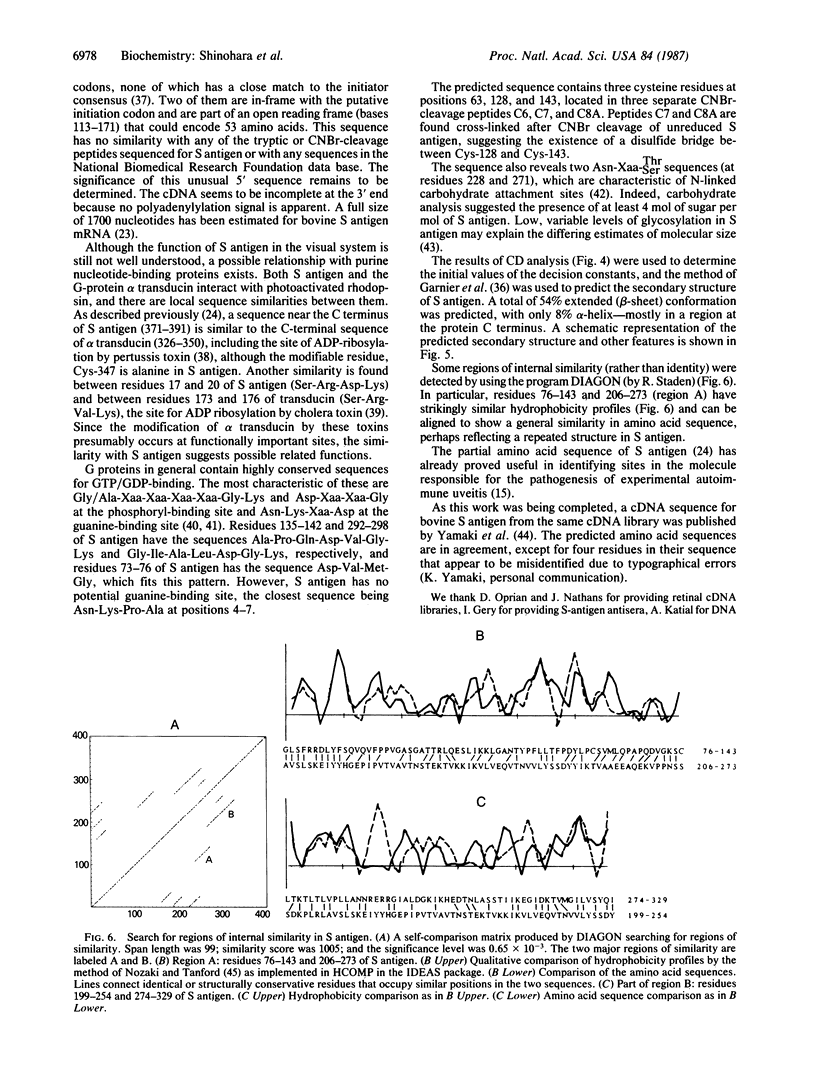
The complete amino acid sequence of bovine S antigen (48-kDa protein) has been determined by cDNA and partial amino acid sequencing. A 1623-base-pair (bp) cDNA contains an open reading frame coding for a protein of 404 amino acids (45,275 Da). Tryptic peptides and cyanogen bromide peptides of native bovine S antigen were purified and partially sequenced. All of these peptides were accounted for in the long open reading frame. Searching of the National Biomedical Research Foundation data bank revealed no extensive sequence homology between S antigen and other proteins. However, there are local regions of sequence similarity with alpha transducin, including the sites subject to ADP-ribosylation by Bordetella pertussis and cholera toxins and the phosphoryl binding-sites. Secondary structure prediction and circular dichroic spectroscopy show that S antigen is composed predominantly of beta-sheet conformation. Acid-catalyzed methanolysis suggests the presence of low levels of carbohydrate in the molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray P., Carter A., Simons C., Guo V., Puckett C., Kamholz J., Spiegel A., Nirenberg M. Human cDNA clones for four species of G alpha s signal transduction protein. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8893–8897. doi: 10.1073/pnas.83.23.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekhuyse R. M., Bessems H. J. The molecular weight of bovine retinal S-antigen. Exp Eye Res. 1985 May;40(5):763–766. doi: 10.1016/0014-4835(85)90146-0. [DOI] [PubMed] [Google Scholar]
- Cassim J. Y., Yang J. T. A computerized calibration of the circular dichrometer. Biochemistry. 1969 May;8(5):1947–1951. doi: 10.1021/bi00833a026. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Wu C. S., Yang J. T. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978 Nov;91(1):13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Dever T. E., Glynias M. J., Merrick W. C. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1814–1818. doi: 10.1073/pnas.84.7.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso L. A., Merryman C. F., Edelberg K. E., Naids R., Kalsow C. S-antigen in the developing retina and pineal gland: a monoclonal antibody study. Invest Ophthalmol Vis Sci. 1985 Apr;26(4):561–567. [PubMed] [Google Scholar]
- Donoso L. A., Merryman C. F., Shinohara T., Dietzschold B., Wistow G., Craft C., Morley W., Henry R. T. S-antigen: identification of the MAbA9-C6 monoclonal antibody binding site and the uveitopathogenic sites. Curr Eye Res. 1986 Dec;5(12):995–1004. doi: 10.3109/02713688608995181. [DOI] [PubMed] [Google Scholar]
- Faure J. P. Autoimmunity and the retina. Curr Top Eye Res. 1980;2:215–302. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Gregerson D. S., Fling S. P., Wohlhueter R. M. Characterization of immunologically active cyanogen bromide peptide fragments of bovine and human retinal S-antigen. Exp Eye Res. 1986 Nov;43(5):803–818. doi: 10.1016/s0014-4835(86)80011-2. [DOI] [PubMed] [Google Scholar]
- Gregerson D. S., Putterman G. J. Preparation, isolation, and immunochemical studies of the cyanogen bromide peptides from a retinal photoreceptor cell autoantigen, S-antigen. J Immunol. 1984 Aug;133(2):843–848. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Higgins R. C., Dahmus M. E. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal Biochem. 1979 Mar;93(2):257–260. doi: 10.1016/s0003-2697(79)80148-7. [DOI] [PubMed] [Google Scholar]
- Horwitz J., Bullard B., Mercola D. Interaction of troponin subunits. The interaction between the inhibitory and tropomyosin-binding subunits. J Biol Chem. 1979 Jan 25;254(2):350–355. [PubMed] [Google Scholar]
- Kalsow C. M., Wacker W. B. Pineal gland involvement in retina-induced experimental allergic uveitis. Invest Ophthalmol Vis Sci. 1978 Aug;17(8):774–783. [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry. 1978 Oct 17;17(21):4389–4395. doi: 10.1021/bi00614a006. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C. Isolation of denatured proteins and peptides by high-performance liquid chromatography. Effect of different perfluorinated acids, column length and large-pore supports. Biochim Biophys Acta. 1982 Jun 4;704(2):284–289. doi: 10.1016/0167-4838(82)90158-3. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Fraser B. A., Kahn R. A., Gilman A. G. ADP-ribosylation of transducin by islet-activation protein. Identification of asparagine as the site of ADP-ribosylation. J Biol Chem. 1984 Jan 25;259(2):749–756. [PubMed] [Google Scholar]
- McCormick F., Clark B. F., la Cour T. F., Kjeldgaard M., Norskov-Lauritsen L., Nyborg J. A model for the tertiary structure of p21, the product of the ras oncogene. Science. 1985 Oct 4;230(4721):78–82. doi: 10.1126/science.3898366. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Kuwabara T., McAllister C., Nussenblatt R. B., Gery I. Adoptive transfer of experimental autoimmune uveoretinitis in rats. Immunopathogenic mechanisms and histologic features. Invest Ophthalmol Vis Sci. 1985 Jan;26(1):1–9. [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Nussenblatt R. B., Gery I., Ballintine E. J., Wacker W. B. Cellular immune responsiveness of uveitis patients to retinal S-antigen. Am J Ophthalmol. 1980 Feb;89(2):173–179. doi: 10.1016/0002-9394(80)90108-7. [DOI] [PubMed] [Google Scholar]
- Nussenblatt R. B., Kuwabara T., de Monasterio F. M., Wacker W. B. S-antigen uveitis in primates. A new model for human disease. Arch Ophthalmol. 1981 Jun;99(6):1090–1092. doi: 10.1001/archopht.1981.03930011090021. [DOI] [PubMed] [Google Scholar]
- Pfister C., Chabre M., Plouet J., Tuyen V. V., De Kozak Y., Faure J. P., Kühn H. Retinal S antigen identified as the 48K protein regulating light-dependent phosphodiesterase in rods. Science. 1985 May 17;228(4701):891–893. doi: 10.1126/science.2988124. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dop C., Tsubokawa M., Bourne H. R., Ramachandran J. Amino acid sequence of retinal transducin at the site ADP-ribosylated by cholera toxin. J Biol Chem. 1984 Jan 25;259(2):696–698. [PubMed] [Google Scholar]
- Wacker W. B., Donoso L. A., Kalsow C. M., Yankeelov J. A., Jr, Organisciak D. T. Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977 Dec;119(6):1949–1958. [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. J., Katial A., Craft C., Shinohara T. Sequence analysis of bovine retinal S-antigen. Relationships with alpha-transducin and G-proteins. FEBS Lett. 1986 Feb 3;196(1):23–28. doi: 10.1016/0014-5793(86)80207-1. [DOI] [PubMed] [Google Scholar]
- Witt P. L., Hamm H. E., Bownds M. D. Preparation and characterization of monoclonal antibodies to several frog rod outer segment proteins. J Gen Physiol. 1984 Aug;84(2):251–263. doi: 10.1085/jgp.84.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaki K., Takahashi Y., Sakuragi S., Matsubara K. Molecular cloning of the S-antigen cDNA from bovine retina. Biochem Biophys Res Commun. 1987 Feb 13;142(3):904–910. doi: 10.1016/0006-291x(87)91499-9. [DOI] [PubMed] [Google Scholar]
- Yatsunami K., Khorana H. G. GTPase of bovine rod outer segments: the amino acid sequence of the alpha subunit as derived from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4316–4320. doi: 10.1073/pnas.82.13.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigler J. S., Jr, Mochizuki M., Kuwabara T., Gery I. Purification of retinal S-antigen to homogeneity by the criterion of gel electrophoresis silver staining. Invest Ophthalmol Vis Sci. 1984 Aug;25(8):977–980. [PubMed] [Google Scholar]



