Abstract
The spatio-temporal attachment site patterns of ticks feeding on their hosts can be of significance if co-feeding transmission (i.e. from tick to tick without a systemic infection of the host) of pathogens affects the persistence of a given disease. Using tick infestation data on roe deer, we analysed preferred attachment sites and niche width of Ixodes ticks (larvae, nymphs, males, females) and investigated the degree of inter- and intrastadial aggregation. The different development stages showed rather consistent attachment site patterns and relative narrow feeding site niches. Larvae were mostly found on the head and on the front legs of roe deer, nymphs reached highest densities on the head and highest adult densities were found on the neck of roe deer. The tick stages feeding (larvae, nymphs, females) on roe deer showed high degrees of intrastadial spatial aggregation, whereas males did not. Male ticks showed large feeding site overlap with female ticks. Feeding site overlap between larval-female and larval-nymphal ticks did occur especially during the months May–August on the head and front legs of roe deer and might allow pathogen transmission via co-feeding. Tick density, niche width and niche overlap on roe deer are mainly affected by seasonality, reflecting seasonal activity and abundance patterns of ticks. Since different tick development stages occur spatially and temporally clustered on roe deer, transmission experiments of tick-borne pathogens are urgently needed.
Keywords: Co-feeding, Competition, Heterogeneity, Host-parasite interaction, Niche overlap, Reverse density-dependence, Tick-borne pathogens
Introduction
For several tick-host associations, it is well known that ticks apparently prefer certain feeding sites (e.g. Nelson et al. 1975; Randolph 1975; Barnard and Morrison 1985; Bloemer et al. 1988; Barnard et al. 1989; Fourie and van Zyl 1991; Fourie et al. 1991; L’Hostis et al. 1994; Fourie and Kok 1995; Mathee et al. 1997; Ogden et al. 1998; Schmidtmann et al. 1998).
In central Europe, roe deer (Capreolus capreolus) are probably the most important free-living host for adult ticks of the Ixodes ricinus complex. Yet, only few studies have investigated patterns of tick infestation on roe deer (Matuschka et al. 1993; Carpi et al. 2008; Vor et al. 2010; Kiffner et al. in press), and none of them explicitly investigated the spatio-temporal distribution of ticks on roe deer individuals. Knowledge about the most infested body parts is, however, crucial for designing acaricide-treated devices to control ticks feeding on deer, a control strategy that turned out to be effective in lowering densities of free-living ticks (Ixodes scapularis and Amblyoma americanum) in the north-eastern USA (e.g. Fish and Childs 2009; Pound et al. 2009).
Besides having a direct effect on the host by inducing a considerable blood loss which might reduce overall fitness of the host (Pfäffle et al. 2009), ticks of the Ixodes ricinus complex are vectors of several bacterial (e.g. Anaplasma phagocytophilum, Borrelia burgdorferi, Rickettsia helvetica), protozoan (e.g. Babesia capreoli, Babesia divergens, Babesia venatorum) and viral (e.g. Louping-ill virus, tick-borne encephalitis virus) pathogens of medical and veterinary importance (Jongejan and Uilenberg 2004; Malandrin et al. 2010). There is a growing body of evidence that, at large scales, numbers of infected ticks (tick-borne encephalitis virus: Hudson et al. 2001) or numbers of human infections (Borrelia burgdorferi: Linard et al. 2007; tick-borne encephalitis virus: Rizzoli et al. 2009) are positively correlated with the density of roe deer. The interpretation of this correlation is, however, not unambiguous: Roe deer have been shown to amplify tick densities (e.g. Jensen et al. 2000; Walker et al. 2001), but considering the tick-borne pathogens of major human health importance such as Borrelia burgdorferi and tick-borne encephalitis virus, they are believed to be dead-end or dilution (Ostfeld and Keesing 2001) hosts. However, it is unclear whether roe deer and other ungulates do provide a platform for non-systemic pathogen transmission among co-feeding ticks (Randolph 2008; Jaenson and Tälleklint 1992; Matuschka et al. 1993; Kimura et al. 1995; Bruno et al. 2000), but it can not be excluded as a possibility (e.g. Gern et al. 1998). If co-feeding transmission does occur between ticks feeding on roe deer, intra- and interstadial aggregation of ticks may enhance co-feeding transmission and might therefore be of considerable interest for models aiming at quantifying basic reproduction numbers of tick-borne pathogens (e.g. Hartemink et al. 2008).
In this paper, we aimed at estimating the preferred feeding sites and tested the ideal free distribution hypothesis at the host level (Fretwell and Lucas 1970), i.e. we investigated whether feeding site selection by ticks is density dependent. This hypothesis predicts that most animals (here ticks) should be found in preferred habitats (in the tick-host context: preferred body parts) and spill over to less preferred habitats when animal density is high (Sutherland 1996). Further on, we aimed at estimating the niche width (i.e. the range of feeding sites) of the different life stages (larvae, nymphs, adults) and sexes (female, male) feeding on roe deer and investigated the degree of niche overlap between different stages/sexes. Ultimately, we investigated whether indices of niche width and overlap are seasonal and whether they depend on the size (body mass) of the host.
Materials and methods
Tick sampling
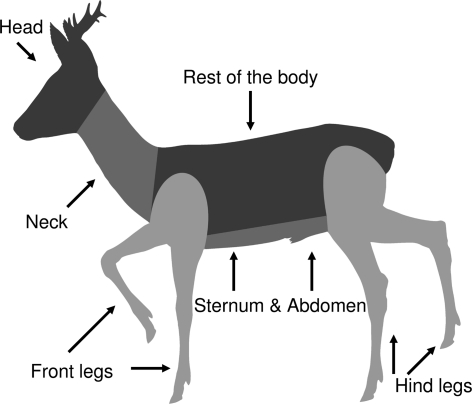
We opportunistically sampled 80 hunter-killed roe deer from forests around the city of Göttingen (centred at, 51°32′2″N, 9°56′8″, radius of ca. 20 km) in central Germany during regular hunting activities. The study area is dominated by mixed deciduous forests comprising mainly of European beech (Fagus sylvatica), Norway maple (Acer pseudoplatanus), European ash (Fraxinus excelsior) and sessile oak (Quercus petraea). The geology of the region is characterized by shallow limestone plateaus with rendzina soils and haplic luvisols, whereas some forest stands grow on sandstone with sandy and loamy cambisols. The altitude above sea level ranges from 151 to 400 m, the mean annual rainfall is 780 mm, and the average annual temperature is 7.8°C (Petritian et al. 2007). Data collection was stratified into 6 distinct sampling seasons: November–December 2007, n = 20; May–June 2008, n = 18; July–August 2008, n = 12; November–December 2008, n = 14, May–June 2009, n = 10 and July–August 2009, n = 6. Roe deer carcasses were disembowelled by the hunters and stored in cooling chambers at 2–8°C until examination. Within 16 h on average (SE: ±2.5 h) after roe deer individuals had been shot, each carcass was examined by two observers wearing latex gloves. The carcass was divided into 6 distinct parts (head, neck, sternum & abdomen, rest of the body, front legs and hind legs, Fig. 1). The roe deer skin was systematically inspected and palpated to detect all ticks. Sites heavily infested were consecutively searched and palpated by both persons. All ticks were removed from each body part with forceps. These ticks were immediately counted and recorded according to life stage and sex (larvae, nymphs, males and females). Finally, they were transferred to sampling tubes and stored at −20°C. All removed ticks belong to the Ixodes ricinus complex; for a more detailed description of the study site and the tick collection see Kiffner et al. (in press). In order to relate the number of ticks to the surface area of each body part, we estimated the surface area of body parts of six roe deer individuals (3 individuals <2 year, 3 individuals >2 years) using basic geometric measurements. Since the absolute surface areas varied considerably among individuals, we used proportional data (Table 1) and allocated these relative measurements to each investigated roe deer individual.
Fig. 1.
Sketch outline of the tick collection sites on a roe deer (Capreolus capreolus) buck. Drawing: W. Tambour, J. Seelig
Table 1.
Mean proportional surface area (±SE) of roe deer body parts
| Head | Neck | Sternum & Abdomen | Rest of the body | Front legs | Hind legs | |
|---|---|---|---|---|---|---|
| >2 years | 0.12 (±0.01) | 0.06 (±0.00) | 0.08 (±0.00) | 0.33 (±0.01) | 0.17 (±0.02) | 0.25 (±0.01) |
| <2 years | 0.12 (±0.01) | 0.05 (±0.01) | 0.10 (±0.01) | 0.28 (±0.02) | 0.20 (±0.01) | 0.26 (±0.02) |
Sample size for each age class is n = 3. For a delineation of the body parts, see Fig. 1
Data analysis
We analysed the relative tick density (i.e. number of ticks of each stage or sex/relative surface area) of each body part with the nonparametric Friedman test in order to provide a density ranking of the body parts infested by ticks. We also analysed whether the relative use of the preferred attachment site varied as a function of the abundance of the considered tick life stage/sex.
To investigate the feeding site specialisation of each tick stage/sex, we calculated Levins index of niche breadth, using each body part as a resource state.
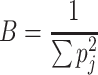 |
where B is the index of niche breadth, p j is the proportion of ticks collected from body part j (Levins 1968). Since B does not follow a normal distribution (B reaches a maximum value if ticks of one life stage/sex were distributed homogenously on all body parts and reaches a minimum value if all ticks of this life stage/sex were concentrated on one body part), we standardised B to B S using the formula:
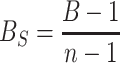 |
where n is the number of body parts (Hurlbert 1978). This standardised index ranges from 0 to 1 (wide niche). Cases with a zero-count of a specific tick life stage/sex were omitted. Using generalised linear models, we tested whether B S varied with sampling season and with body mass (mass of disembowelled body with head in kg) of the host individual.
In order to investigate the overlap in site selection among the different tick life stages and sexes, we calculated a niche overlap index for all tick life stage/sex combinations following Pianka (1973).
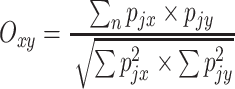 |
where O xy is the index of niche overlap between tick life stage/sex x and tick life stage/sex y, p jx the proportional relative density of tick life stage/sex x on body part j (relative density of this life stage/sex on body part j divided by the total number of this life stage/sex on the entire roe deer), p jy the proportional relative density of tick life stage/sex y on body part j and n the number of body parts (i.e. 6). This index is a symmetrical measure, ranging from 0 (no body parts used in common) to 1 (complete overlap in body part selection). Roe deer individuals with a zero-count of a specific tick life stage/sex were treated as 0 (i.e. no overlap in resource use).
Analogously to the analyses of B S, we ran generalised linear models to test whether O xy is affected by sampling season and host body mass. All calculations were performed for the entire study period and for the 6 distinct sampling seasons. Indices of niche breadth and niche overlap were calculated with Microsoft Excel, statistical tests were performed with R (R Development core team) and SPSS 17.0 (SPSS Inc.).
Results
Preferred attachment sites
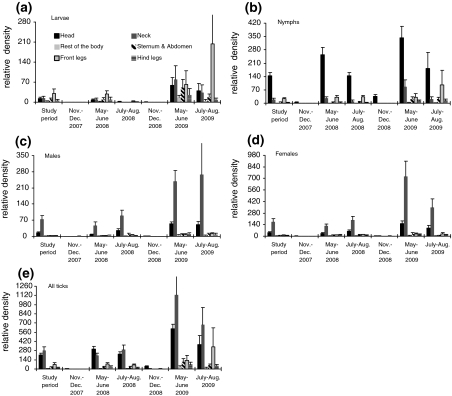
Larvae showed a slightly inconsistent pattern of feeding site selection (Table 2, Fig. 2) across the study period. In two sampling seasons (November–December 2008, May–June 2009), we found no significant (Friedman test P > 0.05) density differences among body parts, and during one sampling period we found no larvae at all (November–December 2007). For the remaining sampling periods, the results of the Friedman test indicate significant (all P < 0.05), but differing density rankings. In May–June 2008 and July–August 2008, the front legs showed the highest larvae density, followed by the head, whereas during the season July–August 2009, larvae density was highest on the head, followed by the front legs.
Table 2.
Ranking (Friedman test) of tick densities on different roe deer body parts (Rest = Rest of the body, St. & Ab. = Sternum & Abdomen) by sampling period and for the entire study period
| Nov.–Dec. 2007 | May–June 2008 | July-Aug. 2008 | Nov.–Dec. 2008 | May–June 2009 | July–Aug. 2009 | Study period | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body part | Mean rank | Body part | Mean rank | Body part | Mean rank | Body part | Mean rank | Body part | Mean rank | Body part | Mean rank | Body part | Mean rank |
| Larvae | |||||||||||||
| – | – | Front legs | 4.86 | Front legs | 4.67 | Head | 3.68 | Head | 4.30 | Head | 4.83 | Front legs | 4.05 |
| – | – | Head | 3.56 | Head | 3.63 | Neck | 3.46 | Neck | 4.05 | Front legs | 4.17 | Head | 3.76 |
| – | – | St. & Ab. | 3.31 | Hind legs | 3.46 | Rest | 3.46 | Front legs | 3.70 | Neck | 3.83 | Neck | 3.46 |
| – | – | Neck | 3.22 | Neck | 3.08 | St. & Ab. | 3.46 | St. & Ab. | 3.45 | St. & Ab. | 3.17 | St. & Ab. | 3.36 |
| – | – | Hind legs | 3.17 | Rest | 3.08 | Front legs | 3.46 | Rest | 2.85 | Rest | 2.50 | Hind legs | 3.23 |
| – | – | Rest | 2.89 | St. & Ab. | 3.08 | Hind legs | 3.46 | Hind legs | 2.65 | Hind legs | 2.50 | Rest | 3.14 |
| Nymphs | |||||||||||||
| Head | 4.35 | Head | 5.89 | Head | 5.92 | Head | 5.64 | Head | 5.70 | Head | 5.58 | Head | 5.42 |
| Front legs | 3.45 | Front legs | 4.47 | Front legs | 4.33 | Front legs | 3.21 | Neck | 4.55 | Front legs | 3.83 | Front legs | 3.78 |
| Neck | 3.30 | Neck | 3.39 | Hind legs | 3.04 | Neck | 3.04 | St. & Ab. | 3.25 | Neck | 3.50 | Neck | 3.36 |
| Rest | 3.30 | Hind legs | 2.72 | St. & Ab. | 3.00 | Rest | 3.04 | Front legs | 3.25 | St. & Ab. | 3.08 | St. & Ab. | 3.03 |
| St. & Ab. | 3.30 | St. & Ab. | 2.58 | Neck | 2.71 | St. & Ab. | 3.04 | Hind legs | 2.65 | Hind legs | 2.83 | Hind legs | 2.97 |
| Hind legs | 3.30 | Rest | 1.94 | Rest | 2.00 | Hind legs | 3.04 | Rest | 1.60 | Rest | 2.17 | Rest | 2.46 |
| Males | |||||||||||||
| Front legs | 3.75 | Neck | 4.75 | Neck | 5.67 | Head | 3.64 | Neck | 6.00 | Neck | 5.50 | Neck | 4.58 |
| Head | 3.45 | Head | 3.92 | Head | 3.96 | Neck | 3.64 | Head | 5.00 | Head | 5.17 | Head | 3.99 |
| Neck | 3.45 | Hind legs | 3.56 | Front legs | 3.21 | Rest | 3.43 | St. & Ab. | 2.80 | Front legs | 3.08 | Front legs | 3.29 |
| Rest | 3.45 | Front legs | 3.22 | Hind legs | 3.04 | St. & Ab. | 3.43 | Hind legs | 2.65 | St. & Ab. | 2.92 | Hind legs | 3.24 |
| St. & Ab. | 3.45 | St. & Ab. | 2.81 | St. & Ab. | 2.63 | Front legs | 3.43 | Front legs | 2.55 | Hind legs | 2.58 | St. & Ab. | 3.06 |
| Hind legs | 3.45 | Rest | 2.75 | Rest | 2.50 | Hind legs | 3.43 | Rest | 2.00 | Rest | 1.75 | Rest | 2.84 |
| Females | |||||||||||||
| Front legs | 3.80 | Neck | 5.56 | Neck | 5.21 | Front legs | 3.82 | Neck | 6.00 | Neck | 6.00 | Neck | 4.66 |
| Hind legs | 3.75 | Head | 3.83 | Head | 4.83 | Head | 3.79 | Head | 4.80 | Head | 4.83 | Head | 4.13 |
| Head | 3.68 | Hind legs | 3.61 | Front legs | 3.50 | Neck | 3.61 | Front legs | 3.20 | Hind legs | 3.33 | Front legs | 3.53 |
| St. & Ab. | 3.38 | Front legs | 3.39 | St. & Ab. | 3.29 | Hind legs | 3.50 | Hind legs | 3.00 | Front legs | 2.92 | Hind legs | 3.39 |
| Neck | 3.20 | St. & Ab. | 2.83 | Hind legs | 2.67 | Rest | 3.14 | Rest | 2.00 | St. & Ab. | 2.25 | St. & Ab. | 2.94 |
| Rest | 3.20 | Rest | 1.78 | Rest | 1.50 | St. & Ab. | 3.14 | St. & Ab. | 2.00 | Rest | 1.67 | Rest | 2.35 |
| Ticks | |||||||||||||
| Head | 4.53 | Head | 5.72 | Head | 5.25 | Head | 5.57 | Neck | 5.60 | Neck | 5.67 | Head | 5.22 |
| Front legs | 3.75 | Neck | 5.11 | Neck | 5.08 | Front legs | 3.57 | Head | 5.20 | Head | 5.17 | Neck | 4.35 |
| Hind legs | 3.50 | Front legs | 3.61 | Front legs | 4.00 | Neck | 3.18 | Front legs | 3.20 | Front legs | 3.67 | Front legs | 3.65 |
| St. & Ab. | 3.18 | Hind legs | 2.75 | St. & Ab. | 2.83 | Hind legs | 3.11 | St. & Ab. | 2.60 | Hind legs | 2.83 | Hind legs | 2.95 |
| Neck | 3.03 | St. & Ab. | 2.47 | Hind legs | 2.50 | Rest | 2.79 | Hind legs | 2.60 | St. & Ab. | 2.58 | St. & Ab. | 2.78 |
| Rest | 3.03 | Rest | 1.33 | Rest | 1.33 | St. & Ab. | 2.79 | Rest | 1.80 | Rest | 1.08 | Rest | 2.05 |
Fig. 2.
The seasonal pattern of mean relative density (number/proportional surface area of each body part) of (a) larvae, (b) nymphs, (c) male, (d) female and (e) all ticks (Ixodes ricinus complex) attached to roe deer (Capreolus capreolus) from the forests of Göttingen, Germany. Note the different scales on the y-axes
Nymphs showed a clear pattern of attachment site selection, at least for the top-ranked body part. During all seasons, significantly highest densities of nymphs were observed on the roe deer’s head. The second highest density was usually observed on the front legs (except for May–June 2009: neck).
Male ticks also exhibited significant density differences between roe deer body parts, except for the winter months (P > 0.05). The highest male tick density was usually found on the roe deer’s neck, followed by its head. Similarly, female ticks reached highest densities on the neck, followed by the head. Again, except for the winter months, female densities were highly significantly different between the distinct body parts.
Testing the ideal free distribution
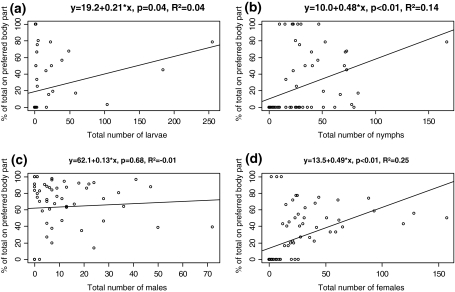
The proportion of tick life stages on the preferred body (i.e. the top ranked body part for the entire study period, Table 2) part increased significantly (larvae: Kendall’s τ = 0.60, P < 0.001, nymphs: τ = 0.42, P < 0.001, females: τ = 0.58, P < 0.001) or did not change significantly (males: τ = −0.05, P = 0.575) with increasing absolute abundance of the same tick life stage/sex (Fig. 3; all correlations n = 80).
Fig. 3.
Use of preferred attachment sites by the four different tick life stages/sexes in relation to total abundance of the same tick life stage/sex on the entire roe deer (n = 80)
Niche breadth
Across the study period, nymphs were most specialised in their feeding site selection, followed by larvae, males and females (Table 3). Niche indices of larvae and nymphs, however, did not differ significantly from each other (paired t-test: t = 0.276, df = 34, P = 0.784). Differences in niche indices were significantly different between larvae and males (t = −2.209, df = 32, P = 0.034), larvae and females (t = −3.951, df = 33, P < 0.001), nymphs and males (t = −3.407, df = 46, P = 0.01), nymphs and females (t = −7.288, df = 55, P < 0.001), and between males and females (t = −4.397, df = 47, P < 0.001). Compared to the niche breadth in the winter months 2008, niche breadth of larvae was significantly larger in May–June 2009 (Table 4). Relative to the same reference season, nymphal niche breadth was significantly larger in all other seasons except for the winter months 2007. Niche breadth of male ticks was significantly larger in May–June 2009, July–August 2008 and 2009 compared to the niche breadth in November–December 2008; niche breadths in other sampling seasons were not significantly different to the reference season. Niche breadth of males was positively associated with host body mass. Female tick niche breadth was also larger during the May–June and July–August seasons compared to the reference season.
Table 3.
Standardised index of niche breadth (B S ± SE) for each tick stage/sex on roe deer (Capreolus capreolus) estimated for each sampling period and for the entire study period
| Nov.–Dec. 2007 | May–June 2008 | July–Aug. 2008 | Nov.–Dec. 2008 | May–June 2009 | July–Aug. 2009 | Study period | |
|---|---|---|---|---|---|---|---|
| Larvae | No larvae found | 0.09 (±0.03) | 0.13 (±0.05) | 0 | 0.40 (±0.08) | 0.22 (±0.19) | 0.18 (±0.17) |
| Nymphs | 0 | 0.14 (±0.01) | 0.16 (±0.01) | 0.01 (±0.00) | 0.15 (±0.06) | 0.29 (±0.18) | 0.11 (±0.11) |
| Males | 0 | 0.22 (±0.02) | 0.27 (±0.03) | 0 | 0.34 (±0.04) | 0.26 (±0.18) | 0.24 (±0.15) |
| Females | 0.07 (±0.03) | 0.41 (±0.02) | 0.42 (±0.02) | 0.11 (±0.01) | 0.29 (±0.04) | 0.39 (±0.12) | 0.31 (±0.16) |
Table 4.
Parameter estimates of generalised linear models explaining the variance of niche breadth of each tick development stage/sex on roe deer (Capreolus capreolus)
| Larvae | Nymphs | Males | Females | |
|---|---|---|---|---|
| Intercept | −0.052 | −0.125 | −0.502 | 0.016 |
| May–June 2008 | 0.068 | 0.131** | 0.287 | 0.280*** |
| May–June 2009 | 0.379* | 0.144** | 0.421* | 0.168* |
| July–August 2008 | 0.111 | 0.161** | 0.379* | 0.306*** |
| July–August 2009 | 0.203 | 0.287*** | 0.343* | 0.267** |
| November–December 2007 | No larvae found | 0.005 | 0.037 | −0.056 |
| Body mass | 0.022 | 0.008 | 0.027* | 0.007 |
* P < 0.05, ** P < 0.01, *** P < 0.001
Spatial niche overlap
Interstadial niche overlap was observed for all tick development/sex combinations (Table 5). During the entire study period, it was highest among adult ticks, followed by nymphs-females, larvae-nymphs, nymphs-males, larvae-males and larvae-females. Larvae-nymph overlap was usually highest in May–June (both years) and in July–August 2009 (Table 6). Overlap between males and larvae and larvae and females roughly followed the same pattern as larvae-nymph overlap. Spatial overlap between nymph-male and female-male ticks was generally higher during the summer months (May–June and July–August) than during the winter months. Spatial female-male tick overlap was positively associated with roe deer body mass.
Table 5.
Pianka’s index of spatial niche overlap on roe deer (Capreolus capreolus) for each tick stage/sex combination estimated for each sampling period and for the entire study period
| Nov.–Dec. 2007 | May–June 2008 | July–Aug. 2008 | Nov.–Dec. 2008 | May–June 2009 | July–Aug. 2009 | Study period | |
|---|---|---|---|---|---|---|---|
| Larvae-Nymphs | 0 | 0.39 (±0.09) | 0.19 (±0.09) | 0.07 (±0.07) | 0.63 (±0.13) | 0.58 (±0.19) | 0.25 (±0.04) |
| Larvae-Males | 0 | 0.27 (±0.09) | 0.04 (±0.03) | 0 | 0.47 (±0.12) | 0.28 (±0.12) | 0.15 (±0.03) |
| Larvae-Females | 0 | 0.29 (+0.07) | 0.06 (±0.02) | 0 | 0.44 (±0.12) | 0.29 (±0.10) | 0.15 (±0.03) |
| Nymphs-Males | 0 | 0.28 (±0.07) | 0.31 (±0.07) | 0.07 (±0.07) | 0.43 (±0.07) | 0.34 (±0.11) | 0.20 (±0.03) |
| Nymphs-Females | 0.04 (±0.04) | 0.32 (±0.05) | 0.43 (±0.08) | 0.15 (±0.09) | 0.43 (±0.07) | 0.42 (+0.11) | 0.26 (±0.03) |
| Males-Females | 0.08 (±0.06) | 0.62 (±0.10) | 0.88 (±0.06) | 0.09 (±0.07) | 0.97 (±0.02) | 0.83 (±0.10) | 0.50 (±0.05) |
Table 6.
Parameter estimates of generalised linear models explaining the variance of spatial niche overlap among tick development stage/sex combinations on roe deer (Capreolus capreolus) (* P < 0.05, ** P < 0.01, *** P < 0.001)
| Larvae-Nymphs | Larvae-Males | Larvae-Females | Nymphs-Males | Nymphs-Females | Males-Females | |
|---|---|---|---|---|---|---|
| Intercept | −0.248 | 0.090 | 0.083 | −0.022 | 0.011 | −0.284 |
| May–June 2008 | 0.271* | 0.281** | 0.301*** | 0.279*** | 0.153 | 0.490*** |
| May–June 2009 | 0.527*** | 0.477*** | 0.448*** | 0.429*** | 0.268* | 0.863*** |
| July–August 2008 | 0.107 | 0.043 | 0.059 | 0.312*** | 0.279** | 0.799*** |
| July–August 2009 | 0.479** | 0.332** | 0.310** | 0.373*** | 0.205 | 0.785*** |
| November–December 2007 | −0.089 | 0.001 | 0.001 | 0.000 | −0.113 | −0.011 |
| Body mass | 0.023 | −0.006 | −0.006 | 0.001 | 0.010 | 0.025* |
Discussion
Feeding site selection and intraspecific aggregation
Overall, ticks were highly aggregated on the roe deer host. Averaged over the study period, roe deer heads were most heavily infested with ticks (Table 2); 54% (SE ± 3) of the total tick burden was found on only 12% (SE ± 1) of the roe deer surface area.
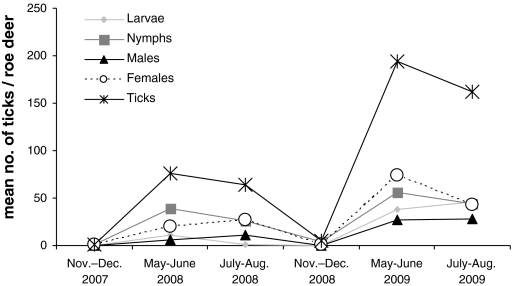
For all sampling seasons combined, tick densities of body parts differed significantly; the pattern of tick density ranking was, however, only consistent for the most (head) and least (rest of the body, i.e. the flanks and the dorsal part of the roe deer) infested body part. The intermediate ranked body parts showed some variation in the density ranking from sampling season to sampling season. The observed distribution patterns and the relative narrow niche widths (Table 3) suggest that each tick life stage apparently selects for certain body parts albeit there are minor derivations from season to season and year to year (cf. Table 2). Larvae appear to prefer mainly the head and the front legs of roe deer. This pattern seems to be roughly similar in Ixodes ricinus larvae feeding on sheep which apparently prefer the head and distal limbs of their hosts (Ogden et al. 1998). Nymphs showed a narrow feeding site niche and had strong preferences for the head and the front legs, again similar to feeding sites of nymphs parasitizing sheep (Ogden et al. 1998). Nymphs were especially clumped on the outside and inside (haired) parts of the roe deer ears. Adult ticks (males and females) apparently prefer the neck and the head which also appears similar in ticks feeding on sheep (Ogden et al. 1998). Adult Ixodes scapularis feeding on white-tailed deer (Odocoileus virginianus) also prefer feeding on the head (incl. the ears) and the neck of their hosts (Schmidtmann et al. 1998). Clearly, any host-targeted tick control device (e.g. Fish and Childs 2009; Pound et al. 2009) should focus the acaricide agent application on these body parts. Feeding site selection and consequently, niche width and niche overlap varied considerably by season but also by year. Ultimately, these variations are mainly due to the strong seasonality in activity patterns of the different stages (Fig. 4).
Fig. 4.
Seasonal pattern of mean numbers of larval, nymphal, male, female and all ticks (Ixodes ricinus complex) attached to roe deer (Capreolus capreolus) from the forests of Göttingen, Germany
Numerous factors might influence the observed spatial aggregation of tick life stages/sexes among which season and year (this paper), but also host/body part characteristics like skin thickness, humidity, blood circulation and de-ticking by grooming behaviour of the host might play an important role (L’Hostis et al. 1994; Ogden et al. 1998). The observed attachment patterns suggest that there is some sort of active selection involved. Contradicting the ideal free distribution hypothesis (Fig. 3), number of ticks feeding on the preferred body part increased significantly (and did not change significantly in males) with increasing abundance of this life stage/sex on the entire roe deer. These results are in line with propositions that aggregation of Ixodes ricinus ticks are caused by pheromone excreted by conspecifics (e.g. Sonenshine 2006; Healy and Bourke 2008) whereas this remains to be further tested for Ixodes ricinus. For the tick Rhipicephalus appendiculatus, gregarious feeding turned out to be beneficial (increased blood feeding rate, reduced time to mating and repletion) for female ticks (Wang et al. 2001). Gregariousness might also be beneficial for Ixodes ticks that attach on the host in order to feed, seeming to be an evolutionary stable strategy. That gregarious feeding might be beneficial is further supported by the fact that apparent intrastadial attraction only occurs in those tick stages that actually feed (larvae, nymphs, females) on the roe deer blood but not in males which ultimately seek a female tick for reproduction. The quest for a mate might explain the strong overlap between the female and male ticks which mainly occurs on the neck of the roe deer. It is notable that niche width of males and female-male overlap are correlated with body mass of the host. This might be due to the positive relationship between adult tick burden and age and body mass of roe deer (Vor et al. 2010). However, until now tick burden on roe deer appears to follow rather unpredictable patterns apart from seasonality and thus impedes straight forward identification of potential super-spreaders (cf. Perkin et al. 2003).
Interstadial aggregation
Next to intraspecific aggregation, interstadial attachment site overlap is of considerable interest as this might facilitate pathogen transmission from one feeding development stage to another (i.e. larvae, nymphs and females). Therefore, larvae-nymph, larvae-female and nymph-female feeding aggregations are of special importance. Of these, niche overlap was greatest between nymphs and females and larvae and nymphs. Nymph-female feeding overlap mainly takes place on the head of the roe deer. Here, distances between the two stages are however relatively large, because most nymphs are located on the pinna whereas most larvae are attached around the muzzle and the eyes. Larvae-female overlap also mainly takes place on the roe deer’s head, whereas both stages feed in close proximity to each other, suggesting that pathogen transmission could be relatively efficient. Overall, niche overlap was significantly affected by season, reflecting seasonal activity peaks of the development stages (Fig. 4) observed during our study. In areas, where larvae and nymphs show even stronger seasonal synchrony (e.g. Randolph et al. 1999, 2000), co-feeding between these immature tick stages might take place, especially on the head and front legs of roe deer, where the two stages feed side by side. For tick-borne encephalitis virus, co-feeding transmission has been observed in forest rodent species such as Apodemus spp., Myodes glareolus (Labuda et al. 1993, 1996); for Borrelia burgdorferi co-feeding transmission has been demonstrated for ticks feeding on several rodent species, medium sized mammals, sheep (Ovis aries) and several bird species (Gern and Rais 1996; Ogden et al. 1997; Gern et al. 1998). Without a systematic infection of the host, Louping-ill virus can be transmitted from tick to tick feeding on hares (Lepus timidus), sheep, red grouse (Lagopus lagopus scoticus), but not by ticks feeding on red deer (Cervus elaphus), rabbits (Oryctolagus cuniculus) and rodents (Apodemus sylvaticus, Myodes glareolus) (Jones et al. 1997; Gilbert et al. 2000). In the absence of experiments testing co-feeding pathogen transmission on roe deer, we strongly recommend conducting such co-feeding transmission experiments for different tick-vectored pathogens.
Acknowledgments
We would like to sincerely thank J. Berger, D. Birke, E. Kreysern, W. Mänz, M. Scholz, and R. Zietlow and all involved hunters and forest rangers for providing the roe deer. We thank K. Hammer, H. Lippe, A. and M. Lödige and M. Scholz for their help during the field work. This study was funded by a research grant from the Federal Ministry of Education and Research (BMBF grant 1363120: Emerging arthropod-borne-viral infections in Germany: Pathogenesis, diagnostics and surveillance).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
C. Kiffner, Phone: +49-551-393583, FAX: +49-551-3922089, Email: ckiffne@gwdg.de
C. Lödige, Email: christinaloedige@hotmail.com
M. Alings, Email: efrafa@web.de
T. Vor, Email: tvor@gwdg.de
F. Rühe, Email: fruehe@gwdg.de
References
- Barnard DR, Morrison RD. Density estimators for populations of the lone star tick, Amblyoma americanum (Acari: Ixodidae), on pastured beef cattle. J Med Entomol. 1985;22:244–249. doi: 10.1093/jmedent/22.3.244. [DOI] [PubMed] [Google Scholar]
- Barnard DR, Morrsion RD, Ervin T. Sites of attachment and density assessment in Amblyoma americanum (Acari: Ixodidae) on nursing beef calves. Exp Appl Acarol. 1989;6:245–252. doi: 10.1007/BF01193983. [DOI] [PubMed] [Google Scholar]
- Bloemer SR, Zimmermann RH, Fairbanks K. Abundance, attachment sites, and density estimators of lone star ticks (Acari: Ixodidae) infesting white tailed deer. J Med Entomol. 1988;25:295–300. doi: 10.1093/jmedent/25.4.295. [DOI] [PubMed] [Google Scholar]
- Bruno P, Bruno G, Peréz-Eid C. Detection of spirochaetes of Borrelia burgdorferi complexe in the skin of cervids by PCR and culture. Eur J Epidemiol. 2000;16:869–873. doi: 10.1023/A:1007646216035. [DOI] [PubMed] [Google Scholar]
- Carpi G, Cagnacci F, Neteler M, Rizzoli A. Tick infestation on roe deer in relation to geographic and remotely sensed climatic variables in a tick-borne encephalitis endemic area. Epidemiol Infect. 2008;136:1416–1424. doi: 10.1017/S0950268807000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish D, Childs JE. Community-based prevention of Lyme disease and other tick-borne diseases through topical application of acaricide to white-tailed deer: background and rationale. Vector-Borne Zoonotic Dis. 2009;9:357–364. doi: 10.1089/vbz.2009.0022. [DOI] [PubMed] [Google Scholar]
- Fourie LJ, Kok DJ. A comparison of Ixodes rubicundus infestations on Friesian and Bonsmara cattle in South Africa. Exp Appl Acarol. 1995;19:529–531. doi: 10.1007/BF00052922. [DOI] [PubMed] [Google Scholar]
- Fourie LJ, van Zyl JM. Interspecific variations in attachment sites and density assessment in female Ixodes rubicundus (Acari: Ixodidae) on domestic and natural hosts. Exp Appl Acarol. 1991;13:1–10. doi: 10.1007/BF01268934. [DOI] [Google Scholar]
- Fourie LJ, Horak IG, van Zyl JM. Sites of attachment and intraspecific infestation densities of the brown paralysis tick Rhipicephalus punctatus on Angora goats. Exp Appl Acarol. 1991;13:243–249. doi: 10.1007/BF01193470. [DOI] [PubMed] [Google Scholar]
- Fretwell SD, Lucas HL. On territorial behaviour and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheor. 1970;19:16–36. doi: 10.1007/BF01601953. [DOI] [Google Scholar]
- Gern L, Rais O. Efficient transmission of Borrelia burgdorferi between cofeeding Ixodes ricinus ticks (Acari: Ixodidae) J Med Entomol. 1996;33:189–192. doi: 10.1093/jmedent/33.1.189. [DOI] [PubMed] [Google Scholar]
- Gern L, Estrada-Pena A, Frandsen F, Gray JS, Jaenson TGT, Jongejan F, Kahl O, Mehl R, Nuttall PA. European reservoir hosts of Borrelia burgdorferi sensu lato. Zbl Bakt. 1998;287:196–204. doi: 10.1016/s0934-8840(98)80121-7. [DOI] [PubMed] [Google Scholar]
- Gilbert L, Jones LD, Hudson PJ, Gould EA. Role of small mammals in the persistence of Louping-ill virus: field survey and tick co-feeding studies. Med Vet Entomol. 2000;14:277–282. doi: 10.1046/j.1365-2915.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- Hartemink NA, Randolph SE, Davis SA, Heesterbeek JAP. The basic reproduction number for complex disease systems: defining R0 for tick-borne infections. Am Nat. 2008;171:743–754. doi: 10.1086/587530. [DOI] [PubMed] [Google Scholar]
- Healy JAE, Bourke P. Aggregation in the tick Ixodes ricinus (Acari: Ixodidae): use and reuse of questing vantage points. J Med Entomol. 2008;45:222–228. doi: 10.1603/0022-2585(2008)45[222:AITTIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hudson PJ, Rizzoli A, Rosà R, Chemini C, Jones LD, Gould EA. Tick-borne encephalitis virus in northern Italy: molecular analysis, relationships with density and seasonal dynamics of Ixodes ricinus. Med Vet Entomol. 2001;15:304–313. doi: 10.1046/j.0269-283x.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Hurlbert SH. The measurement of niche overlap and some relatives. Ecology. 1978;59:67–77. doi: 10.2307/1936632. [DOI] [Google Scholar]
- Jaenson TG, Tälleklint L. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J Med Entomol. 1992;29:813–817. doi: 10.1093/jmedent/29.5.813. [DOI] [PubMed] [Google Scholar]
- Jensen PM, Hansen H, Frandsen F. Spatial risk assessment for lyme borreliosis in Denmark. Scand J Infect Dis. 2000;32:545–550. doi: 10.1080/003655400458857. [DOI] [PubMed] [Google Scholar]
- Jones LD, Gaunt M, Hails RS, Laurenson K, Hudson PJ, Reid H, Henbest P, Gould EA. Transmission of Louping ill virus between infected and uninfected ticks co-feeding on mountain hares. Med Vet Entomol. 1997;11:172–176. doi: 10.1111/j.1365-2915.1997.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(Suppl):S3–S14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kiffner C, Lödige C, Alings M, Vor T, Rühe F (in press) Abundance estimation of Ixodes ticks (Acari: Ixodidae) on roe deer (Capreolus capreolus). Exp Appl Acarol. doi:10.1007/s10493-010-9341-4 [DOI] [PMC free article] [PubMed]
- Kimura K, Isogai E, Kamewaka Y, Nishikawa T, Ishii N, Fujii N. Detection of Lyme-disease spirochetes in the skin of naturally infected wild Sika-deer (Cervus nippon yesoensis) by PCR. Appl Environ Microbiol. 1995;61:1641–1642. doi: 10.1128/aem.61.4.1641-1642.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hostis M, Diarra O, Seegers H. Sites of attachment and density assessment of female Ixodes ricinus (Acari: Ixodidae) on dairy cows. Exp Appl Acarol. 1994;18:681–689. doi: 10.1007/BF00051535. [DOI] [PubMed] [Google Scholar]
- Labuda M, Nuttall PA, Kozuch O, Eleckova E, Zuffova E, Williams T, Sabo A. Non-viraemic transmission of tick-borne encephalitis virus: a mechanism of arbovirus survival in nature. Experientia. 1993;49:802–805. doi: 10.1007/BF01923553. [DOI] [PubMed] [Google Scholar]
- Labuda M, Austyn JM, Zuffova E, Kozuch O, Fuchsberger N, Lysy I, Nuttall PA. Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology. 1996;219:357–406. doi: 10.1006/viro.1996.0261. [DOI] [PubMed] [Google Scholar]
- Levins R. Evolution in changing environments: some theoretical explanations. Princeton: Princeton University Press; 1968. [Google Scholar]
- Linard C, Lamarque P, Heyman P, Ducoffre G, Luyasu V, Tersago K, Vanwambeke SO, Lambin F. Determinants of the geographic distribution of Puumula virus and Lyme borreliosis infections in Belgium. Int J H Geogr. 2007;6:15. doi: 10.1186/1476-072X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandrin L, Jouglin M, Sun Y, Brisseau N, Chauvin A. Redescription of Babesia capreoli (Enigk and Friedhoff, 1962) from roe deer (Capreolus capreolus): Isolation, cultivation, host specificity, molecular characterisation and differentiation from Babesia divergens. Int J Parasitol. 2010;40:277–284. doi: 10.1016/j.ijpara.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Mathee S, Meltzer DGA, Horak IG. Sites of attachment and density assessment of ixodid ticks (Acari: Ixodidae) on impala (Aepyceros melampus) Exp Appl Acarol. 1997;21:179–192. doi: 10.1023/A:1018438719827. [DOI] [PubMed] [Google Scholar]
- Matuschka FR, Heiler M, Eiffert H, Fischer P, Lotter H, Spielman A. Diversionary role of hoofed game in the transmission of Lyme disease spirochetes. Amer J Trop Med Hyg. 1993;48:693–699. doi: 10.4269/ajtmh.1993.48.693. [DOI] [PubMed] [Google Scholar]
- Nelson WA, Keirans JE, Bell JF, Clifford CM. Host-ectoparasite relationship. J Med Entomol. 1975;12:143–166. doi: 10.1093/jmedent/12.2.143. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Nuttal PA, Randolph SE. Natural Lyme disease cycles maintained via sheep by co-feeding ticks. Parasitology. 1997;115:591–599. doi: 10.1017/S0031182097001868. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Hailes RS, Nuttall PA. Interstadial variation in the attachment sites of Ixodes ricinus ticks on sheep. Exp Appl Acarol. 1998;22:227–232. doi: 10.1023/A:1006066315226. [DOI] [PubMed] [Google Scholar]
- Ostfeld R, Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conserv Biol. 2001;14:722–728. doi: 10.1046/j.1523-1739.2000.99014.x. [DOI] [Google Scholar]
- Perkin SE, Cattadori IM, Tangliapietra V, Rizzoli AP, Hudson PJ. Empirical evidence for key hosts in persistence of a tick-borne disease. Int J Parasitol. 2003;33:909–917. doi: 10.1016/S0020-7519(03)00128-0. [DOI] [PubMed] [Google Scholar]
- Petritian AM, von Lüpke B, Petritan IC. Effects of shade on growth and mortality of maple (Acer pseudoplatanus), ash (Fraxinus excelsior) and beech (Fagus sylvatica) saplings. Forestry. 2007;80:397–412. doi: 10.1093/forestry/cpm030. [DOI] [Google Scholar]
- Pfäffle M, Petney T, Elgas M, Skubulla J, Taraschewski H. Tick-induced blood loss leads to regenerative anaemia in the European hedgehog (Erinaceus europaeus) Parasitology. 2009;136:443–452. doi: 10.1017/S0031182009005514. [DOI] [PubMed] [Google Scholar]
- Pianka ER. The structure of lizard communities. Annu Rev Ecol Evol S. 1973;4:53–74. doi: 10.1146/annurev.es.04.110173.000413. [DOI] [Google Scholar]
- Pound JM, Miller JA, George JE, Fish D, Caroll JF, Schulze TL, Daniels TJ, Falco RC, Stafford KC, III, Mather TN. The United States Department of Agriculture’s Northeast Area-Wide Tick Control Project: summary and conclusions. Vector-Borne Zoonotic Dis. 2009;9:439–448. doi: 10.1089/vbz.2008.0200. [DOI] [PubMed] [Google Scholar]
- Randolph SE. Patterns of distribution of the tick Ixodes trianguliceps Birula on its hosts. J Anim Ecol. 1975;44:451–474. doi: 10.2307/3606. [DOI] [Google Scholar]
- Randolph SE. Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology. 2008;129(Suppl):S37–S65. doi: 10.1017/s0031182004004925. [DOI] [PubMed] [Google Scholar]
- Randolph SE, Miklisova D, Lysy J, Rogers DJ, Labuda M. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology. 1999;118:177–186. doi: 10.1017/S0031182098003643. [DOI] [PubMed] [Google Scholar]
- Randolph SE, Green RM, Peacy MF, Rogers DJ. Seasonal synchrony: the key to tick-borne encephalitis foci identified by satellite data. Parasitology. 2000;121:15–23. doi: 10.1017/S0031182099006083. [DOI] [PubMed] [Google Scholar]
- Rizzoli A, Hauffe HC, Tagliapietra V, Neteler M, Rosà R. Forest Structure and roe deer abundance predict tick-borne encephalitis risk in Italy. PLoS ONE. 2009;4:e4336. doi: 10.1371/journal.pone.0004336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtmann ET, Caroll JF, Watson DW. Attachment site patterns of adult blacklegged ticks (Acari: Ixodidae) on white-tailed deer and horses. J Med Entomol. 1998;35:59–63. doi: 10.1093/jmedent/35.1.59. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE. Tick pheromones and their use in tick control. Annu Rev Entomol. 2006;51:557–580. doi: 10.1146/annurev.ento.51.110104.151150. [DOI] [PubMed] [Google Scholar]
- Sutherland WJ. From individual behaviour to Population Biology. Oxford: Oxford University Press; 1996. [Google Scholar]
- Vor T, Kiffner C, Hagedorn P, Niedrig M, Rühe F (2010 online only) Tick burden on European roe deer (Capreolus Capreolus L.). Exp Appl Acarol. doi:10.1007/s10493-010-9337-0 [DOI] [PMC free article] [PubMed]
- Walker AR, Alberdi MP, Urquhart KH, Rose H. Risk factors in habitats of the tick Ixodes ricinus influencing human exposure to Ehrlichia phagocytophila bacteria. Med Vet Entomol. 2001;15:40–49. doi: 10.1046/j.1365-2915.2001.00271.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Hails RS, Cui WW, Nuttal PA. Feeding aggregation of the tick Rhipicephalus appendiculatus (Ixodidae): benefits and costs in the contest with host responses. Parasitology. 2001;123:447–453. doi: 10.1017/S0031182001008654. [DOI] [PubMed] [Google Scholar]