Abstract
OBJECTIVE
To compare the efficacy and safety of the rapid-acting insulin analog glulisine and regular insulin in hyperglycemic hospitalized patients.
RESEARCH DESIGN AND METHODS
A total of 180 hospitalized patients with type 2 diabetes received either glulisine (n = 88) or regular insulin (n = 92) before each meal in combination with insulin glargine at bedtime in a randomized double-blind fashion. All previous diabetes medications were discontinued if applicable. Doses of insulin were adjusted to obtain target blood glucose concentrations of <130 mg/dl before meals and at bedtime while avoiding hypoglycemia.
RESULTS
Overall mean blood glucose concentrations were ∼8 mg/dl lower in the glulisine group than in the regular insulin group (152.6 ± 66.6 vs. 160.4 ± 70.8 mg/dl; P < 0.0002). This improvement was wholly due to ∼22 mg/dl lower levels after 4 days of therapy (140 ± 55 vs. 162 ± 71 mg/dl; P < 0.0007); after day 4, this difference progressively increased such that mean blood glucose concentrations from day 7 onward were ∼31 mg/dl lower in the glulisine group. The mean daily incidence of hypoglycemia was slightly but not significantly lower in the glulisine than the regular insulin group (0.10 ± 0.02 vs. 0.14 ± 0.03 episode/day; P > 0.35).
CONCLUSIONS
In hospitalized type 2 diabetic patients, glulisine may provide better glycemic control than regular insulin, especially in those who have a prolonged length of stay.
Accumulating evidence suggests that hyperglycemia is associated with an increased risk of complications and mortality in hospitalized patients. In critically ill patients, improved glycemic control reduces short- and long-term mortality, rates of multiorgan failure, systemic infections, and length of hospitalization (1–3). Likewise, in patients admitted to general medical and surgical areas hyperglycemia is associated with a prolonged hospital stay, infection, disability, and death (4–6), suggesting that poor glycemic control is associated with poor clinical outcome.
Insulin is the most effective and the most preferred agent for the treatment of hyperglycemia in hospitalized patients (7). However, inpatient insulin therapy is often complicated by variable meal delivery, unpredictable food consumption, and medical conditions, including liver and kidney disease, that predispose to hypoglycemia. Rapid-acting insulin analogs, which have been shown to reduce the risk of hypoglycemia in the outpatient setting (8), may hence be a better choice than regular insulin for the treatment of hyperglycemia in noncritically ill hospitalized patients. The present study was therefore undertaken to compare the efficacy and safety of the rapid-acting insulin analog glulisine and regular insulin in hyperglycemic hospitalized patients.
RESEARCH DESIGN AND METHODS
We enrolled 194 patients with type 2 diabetes who were admitted to a noncritical care medical or surgical unit at the Carl T. Hayden VA Medical Center (Phoenix, AZ). All patients were expected to stay hospitalized ≥3 days. Exclusion criteria included severe hypoglycemia within the past 6 months or hypoglycemia unawareness because of the known risk reduction of hypoglycemia using rapid-acting insulin analogs (9,10), prolonged nothing by mouth status, continuous nutrition (total parenteral nutrition or enteral nutrition), and mental health conditions rendering the patient unable to provide informed written consent. Informed written consent was obtained from all patients after the study had been approved by the local institutional review board.
All prior diabetes medications were discontinued if applicable, and all patients were started on a basal-bolus insulin regimen consisting of insulin glargine (Lantus; sanofi-aventis, Bridgewater, NJ) and glulisine (Apidra; sanofi-aventis, Bridgewater, NJ) or regular insulin (Novolin R, Novo Nordisk, Princeton, NJ). Patients were assigned to receive glulisine or regular insulin in a randomized double-blind fashion. For patients treated only with insulin before the hospitalization, the initial dose of insulin was equal to the total outpatient dose. For all other patients, the initial total daily dose of insulin was 0.4 IU/(kg · day)−1 if BMI was <25 kg/m2, 0.5 IU/(kg · day)−1 if BMI was 25–30 kg/m2, and 0.6 IU/(kg · day)−1 if BMI was >30 kg/m2. One-half of the total daily insulin dose was given as glargine once daily at bedtime, and the other half was given as glulisine or regular insulin in equally divided doses before breakfast, lunch, and dinner. All insulin doses were administered by nurses, who were not blinded to the study medications. Glulisine was administered immediately before meals, regular insulin was administered ∼30 min before meals. Glulisine and regular insulin were not given if a subject was unable to eat to avoid hypoglycemia. Fingerstick blood glucose (FSBG) was tested daily before each meal, at bedtime, and whenever subjects reported symptoms of hypoglycemia; 2-h postprandial and 2:00 a.m. FSBG were tested every 3rd day starting on day 2 of study participation. All FSBG measurements were obtained using the Roche Accu-Chek Inform glucose meter (Roche Diagnostics, Indianapolis, IN) and downloaded to the VA Computerized Patient Record System. Insulin doses were adjusted daily by the investigators (who were blinded to the short-acting insulin) to target blood glucose concentrations of <130 mg/dl before meals and at bedtime while avoiding hypoglycemia using the following guidance: for fasting blood glucose concentrations of 130–160, 161–200, and >200 mg/dl, increase glargine by 10, 20, and 30%, respectively; for prelunch, predinner, and bedtime blood glucose concentrations of 130–160, 161–200, and >200 mg/dl, increase regular insulin or glulisine at the prior meal by 10, 20, and 30%, respectively. Supplemental glulisine or regular insulin was given in addition to the scheduled insulin dose before each meal for blood glucose concentrations >130 mg/dl according to a sliding scale protocol (Table 1). Serum creatinine, white blood cell count, and A1C were measured on the 1st day of hospitalization. Fasting plasma C-peptide with the concurrent plasma glucose concentration was measured on day 1 of study participation. All measurements were performed by the Carl T. Hayden VA Medical Center central laboratory using standard assays.
Table 1.
Supplemental insulin sliding scale
| Blood glucose | Insulin |
||
|---|---|---|---|
| BMI <25 kg/m2 | BMI 25–30 kg/m2 | BMI >30 kg/m2 | |
| 131–170 mg/dl | 1 | 2 | 3 |
| 171–210 mg/dl | 2 | 4 | 5 |
| 211–250 mg/dl | 3 | 6 | 8 |
| 251–290 mg/dl | 5 | 8 | 10 |
| 291–330 mg/dl | 7 | 10 | 13 |
| 331–370 mg/dl | 9 | 12 | 15 |
| >371 mg/dl | 12 | 14 | 18 |
Data are units of insulin.
Hypoglycemia was defined as a blood glucose concentration <60 mg/dl. Severe hypoglycemia was defined as an event that required assistance from another person for recovery. Nocturnal hypoglycemia was defined as an event that occurred between the injection of glargine at bedtime and before the subject awoke in the morning. As an index of β-cell function, homeostasis model assessment of percent β-cell function (HOMA-%B) was calculated as [fasting plasma insulin [picomoles per liter] × 3.33/(fasting plasma glucose [millimoles per liter] – 3.5)] (11).
The primary end points were glycemic control, measured by the mean daily blood glucose concentration, and the incidence of hypoglycemia. Length of stay was the secondary end point. Sample sizes were calculated for 80% power at an α of 0.05 for both primary end points. We assumed an average length of stay of 7 days, a within-group SD of 45 mg/dl in mean daily blood glucose concentrations, and a 30% incidence of subjects experiencing at least one episode of hypoglycemia as found in a previous similar study (12). Under these assumptions, power calculations indicated the need for 80 subjects to detect a 10 mg/dl difference in mean daily blood glucose concentrations and the need for 565 subjects to detect a 25% difference in the incidence of hypoglycemia. However, before reaching the latter sample size, at 194 subjects, the decision was made to terminate the study because of slower than expected enrollment. Baseline characteristics of subjects and outcome variables were compared using the Student t test or the χ2 test as appropriate. Multiple comparisons of blood glucose concentrations over the course of the subjects' study participation were performed using repeated-measures ANOVA. Statistical analyses were performed using the SPSS 16.0 (SPSS, Chicago, IL). P < 0.05 was considered statistically significant. Data are presented as means ± SD unless otherwise indicated.
RESULTS
Patient characteristics
Of the 194 enrolled subjects, 96 were randomly assigned to glulisine and 98 were randomly assigned to regular insulin. Fourteen subjects dropped out for personal nonmedical reasons before receiving any insulin as part of this study: 8 subjects assigned to glulisine and 6 subjects assigned to regular insulin. Therefore, data from 88 subjects in the glulisine group and 92 subjects in the regular insulin group were used for statistical analyses. As shown in Table 2, both groups were well matched for age, sex, BMI, previously unrecognized type 2 diabetes, and prior history of type 2 diabetes, diabetes duration, prior diabetes treatment, blood glucose concentration on admission, A1C, β-cell function, renal function, and white blood cell count. The most common admitting diagnoses were cardiovascular disease, infection, and pulmonary disease (Table 2). The length of time of hospitalization was not significantly different between the glulisine and the regular insulin group (7.3 ± 0.5 vs. 8.4 ± 0.6 days; P > 0.13).
Table 2.
Baseline clinical and biochemical characteristics of the study groups
| Regular insulin | Glulisine | |
|---|---|---|
| n | 92 | 88 |
| Age (years) | 65.1 ± 9.1 | 65.6 ± 10.1 |
| Sex (male/female) | 91/1 | 87/1 |
| BMI (kg/m2) | 32.1 ± 7.0 | 32.6 ± 7.6 |
| Race/ethnicity (white/black/Hispanic) | 72/10/10 | 64/12/12 |
| Unrecognized type 2 diabetes | 4 (4) | 2 (2) |
| History of type 2 diabetes | 88 (96) | 86 (98) |
| Diabetes duration (years) | 11.9 ± 8.0 | 11.6 ± 8.8 |
| Diabetes treatment before hospitalization | ||
| No pharmacological agents | 5 (5.4) | 3 (3.4) |
| Oral agent monotherapy | 12 (13) | 22 (25)* |
| Multiple oral agents | 13 (14) | 18 (20) |
| Insulin plus oral agents | 34 (37) | 27 (31) |
| Insulin only | 28 (30) | 18 (20) |
| A1C (%) | 7.7 ± 1.7 | 7.7 ± 1.8 |
| HOMA-%B | 1.03 ± 0.95 | 0.96 ± 1.06 |
| Admission blood glucose (mg/dl) | 189 ± 86 | 187 ± 95 |
| White blood cell count × 106 | 10.4 ± 4.6 | 10.1 ± 4.4 |
| Serum creatinine (mg/dl) | 1.6 ± 1.3 | 1.6 ± 1.3 |
| Admission diagnosis (%) | ||
| Cardiovascular disease | 30 (32) | 30 (34) |
| Infection | 23 (25) | 18 (20) |
| Pulmonary disease | 11 (12) | 9 (10) |
| Uncontrolled diabetes | 1 (1) | 1 (1) |
| Renal disease | 1 (1) | 3 (3) |
| Amputation/diabetic foot ulcer | 7 (8) | 5 (6) |
| Other | 19 (21) | 22 (25) |
Data are means ± SD or n (%). To convert the values for glucose from milligrams per deciliter to millimoles per liter, multiply by 0.05551.
*P < 0.05.
Insulin doses
In the glulisine and the regular insulin group, the mean total daily dose of insulin was similar (69 ± 33 vs. 71 ± 45 units; NS) and was accounted for by a comparable amount of short-acting insulin (36 ± 18 vs. 38 ± 24 units; NS).
Glycemic control
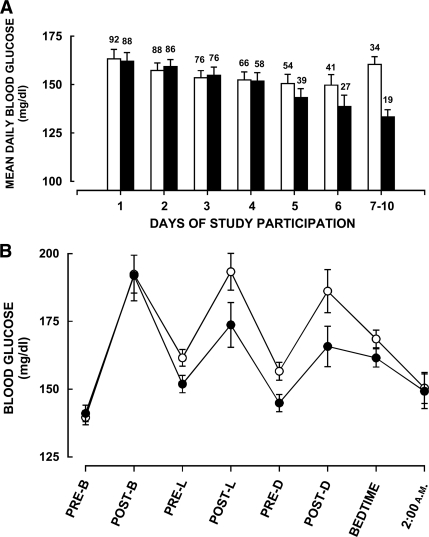
During the entire period of the subjects' study participation, mean blood glucose concentrations were ∼8 mg/dl lower in the glulisine group than in the regular insulin group (152.6 ± 66.6 vs. 160.4 ± 70.8 mg/dl; P < 0.0002). This reduction was wholly due to the on average ∼22 mg/dl lower blood glucose concentrations after 4 days of therapy (140 ± 55 vs. 162 ± 71 mg/dl; P < 0.0007), because levels were virtually identical in both groups during the first 4 days (159 ± 71 vs. 159 ± 71 mg/dl; P > 0.9). After day 4, the target blood glucose level of <130 mg/dl before meals was achieved in 48% of subjects in the glulisine group and in 38% of subjects in the regular insulin group (P < 0.0003); 66% of all blood glucose readings in the glulisine group were 90–180 mg/dl compared with 54% in the regular insulin group (P < 0.0001). The difference in glycemic control between both groups progressively increased such that blood glucose concentrations from 7 day onward were ∼31 mg/dl lower in the glulisine group (133 ± 51 vs. 164 ± 72 mg/dl; P < 0.0001). The time course of mean daily blood glucose concentrations (premeal and bedtime blood glucose concentrations) in both groups of subjects is shown in Fig. 1A.
Figure 1.
A: Time course of mean daily blood glucose concentrations (premeal and bedtime blood glucose concentrations) in patients treated with glulisine (■) or regular insulin (□) in combination with glargine. Numbers on top of each bar indicate the number of subjects in each group. Data are means ± SEM. Overall ANOVA, P < 0.0001. B: Mean blood glucose concentrations prebreakfast (PRE-B), 2-h postbreakfast (POST-B), prelunch (PRE-L), 2-h postlunch (POST-L), predinner (PRE-D), 2-h postdinner (POST-D), at bedtime, and at 2:00 a.m. in patients treated with glulisine (●) or regular insulin (○) in combination with glargine during the entire study. Means of prelunch, predinner, and bedtime blood glucose concentrations (P < 0.0003) and 2-h postprandial blood glucose concentrations (P < 0.03), largely determined by the short-acting insulin, were significantly lower in the glulisine group than in the regular insulin group. In contrast, fasting and 2:00 a.m. blood glucose concentrations, largely determined by glargine, were comparable in both groups (both P > 0.7). Data are means ± SEM.
To examine whether the reduction in glycemia by glulisine could have been due to its direct actions, we separately analyzed blood glucose concentrations that were expected to be predominantly determined by the actions of glargine (e.g., fasting and 2:00 a.m. blood glucose concentrations) and blood glucose concentrations that were expected to be predominantly determined by the actions of glulisine or regular human insulin (e.g., prelunch, predinner, and bedtime blood glucose concentrations, corresponding to ∼4-h postprandial levels and 2-h postprandial blood glucose concentrations). When all blood glucose concentrations throughout the study are used for analysis, means of prelunch, predinner, and bedtime blood glucose concentrations (152 ± 64 vs. 162 ± 70 mg/dl; P < 0.0003) and 2-h postprandial blood glucose concentrations (177 ± 79 vs. 191 ± 77 mg/dl; P < 0.03) were significantly lower in the glulisine group than in the regular insulin group. In contrast, fasting (141 ± 59 vs. 140 ± 57 mg/dl) and 2:00 a.m. blood glucose concentrations (149 ± 67 vs. 150 ± 67 mg/dl) were virtually identical in both groups (both P > 0.7) (Fig. 1B). Similar results were obtained when only blood glucose concentrations past day 4 were examined. Prelunch, predinner, and bedtime blood glucose concentrations (138 ± 51 vs. 162 ± 72 mg/dl; P < 0.000002) and 2-h postprandial blood glucose concentrations (164 ± 70 vs. 191 ± 71 mg/dl; P < 0.0026) were significantly lower in the glulisine group than in the regular insulin group whereas fasting (125 ± 49 vs. 141 ± 54 mg/dl; P = 0.06) and 2:00 a.m. blood glucose concentrations (150 ± 52 vs. 160 ± 67 mg/dl; P > 0.8) were not significantly different.
Subgroup analysis
To examine whether the improved glycemia with glulisine past day 4 could potentially be explained by different characteristics of subjects who stayed hospitalized for a longer period of time, we compared subjects participating >4 days (n = 39 in the glulisine group; n = 54 in the regular insulin group) with subjects participating ≤4 days in separate analyses. Age, sex, BMI, A1C, HOMA-%B, diabetes duration, and admission diagnosis were comparable between these subgroups in both the glulisine group and the regular insulin group (all P > 0.3). Moreover, in subjects who participated >4 days, glycemic control was similar in the glulisine and the regular insulin group during the first 4 days of study participation (P > 0.9) as found when we analyzed data for all subjects.
Hypoglycemia
Throughout the study, there were 123 hypoglycemic events: 56 in the glulisine group and 67 in the regular insulin group. However, neither the number of subjects with one or more hypoglycemic episodes (30 vs. 35%; P > 0.5) nor the average daily incidence of hypoglycemia was significantly different (0.10 ± 0.02 vs. 0.14 ± 0.03 episode/day; P > 0.35). Furthermore, the incidence of hypoglycemia was not significantly different between both groups during the first 4 days of therapy (0.11 ± 0.03 vs. 0.14 ± 0.03 episode/day; P > 0.4), during which glycemia was comparable, and after day 4 (0.07 ± 0.02 vs. 0.06 ± 0.02 episode/day; P > 0.6), during which glycemia was significantly reduced in the glulisine group. The severity and the time of day of hypoglycemic events were also similar in both groups (Table 3). Only one hypoglycemic event, which occurred in the regular insulin group, was severe.
Table 3.
Frequency and severity of hypoglycemia
| Regular insulin | Glulisine | |
|---|---|---|
| Hypoglycemic episodes | 67 | 56 |
| Blood glucose 50–59 mg/dl | 40 | 34 |
| Blood glucose 40–49 mg/dl | 19 | 17 |
| Blood glucose <40 mg/dl | 8 | 5 |
| Subjects with ≥1 hypoglycemic episode | 32 | 26 |
| Incidence of hypoglycemia (episodes/day) | 0.136 ± 0.027 | 0.103 ± 0.020 |
Data are n or means ± SD.
CONCLUSIONS
In hospitalized patients, hyperglycemia is a frequent, serious, and costly problem, and tight glycemic control is recommended by the American Diabetes Association (7). The present trial is the first to compare the efficacy and safety of the rapid-acting insulin analog glulisine and regular insulin in hospitalized patients with type 2 diabetes. Using a randomized double-blind study design, we found that treatment with glulisine resulted in lower blood glucose concentrations than treatment with regular insulin without increasing the risk of hypoglycemia. When all blood glucose readings during the study are considered, the reduction in glycemia by glulisine was ∼8 mg/dl. This reduction was highly significant but arguably modest. However, if only data past day 4 of therapy are considered, glulisine resulted in ∼22 mg/dl reduced blood glucose concentrations, because both groups had similar levels during the first 4 days. Thereafter, the difference in glycemic control progressively increased such that after 7 days blood glucose concentrations were ∼31 mg/dl lower in the glulisine group.
It is of note that subjects participating for >4 days did not differ from those participating for ≤4 days in demographic, physical, and medical characteristics and that glycemic control was similar in the glulisine and the regular insulin group during their first 4 days of study participation as we had found when we analyzed data for all subjects. Furthermore, the incidence of hypoglycemia was, if anything, slightly higher in the regular insulin group than in the glulisine group. These findings suggest that the improvement in glycemic control by glulisine after but not during the first 4 days of therapy was due to the length of time required for dose titration and not due to unique characteristics of subjects who were hospitalized for a longer period of time. Moreover, they denote that insulin was titrated at least equally aggressively in the regular insulin group as in the glulisine group by the blinded investigators, suggesting that development of hypoglycemia prevented further up-titration of regular insulin and achievement of glycemic control similar to that in the glulisine group and that, for a given aggressiveness in dose titration with similar frequency of hypoglycemia, glulisine may provide better glycemic control than regular insulin in hospitalized patients with type 2 diabetes.
The results of the present study are very much consistent with those of Dailey et al. (8) in that twice-daily glulisine in combination with NPH insulin improves glycemic control without increasing the frequency of hypoglycemia in outpatient type 2 diabetic patients. In contrast, our results disagree somewhat with those of Umpierrez et al. (12) in hospitalized patients with type 2 diabetes. In this study, the insulin analogs detemir given once daily and aspart given before meals resulted in glycemic control and frequency of hypoglycemia similar to that with a split-mixed regimen with NPH and regular insulin despite use of a dose titration schedule similar to that in the present study. However, contrary to our study, subjects were younger and were excluded for clinically relevant liver and kidney disease, suggesting that differences in study populations, specifically the risk of hypoglycemia, may be an explanation for the differing results.
In the present study both groups of subjects were well matched for age, diabetes duration, prior diabetes treatment, prior glycemic control, β-cell function, renal function, and white blood cell count. Furthermore, the study personnel, making adjustments to the insulin doses, was blinded to the short-acting insulin. Only nurses, who administered the insulin, were unblinded to the types of short-acting insulin because of the different administration times in relation to meals. Therefore, differences in subjects' characteristics between the glulisine and regular insulin groups or bias of the investigators is an unlikely explanation of our results. Nevertheless, we acknowledge the following limitations of our study. First, it was performed in a single center in an elderly veteran population involving nearly exclusively males. Thus, whether the results can be generalized to other hospital settings and populations needs to be examined. Second, only individuals highly trained in insulin treatment made adjustments in insulin doses, and blood glucose levels were carefully reviewed at least once a day, exceeding the usual standard of care. Therefore, whether treatment with glulisine provides superior control compared with regular insulin in hospitalized patients in the hands of less well-trained providers and with less careful blood glucose monitoring may need to be tested. Third, it is possible that some of our study patients were not completely blinded regarding the type of mealtime insulin because of their knowledge of the different administration times of regular insulin and glulisine relative to meals. And fourth, we unfortunately did not achieve our estimated sample size needed for detecting differences in the incidence of hypoglycemia because of slower than expected patient recruitment.
In critically ill patients, hypoglycemia has been found to be associated with poor clinical outcome (13). Although the causality of hypoglycemia leading to poor clinical outcome has been questioned (14), hypoglycemia or the fear of it in health care providers and patients is undoubtedly the major barrier for the control of glycemia. In the present study, 30–35% of patients experienced at least one episode of hypoglycemia with an incidence of ∼0.1–0.14 episode/day, comparable to the rates of hypoglycemia in the previous similar study by Umpierrez et al. (12). This observation demonstrates the difficulty of achieving tight glycemic control and underscores the importance of improving our treatment modalities in hospitalized patients with type 2 diabetes.
In summary, the present study provides evidence suggesting that treatment with glulisine can provide superior glycemic control compared with regular insulin in hospitalized patients with type 2 diabetes, especially in those who have a prolonged length of stay. Further studies are needed to examine whether these results can be generalized to other populations and hospital settings, and whether the benefits of glulisine persevere with the usual standard of care for glycemic control.
Acknowledgments
This investigator-initiated study was supported by the office of Research and Development, Medical Research Service, Department of Veterans Affairs and by an unrestricted grant from sanofi-aventis (to C.M.).
No other potential conflicts of interest relevant to this article were reported.
C.M. researched data, contributed to discussion, and wrote the manuscript. A.B., E.P., M.V., and R.V. researched data and contributed to discussion.
Footnotes
Clinical trial reg. no. NCT00528918, clinicaltrials.gov
The study sponsors were not involved in the design of the study; the collection, analysis, or interpretation of the data; or the preparation of the manuscript. The contents of this article do not represent the views of the Department of Veterans Affairs or the U.S. government.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R: Intensive insulin therapy in the critically ill patients. N Engl J Med 2001;345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 2.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC: Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001;32:2426–2432 [DOI] [PubMed] [Google Scholar]
- 3.Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, Fineberg SE, Tierney WR: Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology 2002;59:67–71 [DOI] [PubMed] [Google Scholar]
- 4.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE: Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 [DOI] [PubMed] [Google Scholar]
- 5.Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, Pomfret EA, Driscoll DF, Forse RA, Bistrian BR: Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr 1998;22:77–81 [DOI] [PubMed] [Google Scholar]
- 6.Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, Hirsch IB, Hirsh IB: American Diabetes Association Diabetes in Hospitals Writing Committee Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004;27:553–591 [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Standards of medical care in diabetes—2009. Diabetes Care 2009;32:S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dailey G, Rosenstock J, Moses RG, Ways K: Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care 2004;27:2363–2368 [DOI] [PubMed] [Google Scholar]
- 9.Brunelle BL, Llewelyn J, Anderson JH, Jr, Gale EA, Koivisto VA: Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care 1998;21:1726–1731 [DOI] [PubMed] [Google Scholar]
- 10.Velussi M: Lispro insulin treatment in comparison with regular human insulin in type 2 diabetic patients living in nursing homes. Diabetes Nutr Metab 2002;15:96–100 [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 12.Umpierrez GE, Hor T, Smiley D, Temponi A, Umpierrez D, Ceron M, Munoz C, Newton C, Peng L, Baldwin D: Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krinsley JS, Grover A: Severe hypoglycemia in critically ill patients: risk factors and outcomes. Crit Care Med 2007;35:2262–2267 [DOI] [PubMed] [Google Scholar]
- 14.Vanhorebeek I, Langouche L, Van den Berghe G: Tight blood glucose control: what is the evidence? Crit Care Med 2007;35:S496–S502 [DOI] [PubMed] [Google Scholar]