Abstract
OBJECTIVE
Injected volume and subcutaneous adipose tissue blood flow (ATBF) affect insulin absorption. Pharmacokinetics of short-acting insulin analogs were established by assessing injection of small doses in lean subjects, healthy or with type 1 diabetes. In obese patients, however, daily dosages are larger and ATBF is decreased. This study assessed the kinetics of a short-acting insulin analog in obese subjects with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Euglycemic clamps after subcutaneous lispro injections were performed. Six healthy control subjects received 10 units. Seven obese (BMI 38.3 ± 7.0 kg/m2) subjects with type 2 diabetes received 10, 30, and 50 units. Plasma lispro was measured by specific radioimmunoassay and ATBF by the 133Xe-washout technique.
RESULTS
ATBF was 64% lower in subjects with type 2 diabetes than in control subjects. After 10 units injection, time to lispro plasma peak (Tmax) was similar (48.3 vs. 55.7 min; control subjects versus type 2 diabetic subjects), although maximal concentration (Cmax)/dose was 41% lower in subjects with type 2 diabetes, with lower and delayed maximal glucose infusion rate (GIRmax: 9.0 vs. 0.6 mg/kg/min, P < 0.0001, 69 vs. 130 min, P < 0.0001, respectively). After 30- and 50-unit injections, Tmax (88.6 and 130.0 min, respectively) and time to GIRmax (175 and 245 min) were further delayed and dose related (r2 = 0.51, P = 0.0004 and r2 = 0.76, P < 0.0001, respectively).
CONCLUSIONS
Absorption and hypoglycemic action of increasing dosages of lispro are critically delayed in obese subjects with type 2 diabetes.
The general purpose of intensive insulin regimens is to achieve postprandial glucose control. Short-acting insulin analogs were originally designed to fit this premise by synchronizing plasma insulin increase and food absorption (1). They are indeed absorbed more quickly than regular human insulin. However, this was demonstrated in normal-weight healthy subjects or lean subjects with type 1 diabetes in studies assessing small subcutaneous injections (i.e., 4–12 units) (2–4). Paradoxically, few studies (5,6) have either assessed such dosages or been conducted in overweight subjects with or without type 2 diabetes, although clinical practice shows that most patients with type 2 diabetes on insulin are distinctly overweight or obese and on much larger insulin dosages (7,8).
Multiple factors affect insulin absorption including its physicochemical properties, excipients, concentration, and dosage as well as the clinical conditions under which it is injected, e.g., orthostatic position, injection site, depth, exercises, massage, temperature, and smoking (1,4,9,10). Injected volume (11) and subcutaneous adipose tissue blood flow (ATBF) are two other major absorption factors (12,13). Although it is well recognized that ATBF is dramatically altered in obese insulin-resistant individuals and in subjects with type 2 diabetes, baseline values are 50–70% lower than values for lean healthy subjects and physiological postprandial doubling is blunted (14,15). Nonetheless, most studies have been conducted in lean subjects.
In view of the above, we hypothesized that absorption rate and activity of short-acting insulin analogs would be substantially lower in obese subjects with type 2 diabetes than pharmacokinetics reported in the literature. This study thus assessed the pharmacokinetic and pharmacodynamic responses of obese subjects with type 2 diabetes to subcutaneous injections of lispro at incrementally larger dosages (10, 30, and 50 units) during euglycemic clamps.
RESEARCH DESIGN AND METHODS
Study design
This single-dose single-blinded controlled three-way randomized sequential study in subjects with type 2 diabetes was conducted at the clinical research center.
Nonsmoking patients aged 18–75 years, with a BMI ≥30 kg/m2 and an A1C ≤10%, taking over 100 units insulin daily, with or without oral hypoglycemic agents, were recruited. All were asked to maintain a stable diet and physical activity level between experiments and to refrain from strenuous exercise, alcohol, and caffeine intake for 48 h before each experiment.
The experimental protocol was duly approved by the Research Ethics Committee, conducted according to the Declaration of Helsinki principles, and all subjects signed the consent form.
Protocol
Experiments were performed 3 weeks apart in randomized order (10, 30, or 50 units). Subjects with type 2 diabetes were admitted at 8:00 p.m. on the evening preceding each experimental day, after having their evening meal and their usual insulin injection. An intravenous antecubital cannula was inserted into each arm: one for venous sampling and glucose measurements (Beckman Instruments, Diagnostic Systems Group, Brea, CA) and the other for dual administration of human insulin (Toronto R, Novo Nordisk Canada, Mississauga, ON) and dextrose as needed. Plasma glucose level was brought progressively into the normal target range (i.e., 5–6 mmol/l overnight).
Experiments started at 8:00 a.m. Anthropometric data were recorded: height, weight, and body composition by bioelectrical impedance (Tanita, Arlington Heights, IL). Subjects were kept fasting (drinking water permitted) during the entire 8-h clamp study. A venous catheter was retrogradely inserted into the hand of the same arm used for nighttime blood samplings, with the hand kept warm in a heating pad. Euglycemic clamp was performed after subcutaneous injection of lispro, 20 min after interruption of the overnight insulin infusion. Lispro was administered with a pen device (HumaPenErgo, Eli Lilly Canada, Toronto, ON) with an 8-mm needle (30 G × 0.3 × 8 mm) into subcutaneous adipose tissue 8 cm above the umbilicus and 10 cm from the medial line.
Plasma glucose was measured every 5 min to clamp glucose levels between 5 and 6 mmol/l with a 20% dextrose infusion via the antecubital catheter already in place. Blood samples were collected at 10-min intervals for the first 3 h and at 20-min intervals thereafter. Study procedures ended at 4:00 p.m. Subjects received a meal and their usual dose of insulin. They were discharged once glucose stabilized over 6 mmol/l.
Healthy control subjects were admitted on the experimental day at 7:30 a.m., fasting from 8:00 p.m. the prior evening. Each received a single dose 10 units lispro; all other procedures were identical to those described above.
ATBF was measured once, on the first experimental day, in each subject using the gold standard method, i.e., the 133Xe washout technique, a routinely used technique in our hands (16). Briefly, 133Xe (Bristol-Myers Squibb Canada, Dorval, Quebec) was injected in the subcutaneous adipose tissue of the abdomen, at the opposite side of the insulin injection site. ATBF was measured quantitatively using a Mediscint System (John Caunt Scientific, Oxford, U.K.).
Sample analysis
Blood samples were collected in tubes containing sodium citrate and a protease inhibitor cocktail (Complete, EDTA-free; Roche Diagnostics, Mannheim, Germany). Blood was promptly centrifuged at 4°C, and the resultant plasma aliquots were frozen immediately in liquid nitrogen and stored at −80°C until assaying. Plasma lispro was measured in duplicate with a specific radioimmunoassay kit (Linco Research, St. Charles, MO).
Calculations and statistical analyses
Plasma lispro measurements were used to estimate absorption rate constant (ka), maximum plasma concentration (Cmax), time to maximal concentration (Tmax), area under the lispro plasma concentration curve (AUC0-∞), Cmax to dose ratio (Cmax/D), AUC0-∞ to dose ratio (AUC0-∞/D), volume of distribution (Vz), clearance (Cl), half-life (t½), and mean residence time. Calculations were performed assuming a noncompartmental distribution using the WinNonlin 5.2 software (Pharsight, Mountain View, CA).
Using glucose infusion rate (GIR) versus time data, the maximum glucose infusion rate (GIRmax), time to maximum glucose infusion rate (tGIRmax), and total glucose infusion from injection to end of clamp (GItot) were calculated.
The study comprised one experiment in healthy subjects and three in obese subjects with type 2 diabetes; in the latter subjects, 10-unit experiments were used as control for comparison with larger dosages. Results not normally distributed, based on the Normal Quintile Plot, were log-transformed for all statistical analyses and reported back-transformed in their original units. Values of P < 0.05 were considered significant.
Fisher exact tests, for categorical variables, and unpaired t tests, for continuous variables, were used to compare characteristics between groups. Unpaired t tests were used for comparison between groups of pharmacokinetic and pharmacodynamic variables with 10-unit injections. Repeated-measures ANOVA tests were used to compare differences in pharmacokinetic and pharmacodynamic variables at different dosages in subjects with type 2 diabetes, with Tukey honestly significance difference tests for post hoc multiple comparisons.
For correlations between parameters that were repeatedly assessed at multiple insulin dosages in the same patients, repeated-measures ANOVA tests considering clustering of multiple measurements were used. All adjustments were performed again by multivariate ANOVA tests. Data calculations and statistical analyses were performed using JMP 7.0 software (SAS Institute, Cary, NC).
RESULTS
Six healthy subjects and seven obese subjects with type 2 diabetes were enrolled (A1C 8.1 ± 1.2%, duration of diabetes 20.2 ± 8.6 years, insulin therapy 5.1 ± 4.2 years). Subjects with type 2 diabetes participated in all three experiments (10, 30, and 50 units). Their age, BMI, weight, and adiposity indexes were higher although ATBF was blunted (Table 1). Heart rate, blood pressure, and ATBF remained stable in both groups during experiments (data not shown).
Table 1.
Characteristics of study groups
| Healthy subjects | Subjects with type 2 diabetes | P | |
|---|---|---|---|
| n (men/women) | 6 (3/3) | 7 (6/1) | 0.266 |
| Age (years) | 23.7 ± 2.4 | 60.3 ± 7.6 | <0.0001 |
| BMI (kg/m2) | 22.1 ± 1.4 | 38.3 ± 7.0 | 0.0002 |
| Weight (kg) | 70.0 ± 7.6 | 111.0 ± 14.3 | 0.0002 |
| Fat (%) | 22.4 ± 7.9 | 32.6 ± 5.1 | 0.017 |
| Fat mass (kg) | 15.4 ± 4.5 | 36.5 ± 9.6 | 0.0005 |
| Fat-free mass (kg) | 54.6 ± 10.2 | 74.5 ± 7.7 | 0.002 |
| Total body water (kg) | 40.0 ± 7.5 | 54.5 ± 5.7 | 0.002 |
| ATBF (ml/min/100 g tissue) | 4.2 ± 0.7 | 1.5 ± 0.5 | <0.0001 |
Data are means ± SD.
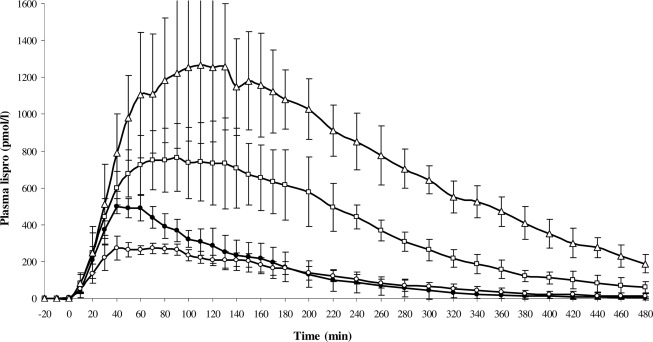
After the 10-unit injection, the ratio Cmax/D was 41% lower (P < 0.001) in subjects with type 2 diabetes than in healthy subjects, but Cmax, Tmax, AUC0-∞, AUC0-∞/D, ka, and Cl were similar in both groups. Mean residence time, Vz, and t½ tended to be greater in subjects with type 2 diabetes than in control subjects (Fig. 1, Table 2). After the 30- and 50-unit injections, ka dropped by 60% (P = 0.035) and Tmax was delayed by 33 (P = 0.118) and 74 min (P < 0.001), respectively. Cmax/D, Cl, Vz, and t½ were not affected by the dose, although mean residence time tended to be greater. Tmax (r2 = 0.51, P = 0.0004), Cmax (r2 = 0.90, P < 0.0001), and AUC0-∞ (r2 = 0.94, P < 0.0001) were associated with dosage.
Figure 1.
Mean (± SD) plasma lispro concentration over 480-min euglycemic clamps after subcutaneous injection of 10 units in healthy subjects (●) and 10 units (○), 30 units (□), and 50 units (▵) in obese subjects with type 2 diabetes.
Table 2.
Pharmacokinetic and pharmacodynamic parameters after subcutaneous injection of lispro
| Healthy subjects (10 units) | Subjects with type 2 diabetes (10 units) | Subjects with type 2 diabetes (30 units) | Subjects with type 2 diabetes (50 units) | |
|---|---|---|---|---|
| ka (min) | 0.0531 ± 0.0236 | 0.0455 ± 0.0242 | 0.0184 ± 0.0076§ | 0.0179 ± 0.0091§ |
| Tmax (min) | 48.3 ± 4.1 | 55.7 ± 14.0 | 88.6 ± 21.9 | 130. 0 ± 46.0‖¶ |
| Cmax (pmol/l) | 523 ± 42 | 310 ± 28 | 808 ± 218‖ | 1,313 ± 346‖# |
| Cmax/D (liters) | 0.0091 ± 0.0007 | 0.0054 ± 0.0005† | 0.0047 ± 0.0012 | 0.0046 ± 0.0012 |
| AUC0-∞ (pmol/min/l) | 68,462 ± 17,346 | 60,683 ± 15,191 | 192,155 ± 46,873‖ | 372,571 ± 59,578‖# |
| AUC0-∞/D (min/l) | 1.190 ± 0.302 | 1.056 ± 0.264 | 1.140 ± 0.188 | 1.296 ± 0.208 |
| Vz (liters) | 67 ± 16 | 118 ± 34 | 104 ± 53 | 107 ± 46 |
| Cl (l/min) | 0.88 ± 0.21 | 0.99 ± 0.22 | 0.90 ± 0.14 | 0.79 ± 0.13 |
| t½ (min) | 67 ± 15 | 100 ± 34 | 97 ± 38 | 136 ± 72 |
| Mean resistance time (min) | 119 ± 21 | 180 ± 65 | 196 ± 30 | 236 ± 49 |
| tGIRmax (min) | 69 ± 12 | 130 ± 23‡ | 175 ± 21 | 245 ± 64‖# |
| GIRmax* (mg/kg/min) | 9.0 (7.1–11.4) | 0.6 (0.4–0.9)‡ | 2.0 (1.4–2.7)‖ | 2.5 (1.7–3.7)‖ |
| GItot* (mg/kg) | 2,299 (1,881–2,811) | 92 (49–174)‡ | 364 (249–533)‖ | 678 (462–994)‖ |
Data are means ± SD unless otherwise indicated. There were 10 units administered in healthy subjects and 10, 30, and 50 units in obese subjects with type 2 diabetes.
*Geometric means with 95% CI;
†P < 0.001 compared with healthy controls using unpaired t test;
‡P < 0.0001 compared with healthy controls using unpaired t test;
§P < 0.04 compared with 10 units in subjects with type 2 diabetes using repeated-measures ANOVA;
‖P ≤0.002 compared with 10 units in subjects with type 2 diabetes using repeated-measures ANOVA;
¶P < 0.05 compared with 30 units in subjects with type 2 diabetes using repeated-measures ANOVA;
#P ≤ 0.002 compared with 30 units in subjects with type 2 diabetes using repeated-measures ANOVA.
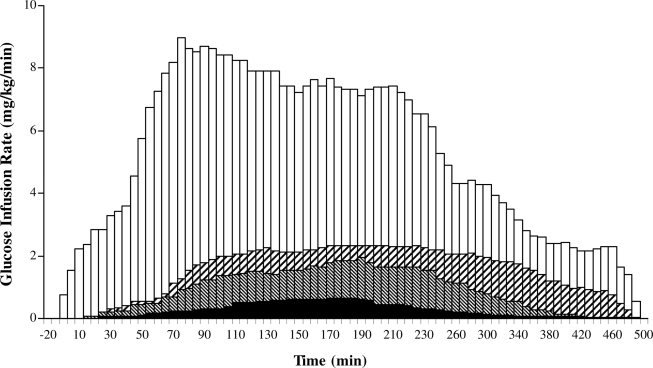
The glucodynamic differences between healthy subjects and type 2 diabetic subjects after 10 units of lispro were considerable (Fig. 2, Table 2). GIRmax and GItot were, respectively, 7% (P < 0.0001) and 4% (P < 0.0001) of the value measured in healthy subjects, and tGIRmax was prolonged by 1 h (P < 0.0001). After the 30- and 50-unit injections, GIRmax and GItot were different from the 10-unit values (P < 0.0001 for both). After the 50-unit lispro injection, tGIRmax was longer than after the 10- and 30-unit injections (P = 0.002). GIRmax (r2 = 0.67, P < 0.0001), GItot (r2 = 0.73, P < 0.0001), and tGIRmax (r2 = 0.76, P < 0.0001) were strongly correlated with dosage. After the 10-unit injection, the average difference between Tmax and tGIRmax was 19 min in healthy subjects and 74 min (P < 0.0007) in subjects with type 2 diabetes. The gap increased further when subjects received 30 and 50 units (86 and 115 min, respectively).
Figure 2.
Glucose infusion rate over 480-min euglycemic clamps after subcutaneous injection of 10 units in healthy subjects (□) and 10 units (■), 30 units (▧), and 50 units (▨) in obese subjects with type 2 diabetes.
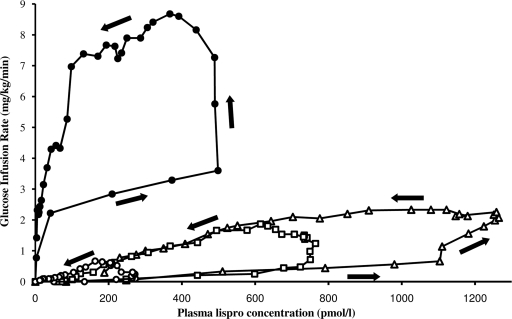
When GIR was plotted as a function of lispro plasma concentrations, the sequential response-concentration relationship depicted a counterclockwise hysteresis for both healthy subjects and subjects with type 2 diabetes (Fig. 3). In healthy subjects receiving 10 units of insulin, an initial GIR response of 2.22 mg/kg/min was seen with insulin concentrations nearing 40 pmol/l. Thereafter, large increases of insulin concentrations were required to increase GIR, although once the response was triggered, it was maintained while plasma concentrations decreased to 20% of the Cmax. In obese subjects with type 2 diabetes, after a 10-unit injection, much greater concentrations of insulin were required to produce even a minimal effect (e.g., 273 pmol/l of insulin elicited a GIR of 0.1 mg/kg/min). The response later increased abruptly to attain GIRmax when plasma concentrations of insulin were already dropping; once GIRmax was attained, the response decreased linearly with insulin plasma concentrations. The same pattern was observed for 30- and 50-unit injections.
Figure 3.
Plot of mean glucose infusion rate as a function of insulin plasma concentrations in healthy subjects receiving subcutaneously 10 units of lispro (●) and in obese subjects with type 2 diabetes receiving 10 units (○), 30 units (□), and 50 units (▵) of lispro. Data points are connected in chronological order; as depicted by the arrows, the resulting relationship denotes a counterclockwise hysteresis.
CONCLUSIONS
This study characterizes the pharmacokinetic and pharmacodynamic proprieties of the short-acting insulin analog lispro in obese subjects with type 2 diabetes. After low-dose injection (10 units), lispro absorption in subjects with type 2 diabetes was as comparable as in control subjects, although the hypoglycemic effect was blunted. However, both absorption and activity were severely delayed and blunted at higher dosages (30 and 50 units) in subjects with type 2 diabetes, featuring a dose-response effect. Kinetic and dynamic parameters estimated in control subjects confirmed those published elsewhere (2–4) and support the value of our findings.
It has been repeatedly proposed, from correlations with pharmacokinetic parameters, that subcutaneous fat thickness, obesity, and low ATBF reduce insulin absorption (12,13). Conversely, the present study does not confirm these facts when small dosages are administered. Insulin Vz and Cl depend on fat-free mass (17). Conversely, adipose tissue is essentially water free. Therefore, higher fat-free mass and total body water in our subjects with type 2 diabetes could explain the increment tendency in Vz, which should account for the decrease in Cmax and Cmax/D when comparing with control subjects.
Within our obese subjects with type 2 diabetes presenting high daily insulin needs, we indeed expected to observe a blunted pharmacodynamic profile compared with control subjects. Moreover, we showed a dose-dependent delay of Tmax and GIRmax at high doses in obese type 2 diabetic subjects. Similar results were found in a study done with healthy subjects using lower lispro doses (18) and in another study using inhaled insulin in subjects with type 1 diabetes (19). These effects observed at lower dose were expected to be more pronounced with higher doses in insulin-resistant subjects. Interindividual variation in insulin requirements was evaluated in overweight subjects with type 2 diabetes (20). The 8-h clamp period was not long enough to determine the entire absorption and action profile of 36 units of regular human insulin. Authors attributed these results to the possible slow insulin absorption in obese subjects with type 2 diabetes and to decreasing insulin absorption with increasing doses. They also correlated the absorbed insulin amount to daily insulin requirements. Herein, at low dosage, we did not observe a slower absorption of lispro in obese subjects with type 2 diabetes, but indeed confirmed that higher doses have a reduced effect. Thus, in obese subjects with type 2 diabetes, as ours, high insulin needs may account in part for low absorption efficiency with high doses.
As shown in Fig. 3, both groups exhibited a counterclockwise hysteresis, although the magnitude was severely blunted in subjects with type 2 diabetes after 10-, 30-, and 50-unit injections. Meanwhile, GIR remained low compared with control subjects. These findings illustrate the insulin resistance expected in our obese subjects with type 2 diabetes.
Fast prandial rise in plasma and fast action of insulin are both key to adequate postprandial metabolic control. The importance of determining whether short-acting insulin analogs are efficient was recently brought into question (21–23). Several studies (rev. in 22) have noted no or few benefits for these analogs relatively to human insulin in patients with type 2 diabetes as opposed to type 1 diabetes. Recent large studies (24,25) provided no evidence supporting the use of preprandial insulins compared with basal insulins. The prolonged time-action profile of short-acting insulin analogs shown in this study could provide an explanation to why preprandial insulins have not had the expected benefits. In daily life, the delay in pharmacodynamic responses after short-acting analog injections may hamper postprandial metabolic control, especially when large dosages are used.
The limitation of this study relates to the impossibility to distinguish between group and dose effect, since high dosages were not tested in the control group. Testing high dosages in control subjects would indeed require intensive care management.
In summary, this study shows that absorption and hypoglycemic action of short-acting insulin analogs are critically delayed at incrementally larger dosages in obese subjects with type 2 diabetes.
Acknowledgments
This work was supported by grants from Diabète Québec, Fonds de la Recherche en Santé du Québec (FRSQ), and Eli Lilly (Investigator-Initiated Research Program). J.-L.A. is a Canadian Institutes of Health Research (CIHR) scholar (New Investigator award) and member of the FRSQ-funded Centre de Recherche Clinique Étienne-Le Bel. M.G.-A. received a scholarship from Diabète Québec.
No other potential conflicts of interest relevant to this article were reported.
M.G.-A. contributed to study concept and discussion, researched data, and wrote the manuscript. P.d.S. contributed to discussion and data analysis and reviewed/edited the manuscript. J.-P.B. contributed to discussion, data analysis, and statistics and reviewed/edited the manuscript. E.M. researched ATBF data. P.B. contributed to discussion and data analysis and wrote the manuscript. J.M. contributed to study concept and discussion and reviewed/edited the manuscript. J.-L.A. was the principal investigator, contributed to the study concept and discussion, and wrote the manuscript.
Preliminary data of this study were published as abstracts in congress reports by the American Diabetes Association, European Association for the Study of Diabetes, and Canadian Diabetes Association.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Hirsch IB: Insulin analogues. N Engl J Med 2005;352:174–183 [DOI] [PubMed] [Google Scholar]
- 2.Homko C, Deluzio A, Jimenez C, Kolaczynski JW, Boden G: Comparison of insulin aspart and lispro: pharmacokinetic and metabolic effects. Diabetes Care 2003;26:2027–2031 [DOI] [PubMed] [Google Scholar]
- 3.Mudaliar SR, Lindberg FA, Joyce M, Beerdsen P, Strange P, Lin A, Henry RR: Insulin aspart (B28 asp-insulin): a fast-acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care 1999;22:1501–1506 [DOI] [PubMed] [Google Scholar]
- 4.ter Braak EW, Woodworth JR, Bianchi R, Cerimele B, Erkelens DW, Thijssen JH, Kurtz D: Injection site effects on the pharmacokinetics and glucodynamics of insulin lispro and regular insulin. Diabetes Care 1996;19:1437–1440 [DOI] [PubMed] [Google Scholar]
- 5.Becker RH, Frick AD, Burger F, Potgieter JH, Scholtz H: Insulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjects. Exp Clin Endocrinol Diabetes 2005;113:435–443 [DOI] [PubMed] [Google Scholar]
- 6.Clauson PG, Linde B: Absorption of rapid-acting insulin in obese and non obese NIDDM patients. Diabetes Care 1995;18:986–991 [DOI] [PubMed] [Google Scholar]
- 7.Lane WS, Cochran EK, Jackson JA, Scism-Bacon JL, Corey IB, Hirsch IB, Skyler JS: High-dose insulin therapy: is it time for U-500 insulin? Endocr Pract 2009;15:71–79 [DOI] [PubMed] [Google Scholar]
- 8.Yki-Jarvinen H: Combination therapies with insulin in type 2 diabetes. Diabetes Care 2001;24:758–767 [DOI] [PubMed] [Google Scholar]
- 9.Hildebrandt P: Subcutaneous absorption of insulin in insulin-dependent diabetic patients: influence of species, physico-chemical properties of insulin and physiological factors. Dan Med Bull 1991;38:337–346 [PubMed] [Google Scholar]
- 10.Sindelka G, Heinemann L, Berger M, Frenck W, Chantelau E: Effect of insulin concentration, subcutaneous fat thickness and skin temperature on subcutaneous insulin absorption in healthy subjects. Diabetologia 1994;37:377–380 [DOI] [PubMed] [Google Scholar]
- 11.Hildebrandt P, Birch K, Sestoft L, Volund A: Dose-dependent subcutaneous absorption of porcine, bovine and human NPH insulins. Acta Med Scand 1984;215:69–73 [DOI] [PubMed] [Google Scholar]
- 12.Hildebrandt P: Skinfold thickness, local subcutaneous blood flow and insulin absorption in diabetic patients. Acta Physiol Scand Suppl 1991;603:41–45 [PubMed] [Google Scholar]
- 13.Vora JP, Burch A, Peters JR, Owens DR: Relationship between absorption of radiolabeled soluble insulin, subcutaneous blood flow, and anthropometry. Diabetes Care 1992;15:1484–1493 [DOI] [PubMed] [Google Scholar]
- 14.Blaak EE, van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, Saris WH: Beta-adrenergic stimulation and abdominal subcutaneous fat blood flow in lean, obese, and reduced-obese subjects. Metabolism 1995;44:183–187 [DOI] [PubMed] [Google Scholar]
- 15.Dimitriadis G, Lambadiari V, Mitrou P, Maratou E, Boutati E, Panagiotakos DB, Economopoulos T, Raptis SA: Impaired postprandial blood flow in adipose tissue may be an early marker of insulin resistance in type 2 diabetes. Diabetes Care 2007;30:3128–3130 [DOI] [PubMed] [Google Scholar]
- 16.Ardilouze JL, Fielding BA, Currie JM, Frayn KN, Karpe F: Nitric oxide and beta adrenergic stimulation are major regulators of pre- and postprandial subcutaneous adipose tissue blood flow in humans. Circulation 2004;109:47–52 [DOI] [PubMed] [Google Scholar]
- 17.Yki-Järvinen H, Koivisto VA, Karonen SL: Influence of body composition on insulin clearance. Clin Physiol 1985;5:45–52 [DOI] [PubMed] [Google Scholar]
- 18.Rave KM, Nosek L, de la Pena A, Seger M, Ernest CS, II, Heinemann L, Batycky RP, Muchmore DB: Dose response of inhaled dry-powder insulin and dose equivalence to subcutaneous insulin lispro. Diabetes Care 2005;28:2400–2405 [DOI] [PubMed] [Google Scholar]
- 19.Brunner GA, Balent B, Ellmerer M, Schaupp L, Siebenhofer A, Jendle JH, Okikawa J, Pieber TR: Dose-response relation of liquid aerosol inhaled insulin in type I diabetic patients. Diabetologia 2001;44:305–308 [DOI] [PubMed] [Google Scholar]
- 20.Ryysy L, Hakkinen AM, Goto T, Vehkavaara S, Westerbacka J, Halavaara J, Yki-Järvinen H: Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 2000;49:749–758 [DOI] [PubMed] [Google Scholar]
- 21.Barnett AH: How well do rapid-acting insulins work in obese individuals? Diabetes Obes Metab 2006;8:388–395 [DOI] [PubMed] [Google Scholar]
- 22.Singh SR, Ahmad F, Lal A, Yu C, Bai Z, Bennett H: Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis. CMAJ 2009;180:385–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plank J, Siebenhofer A, Berghold A, Jeitler K, Horvath K, Mrak P, Pieber TR: Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med 2005;165:1337–1344 [DOI] [PubMed] [Google Scholar]
- 24.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T: Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084 [DOI] [PubMed] [Google Scholar]
- 25.Holman RR, Farmer AJ, Davies MJ, Levy JC, Darbyshire JL, Keenan JF, Paul SK: Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 [DOI] [PubMed] [Google Scholar]