Abstract
OBJECTIVE
Plasma osteoprotegerin (OPG) is an emerging strong and independent predictor of cardiovascular disease (CVD) in high-risk populations. OPG is a bone-related glycopeptide produced by vascular smooth muscle cells, and increased plasma OPG levels may reflect arterial vascular damage. We aimed to investigate the prognostic value of OPG in relation to all-cause and cardiovascular mortality in a cohort of type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
In a prospective observational follow-up study, 283 type 2 diabetic patients (172 men; aged 53.9 ± 8.8 years) were followed for a median of 16.8 years (range 0.2–23.0). Baseline plasma OPG concentrations were determined by immunoassay.
RESULTS
During follow-up, 193 (68%) patients died. High versus low levels of OPG predicted all-cause mortality (covariate-adjusted for urinary albumin excretion rate [UAER], estimated glomerular filtration rate, and conventional risk factors); hazard ratio (HR) 1.81 [95% CI 1.21–2.69]. The all-cause predictive effect of OPG was independent of NH2-terminal pro-brain natriuretic peptide (NT-proBNP) and was also useful within groups divided according to level of UAER. In total, 103 (73%) patients died because of CVD. High and medium versus low levels of OPG predicted cardiovascular mortality (unadjusted HR 1.86 [95% CI 1.07–3.23] and 3.51 [2.10–5.85], respectively). However, after adjustment for the covariates, HRs were no longer significant.
CONCLUSIONS
Elevated plasma OPG is a strong predictor of all-cause mortality in type 2 diabetic patients. The effect of OPG on all-cause mortality was independent of conventional cardiovascular risk factors, UAER, and NT-proBNP levels.
Plasma osteoprotegerin (OPG) is a promising strong and independent predictor of cardiovascular disease (CVD) in high-risk individuals, such as type 1 diabetic patients with nephropathy and nondiabetic patients after kidney transplantation or myocardial infarction (1–4). OPG is a member of the tumor necrosis factor receptor superfamily acting as a soluble decoy receptor for the receptor activator of nuclear factor-κβ ligand (RANKL) to prevent osteoclast activation and bone resorption (5). OPG mRNA has been detected in a variety of human tissues, including the lung, heart, and kidney (5). This bone-related glycoprotein is present in the arterial wall, and plasma OPG has been suggested to reflect the increased OPG content in arterial tissue observed in diabetic patients (6). OPG is upregulated in calcified coronary plaques (7) and associated with angiographic disease severity and cardiovascular events independent of conventional risk factors (8,9). Therefore, increased plasma OPG levels are suggested to be a marker of arterial vascular damage.
CVD is the major determinant of morbidity and mortality in patients with type 2 diabetes and, in particular, patients with an elevated urinary albumin excretion rate (UAER) (10). Increased OPG levels are associated with diabetes (11). Recently, an elevated plasma OPG level was shown to predict increased mortality in patients with type 1 diabetes and diabetic nephropathy (4) and also to predict increased incidence of cardiovascular events among patients with uncomplicated type 2 diabetes who were followed for 18 months (12). However, the prognostic importance of OPG in type 2 diabetic patients with long follow-up and elevated UAER is unknown. Therefore, this study examines the predictive value of plasma OPG in relation to all-cause and cardiovascular mortality in a large cohort of type 2 diabetic patients followed prospectively for 17 years.
RESEARCH DESIGN AND METHODS
The study population included all type 2 diabetic patients (n = 363) younger than 66 years of age attending a tertiary referral hospital during 1987. Thirty-six non-Caucasian patients were excluded as well as 4 patients without urine collections at baseline and 40 patients without OPG measurements. Thus, in total, the cohort consisted of 283 patients. Of these, 167 had normoalbuminuria, 74 had microalbuminuria, and 42 had macroalbuminuria.
In a prospective observational study design, the patients were followed for a median of 16.8 years (range 0.2–23.0) until 1 January 2010, until death (n = 193), or until emigration (n = 2). A follow-up of the patients until 2004 has previously been published (13). This study was performed in accordance with the Helsinki Declaration. The local ethics committee approved the study, and all patients gave their informed written consent.
Baseline clinical and laboratory investigations
Baseline measurements have previously been described (13). In brief, urinary albumin concentration was measured by radioimmunoassay from 24-h urine collections. Patients were classified as having normoalbuminuria (albumin excretion <30 mg/24 h), microalbuminuria (30–299 mg/24 h), or macroalbuminuria (≥300 mg/24 h) according to established guidelines. Glomerular filtration rate was estimated (eGFR) by the Modification of Diet in Renal Disease equation (14). Plasma NH2-terminal pro-brain natriuretic peptide (NT-proBNP) was measured as previously described (13).
Measurement of OPG
Blood samples for determination of OPG were collected in EDTA-citrated tubes and centrifuged, and the isolated plasma was stored at −80°C until analysis. Plasma OPG was measured in random order by a sandwich ELISA using commercially available antibodies (R&D Systems, Minneapolis, MN) as previously described (15). Briefly, mouse anti-human OPG was used as capture antibody, and a biotinylated goat anti-human OPG was used for detection. Recombinant human OPG was used for calibration, and the analytical range of the assay was 62.5–4,000 pg/ml. Our intra-assay coefficient of variation is 3%, and the interassay variation is <8% in duplicate measurements as previously reported (4). The OPG stability after >15 years of storage is not known, but all samples were treated and stored under the same conditions, and repeated freeze-thawing of test samples did not have any effect on results.
Follow-up
Vital status for all patients was traced through the National Register until 1 January 2010. Information on the cause of death obtained from the death certificate was only accessed until 2004, thus analysis of cardiovascular mortality was evaluated until 2004. All death certificates were reviewed independently by two observers, and the primary cause of death was recorded. Additional available information from necropsy reports was included in 24% of patients. All deaths were classified as cardiovascular deaths unless an unequivocal noncardiovascular cause was established (16).
Statistical analysis
Normally distributed variables are given as means ± SD, whereas nonnormally distributed variables were log-transformed before analysis and are given as medians (range).
Mortality was displayed as Kaplan-Meier (log-rank test) plots according to tertiles of OPG level. Cox regression enter model analysis was used to analyze the relationships between tertiles of baseline OPG levels and mortality/cardiovascular mortality by univariate and multivariate analyses displayed as unadjusted hazard ratios (HRs) (95% CIs) or adjusted for prespecified baseline cardiovascular risk factors (sex, age, smoking status, systolic blood pressure, serum cholesterol, UAER, eGFR, known ischemic heart disease, and plasma NT-proBNP). A two-tailed P-value ≤ 0.05 was considered statistically significant. All calculations were performed using SPSS for Windows, version 14.0 (Chicago, IL).
RESULTS
Among the 283 type 2 diabetic patients, the median (range) OPG was 1,963 pg/ml (439–5,400). Plasma OPG levels at baseline were positively associated with age (r = 0.36, P < 0.001), systolic blood pressure (r = 0.25, P < 0.001), cholesterol (r = 0.20, P = 0.001), UAER (r = 0.23, P < 0.001), known diabetes duration (r = 0.13, P = 0.028), and plasma NT-proBNP levels (r = 0.15, P < 0.001) and inversely related to increased eGFR (r = −0.27, P < 0.001). OPG levels did not differ between men and women (1,981 pg/ml [439–4,836] and 1,903 pg/ml [477–5,400], respectively) (P = 0.65) and were not associated with A1C levels (P = 0.069).
Patients divided into tertiles according to OPG levels (1st tertile <1,691 pg/ml, 2nd tertile 1,691–2,219 pg/ml, and 3rd tertile >2,219 pg/ml) are shown in Table 1. The patients with OPG in the highest tertile were older (P < 0.001) and had more retinopathy (P < 0.001), higher UAER (P = 0.001), and lower eGFR (P < 0.001). Similarly, cholesterol and NT-proBNP levels were higher (P = 0.006 and P < 0.001, respectively), and ischemic heart disease at baseline (P = 0.004) was more frequent in the 3rd tertile.
Table 1.
Baseline clinical and laboratory characteristics of 283 type 2 diabetic patients divided according to tertiles of OPG (<1,691; 1,691–2,219; and >2,219 pg/ml)
| 1st tertile | 2nd tertile | 3rd tertile | P | |
|---|---|---|---|---|
| n | 94 | 95 | 94 | |
| Male (%) | 57 | 63 | 62 | 0.71 |
| Age (years) | 50 ± 10 | 54 ± 8 | 59 ± 6 | <0.001 |
| Duration of diabetes (years) | 5 (1–39) | 7 (1–31) | 8 (1–34) | 0.15 |
| BMI (kg/m2) | 28.6 ± 5.4 | 28.7 ± 5.0 | 28.3 ± 4.7 | 0.86 |
| A1C (%) | 7.7 ± 1.7 | 8.0 ± 1.8 | 8.4 ± 2.0 | 0.052 |
| Retinopathy (%) | 28 | 31 | 43 | 0.038 |
| Nephropathy (normo-/micro-/macroalbuminuria) (%) | 73/15/12 | 57/30/13 | 48/33/19 | 0.007 |
| UAER (mg/24 h) | 10 (1–5,108) | 23 (1–3,969) | 38 (1–8,019) | <0.001 |
| eGFR (ml/min/1.73 m2) | 99 (58–159) | 94 (52–239) | 81 (12–131) | <0.001 |
| Smoking (%) | 35 | 32 | 32 | 0.77 |
| Systolic blood pressure (mmHg) | 145 ± 22 | 147 ± 19 | 160 ± 24 | <0.001 |
| Diastolic blood pressure (mmHg) | 85 ± 11 | 84 ± 11 | 87 ± 13 | 0.37 |
| Total cholesterol (mmol/l) | 5.9 ± 1.2 | 6.4 ± 1.6 | 6.7 ± 2.0 | 0.006 |
| NT-proBNP (ng/ml) | 52 (5–544) | 53 (5–3,768) | 93 (12–3,023) | <0.001 |
| Known ischemic heart disease (%) | 12 | 18 | 31 | 0.004 |
| OPG (pg/ml) | 1,428 (439–1,691) | 1,962 (1,695–2,218) | 2,743 (2,235–5,400) | <0.001 |
Data are given as (%), means ± SD, or medians (range).
Follow-up data
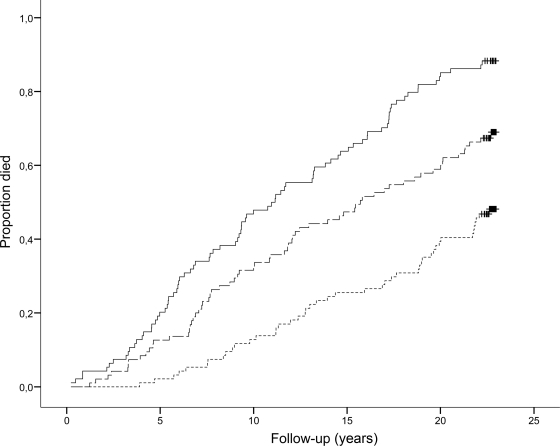
During follow-up, 45 (48%) patients in the 1st tertile died, whereas 65 (68%) patients in the 2nd tertile and 83 (88%) patients in the 3rd tertile died. Figure 1 shows the Kaplan-Meier curve for all-cause mortality in patients with type 2 diabetes (P < 0.001). As shown in Table 2, the unadjusted HRs (95% CI) for all-cause mortality were 1.92 [1.31–2.81] and 3.48 (2.41–5.04) for the 2nd and 3rd vs. 1st tertile, respectively. Third tertile OPG levels remained an independent predictor of all-cause mortality in the Cox regression model (covariate-adjusted [sex, age, smoking, systolic blood pressure, cholesterol, UAER, eGFR, and ischemic heart disease] 1.81 [1.21–2.69] [3rd vs. 1st tertile, P = 0.005]). Furthermore, as shown in Table 2, when adding NT-proBNP levels to the model, 3rd tertile OPG levels remained predictive (1.66 [1.10–2.50]). Cox regression analyses including OPG concentrations as a continuous variable revealed an adjusted HR (including NT-proBNP adjustment) for all-cause for a 10-fold increase in OPG of 5.83 (2.02–16.78).
Figure 1.
Kaplan-Meier curves of all-cause mortality in 283 type 2 diabetic patients according to tertiles of plasma OPG. Bottom line, 1st tertile (OPG <1,691 pg/ml); center line, 2nd tertile (OPG 1,691–2,219 pg/ml); top line 3rd tertile (OPG >2,219 pg/ml). Log-rank test for overall difference, P < 0.0001.
Table 2.
Adjusted and unadjusted HRs of long-term cumulative mortality and cardiovascular mortality among the 283 type 2 diabetic patients
| All-cause mortality |
CVD mortality |
|||
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI)* | HR (95% CI) | HR (95% CI)* | |
| Age (years) | 1.09 (1.06–1.11) | 1.05 (1.03–1.08) | 1.10 (1.07–1.13) | 1.07 (1.03–1.11) |
| Sex (male vs. female) | 1.29 (0.96–1.73) | 1.26 (0.92–1.74) | 1.24 (0.83–1.86) | 1.32 (0.83–2.10) |
| History of cardiovascular disease | 2.54 (1.83–3.53) | 1.43 (0.98–2.09) | 3.83 (2.55–5.76) | 2.56 (1.63–4.02) |
| Systolic blood pressure (mmHg) | 1.02 (1.01–1.03) | 1.01 (1.00–1.02) | 1.02 (1.01–1.03) | 1.00 (0.99–1.01) |
| Cholesterol (mmol/l) | 1.06 (0.97–1.15) | 0.94 (0.85–1.04) | 1.12 (1.01–1.25) | 1.10 (0.96–1.24) |
| eGFR (ml/min/1.73 m2) | 0.98 (0.98–0.99) | 1.00 (0.99–1.01) | 0.98 (0.97–0.99) | 1.00 (0.99–1.01) |
| UAER (mg/24 h) | 1.55 (1.32–1.82) | 1.30 (1.07–1.59) | 1.72 (1.39–2.13) | 1.52 (1.18–1.97) |
| Smoking (yes vs. no) | 0.97 (0.73–1.29) | 1.17 (0.86–1.57) | 1.08 (0.73–1.59) | 1.14 (0.91–2.09) |
| NT-proBNP (2nd vs. 1st tertile) | 1.82 (1.22–2.71) | 1.62 (1.07–2.45) | 1.69 (0.91–3.01) | 1.39 (0.74–2.60) |
| NT-proBNP (3rd vs. 1st tertile) | 5.02 (3.43–7.36) | 2.44 (1.57–3.78) | 5.59 (3.26–5.59) | 2.28 (1.22–4.27) |
| OPG (2nd vs. 1st tertile) | 1.92 (1.31–2.81) | 1.33 (0.89–2.01) | 1.86 (1.07–3.23) | 1.09 (0.61–1.94) |
| OPG (3rd vs. 1st tertile) | 3.48 (2.41–5.04) | 1.66 (1.10–2.50) | 3.51 (2.10–5.85) | 1.25 (0.71–2.21) |
Columns 2 and 3 show unadjusted and adjusted HRs, respectively, for predictors of all-cause mortality, and columns 4 and 5 show HRs for predictors of CVD mortality.
*Adjusted for the other variables in the table. UAER, log transformed UAER.
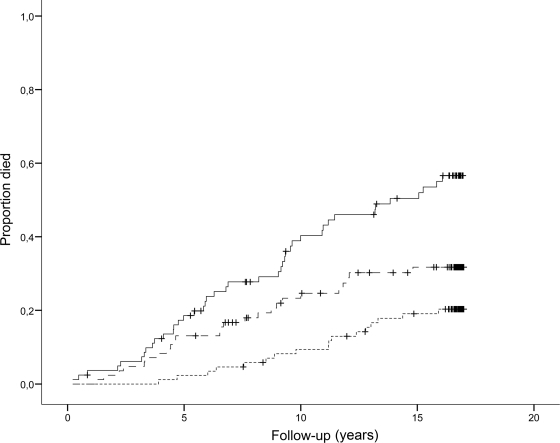
In total, 103 patients died of CVD during follow-up until 2004. As shown in Fig. 2, 21 (22%) were in the 1st, 32 (34%) in the 2nd, and 50 (53%) in the 3rd tertile (P < 0.001). The unadjusted HRs for cardiovascular mortality were 1.86 (95% CI 1.07–3.23) and 3.51 (2.10–5.85), for the 2nd and 3rd versus 1st tertile, respectively. However, when including the above mentioned covariates, plasma OPG was no longer predictive of cardiovascular mortality (1.16 [0.66–2.05] and 1.36 [0.78–2.38], respectively). Cox regression analyses including OPG concentration as a continuous variable revealed adjusted HR (including NT-proBNP adjustment) for cardiovascular mortality for a 10-fold increase in OPG of 4.90 (1.20–19.98).
Figure 2.
Kaplan-Meier curves of cardiovascular mortality in 283 type 2 diabetic patients according to tertiles of plasma OPG. Bottom line, 1st tertile (OPG <1,691 pg/ml); center line, 2nd tertile (OPG 1,691–2,219 pg/ml); top line, 3rd tertile (OPG >2,219 pg/ml). Log-rank test for overall difference, P < 0.0001.
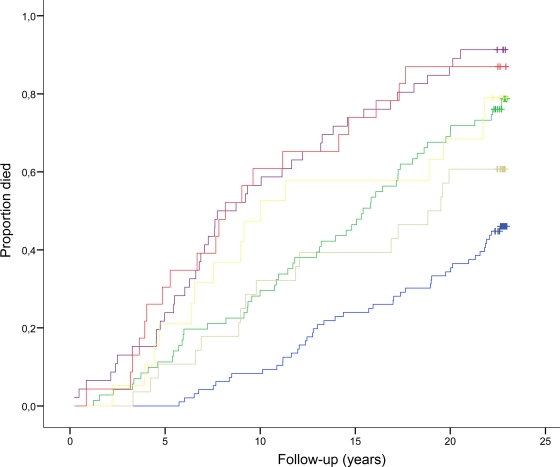
In the normoalbuminuric group, 99 (59%) patients died during follow-up, whereas in the micro- and macroalbuminuric groups 59 (80%) and 35 (83%) patients died, respectively (P < 0.001). OPG levels in the normoalbuminuric group were lower than those in patients with micro- and macroalbuminuria: median 1,816 pg/ml (range 477–5,400); 2,079 pg/ml (439–4,791); and 2,098 pg/ml (1,044–4,836), respectively (P = 0.003). As shown in Fig. 3, OPG also had predictive effect within groups divided according to UAER. Furthermore, patients with macroalbuminuria and median OPG values <1,963 pg/ml had a similar survival rate to the type 2 diabetic patients with normoalbuminuria and OPG levels >1,963 pg/ml (79 vs. 77%, P < 0.001).
Figure 3.
Kaplan-Meier curves of all-cause mortality in 283 type 2 diabetic patients according to UAER and median plasma OPG (1,963 pg/ml). Patients with normoalbuminuria and OPG <1,963 pg/ml (blue line) or OPG >1,963 pg/ml (green line); patients with microalbuminuria and OPG <1,963 pg/ml (gray line) or OPG >1,963 pg/ml (purple line); and patients with macroalbuminuria and OPG <1,963 pg/ml (yellow line) or OPG >1,963 pg/ml (red line). Log-rank test for overall difference, P < 0.0001.
CONCLUSIONS
This 17-year prospective observational follow-up study in type 2 diabetic patients showed a strong predictive value of plasma OPG for all-cause mortality. The effect was shown to be independent of conventional cardiovascular risk as well as UAER and kidney function. Furthermore, when adding baseline NT-proBNP levels to the analysis, elevated OPG was still independently predictive of all-cause mortality. In addition, elevated levels of OPG were associated with an increased risk of cardiovascular mortality.
Some prospective studies have investigated plasma OPG as a cardiovascular risk marker in high-risk diabetic and nondiabetic populations (1, 2, 9, 11, 12, 17, 18). In a large healthy, middle-aged population (mean age 59 years), increased OPG levels were associated with baseline carotid intima-media thickness and carotid artery plaque progression during 10 years of follow-up (9). In the latter study, OPG was also an independent risk factor for vascular mortality. Furthermore, increased OPG levels are associated with diabetes, and patients in the highest OPG quintile (vs. lowest) showed a fourfold adjusted risk of CVD among 490 elderly women (aged >65 years) followed for 10 years (11). OPG also independently predicted an adverse outcome in 243 post–acute myocardial infarction heart failure patients followed for 2 years and in 897 patients with acute coronary syndrome (median age 66 years, 71% men). Baseline OPG concentrations were strongly associated with increased mortality during 89 months of follow-up (2,17). In an 18-month follow-up study of 510 patients with uncomplicated type 2 diabetes (78% with normoalbuminuria), elevated levels of OPG predicted cardiovascular events (12). Furthermore, high levels of OPG predicted all-cause and cardiovascular mortality in type 1 diabetic patients with diabetic nephropathy independently of known cardiovascular risk markers including NT-proBNP (4). Thus, our results confirm and extend the current evidence of OPG as a marker of all-cause mortality risk in high-risk diabetic populations. Previous similar studies did not investigate the effect of OPG in patients with elevated UAER (11,12). Our study revealed a strong association between OPG and mortality independent of well-established cardiovascular risk predictors such as elevated UAER and NT-proBNP in type 2 diabetic patients (10,13). The independent predictive effect of OPG was also useful within groups according to UAER (normo-, micro-, and macroalbuminuric). Furthermore, patients with macroalbuminuria and OPG <1,963 pg/ml had a survival rate similar to those with normoalbuminuria and type 2 diabetes with OPG >1,963 pg/ml.
Our study was not designed to explain the mechanisms for increased OPG levels or the possible causal link between these raised OPG levels and cardiovascular death, but the elevated levels of OPG may reflect increased synthesis or reduced OPG elimination. Firstly, the increased OPG levels may result from increased OPG synthesis as part of a protective response to or a causal part of increased arteriosclerosis, including coronary artery disease. OPG is present in the arterial wall in diabetic patients, and plasma OPG has been suggested to reflect the arterial OPG content and therefore express arterial vascular damage (6). OPG is upregulated in calcified coronary plaques (7) and has been associated with angiographic disease severity and cardiovascular events, independent of conventional risk factors in other high-risk populations (8,19).
Secondly, the pathway of elimination for the OPG molecule is unknown, but a reduced renal excretion of OPG has been shown to explain part of the increased levels in type 1 diabetic patients (4). Thus, among several confounding factors, renal dysfunction may be important because impaired renal function represents a cardiovascular risk factor by itself (20). Therefore, we adjusted for eGFR and UAER at baseline in addition to conventional CVD risk factors.
OPG may be considered an additional marker of the atherosclerosis involved in the pathogenesis of ischemic heart disease, but the possible therapeutic benefit from lowering OPG remains to be shown in intervention studies.
Some limitations of the current study should be mentioned. The study includes a relatively small sample size, and 40 patients were without OPG measurements. Furthermore, the follow-up of vital status was done until 2010, but the follow-up of CVD mortality was only possible to access until 2004, and we did not include nonfatal events in our follow-up. Our study, however, is strengthened by the long follow-up period, the unselected clinic-based diabetes population, the additional available information from necropsy reports in 24% of patients, and the completeness of follow-up. The stability of OPG after more than 15 years of storage is not known. However, all samples were treated and stored at −80°C under the same conditions and were analyzed in random order. Finally, changes in treatment guidelines during follow-up have changed the cardiovascular risk profile of patients today compared with that of our patients.
In conclusion, elevated plasma OPG is a strong predictor of all-cause mortality in type 2 diabetic patients. The effect of OPG was independent of conventional cardiovascular risk factors, including UAER, eGFR, and NT-proBNP.
Acknowledgments
M.-A.G. is an employee and shareholder of Novo Nordisk. P.R. has received lecture fees from Novartis and Boehringer Ingelheim, and research grant from Novartis, has served as a consultant for Merck, and has equity interest in Novo Nordisk. H.-H.P has served as a consultant for Novartis, Merck, Pfizer, and sanofi-aventis; has equity interest in Merck and Novo Nordisk; has received lecture fees from Novartis, Merck, Pfizer, and sanofi-aventis; and has received grant support from Novartis, AstraZeneca, and sanofi-aventis. No other potential conflicts of interest relevant to this article were reported.
H.R. researched data, contributed to the discussion, and wrote the manuscript. P.R., H.-H.P., L.M.R., M.-A.G., and L.T. researched data, contributed to the discussion, and reviewed/edited the manuscript. M.L. contributed to the discussion and reviewed/edited the manuscript.
The authors acknowledge the work of lab technicians Ulla M. Smidt and Berit R. Jensen, employees at Steno Diabetes Center.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Hjelmesaeth J, Ueland T, Flyvbjerg A, Bollerslev J, Leivestad T, Jenssen T, Hansen TK, Thiel S, Sagedal S, Røislien J, Hartmann A: Early posttransplant serum osteoprotegerin levels predict long-term (8-year) patient survival and cardiovascular death in renal transplant patients. J Am Soc Nephrol 2006;17:1746–1754 [DOI] [PubMed] [Google Scholar]
- 2.Ueland T, Jemtland R, Godang K, Kjekshus J, Hognestad A, Omland T, Squire IB, Gullestad L, Bollerslev J, Dickstein K, Aukrust P: Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol 2004;44:1970–1976 [DOI] [PubMed] [Google Scholar]
- 3.Nybo M, Rasmussen LM: The capability of plasma osteoprotegerin as a predictor of cardiovascular disease: a systematic literature review. Eur J Endocrinol 2008;159:603–608 [DOI] [PubMed] [Google Scholar]
- 4.Jorsal A, Tarnow L, Flyvbjerg A, Parving HH, Rossing P, Rasmussen LM: Plasma osteoprotegerin levels predict cardiovascular and all-cause mortality and deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetologia 2008;51:2100–2107 [DOI] [PubMed] [Google Scholar]
- 5.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ: Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997;89:309–319 [DOI] [PubMed] [Google Scholar]
- 6.Olesen P, Ledet T, Rasmussen LM: Arterial osteoprotegerin: increased amounts in diabetes and modifiable synthesis from vascular smooth muscle cells by insulin and TNF-alpha. Diabetologia 2005;48:561–568 [DOI] [PubMed] [Google Scholar]
- 7.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ: Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2001;21:1998–2003 [DOI] [PubMed] [Google Scholar]
- 8.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, Nishizawa Y: Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation 2002;106:1192–1194 [DOI] [PubMed] [Google Scholar]
- 9.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J: Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 2004;109:2175–2180 [DOI] [PubMed] [Google Scholar]
- 10.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J: ADVANCE Collaborative Group Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browner WS, Lui LY, Cummings SR: Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab 2001;86:631–637 [DOI] [PubMed] [Google Scholar]
- 12.Anand DV, Lahiri A, Lim E, Hopkins D, Corder R: The relationship between plasma osteoprotegerin levels and coronary artery calcification in uncomplicated type 2 diabetic subjects. J Am Coll Cardiol 2006;47:1850–1857 [DOI] [PubMed] [Google Scholar]
- 13.Tarnow L, Gall MA, Hansen BV, Hovind P, Parving HH: Plasma N-terminal pro-B-type natriuretic peptide and mortality in type 2 diabetes. Diabetelogia 2006;49:2256–2262 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470 [DOI] [PubMed] [Google Scholar]
- 15.Knudsen ST, Foss CH, Poulsen PL, Andersen NH, Mogensen CE, Rasmussen LM: Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. Eur J Endocrinol 2003;149:39–42 [DOI] [PubMed] [Google Scholar]
- 16.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S: CHARM Investigators and Committees Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003;362:759–766 [DOI] [PubMed] [Google Scholar]
- 17.Omland T, Ueland T, Jansson AM, Persson A, Karlsson T, Smith C, Herlitz J, Aukrust P, Hartford M, Caidahl K: Circulating osteoprotegerin levels and long-term prognosis in patients with acute coronary syndromes. J Am Coll Cardiol 2008;51:627–633 [DOI] [PubMed] [Google Scholar]
- 18.Morena M, Terrier N, Jaussent I, Leray-Moragues H, Chalabi L, Rivory JP, Maurice F, Delcourt C, Cristol JP, Canaud B, Dupuy AM: Plasma osteoprotegerin is associated with mortality in hemodialysis patients. J Am Soc Nephrol 2006;17:262–270 [DOI] [PubMed] [Google Scholar]
- 19.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J: Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 2004;109:2175–2180 [DOI] [PubMed] [Google Scholar]
- 20.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305 [DOI] [PubMed] [Google Scholar]