Abstract
OBJECTIVE
Previous observational studies have found an increased risk of acute pancreatitis among type 2 diabetic patients. However, limited information is available on this association and specifically on the role of antidiabetic treatment. Our aim, therefore, was to further assess the risk of acute pancreatitis in adult patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
We performed a population-based case-control analysis nested in a cohort of 85,525 type 2 diabetic patients and 200,000 diabetes-free individuals from the general population using data from The Health Improvement Network database. Subjects were followed up to ascertain incident cases of acute pancreatitis.
RESULTS
We identified 419 cases of acute pancreatitis, 243 in the general population and 176 in the diabetes cohort. Incidence rates were 30.1 and 54.0 per 100,000 person-years in the general population and the diabetes cohort, respectively. In the cohort analysis, the adjusted incidence rate ratio of acute pancreatitis in diabetic patients versus that in the general population was 1.77 (95% CI 1.46–2.15). The magnitude of this association decreased with adjustment for multiple factors in the nested case-control analysis (adjusted odds ratio 1.37 [95% CI 0.99–1.89]). Furthermore, we found that the risk of acute pancreatitis was decreased among insulin-treated diabetic patients (0.35 [0.20–0.61]).
CONCLUSIONS
Type 2 diabetes may be associated with a slight increase in the risk of acute pancreatitis. We also found that insulin use in type 2 diabetes might decrease this risk. Further research is warranted to confirm these associations.
Acute pancreatitis is defined as an acute inflammatory process of the pancreas. The incidence of acute pancreatitis in the general population shows geographical variation. Incidence rates reported in the literature range between 4 and up to >100 cases per 100,000 person-years in the western world (1–3). Data from western countries suggest that the incidence of acute pancreatitis has been increasing over the last 40 years (3).
The reason for this increase is unknown. However, a concurrent trend has been the rapid, worldwide increase in type 2 diabetes and obesity. Several clinical factors associated with type 2 diabetes and obesity are known or putative risk factors for acute pancreatitis (e.g., gallstone disease). Therefore, it can be hypothesized that in type 2 diabetic patients the risk of acute pancreatitis might be higher than that for the general population (2). Studies exploring whether diabetes or antidiabetic treatment may act as risk factors for the development of acute pancreatitis have been limited so far (2,4–6). Three observational studies reported an approximately two- to threefold increased risk of acute pancreatitis among diabetic patients (2,4,5). The purpose of this study was to further assess the risk of acute pancreatitis in association with type 2 diabetic patients and antidiabetic treatment.
RESEARCH DESIGN AND METHODS
This population-based cohort study with a nested case-control analysis was based on information from The Health Improvement Network (THIN). THIN is a U.K. longitudinal primary care medical record database that includes diagnostic and prescribing data recorded by general practitioners as part of their routine clinical care. The population in THIN is representative of the general population in the U.K.
The source population included all individuals aged 20–79 years in THIN enrolled for at least 2 years with their general practitioner, who had the first prescription ever recorded in the computer files >1 year before entering the study and at least one health contact in the last 2 years. The study period was from 1 January 1996 to 31 December 2006. We excluded all individuals with a history of cancer (other than nonmelanoma skin cancer) or pancreatic disease before entering the study. Individuals aged ≥70 years with a follow-up >1 year and less than two health contacts during their follow-up period were removed because data completeness is most likely seriously deficient in this subgroup. We identified two cohorts within this source population: the type 2 diabetes cohort and the (diabetes-free) general population cohort.
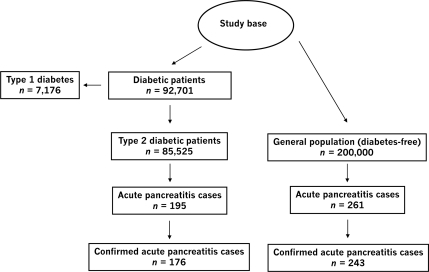
We ascertained all patients within the study base with a READ code of diabetes recorded in the database. Based on type-specific READ codes (i.e., those that denote explicitly the type of diabetes), the age at diagnosis, and the lifetime history of antidiabetic pharmacological treatment we were able to classify the initial 92,701 patients with a recorded diagnosis of diabetes into 7,176 (7.74%) patients with type 1 and 85,525 (92.26%) with type 2 diabetes. The latter comprised the type 2 diabetes cohort (Fig. 1).
Figure 1.
Study cohorts and acute pancreatitis ascertainment.
The general population cohort included 200,000 individuals randomly sampled from the source population and frequency-matched by age, sex, and calendar year to the diabetes cohort. The same eligibility criteria as for the diabetes cohort were applied with the additional condition that patients had to be free of a recorded diagnosis of diabetes.
Follow-up for acute pancreatitis
We followed all members from the diabetes and the general population cohort from the date when an individual met all eligibility criteria until the earliest occurrence of one of the following end points: a recorded diagnosis of acute pancreatitis, cancer, his or her 80th birthday, death, or the end of the study period. We reviewed the computerized patient profiles with free text comments of all individuals identified as potentially having cases of pancreatitis. After this manual review of all potential cases, we classified cases as “not confirmed” (and subsequently excluded them from the study) in situations in which acute pancreatitis was initially suspected but ruled out later on, and in individuals who met an exclusion criterion (i.e., cancer or prevalent case of pancreatic disease). All remaining cases were considered as “confirmed” incident cases, most cases (88%) being well documented; i.e., the episode of acute pancreatitis either included a documented emergency admission and/or hospitalization or the diagnosis had been made by a specialist.
For a random sample of 50 case subjects, we also sent out a questionnaire to the patient's general practitioners to confirm the diagnosis and to provide all available additional data related to that event. We received valid information (questionnaires and additional clinical documents) for 44 case subjects, corresponding to a 88% response rate.
Statistical analyses
Cohort analysis.
Person-time at risk in each study cohort was classified across strata by age, sex, and calendar year. Age- and sex-specific incidence rates of acute pancreatitis were calculated using the corresponding person-time at risk in each cohort as denominator.
Crude and adjusted incidence rate ratios (IRRs) with 95% CIs associated with diabetes were computed using a Poisson regression model with age, sex, and calendar year included in the model. Potential interactions were studied by including these variables in the model as interaction terms.
Nested case-control analysis.
In addition, a nested case-control analysis was performed to evaluate in more detail the role of diabetes and of antidiabetic drugs on the risk of acute pancreatitis. The individuals with confirmed cases of acute pancreatitis from both cohorts were used as case subjects and their date of diagnosis was used as the index date.
Control subjects were randomly sampled from the two study cohorts in which pancreatitis case subjects were ascertained. A group of 5,000 control subjects was randomly selected from the list of eligible person-time and frequency-matched to the case subjects on sex, same age (±1 year), and calendar year.
For all case and control subjects we ascertained demographic (age, sex, Townsend deprivation index [7,8], and BMI) and lifestyle factors (e.g., smoking and alcohol intake) as well as general comorbidity (e.g., chronic conditions and previous gastrointestinal diseases) at the index date. In addition, we ascertained exposure to antidiabetic drugs using separate exposure variables for insulin, metformin, sulfonylureas, thiazolidinediones, and other antidiabetic drugs (i.e., acarbose, repaglinide, and nateglinide) and other common drugs or drug classes including antibiotics, antidepressants, corticosteroids, acid-suppressing drugs, nonsteroidal anti-inflammatory drugs (NSAIDs), aspirin, paracetamol, antihypertensive agents, lipid-lowering drugs, and hormone replacement therapy. Individuals were classified as current users if they were taking the drug at the index date or had taken it within the previous 30 days. Those not currently exposed but who had used the drug within the year before the index date were classified as past users. Everyone else was classified as nonuser. We also explored the effect of drug duration (<1 year, 1–3 years, and >3 years) on the risk of acute pancreatitis.
Unconditional regression analyses were used to assess odds ratios (ORs) together with 95% CIs. The fully adjusted model included the matching variables age and sex and the following predictors of acute pancreatitis: smoking status, alcohol intake, ischemic heart disease, previous gastrointestinal disease, exposure to antidiabetic drugs, antibiotics, acid-suppressing drugs, NSAIDs (including aspirin and coxibs), paracetamol, and antihypertensive drugs, BMI, and Townsend deprivation index. All analyses were done using Stata SE 10.0 (StataCorp, College Station, TX).
RESULTS
Cohort analysis
A total of 85,525 patients with type 2 diabetes were included for the cohort analyses together with the 200,000 frequency-matched individuals from the general population. The average ± SD age at start date in the type 2 diabetes and the general population cohort was 61.2 ± 11.4 and 59.5 ± 12.8 years, respectively; 43.7% of subjects in the diabetes and 43.5% in the general population cohort were female. The total amount of follow-up time was 325,990 person-years in the type 2 diabetes and 807,453 person-years in the general population cohort, corresponding to an average follow-up time of 3.8 and 4.0 years, respectively. During follow-up, we identified a total of 456 potential cases of acute pancreatitis, 261 in the general population and 195 in the diabetes cohort.
After manual review of the patients' computerized profiles, we classified 35 cases as not confirmed cases (7.7%). The remaining 421 cases were classified as confirmed cases.
In the validation study, 2 of the 44 cases in individuals for whom we received valid information were considered as not confirmed by the general practitioners. This resulted in a confirmation rate of 95.5% in the validation study. These 2 not confirmed cases were subsequently excluded, leaving 419 cases of acute pancreatitis included in the final analyses (243 cases in the general population and 176 cases in the diabetes cohort). The corresponding crude incidence rate of acute pancreatitis was 30.1 per 100,000 person-years in the general population and 54.0 per 100,000 person-years in the diabetes cohort. Incidence rates stratified by age and sex for the diabetic and the general population cohort are presented in Table 1.
Table 1.
Incidence rate of acute pancreatitis by age and sex in the general population without diabetes and in type 2 diabetic patients (cohort analysis)
| Age strata | Person-years | Cases | Incidence rate per 100,000 person-years (95% CI) |
|---|---|---|---|
| General population (n = 200,000) | |||
| Women | |||
| 20–39 years | 25,300 | 5 | 19.8 (6.4–46.1) |
| 40–59 years | 105,655 | 22 | 20.8 (13–31.5) |
| 60–69 years | 105,824 | 27 | 25.5 (16.8–37.1) |
| 70–79 years | 116,742 | 41 | 35.1 (25.2–47.6) |
| Overall | 353,521 | 95 | 26.9 (21.7–32.8) |
| Men | |||
| 20–39 years | 25,732 | 5 | 19.4 (6.3–45.3) |
| 40–59 years | 157,241 | 46 | 29.3 (21.4–39) |
| 60–69 years | 146,542 | 41 | 28.0 (20–37.9) |
| 70–79 years | 124,416 | 56 | 45.0 (34–58.4) |
| Overall | 453,932 | 148 | 32.6 (27.5–38.3) |
| Total | 807,453 | 243 | 30.1 (26.4–34.1) |
| Type 2 diabetes (n = 85,525) | |||
| Women | |||
| 20–39 years | 5,105 | 4 | 78.4 (21.3–200.6) |
| 40–59 years | 40,585 | 21 | 51.7 (32–79.1) |
| 60–69 years | 45,377 | 27 | 59.5 (39.2–86.5) |
| 70–79 years | 50,057 | 22 | 43.9 (27.5–66.5) |
| Overall | 141,125 | 74 | 52.4 (41.1–65.8) |
| Men | |||
| 20–39 years | 4,479 | 2 | 44.7 (5.4–161.3) |
| 40–59 years | 61,109 | 35 | 57.3 (39.8–79.6) |
| 60–69 years | 63,979 | 32 | 50.0 (34.2–70.6) |
| 70–79 years | 55,299 | 33 | 59.7 (41–83.8) |
| Overall | 184,865 | 102 | 55.2 (44.9–66.9) |
| Total | 325,990 | 176 | 54.0 (46.3–62.5) |
| IRRdiabetes 1.79 (1.48–2.18)* | |||
*Unadjusted estimate of acute pancreatitis in type 2 diabetic patients compared with the general population.
These results translate into a 79% increased risk of a first-ever episode of acute pancreatitis among type 2 diabetic patients compared with the general population (crude IRR 1.79 [95% CI 1.48–2.18]). Because the two cohorts were frequency matched, we obtained very similar results when we estimated the age-, sex-, and calendar year–adjusted IRR by fitting a Poisson regression model (1.77 [1.46–2.15]).
Increasing age was associated with a higher risk of acute pancreatitis in the overall cohort. Compared with those aged <40 years, the risk of acute pancreatitis was increased in those aged 40–59, 60–69, and ≥70 years by 17, 20, and 50%, respectively (Ptrend = 0.02).
Furthermore, we explored the potential effect modification of the association between diabetes and acute pancreatitis by age. We found a significant trend toward a decreasing association between diabetes and acute pancreatitis with increasing age (P = 0.019). We also evaluated whether sex could modify the association between diabetes and acute pancreatitis, but the corresponding statistical test was not significant.
Nested case-control analysis
After adjustment for various demographic and lifestyle variables, comorbidities, and drug exposure, patients with type 2 diabetes had a nonsignificantly increased risk of acute pancreatitis compared with those free of diabetes (adjusted OR 1.37 [95% CI 0.99–1.89]) (Table 2). When we analyzed separately the risk associated with incident diabetes (diabetes first diagnosed during the study period) and prevalent diabetes (diabetes first diagnosed before the study period), the adjusted ORs were virtually the same (1.38 [0.98–1.94] and 1.34 [0.90–2.01], respectively). Furthermore, to explore the possibility of an increased risk of acute pancreatitis around the time of the first diagnosis of diabetes, we further divided our cohort of incident diabetic patients according to the time elapsed since the first diagnosis of diabetes. During the 1st year after the diagnosis of diabetes, the risk was somewhat higher (1.61 [1.00–2.60]) than thereafter (1.26 [0.85–1.88]). This difference, however, did not reach statistical significance (P = 0.38).
Table 2.
Risk of acute pancreatitis associated with diabetes, antidiabetic drugs, and other factors (nested case-control analysis)
| Case subjects | Control subjects | Odds ratio (95% CI) |
||
|---|---|---|---|---|
| Univariate | Adjusted* | |||
| n | 419 | 5,000 | ||
| Smoking | ||||
| Nonsmoker | 128 (30.5) | 1,795 (35.9) | 1 (referent) | 1 (referent) |
| Smoker | 71 (16.9) | 623 (12.5) | 1.60 (1.18–2.17) | 1.48 (1.06–2.06) |
| Former smoker | 175 (41.8) | 1,909 (38.2) | 1.29 (1.01–1.63) | 1.14 (0.89–1.47) |
| Unknown | 45 (10.7) | 673 (13.5) | 0.94 (0.66–1.33) | 1.18 (0.81–1.72) |
| Alcohol use | ||||
| Nondrinker | 200 (47.7) | 2,082 (41.6) | 1 (referent) | 1 (referent) |
| Between 1 and 7/week | 92 (22.0) | 1,236 (24.7) | 0.77 (0.60–1.00) | 0.86 (0.66–1.13) |
| Between 8 and 29/week | 58 (13.8) | 895 (17.9) | 0.67 (0.50–0.91) | 0.75 (0.54–1.03) |
| ≥30/week | 45 (10.7) | 291 (5.8) | 1.61 (1.14–2.27) | 1.49 (1.02–2.18) |
| Unknown | 24 (5.7) | 496 (9.9) | 0.50 (0.33–0.78) | 0.72 (0.43–1.19) |
| Diabetes | ||||
| No | 243 (58.0) | 3,489 (69.8) | 1 (referent) | 1 (referent) |
| Yes | 176 (42.0) | 1,511 (30.2) | 1.67 (1.36–2.05) | 1.37 (0.99–1.89) |
| Antidiabetic drugs | ||||
| Insulin | ||||
| Nonuse (366+) | 399 (95.2) | 4,650 (93.0) | 1 (referent) | 1 (referent) |
| Current use (0–30) | 19 (4.5) | 328 (6.6) | 0.68 (0.42–1.08) | 0.35 (0.20–0.61) |
| Past use (31–365) | 1 (0.2) | 22 (0.4) | 0.53 (0.07–3.94) | 0.31 (0.04–2.43) |
| Metformin | ||||
| Nonuse (366+) | 328 (78.3) | 4,261 (85.2) | 1 (referent) | 1 (referent) |
| Current use (0–30) | 75 (17.9) | 655 (13.1) | 1.49 (1.14–1.94) | 0.81 (0.56–1.15) |
| Past use (31–365) | 16 (3.8) | 84 (1.7) | 2.47 (1.43–4.27) | 1.18 (0.62–2.25) |
| Sulfonylureas | ||||
| Nonuse (366+) | 334 (79.7) | 4,460 (89.2) | 1 (referent) | 1 (referent) |
| Current use (0–30) | 69 (16.5) | 474 (9.5) | 1.94 (1.48–2.56) | 1.25 (0.86–1.80) |
| Past use (31–365) | 16 (3.8) | 66 (1.3) | 3.24 (1.85–5.65) | 2.58 (1.34–4.96) |
| Thiazolidinediones | ||||
| Nonuse (366+) | 396 (94.5) | 4,864 (97.3) | 1 (referent) | 1 (referent) |
| Current use (0–30) | 20 (4.8) | 117 (2.3) | 2.10 (1.29–3.41) | 1.25 (0.72–2.16) |
| Past use (31–365) | 3 (0.7) | 19 (0.4) | 1.94 (0.57–6.58) | 1.23 (0.33–4.66) |
| Other antidiabetic drugs | ||||
| Nonuse (366+) | 412 (98.3) | 4,962 (99.2) | 1 (referent) | 1 (referent) |
| Current use (0–30) | 4 (1.0) | 27 (0.5) | 1.78 (0.62–5.12) | 1.23 (0.40–3.78) |
| Past use (31–365) | 3 (0.7) | 11 (0.2) | 3.28 (0.91–11.82) | 2.56 (0.61–10.66) |
| Gastrointestinal disease† | ||||
| No | 107 (25.5) | 2,318 (46.4) | 1 (referent) | 1 (referent) |
| Yes | 312 (74.5) | 2,682 (53.6) | 2.52 (2.01–3.16) | 1.91 (1.50–2.45) |
| BMI | ||||
| 20–24 kg/m2* | 95 (22.7) | 1,242 (24.8) | 1 (referent) | 1 (referent) |
| <20 kg/m2 | 15 (3.6) | 164 (3.3) | 1.20 (0.68–2.11) | 1.13 (0.63–2.02) |
| 25–29 kg/m2 | 154 (36.8) | 1,809 (36.2) | 1.11 (0.85–1.45) | 0.99 (0.75–1.31) |
| ≥30 kg/m2 | 131 (31.3) | 1,337 (26.7) | 1.28 (0.97–1.69) | 0.92 (0.68–1.24) |
| Unknown | 24 (5.7) | 448 (9.0) | 0.70 (0.44–1.11) | 1.01 (0.60–1.72) |
| Paracetamol | ||||
| Nonuse (366+) | 240 (57.3) | 3,620 (72.4) | 1 (referent) | 1 (referent) |
| Current use (0–30) | 105 (25.1) | 732 (14.6) | 2.16 (1.70–2.76) | 1.65 (1.25–2.18) |
| Past use (31–365) | 74 (17.7) | 648 (13.0) | 1.72 (1.31–2.26) | 1.38 (1.02–1.86) |
| ACE inhibitors | ||||
| Nonuse (366+) | 3,892 (77.8) | 280 (66.8) | 1 (referent) | 1 (referent) |
| Current use (0–30) | 978 (19.6) | 124 (29.6) | 1.76 (1.41–2.20) | 1.37 (1.04–1.80) |
| Past use (31–365) | 130 (2.6) | 15 (3.6) | 1.60 (0.93–2.77) | 1.12 (0.61–2.04) |
Data are n (%) unless otherwise indicated.
*Adjusted for all variables included in the table plus age, sex, Townsend index, ischemic heart disease, and exposure to antibiotics, H2 blockers, proton pump inhibitors, NSAIDs (including aspirin and coxibs), and other antihypertensive drugs.
†Includes gallstones, biliary tract disease, cholecystitis, gastroenteritis, abdominal pain, and others.
Among users of antidiabetic drugs, current users of insulin were at a decreased risk of acute pancreatitis (adjusted OR 0.35 [95% CI 0.20–0.61]) compared with nonusers. Moreover, past use of sulfonylureas was associated with a significant risk increase of acute pancreatitis compared with that for nonusers (2.58 [1.34–4.96]). Otherwise, exposure to antidiabetic drugs was not materially associated with the risk of acute pancreatitis (Table 2).
We also explored the impact of treatment duration among current antidiabetic drug users (Table 3). A reduced risk of acute pancreatitis was observed across all different strata of insulin duration with a similar magnitude of association, corresponding to a 60–70% reduction in the acute pancreatitis risk. Of interest, metformin and sulfonylureas, which overall did not seem to be associated with acute pancreatitis, were associated with decreased and increased risks, respectively, but only among long-term users of these drugs. The other antidiabetic drugs studied (thiazolidinediones and others) were not found to be associated with acute pancreatitis in any of these analyses although their numbers were considerably smaller.
Table 3.
Risk of acute pancreatitis and duration of current use of antidiabetic drugs (nested case-control analysis)
| Case subjects | Control subjects | OR (95% CI)* | |
|---|---|---|---|
| n | 419 | 5,000 | |
| Insulin | |||
| Nonuse† | 399 (95.2) | 4,650 (93.0) | 1 (referent) |
| Short duration (<1 year) | 7 (1.7) | 82 (1.6) | 0.41 (0.17–1.00) |
| Mid duration (1–3 years) | 5 (1.2) | 88 (1.8) | 0.34 (0.13–0.91) |
| Long duration (>3 years) | 7 (1.7) | 158 (3.2) | 0.30 (0.13–0.68) |
| Metformin | |||
| Nonuse† | 328 (78.3) | 4,261 (85.2) | 1 (referent) |
| Short duration (<1 year) | 26 (6.2) | 213 (4.3) | 0.88 (0.53–1.47) |
| Mid duration (1–3 years) | 31 (7.4) | 237 (4.7) | 0.93 (0.58–1.49) |
| Long duration (>3 years) | 18 (4.3) | 205 (4.1) | 0.50 (0.28–0.91) |
| Sulfonylureas | |||
| Nonuse† | 334 (79.7) | 4,460 (89.2) | 1 (referent) |
| Short duration (<1 year) | 13 (3.1) | 125 (2.5) | 0.81 (0.42–1.57) |
| Mid duration (1–3 years) | 25 (6.0) | 156 (3.1) | 1.20 (0.70–2.03) |
| Long duration (>3 years) | 31 (7.4) | 193 (3.9) | 1.66 (1.01–2.74) |
| Thiazolidinediones | |||
| Nonuse† | 396 (94.5) | 4,864 (97.3) | 1 (referent) |
| Short duration (<1 year) | 11 (2.6) | 57 (1.1) | 1.28 (0.61–2.68) |
| Mid duration (1–3 years) | 7 (1.7) | 46 (0.9) | 1.19 (0.49–2.90) |
| Long duration (>3 years) | 2 (0.5) | 14 (0.3) | 1.27 (0.23–6.89) |
| Other antidiabetic drugs | |||
| Nonuse† | 412 (98.3) | 4,962 (99.2) | 1 (referent) |
| Short duration (<1 year) | 1 (0.2) | 9 (0.2) | 1.24 (0.13–11.82) |
| Mid duration (1–3 years) | 1 (0.2) | 8 (0.2) | 0.94 (0.11–8.14) |
| Long duration (>3 years) | 2 (0.5) | 10 (0.2) | 1.85 (0.34–10.10) |
Data are n (%) unless otherwise indicated.
*Adjusted for all variables included in the table plus those in the fully adjusted model of Table 2.
†Baseline category.
Among individuals with diabetes, metformin and sulfonylureas were the two most commonly prescribed drugs, followed by insulin. Among control subjects, a total of 73% of diabetic patients were receiving specific antidiabetic drug treatment at the index date. The remaining 27% were not currently receiving antidiabetic drug therapy. Treated diabetic patients had an adjusted OR of 1.19 [95% CI 0.91–1.55] for acute pancreatitis compared with that for the general population, whereas those not receiving antidiabetic drug treatment (1.49 [1.06–2.08]) seemed to concentrate the overall increased risk associated with diabetes. However, this difference was not statistically significant (P = 0.21). In addition, the risk of acute pancreatitis was significantly increased among current smokers, those taking ≥30 units of alcohol per week, individuals with a previous history of gastrointestinal disease, and current users of paracetamol and ACE inhibitors (Table 2).
CONCLUSIONS
The results of this study confirm the excess risk of acute pancreatitis associated with type 2 diabetes previously reported in other observational studies (2,4,5). In fact, the cohort analysis yielded a statistically significant 77% increased risk of acute pancreatitis associated with a prior history of diabetes. This translates into ∼23 additional cases for every 100,000 patients with diabetes each year. However, the magnitude of this association was reduced when it was adjusted for other risk factors in a multivariate model and became borderline significant in the nested-case control analysis.
This association of an increased risk of acute pancreatitis and type 2 diabetes seems more pronounced at younger ages and has already been observed in a recent retrospective cohort study based on information from a U.S, health claims database (2). We were also able to assess how antidiabetic drugs might influence this association. Interestingly, use of insulin and long-term use of metformin were associated with a decreased risk of pancreatitis, as opposed to long-term use of sulfonylureas, which seems to increase the risk. In a previous case-control study, Blomgren et al. (4) found that the sulfonylurea glyburide increased the risk of acute pancreatitis, but neither insulin nor metformin seemed to lower the risk. In fact, there are reports of cases of acute pancreatitis in patients using metformin after an episode of acute renal failure (9,10). To the best of our knowledge, this is the first study suggesting a reduced risk associated with these antidiabetic drugs. We think it is premature to propose potential mechanisms before these findings are replicated. Overall, when we analyzed the risk of pancreatitis among treated and not treated diabetic patients separately, we observed that the greatest risk appeared among those without antidiabetic pharmacotherapy, who represent a quarter of our diabetic population. This result may be partially due to a slight increased risk of acute pancreatitis immediately after the diagnosis of diabetes, although this assumption could not be confirmed in our study.
Our data from the general population cohort could replicate results from previous epidemiological studies showing that the incidence of acute pancreatitis rises with increasing age and tends to be higher in men than women (1,11,12). Furthermore, the incidence rate found in the general population is also in line with results from a recent study from England (13).
Among other risk factors studied, we found that previously reported risk factors such as smoking (5), alcohol use (1,14), or use of ACE inhibitors (15,16) were replicated in our study. Exposure to paracetamol was also associated with an increased pancreatitis risk as in previous studies (15).
The current study has several strengths. First, it is based on a large database with documented high data quality and completeness (17–19). Second, detailed information on important lifestyle factors, comorbidities, concomitant drug therapies, and BMI was available. Third, we reviewed the patient profiles of computer-detected potential cases with all additional information included in free text comments and validated the case status in a random sample by accessing the original medical records available in general practitioner offices. Fourth, we could replicate results from other studies regarding the association of various comorbidities and/or exposures and the risk of acute pancreatitis.
However, the study also has its limitations. Although we adjusted our analyses for various potential risk factors of acute pancreatitis, it is possible that there is still some residual confounding we did not account for. Moreover, throughout this report we present results for a large number of factors including lifestyle, comorbidity, and drug therapies (as well as different drug durations). This implicit multiple testing could be inflating the study-wide type I error. One should keep this in mind, when interpreting these results, and even more so in the case of subanalyses that do not belong to the main purpose of the study. However, because of the exploratory nature of these findings, we did not deem it necessary to adjust for multiple comparisons.
In summary, we have shown that type 2 diabetes may be associated with a slight increase in the risk of acute pancreatitis. We have also found that use of insulin in type 2 diabetes might be associated with a reduced risk. Further research is warranted to confirm these observed associations.
Acknowledgments
This study was sponsored by Novartis Global Clinical Epidemiology.
No other potential conflicts of interest relevant to this article were reported.
A.G.P. and L.A.G.R. researched data, contributed to discussion, wrote the manuscript, and reviewed/edited the manuscript. R.G.S. contributed to discussion, wrote the manuscript, and reviewed/edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Spanier BW, Dijkgraaf MG, Bruno MJ: Epidemiology, aetiology and outcome of acute and chronic pancreatitis: an update. Best Pract Res Clin Gastroenterol 2008;22:45–63 [DOI] [PubMed] [Google Scholar]
- 2.Noel RA, Braun DK, Patterson RE, Bloomgren GL: Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care 2009;32:834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yadav D, Lowenfels AB: Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas 2006;33:323–330 [DOI] [PubMed] [Google Scholar]
- 4.Blomgren KB, Sundström A, Steineck G, Wiholm BE: Obesity and treatment of diabetes with glyburide may both be risk factors for acute pancreatitis. Diabetes Care 2002;25:298–302 [DOI] [PubMed] [Google Scholar]
- 5.Eland IA, Sundström A, Velo GP, Andersen M, Sturkenboom MC, Langman MJ, Stricker BH, Wiholm B: EDIP Study Group of the European Pharmacovigilance Research Group Antihypertensive medication and the risk of acute pancreatitis: the European case-control study on drug-induced acute pancreatitis (EDIP). Scand J Gastroenterol 2006;41:1484–1490 [DOI] [PubMed] [Google Scholar]
- 6.Dore DD, Seeger JD, Arnold Chan K: Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 2009;25:1019–1027 [DOI] [PubMed] [Google Scholar]
- 7.Morris R, Carstairs V: Which deprivation? A comparison of selected deprivation indexes J Public Health Med 1991;13:318–326 [PubMed] [Google Scholar]
- 8.Shohaimi S, Welch A, Bingham S, Luben R, Day N, Wareham N, Khaw KT: Area deprivation predicts lung function independently of education and social class. Eur Respir J 2004;24:157–161 [DOI] [PubMed] [Google Scholar]
- 9.Fimognari FL, Corsonello A, Pastorell R, Antonelli-Incalzi R: Metformin-induced pancreatitis: a possible adverse drug effect during acute renal failure. Diabetes Care 2006;29:1183. [DOI] [PubMed] [Google Scholar]
- 10.Mallick S: Metformin induced acute pancreatitis precipitated by renal failure. Postgrad Med J 2004;80:239–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eland IA, Sturkenboom MJ, Wilson JH, Stricker BH: Incidence and mortality of acute pancreatitis between 1985 and 1995. Scand J Gastroenterol 2000;35:1110–1116 [DOI] [PubMed] [Google Scholar]
- 12.Eland IA, Sturkenboom MC, van der Lei J, Wilson JH, Stricker BH: Incidence of acute pancreatitis. Scand J Gastroenterol 2002;37:124. [DOI] [PubMed] [Google Scholar]
- 13.Roberts SE, Williams JG, Meddings D, Goldacre MJ: Incidence and case fatality for acute pancreatitis in England: geographical variation, social deprivation, alcohol consumption and aetiology—a record linkage study. Aliment Pharmacol Ther 2008;28:931–941 [DOI] [PubMed] [Google Scholar]
- 14.Steinberg W, Tenner S: Acute pancreatitis. N Engl J Med 1994;330:1198–1210 [DOI] [PubMed] [Google Scholar]
- 15.Badalov N, Baradarian R, Iswara K, Li J, Steinberg W, Tenner S: Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol 2007;5:648–661; quiz 644 [DOI] [PubMed] [Google Scholar]
- 16.Balani AR, Grendell JH: Drug-induced pancreatitis : incidence, management and prevention. Drug Saf 2008;31:823–837 [DOI] [PubMed] [Google Scholar]
- 17.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL: Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007;16:393–401 [DOI] [PubMed] [Google Scholar]
- 18.Meal A, Leonardi-Bee J, Smith C, Hubbard R, Bath-Hextall F: Validation of THIN data for non-melanoma skin cancer. Qual Prim Care 2008;16:49–52 [PubMed] [Google Scholar]
- 19.Lo Re V, 3rd, Haynes K, Forde KA, Localio AR, Schinnar R, Lewis JD: Validity of The Health Improvement Network (THIN) for epidemiologic studies of hepatitis C virus infection. Pharmacoepidemiol Drug Saf 2009;18:807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]