Abstract
OBJECTIVE
To investigate the long-term associations of magnesium intake with incidence of diabetes, systemic inflammation, and insulin resistance among young American adults.
RESEARCH DESIGN AND METHODS
A total of 4,497 Americans, aged 18–30 years, who had no diabetes at baseline, were prospectively examined for incident diabetes based on quintiles of magnesium intake. We also investigated the associations between magnesium intake and inflammatory markers, i.e., high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), and fibrinogen, and the homeostasis model assessment of insulin resistance (HOMA-IR).
RESULTS
During the 20-year follow-up, 330 incident cases of diabetes were identified. Magnesium intake was inversely associated with incidence of diabetes after adjustment for potential confounders. The multivariable-adjusted hazard ratio of diabetes for participants in the highest quintile of magnesium intake was 0.53 (95% CI, 0.32–0.86; Ptrend < 0.01) compared with those in the lowest quintile. Consistently, magnesium intake was significantly inversely associated with hs-CRP, IL-6, fibrinogen, and HOMA-IR, and serum magnesium levels were inversely correlated with hs-CRP and HOMA-IR.
CONCLUSIONS
Magnesium intake was inversely longitudinally associated with incidence of diabetes in young American adults. This inverse association may be explained, at least in part, by the inverse correlations of magnesium intake with systemic inflammation and insulin resistance.
Although obesity is an important risk factor for diabetes, certain foods or nutrients may also be associated with an increased risk of diabetes (1). Magnesium, found in whole grains, is an essential cofactor for multiple enzymes involved in glucose metabolism (2). Several cohort studies have investigated magnesium intake in relation to risk of diabetes, but the findings have been inconsistent (3). Some (4–6), but not all (7–9), studies found an inverse association between magnesium intake and diabetes risk. Of note, all previous studies except one (7) used self-reported cases, and all studies were conducted among middle-aged or elderly individuals.
In addition, the pathophysiological mechanisms underlying the beneficial effects of magnesium intake on diabetes are not fully understood. Cross-sectional studies have suggested an inverse correlation between magnesium intake and inflammatory markers (10,11), and some clinical and experimental studies have suggested that magnesium may improve insulin sensitivity (12,13). Therefore, we investigated magnesium intake in relation to the incidence of diabetes in a large cohort of young American adults participating in the Coronary Artery Risk Development in Young Adults (CARDIA) study. To explore possible mechanisms, we also examined whether magnesium intake is inversely associated with systemic inflammation markers, i.e., high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), and fibrinogen, and with the homeostasis model assessment of insulin resistance (HOMA-IR).
RESEARCH DESIGN AND METHODS
The CARDIA study is an ongoing, multicenter, prospective cohort study investigating the role of lifestyle and other factors in the development of risk factors for cardiovascular diseases among young adults (14). In brief, 5,115 African American and Caucasian men and women, aged 18–30 years, were enrolled from 1985 to 1986. To date, six follow-up examinations have been completed. The average follow-up rate was 94.1%, and ∼70% of participants in the original cohort completed the follow-up examination at year 20 (2005–2006).
For analyses, we excluded participants who had taken antidiabetes medications and/or had a fasting plasma glucose ≥7 mmol/l at baseline (n = 35), those who did not participate in any follow-up examinations (n = 204), those who had missing data on magnesium or total energy intake or who reported implausible total energy intake (<800 or >8,000 kcal/day for men or <600 or >6,000 kcal/day for women, n = 74), and women who were pregnant at any examination (n = 233). We further excluded participants who had missing data on smoking status (n = 33), alcohol consumption (n = 18), physical activity (n = 1), BMI (n = 14), or waist circumference (n = 6). After these exclusions, a total of 4,497 participants (87.9% of 5,115) remained in the analyses.
All participants gave written informed consent. The study design, data collection, and analyses were approved by the institutional review boards of the centers involved.
Assessment of magnesium intake and other dietary factors
The details of dietary assessment and validation of magnesium intake in CARDIA have been described previously (15). In brief, we collected dietary data, including magnesium intake at baseline and examination years 7 and 20, using a validated interviewer-administered CARDIA Diet History Questionnaire (16). Information on supplement use was also collected. Magnesium intake represented the sum of dietary magnesium intake and magnesium supplement.
Measurement of other covariates
Age, sex, ethnicity, years of education, smoking status, and alcohol consumption were self-reported, obtained by interview or self-administered questionnaire. Smoking status was classified as never, former, and current. Alcohol consumption was classified into four groups based on daily amount ingested: none, 0.1–9.9, 10–19.9, and ≥20 g/day. BMI was calculated as weight in kilograms divided by the square of height in meters and was classified into three groups: <25, 25–29.9, and ≥30 kg/m2 (17). Waist circumference was measured at the maximum abdominal girth, and all anthropometric measures were taken in duplicate and averaged. Three measurements of resting systolic and diastolic fifth-phase blood pressures were taken using a random-zero sphygmomanometer. The average of the second and third measurements was used in the analyses. Physical activity was assessed using the interview-based, validated, CARDIA Physical Activity History Questionnaire (18). A score of 100 exercise units is approximately equivalent to participation in vigorous activity for 2–3 h/week during 6 months of the year. Family history of diabetes was defined as either mother or father having diabetes.
Assessment of inflammatory markers, HOMA-IR, oral glucose tolerance test, and A1C
Serum hs-CRP was measured at years 7, 15, and 20, with a nephelometry-based high throughput assay. Intra- and interassay coefficients of variations (CVs) were 2.3–4.4 and 2.1–5.7%, respectively. IL-6 was analyzed at year 20 using a high-sensitivity enzyme-linked immunosorbent assay. A routine CV was 6.3%. Fibrinogen was assessed at years 5, 7, and 20 by the Clauss method (year 5) and a BNII nephelometer (years 7 and 20) calibrated with standard normal plasma (19). Intra- and interassay CVs were 2.7 and 2.6% at year 7 and 3.1 and 4.2% at year 20. Fasting plasma glucose and insulin levels were determined by the hexokinase ultraviolet method and radioimmunoassay, respectively, at years 0, 7, 10, 15, and 20. Masked analysis of split serum samples resulted in a technical error of 16.6% for mean insulin levels (r = 0.98). Between-method correlation was 0.83. HOMA-IR was calculated as glucose (millimoles per liter) × insulin (milliunits per liter)/22.5 (20).
Two-hour plasma glucose levels were measured from a standard 2-h oral glucose tolerance test (OGTT) at years 10 and 20. A1C was assessed using a Tosoh G7 high-performance liquid chromatography instrument at year 20. The interassay CVs were 2.0–3.0%.
Ascertainment of diabetes
Participants with one or more of the following were determined to have diabetes: 1) fasting plasma glucose ≥7.0 mmol/l (years 0, 7, 10, 15, and 20); 2) nonfasting plasma glucose ≥11.1 mmol/l (years 0, 7, 10, 15, and 20); 3) postprandial 2-h plasma glucose ≥11.1 mmol/l from an OGTT (years 10 and 20); 4) A1C ≥6.5%; (year 20); or 5) reported use of antidiabetes medications (all examinations), which were verified by medication names (21). We could not clearly distinguish diabetes type, because some participants were young at diagnosis and used insulin as treatment. Therefore, we used the term “diabetes” rather than “type 2 diabetes,” but the great majority of participants had type 2 diabetes.
Statistical analysis
Participants were divided into quintiles according to magnesium intake (milligrams/1,000 kcal). Baseline characteristics of participants were calculated as mean and SD, median and interquartile range, or percentage and were compared according to quintiles of magnesium intake using ANOVA, a Kruskal-Wallis test, or a χ2 test as appropriate.
We used the Cox proportional hazards model to estimate the hazard ratios (HRs) and 95% CIs of incident diabetes. Follow-up time was calculated as the difference between the baseline examination and the year in which diabetes was first identified, year 20, or the year a participant was censored. To best represent long-term dietary intake and to minimize measurement error and the effect of diagnosed diabetes or other conditions on diet, we used only baseline magnesium intake in relation to cases identified at years 2, 5, and 7 and used the average magnesium intake of baseline and year 7 in relation to cases occurring at years 10, 15, and 20 (15). If year 7 data were missing, we imputed by multiplying baseline magnesium intake and the ratio of the mean values at two examinations. In addition, because the cutoff point of fasting glucose for defining diabetes was changed from 140 to 126 mg/dl in 1997, we used a cutoff point of 140 mg/dl at years 0, 7, and 10 in sensitivity analysis to test the robustness of our results.
The initial analysis (model 1) was adjusted for age, sex, ethnicity, and study center. In model 2, we further adjusted for education, smoking status, alcohol consumption, physical activity, family history of diabetes, BMI, systolic blood pressure, and total energy intake. In model 3, we additionally adjusted for dietary intake of saturated fat and crude fiber. Continuous variables using the median value in each quintile were created for trend tests.
In addition, we tested for possible interactions between magnesium intake and sex, ethnicity, family history of diabetes, and BMI by adding corresponding multiplicative interaction terms in the models, followed by the likelihood ratio test. We also stratified the data according to these variables to determine whether they modified the associations.
We examined the association between magnesium intake and serum levels of inflammatory markers and HOMA-IR. A logarithmic transformation was used to improve the normality of hs-CRP, IL-6, fibrinogen, and HOMA-IR distributions. Because hs-CRP, fibrinogen, and HOMA-IR were measured multiple times, generalized estimating equations with exchangeable correlation structure for simplicity were used. Analogous to the accumulative diet method used in the Cox models, we used the average magnesium intake of years 0 and 7 for all repeated measures that occurred at years 10, 15, or 20. Because IL-6 was measured once, the general linear model was used. The adjusted covariates in the models were the same as those listed in Table 3.
Table 3.
Multivariable-adjusted associations of total magnesium intake (quintiles) with inflammatory markers and HOMA-IR
| Quintiles of total magnesium intake |
Ptrend | |||||
|---|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 | 5 (highest) | ||
| hs-CRP (n = 4,157) | ||||||
| Median (mg/l) | 1.60 | 1.37 | 1.27 | 1.00 | 0.86 | |
| Model 1 | 0 | −0.071 (−0.153 to 0.012) | −0.008 (−0.093 to 0.077) | −0.151 (−0.245 to −0.057) | −0.270 (−0.368 to −0.172) | <0.01 |
| Model 2 | 0 | −0.069 (−0.149 to 0.012) | 0.012 (−0.071 to 0.095) | −0.095 (−0.186 to −0.004) | −0.190 (−0.286 to −0.094) | <0.01 |
| Model 3 | 0 | −0.058 (−0.140 to 0.024) | 0.028 (−0.057 to 0.114) | −0.071 (−0.166 to 0.024) | −0.160 (−0.262 to −0.058) | <0.01 |
| IL-6 (n = 3,254) | ||||||
| Median (mg/l) | 2.26 | 1.96 | 1.66 | 1.54 | 1.39 | |
| Model 1 | 0 | −0.097 (−0.185 to −0.009) | −0.154 (−0.245 to −0.062) | −0.201 (−0.296 to −0.105) | −0.284 (−0.383 to −0.186) | <0.01 |
| Model 2 | 0 | −0.088 (−0.174 to −0.002) | −0.134 (−0.223 to −0.044) | −0.141 (−0.236 to −0.045) | −0.217 (−0.317 to −0.118) | <0.01 |
| Model 3 | 0 | −0.082 (−0.169 to 0.005) | −0.121 (−0.214 to −0.029) | −0.125 (−0.225 to −0.025) | −0.200 (−0.306 to −0.094) | <0.01 |
| Fibrinogen (n = 4,325) | ||||||
| Median (mg/l) | 314 | 319 | 319 | 316 | 306 | |
| Model 1 | 0 | −0.010 (−0.023 to 0.004) | −0.003 (−0.018 to 0.011) | −0.018 (−0.033 to −0.002) | −0.038 (−0.055 to −0.021) | <0.01 |
| Model 2 | 0 | −0.007 (−0.020 to 0.007) | 0.005 (−0.009 to 0.019) | −0.003 (−0.018 to 0.012) | −0.015 (−0.032 to 0.002) | 0.08 |
| Model 3 | 0 | −0.007 (−0.021 to 0.006) | 0.003 (−0.012 to 0.017) | −0.006 (−0.022 to 0.010) | −0.020 (−0.038 to −0.002) | 0.03 |
| HOMA-IR (n = 4,239) | ||||||
| Median (mg/l) | 2.66 | 2.63 | 2.50 | 2.36 | 2.11 | |
| Model 1 | 0 | −0.046 (−0.081 to −0.012) | −0.040 (−0.077 to −0.003) | −0.085 (−0.123 to −0.047) | −0.153 (−0.195 to −0.112) | <0.01 |
| Model 2 | 0 | −0.045 (−0.077 to −0.013) | −0.036 (−0.070 to −0.002) | −0.068 (−0.103 to −0.033) | −0.122 (−0.160 to −0.084) | <0.01 |
| Model 3 | 0 | −0.046 (−0.078 to −0.013) | −0.036 (−0.071 to −0.000) | −0.065 (−0.102 to −0.029) | −0.117 (−0.157 to −0.077) | <0.01 |
Data are β coefficients (95% CIs) unless otherwise indicated. A logarithmic transformation was used to improve the normality of distribution for dependent variables. Generalized estimating equations were used for repeated measurements of hs-CRP, fibrinogen, and HOMA-IR, and a general linear regression model was used for IL-6. The median magnesium level in each quintile was created for the trend tests. The adjusted covariates in the models were the same as those listed in Table 2.
We examined the correlation between serum and magnesium intake at year 20 and also analyzed the relations between serum magnesium and inflammatory markers and HOMA-IR using the general linear model, after adjustment for covariates in the same year in the manner described above.
All analyses were performed with SAS (version 9.1.3; SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of 4,497 participants according to quintiles of magnesium intake are shown in Table 1. Compared with participants in the lowest quintile of magnesium intake, those in the highest quintile were slightly older and more likely to be female, Caucasian, and not current smokers. They also had lower BMI and waist circumference and were less likely to have a family history of diabetes. In addition, they had higher rates of magnesium supplement use and crude fiber intake and lower intakes of total energy and saturated fat. The correlation coefficients of total magnesium intake with magnesium supplementation and whole grain consumption were 0.58 and 0.25 at year 0 and 0.71 and 0.25 at year 7 (all P < 0.001).
Table 1.
Baseline characteristics of CARDIA study participants according to total magnesium intake quintiles
| Characteristics | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P values* |
|---|---|---|---|---|---|---|
| n | 899 | 900 | 899 | 900 | 899 | |
| Magnesium intake (mg/1,000 kcal) | 99.9 (91.7–106.1) | 121.0 (116.5–125.2) | 140.1 (134.8–145.5) | 162.1 (155.8–169.3) | 201.5 (187.7–233.3) | — |
| Age (years) | 23.9 ± 3.8 | 24.3 ± 3.7 | 25.0 ± 3.6 | 25.5 ± 3.4 | 26.0 ± 3.2 | <0.01 |
| Female sex (%) | 52.4 | 46.8 | 46.8 | 53.0 | 64.7 | <0.01 |
| African American (%) | 82.7 | 67.7 | 48.5 | 31.1 | 23.0 | <0.01 |
| Education (years) | 12.8 ± 1.8 | 13.3 ± 2.1 | 13.9 ± 2.1 | 14.4 ± 2.3 | 14.8 ± 2.3 | <0.01 |
| Current smoker (%) | 36.4 | 33.0 | 28.1 | 27.2 | 24.7 | <0.01 |
| Alcohol intake (g/day) | 10.2 ± 20.1 | 12.9 ± 22.2 | 12.2 ± 19.6 | 13.4 ± 22.6 | 10.6 ± 16.2 | <0.01 |
| Physical activity score (units) | 354.0 ± 293.5 | 407.7 ± 305.2 | 421.4 ± 289.5 | 435.7 ± 289.3 | 474.8 ± 288.5 | <0.01 |
| Family history of diabetes (%) | 16.2 | 14.0 | 14.6 | 12.3 | 11.4 | 0.02 |
| BMI (kg/m2) | 25.0 ± 5.9 | 25.0 ± 5.5 | 24.5 ± 4.7 | 24.1 ± 4.4 | 24.0 ± 4.3 | <0.01 |
| Waist circumference (cm) | 78.5 ± 12.9 | 79.1 ± 11.7 | 78.4 ± 10.6 | 77.3 ± 10.7 | 76.2 ± 10.0 | <0.01 |
| Glucose (mg/dl) | 81.3 ± 8.8 | 81.9 ± 9.0 | 81.6 ± 8.5 | 82.2 ± 7.7 | 82.0 ± 7.4 | 0.29 |
| Insulin (μU/ml) | 12.8 ± 6.9 | 12.5 ± 6.5 | 11.2 ± 4.9 | 10.6 ± 4.7 | 10.5 ± 4.8 | <0.01 |
| Systolic blood pressure (mmHg) | 111.2 ± 10.8 | 111.5 ± 11.2 | 110.8 ± 10.9 | 109.9 ± 10.2 | 109.2 ± 11.3 | <0.01 |
| Diastolic blood pressure (mmHg) | 68.2 ± 10.2 | 68.8 ± 10.2 | 69.2 ± 9.5 | 68.8 ± 9.0 | 68.2 ± 9.3 | 0.11 |
| Total energy intake (kcal) | 3,264 ± 1,650 | 3,170 ± 1,571 | 2,943 ± 1,363 | 2,652 ± 1,189 | 2,374 ± 1,127 | <0.01 |
| Total saturated fat (g) | 53.4 ± 31.3 | 51.6 ± 28.3 | 47.8 ± 25.0 | 42.3 ± 21.6 | 35.2 ± 20.6 | <0.01 |
| Crude fiber (g) | 4.7 ± 2.9 | 5.4 ± 3.0 | 5.6 ± 3.1 | 6.0 ± 3.2 | 6.7 ± 4.0 | <0.01 |
| Whole grain (times/week) | 4.9 ± 7.3 | 7.4 ± 8.6 | 9.7 ± 9.7 | 11.0 ± 9.5 | 13.1 ± 11.1 | <0.01 |
| Magnesium supplementation (mg) | 1.6 ± 9.4 | 4.0 ± 15.7 | 9.5 ± 25.2 | 17.9 ± 35.9 | 69.4 ± 137.1 | <0.01 |
Data are median (interquartile range), means ± SD, or %.
*P values are for any difference across the quintiles of magnesium intake using ANOVA, Kruskal-Wallis test, or χ2 test as appropriate.
During the 20-year follow-up, 330 incident cases of diabetes were identified, including 212 cases determined by fasting glucose criteria and 3, 37, 35, and 43 cases determined by nonfasting glucose, 2-h glucose after OGTT, A1C, and antidiabetes medication use criteria, respectively. Magnesium intake was inversely associated with incidence of diabetes. The incidence of diabetes was 47% lower (HR 0.53 [95% CI 0.32–0.86]; Ptrend < 0.01) for participants in the highest quintile compared with that for those in the lowest quintile, after adjustment for age, sex, ethnicity, study center, and other potential confounders (Table 2). When data were stratified according to magnesium supplementation, the observed inverse associations were generally consistent but were somewhat attenuated among supplement nonusers because the range of magnesium intake was substantially narrowed (Table 2).
Table 2.
Incident diabetes according to total magnesium intake quintiles
| Quintiles of total magnesium intake |
Ptrend* | |||||
|---|---|---|---|---|---|---|
| 1 (lowest) | 2 | 3 | 4 | 5 (highest) | ||
| Total cohort (n = 4,497) | ||||||
| Magnesium intake (mg/1,000 kcal) | 99.9 (91.7–106.1) | 121.0 (116.5–125.2) | 140.1 (134.8–145.5) | 162.1 (155.8–169.3) | 201.5 (187.7–233.3) | — |
| No. of events/participants | 81/899 | 85/900 | 77/899 | 52/900 | 35/899 | — |
| Model 1 | 1.00 | 0.95 (0.70–1.30) | 0.91 (0.66–1.26) | 0.63 (0.43–0.92) | 0.46 (0.30–0.71) | <0.01 |
| Model 2 | 1.00 | 0.89 (0.65–1.22) | 0.88 (0.63–1.24) | 0.72 (0.49–1.05) | 0.52 (0.33–0.82) | <0.01 |
| Model 3 | 1.00 | 0.96 (0.70–1.32) | 0.97 (0.68–1.38) | 0.77 (0.51–1.18) | 0.53 (0.32–0.86) | <0.01 |
| Supplement users (n = 1,205) | ||||||
| Magnesium intake (mg/1,000 kcal) | 123.6 (112.6–133.0) | 151.0 (146.1–156.0) | 172.6 (166.6–179.0) | 196.0 (188.9–203.7) | 248.8 (233.3–290.4) | |
| No. of events/participants | 32/241 | 16/241 | 17/241 | 6/241 | 6/241 | |
| Model 3 | 1.00 | 0.66 (0.33–1.30) | 0.70 (0.34–1.44) | 0.24 (0.09–0.67) | 0.32 (0.10–0.96) | 0.01 |
| Supplement nonusers (n = 3,292) | ||||||
| Magnesium intake (mg/1,000 kcal) | 97.0 (89.4–102.0) | 115.1 (111.0–119.0) | 130.1 (126.5–134.2) | 149.7 (144.2–154.5) | 180.0 (169.3–196.6) | |
| No. of events/participants | 59/658 | 58/659 | 54/658 | 45/659 | 37/658 | |
| Model 3 | 1.00 | 0.96 (0.66–1.41) | 0.79 (0.53–1.18) | 0.79 (0.50–1.26) | 0.74 (0.44–1.26) | 0.21 |
Data are medians (interquartile range) for magnesium intake and HRs (95% CI) for models.
*All models were constructed by the Cox proportional hazards model. The median magnesium level in each quintile was created for the trend tests. Model 1: adjustment for age (continuous), sex, ethnicity (African American or Caucasian), and study center; model 2: model 1 with additional adjustment for education (continuous), smoking status (never, former, or current), alcohol consumption (none, 0.1–9.9, 10–19.9, or ≥20 g/day), physical activity (quintiles), family history of diabetes, BMI (<25, 25–29.9, or ≥30 kg/m2), systolic blood pressure (continuous), and total energy intake (quintiles); model 3: model 2 with additional adjustment for dietary intakes (quintiles) of saturated fat and crude fiber.
When we substituted waist circumference for BMI in model 3, a similar inverse association was observed (HR 0.59 [95% CI 0.36–0.98]; Ptrend = 0.04). In addition, when we used different definitions of diabetes based on the time period of the examination, a total of 327 incident cases of diabetes were identified. These results were essentially the same as findings from the primary analysis (data not shown).
In addition, we examined a few food groups that are rich in magnesium. These associations were qualitatively consistent with magnesium intake. For example, the multivariable-adjusted HRs of incident diabetes were 1.00, 1.26 (95% CI 0.93–1.71), 0.82 (0.57–1.17), 0.71 (0.48–1.04), and 0.81 (0.55–1.20) (Ptrend = 0.03) across quintiles of whole grain consumption.
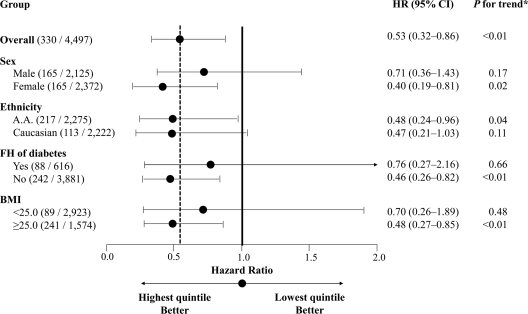
By stratifying data according to sex, ethnicity, family history of diabetes, and BMI, respectively, the observed inverse associations were more pronounced in women, overweight individuals, and those without a family history of diabetes. However, none of the tests for interactions among these variables showed statistical significance (Fig. 1).
Figure 1.
HRs (95% CI) for incident diabetes in participants in the highest magnesium intake quintile compared with those in the lowest quintile, stratified by sex, ethnicity, family history of diabetes, and BMI. Data in the parentheses in the group column are the ratio of the number of events to number of participants. The adjusted covariates in the models were the same as those for model 3 in Table 2. *Continuous variables using the median value in each quintile were created for trend tests. A.A., African American; FH, family history.
Magnesium intake was also inversely associated with hs-CRP, IL-6, and fibrinogen levels after adjustment for potential confounders. Moreover, a significant inverse association between magnesium intake and HOMA-IR was observed (Table 3). Furthermore, although the Spearman correlation coefficient between serum and total magnesium intake at year 20 was 0.07, serum magnesium levels were significantly inversely correlated with hs-CRP and HOMA-IR (supplementary Table, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0994/DC1).
CONCLUSIONS
In this longitudinal study, we found a significant inverse association between magnesium intake and incidence of diabetes in young American adults. Our findings are generally consistent with results from some previous studies (1,4–6). Conversely, three other studies found no significant associations between magnesium intake and risk of diabetes (7–9), although one of these reported that a low serum magnesium level was a strong, independent predictor of incident type 2 diabetes (7). Of note, in a meta-analysis of cohort studies (3), the pooled relative risk of type 2 diabetes per 100 mg/day increment of magnesium intake was 0.85 (95% CI 0.79–0.92).
We observed an inverse association between magnesium intake and HOMA-IR. This finding indicates that the beneficial effects of magnesium intake on risk of diabetes may be partially due to role of magnesium in improving insulin sensitivity (2,8,22) and suggests that low magnesium intake and insulin resistance may detrimentally influence each other. Magnesium is important as a second messenger for insulin action. A reduced intracellular magnesium concentration may result in defective tyrosine kinase activity during insulin signaling and decreased insulin sensitivity (12,13). Moreover, insulin itself is an important regulatory factor for intracellular magnesium accumulation (12).
An inverse association between magnesium intake and hs-CRP level was reported in three cross-sectional studies (10,11,23). In addition, an inverse association between magnesium intake and IL-6 level was found in one (23) but not in another study (11). In the present study, magnesium intake was inversely associated with levels of hs-CRP, IL-6, and fibrinogen, and serum magnesium was inversely associated with hs-CRP levels. These findings support another possible pathway for altering systemic inflammation and may explain, at least in part, the potential beneficial effect of magnesium intake on diabetes risk.
The strengths of our study include a long-term follow-up period, a large sample size of young adults, and a sample well balanced for sex and ethnicity. Most previous findings are from studies in middle-aged or elderly individuals, who were likely to have already had disease onset and may have made lifestyle changes for disease prevention or treatment. Furthermore, we longitudinally measured several inflammatory markers and fasting insulin level (HOMA-IR), which enabled us to explore potential pathophysiological mechanisms for the beneficial effects of magnesium intake on diabetes risk. Moreover, we defined diabetes based mainly on fasting and postprandial glucose levels from an OGTT and A1C measurements, in addition to a self-reported questionnaire. In previous studies (4–6,8,9), except the Atherosclerosis Risk in Communities (ARIC) study (7), all diabetes cases were self-reported. Our study was further strengthened by our consistent findings across dietary magnesium intake, serum magnesium level, and consumption of whole grains, a major source of magnesium.
Our study also had limitations. First, the possibility of confounding from unknown or unmeasured factors cannot be completely ruled out. Participants in the highest quintile of magnesium intake in particular were most likely to take supplements, meaning that these participants may be more health-conscious and therefore different from participants in other groups. However, our results are unlikely to be substantially biased, given our extensive data analysis, consistent findings in sensitivity analysis (e.g., stratified analysis based on supplementation) and supportive biological mechanisms. Second, there is an inherent limitation in dietary assessment of individual nutrients including magnesium. Thus, observed associations are likely to reflect consumption of foods rich in magnesium such as whole grains, nuts, legumes, fruits, and vegetables but may not be the result of an isolated effect of magnesium intake. Third, although the inverse association of magnesium intake with inflammatory markers remained after further adjustment for some health conditions including asthma and serum cholesterol levels, we could not fully control for all possible sources of systemic inflammation (e.g., acute infection) because of lack of information. Fourth, lack of fasting glucose data for years 2 and 5 may have resulted in underestimation and misclassification of diabetes at these times. However, this limitation should not substantially bias our results, because diabetes incidence was relatively low for these years, when most participants were in their 20s, and many cases of diabetes diagnosed between years 0 and 7 would have been detected at year ≤7.
A recent clinical trial suggested that magnesium supplementation improves insulin sensitivity in hypomagnesemic nondiabetic participants (24). In a crossover trial among healthy individuals, magnesium supplementation inhibited fat absorption and decreased postprandial triglyceride levels (25). In our data, total magnesium intake was important regardless of its source. Therefore, increasing magnesium intake may be important for improving insulin sensitivity, reducing systemic inflammation, and decreasing diabetes risk.
In summary, magnesium intake was inversely associated with incidence of diabetes. The potential beneficial effects of magnesium intake on the risk of diabetes may be explained by the favorable effects of magnesium on systemic inflammation and insulin resistance. Further large-scale clinical trials are needed to establish causal inference and elucidate the mechanisms behind this potential benefit.
Supplementary Material
Acknowledgments
CARDIA is funded by the National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI) through contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095. This study was supported by NHLBI grants R01-HL-081572 and partially by R01-HL-53560. D.J.K. was supported by grant A050463 from the Korean Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea.
No potential conflicts of interest relevant to this article were reported.
D.J.K. researched data, contributed to discussion, wrote the manuscript, and reviewed/edited the manuscript. P.X. researched data, contributed to discussion, and reviewed/edited the manuscript. K.L., C.L., K.Y., and D.R.J. contributed to discussion and reviewed/edited the manuscript. K.H. contributed to discussion, wrote the manuscript, and reviewed/edited the manuscript.
We thank Dr. Winston Choi of the University of Alabama at Birmingham for verifying SAS programming and thank Dr. Atsushi Hozawa of the University of Minnesota for his valuable comments.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer FE: Diet and risk of clinical diabetes in women. Am J Clin Nutr 1992;55:1018–1023 [DOI] [PubMed] [Google Scholar]
- 2.Belin RJ, He K: Magnesium physiology and pathogenic mechanisms that contribute to the development of the metabolic syndrome. Magnes Res 2007;20:107–129 [PubMed] [Google Scholar]
- 3.Larsson SC, Wolk A: Magnesium intake and risk of type 2 diabetes: a meta-analysis. J Intern Med 2007;262:208–214 [DOI] [PubMed] [Google Scholar]
- 4.Meyer KA, Kushi LH, Jacobs DR, Jr, Slavin J, Sellers TA, Folsom AR: Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–930 [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Ridaura R, Willett WC, Rimm EB, Liu S, Stampfer MJ, Manson JE, Hu FB: Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care 2004;27:134–140 [DOI] [PubMed] [Google Scholar]
- 6.van Dam RM, Hu FB, Rosenberg L, Krishnan S, Palmer JR: Dietary calcium and magnesium, major food sources, and risk of type 2 diabetes in U.S. black women. Diabetes Care 2006;29:2238–2243 [DOI] [PubMed] [Google Scholar]
- 7.Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL: Serum and dietary magnesium and the risk for type 2 diabetes mellitus: the Atherosclerosis Risk in Communities Study. Arch Intern Med 1999;159:2151–2159 [DOI] [PubMed] [Google Scholar]
- 8.Song Y, Manson JE, Buring JE, Liu S: Dietary magnesium intake in relation to plasma insulin levels and risk of type 2 diabetes in women. Diabetes Care 2004;27:59–65 [DOI] [PubMed] [Google Scholar]
- 9.Hodge AM, English DR, O'Dea K, Giles GG: Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004;27:2701–2706 [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S: Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care 2005;28:1438–1444 [DOI] [PubMed] [Google Scholar]
- 11.Song Y, Li TY, van Dam RM, Manson JE, Hu FB: Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr 2007;85:1068–1074 [DOI] [PubMed] [Google Scholar]
- 12.Paolisso G, Scheen A, D'Onofrio F, Lefèbvre P: Magnesium and glucose homeostasis. Diabetologia 1990;33:511–514 [DOI] [PubMed] [Google Scholar]
- 13.Takaya J, Higashino H, Kobayashi Y: Intracellular magnesium and insulin resistance. Magnes Res 2004;17:126–136 [PubMed] [Google Scholar]
- 14.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ: CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–1116 [DOI] [PubMed] [Google Scholar]
- 15.He K, Liu K, Daviglus ML, Morris SJ, Loria CM, Van Horn L, Jacobs DR, Jr, Savage PJ: Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006;113:1675–1682 [DOI] [PubMed] [Google Scholar]
- 16.Liu K, Slattery M, Jacobs D, Jr, Cutter G, McDonald A, Van Horn L, Hilner JE, Caan B, Bragg C, Dyer A: A study of the reliability and comparative validity of the CARDIA dietary history. Ethn Dis 1994;4:15–27 [PubMed] [Google Scholar]
- 17.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report [article online], 1998. National Institutes of Health; National Heart, Lung, and Blood Institute; (Publ. no. 98-4083). Available from: http://www.nhlbi.nih.gov/guidelines/obesity/ob_gdlns.pdf Accessed 12 November 2009 [Google Scholar]
- 18.Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM: A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc 1997;29(6 Suppl.):S1–S205 [PubMed] [Google Scholar]
- 19.Hozawa A, Jacobs DR, Jr, Steffes MW, Gross MD, Steffen LM, Lee DH: Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) study. Clin Chem 2007;53:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33 (Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung TT, Manson JE, Solomon CG, Liu S, Willett WC, Hu FB: The association between magnesium intake and fasting insulin concentration in healthy middle-aged women. J Am Coll Nutr 2003;22:533–538 [DOI] [PubMed] [Google Scholar]
- 23.Chacko SA, Song Y, Nathan L, Tinker L, de Boer IH, Tylavsky F, Wallace R, Liu S: Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care 2010;33:304–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerrero-Romero F, Tamez-Perez HE, González-González G, Salinas-Martínez AM, Montes-Villarreal J, Treviño-Ortiz JH, Rodríguez-Morán M: Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomized trial. Diabetes Metab 2004;30:253–258 [DOI] [PubMed] [Google Scholar]
- 25.Kishimoto Y, Tani M, Uto-Kondo H, Saita E, Iizuka M, Sone H, Yokota K, Kondo K: Effects of magnesium on postprandial serum lipid responses in healthy human subjects. Br J Nutr 2010;103:469–472 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.