Abstract
OBJECTIVE
To determine the relationships among large, small, and autonomic fiber neurophysiological measures in a cross-sectional study of patients with diabetes.
RESEARCH DESIGN AND METHODS
We assessed 130 individuals: 25 healthy subjects and 105 subjects with diabetes. Subjects were classified by the presence or absence of neuropathy by physical examination. All subjects underwent autonomic testing, nerve conduction studies, quantitative sensory testing, and nerve-axon reflex vasodilation in addition to quantifiable neurological examination and symptom scores. Correlation and cluster analysis were used to determine relationships between and among different neurophysiological testing parameters.
RESULTS
Results of neurophysiological tests were abnormal in patients with clinical evidence of diabetic neuropathy compared with results in healthy control subjects and in those without neuropathy (P < 0.01, all tests). The correlations among individual tests varied widely, both within (r range <0.5–>0.9, NS to <0.001) and between test groups (r range <0.2–>0.5, NS to <0.01). A two-step hierarchical cluster analysis revealed that neurophysiological tests do not aggregate by typical “small,” “large,” or “autonomic” nerve fiber subtypes.
CONCLUSIONS
The modest correlation coefficients seen between the different testing modalities suggest that these techniques measure different neurophysiological parameters and are therefore not interchangeable. However, the data suggest that only a small number of neurophysiological tests are actually required to clinically differentiate individuals with neuropathy from those without. The natural clustering of both patients and healthy control subjects suggests that variations in the population will need to be considered in future studies of diabetic neuropathy.
Microvascular complications of diabetes, which include retinopathy, neuropathy, and nephropathy are major contributors to morbidity and mortality. Although neuropathy severity is related to duration and degree of glycemic control, individual subjects may have widely disparate clinical presentations despite similar risk factors. Neuropathy progression preferentially affecting nerve fiber subtypes may explain some clinical heterogeneity, but different neurophysiological tests are required to identify dysfunction of different nerve populations in diabetes.
Nerve conduction studies, assessing large myelinated fibers, are widely used both in clinical practice and as end points in longitudinal investigations of diabetic neuropathy (1,2). Damage to small thinly and unmyelinated nerves or autonomic fibers can be measured by quantitative sensory testing, autonomic testing, and laser Doppler flowmetry (3–6). To date, few investigators have examined the relationships between different measures of neurophysiological function in diabetic and other peripheral neuropathies (4,7–10).
A cross-sectional study of diabetic neuropathy was used to determine the relationships among large, small, and autonomic fiber neurophysiological measures. We hypothesized that measures assessing similar neurophysiological functions would correlate and cluster together along typical nerve fiber subtypes (small, large, or autonomic). In addition, we determined the sensitivity and specificity of these neurophysiological measures using the Neuropathy Disability Score (NDS) as a gold standard (11). Because this clinical measure is weighted toward large fiber assessment, we hypothesized that neurophysiological tests of large fiber function would have higher sensitivity and specificity than measures of small fiber or autonomic function.
RESEARCH DESIGN AND METHODS
We studied a total of 130 subjects: 25 healthy subjects without diabetes and 105 subjects with diabetes. The study protocol was approved by the Beth Israel Deaconess Medical Center Institutional Review Board, and all subjects gave their informed consent. Exclusion criteria included symptomatic peripheral arterial disease, congestive heart failure, cardiac arrhythmias, stroke, end-stage renal failure, uncontrolled hypertension, severe hyperlipidemia, chronic liver disease, or other chronic medical condition requiring ongoing active treatment. All subjects were studied at a single institution, with the same examiners at each visit. Each test was administered by a trained technician, in a random testing order, without knowledge of other test results to reduce bias. Detailed anthropomorphic measurements were taken at each visit, and peripheral venous blood was sent for routine hematology and chemistry tests (including complete hepatic, renal, and other metabolic testing panels) under fasting conditions.
Symptoms and examination
Subjects completed the Neuropathy Symptom Score (NSS) questionnaire (12) and had physical examinations quantified by the NDS (11,13). In brief, the NDS grades neuropathy from scores of 0 (no neuropathy) to 28 (severe neuropathy). Reflexes are graded at the knee and ankle for a maximum of eight points if areflexic. Sensory tests include pinprick sensation, light touch, vibration, and temperature perception. A score is given according to the anatomic location at which the patient can identify the introduced stimulus. If the patient perceives the stimulus at all levels, a score of 0 is given. A score of 1 is given if the patient fails to perceive the stimulus at the base of the toe, 2 at the midfoot, 3 at the heel, 4 at the lower leg, and 5 if at or above the knee level. The average score of both feet is entered as the sensory score. An NDS score of 0–2 was defined as “no neuropathy,” scores ≥3 were considered to indicate “neuropathy.” The NDS score was used as the gold standard against which other tests were compared. A set of 12 Semmes-Weinstein monofilaments were used (ranging from 2.83 to 10 g; Stoelting, Wood Dale, IL) to determine the cutaneous perception threshold at the plantar surface of both great toes. Starting with the 2.83 g, each monofilament was applied a single time to the plantar surface of the great toe, held for 1.5 s, and removed. The monofilament weight was increased until the subject reported detecting the monofilament. This testing was repeated for both legs.
Quantitative sensory testing
Thermal testing was performed using a Medoc TSA-II thermal and vibratory analyzer (WR Medical Electronics, Stillwater, MN). The method of limits was used for detection of 1) warmth, 2) cool, 3) heat pain, 4) cold pain on the right thenar eminence of the hand and dorsum of the foot, and 5) vibration detection on the right thumb and right great toe (14,15). In brief, three repetitions of the same stimulus were applied with standard instructions with maximal heat stimulation of 50°C and cold of 0°C to avoid cutaneous injury. If response variability was >10%, subjects were given a break before retesting.
Nerve conduction studies
Nerve conduction studies were performed using a Viking IIIP electromyograph (Viasys Healthcare, Madison, WI) by the same trained technician for all patients. Peroneal and sural nerve conduction studies were performed on the left leg using standard methodologies (1). Results of the peroneal motor conduction velocity, peroneal compound muscle action potential, sural sensory conduction velocity, and sural sensory nerve action potential (SNAP) were reported for all individuals. Individuals without recordable responses were assigned amplitudes of 0 and the lowest measured velocity and longest distal latency of any subject.
Autonomic testing
Subjects had continuous beat-to-beat blood pressure and electrocardiography monitoring during autonomic testing. Cardiovagal function was assessed by the heart rate response to deep respiration and the heart rate response to the Valsalva maneuver (16). Sympathetic adrenergic function was measured by the blood pressure response during phase 2 and phase 4 of the Valsalva maneuver, and the hemodynamic response to passive 60° upright tilt for 10 min (16,17).
Laser Doppler flowmetry
After 20 min of adjustment to the ambient temperature (23–24°C), the blood flow responses to iontophoresis of 1% acetylcholine chloride solution were assessed at the volar surface of the forearm and at the dorsum of the foot with two single point laser Doppler probes and a DRT4 laser Doppler blood flow monitor (Moor Instruments, Millwey, Devon, U.K.) using standard protocols (18,19). The baseline blood flow was measured for 40 s, followed by 60 s of iontophoresis at 200 μA. The vasodilatory response was measured for 90 s after iontophoresis in endothelial-dependent (direct blood flow) and -independent (axon reflex–mediated blood flow) regions.
Statistical analysis
Results are expressed as means ± SD. Variables were compared by ANOVA with Tamhane T2 post hoc tests. Receiver operator characteristic (ROC) curves were generated for each test to determine sensitivity and specificity versus the clinical diagnosis of neuropathy (NDS >2). Groups of subjects (independent of the diagnosis of diabetes and neuropathy) with similar responses to neurophysiological variables and physical examination findings were categorized using two-step hierarchical cluster analysis. Clustering was performed using the Schwarz Bayesian information criterion with log-likelihood measures for probability distribution of variables. The contribution of each variable to the likelihood of cluster assignment was determined. Dendrograms of the cluster linkage between variables were calculated using the Ward method, with z scores calculated for each variable. Statistical analysis was performed using SPSS (version 15.0; SPSS, Chicago, IL).
RESULTS
Demographics
The basic demographic information for subjects is shown in Table 1. A total of 25 healthy control subjects, 47 subjects with diabetes without neuropathy, and 58 subjects with diabetes and neuropathy (NDS >2) were included. Subjects with diabetic neuropathy were older and had higher systolic blood pressures that those without neuropathy or healthy control subjects (P < 0.01). In addition, all subjects with diabetes (with and without neuropathy) had lower cholesterol and lower LDL cholesterol levels than the healthy control subjects (P < 0.01). There were no differences in BMI, weight, height, or sex between groups. Diabetic subjects with and without neuropathy had no difference in A1C or duration of diabetes. Control subjects had lower body weights and lower BMI and were younger than diabetic subjects, although only age was statistically different from subjects with diabetes and neuropathy.
Table 1.
Subject demographics and characteristics
| Healthy control | Diabetes with no neuropathy | Diabetes with neuropathy | |
|---|---|---|---|
| Age (years) | 49 ± 16.6 | 52.3 ± 15.8 | 63.7 ± 9.5*† |
| Sex (male/female) | 12/13 | 20/26 | 2326 |
| BMI (kg/m2) | 28.2 ± 6.2 | 32.4 ± 8.6 | 31.2 ± 6.3 |
| Height (m) | 1.70 ± 0.09 | 1.68 ± 0.08 | 1.69 ± 0.10 |
| Weight (kg) | 82 ± 19 | 92 ± 23 | 89 ± 19 |
| Systolic blood pressure (mmHg) | 118 ± 12 | 122 ± 13 | 130 ± 16*† |
| Diastolic blood pressure (mmHg) | 73 ± 9 | 73 ± 8 | 73 ± 8 |
| Heart rate (bpm) | 66 ± 9 | 70 ± 10 | 69 ± 11 |
| Diabetes type (1/2) | NA | 33/14 | 37/11 |
| Duration diabetes (years) | NA | 13.1 ± 10.3 | 16.4 ± 11.9 |
| A1C (%) | 5.5 ± 0.3 | 7.2 ± 1.0* | 7.0 ± 0.9* |
| Total cholesterol (mmol/l) | 195 ± 31 | 160 ± 38* | 168 ± 40* |
| Triglycerides (mmol/l) | 107 ± 50 | 124 ± 94 | 135 ± 84 |
| HDL cholesterol (mmol/l) | 61 ± 17 | 57 ± 19 | 57 ± 14 |
| LDL cholesterol (mmol/l) | 114 ± 31 | 78 ± 31* | 86 ± 34* |
| NSS | 0.2 ± 0.5 | 0.7 ± 1.0 | 4.5 ± 3.7*† |
| NDS | 0.2 ± 0.5 | 0.4 ± 0.8 | 6.3 ± 5.3*† |
Data are means ± SD. Characteristics of the study groups were compared by one-way ANOVA with Tamhane T2 post hoc tests. Diabetes type and duration were analyzed by χ2 and unpaired t tests accordingly. NA, not applicable.
*P < 0.01 vs. healthy control subjects;
†P < 0.01 vs. diabetes without neuropathy.
Neurophysiological testing
The results of all neurophysiological tests by group are shown in Table 2. All tests of nerve conduction studies, 4 of 6 measures of autonomic function, and 9 of 11 tests of quantitative sensation differentiated diabetic subjects with neuropathy from those without. Tests of cutaneous blood flow did not reliably differentiate diabetic subjects with neuropathy from those without. As revealed in Table 2, every test differentiated healthy control subjects from individuals with diabetic neuropathy, except the change in systolic and diastolic blood pressures during tilt table testing, the pain detection thresholds in the upper limb, and the cutaneous blood flow changes (both endothelial dependent and axon reflex mediated) in the upper limb.
Table 2.
Neurophysiological function
| Healthy control | Diabetes with no neuropathy | Diabetes with neuropathy | ROC threshold | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Autonomic testing | ||||||
| Phase 2 Valsalva maneuver blood pressure (mmHg) | 1.80 ± 0.40 | 1.33 ± 0.71* | 0.73 ± 0.67*† | <2 | 89 | 57 |
| Valsalva ratio | 1.54 ± 0.23 | 1.50 ± 0.29 | 1.26 ± 0.21*† | <1.3 | 73 | 78 |
| Heart rate variability (bpm) | 16.3 ± 6.7 | 12.5 ± 5.9 | 8.2 ± 5.0*† | <9.6 | 73 | 74 |
| Valsalva phase 4 blood pressure overshoot | 1.92 ± 0.28 | 1.41 ± 0.75* | 0.86 ± 0.87*† | <2 | 70 | 67 |
| Fall in systolic blood pressure during tilt (mmHg) | 15.9 ± 13.9 | 14.9 ± 12.0 | 19.6 ± 16.4 | >16 | 52 | 53 |
| Change in heart rate during tilt (bpm) | 16.0 ± 8.5 | 14.6 ± 8.5 | 7.7 ± 13.7* | <12 | 35 | 39 |
| Nerve conduction studies | ||||||
| Peroneal amplitude (mV) | 6.7 ± 2.6 | 5.5 ± 2.6 | 3.5 ± 2.4*† | <4.5 | 73 | 78 |
| Sural amplitude (μV) | 10.7 ± 8.0 | 7.5 ± 6.5* | 3.4 ± 4.1*† | <4 | 68 | 70 |
| Peroneal velocity (m/s) | 47.7 ± 4.4 | 45.0 ± 4.9 | 42.0 ± 5.4*† | <44 | 64 | 72 |
| Peroneal distal latency (ms) | 4.7 ± 0.8 | 4.7 ± 0.6 | 5.0 ± 0.7*† | >4.5 | 61 | 53 |
| Sural velocity (m/s) | 47.4 ± 5.8 | 45.7 ± 6.3 | 42.6 ± 6.5*† | <43 | 59 | 70 |
| Sensory testing | ||||||
| Cold-pain foot (°C) | 6.4 ± 10.1 | 7.3 ± 9.3 | 2.1 ± 4.7*† | <10 | 89 | 29 |
| Heat-pain hand (°C) | 46.0 ± 4.5 | 45.5 ± 4.5 | 46.8 ± 3.2 | >45.1 | 80 | 41 |
| Cold detection foot (°C) | 25.7 ± 7.0 | 25.3 ± 6.4 | 17.2 ± 10.5*† | <25.5 | 77 | 70 |
| Monofilament threshold right (g) | 3.76 ± 0.4 | 3.8 ± 0.5 | 4.7 ± 1.3*† | >4.1 | 76 | 75 |
| Vibratory detection toe | 4.8 ± 7.4 | 5.2 ± 7.6 | 20.6 ± 26.0*† | >4.3 | 76 | 72 |
| Heat-pain foot (°C) | 47.4 ± 3.5 | 47.7 ± 2.5 | 49.0 ± 1.7*† | >48.3 | 69 | 62 |
| Heat detection hand (°C) | 34.3 ± 2.9 | 35.1 ± 1.9 | 36.7 ± 3.8*† | >34.5 | 69 | 49 |
| Heat detection foot (°C) | 39.1 ± 5.9 | 40.3 ± 4.2 | 44.4 ± 4.2*† | >43 | 67 | 72 |
| Vibratory detection thumb | 1.0 ± 0.5 | 2.0 ± 2.5* | 3.8 ± 6.3*† | >1.5 | 67 | 70 |
| Cold detection hand (°C) | 30.2 ± 2.0 | 31.1 ± 4.9 | 28.9 ± 3.7† | <30.2 | 61 | 72 |
| Cold-pain hand (°C) | 6.2 ± 6.5 | 7.7 ± 8.9 | 4.2 ± 6.9* | <3 | 61 | 54 |
| Cutaneous blood flow | ||||||
| Direct leg % change | 248 ± 285 | 202 ± 205 | 114 ± 159* | <125% | 77 | 60 |
| Direct forearm % change | 490 ± 435 | 372 ± 366 | 344 ± 314 | <315% | 55 | 50 |
| Axon reflex leg % change | 146 ± 212 | 150 ± 257 | 63 ± 74* | <75% | 69 | 49 |
| Axon reflex arm % change | 291 ± 354 | 412 ± 920 | 720 ± 3,370 | <220% | 71 | 43 |
Data are means ± SD or % for sensitivity and specificity. Characteristics of the study groups were compared by one-way ANOVA with Tamhane T2 post hoc tests. ROC curves were generated with the selected maximal sensitivity and specificity for each test shown, and test values for the selection are reported as the ROC threshold.
*P < 0.01 vs. healthy control subjects;
†P < 0.01 vs. diabetes without neuropathy.
Sensitivity and specificity
The tests with the highest sensitivity included the blood pressure fall during phase 2 of the Valsalva maneuver (89%) and the cold-pain detection threshold in the foot (89%). Tests with the highest specificity included the Valsalva ratio (78%), the peroneal amplitude (78%), and the monofilament detection threshold (75%). The tests with the highest overall sensitivity and specificity among each testing modality included the Valsalva ratio (73% sensitivity and 78% specificity) for autonomic studies, the peroneal compound muscle action potential amplitude (73 and 78%) for nerve conduction studies, monofilament detection threshold (76 and 75%) for sensory studies, and the direct blood flow change in the arm (77 and 60%) for cutaneous blood flow studies. The sensitivity and specificity for each test (against the NDS score), categorized by neurophysiological function is reported in Table 2.
Correlations between tests
The highest correlation within autonomic tests was the heart rate variability and the Valsalva ratio (r = 0.64). The highest correlation between an autonomic test and any other testing modality was the Valsalva ratio and the sural SNAP (r = 0.52). The highest correlation within nerve conduction studies was the sural SNAP amplitude and sural nerve conduction velocity (r = 0.73). The highest correlation with any other test was the sural SNAP amplitude and Valsalva ratio (r = 0.52). The highest correlation within sensory studies was the heat-pain detection threshold in the hand and the cold-pain detection threshold in the hand (r = 0.59). The highest correlation with any other test was the sural amplitude and the monofilament detection threshold (r = −0.44) and the cold detection in the foot with the blood pressure overshoot during phase 4 of the Valsalva maneuver (r = 0.44). The highest correlation within cutaneous blood flow testing was the direct and axon reflex–mediated blood flow in the leg (r = 0.34). The highest correlation with any other test was the heat-pain detection in the foot and the axon reflex–mediated blood flow in the leg (r = −0.29). A detailed matrix of the correlations between and within groups is reported in supplementary Tables 1–4, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc10-0763/DC1.
Cluster analysis
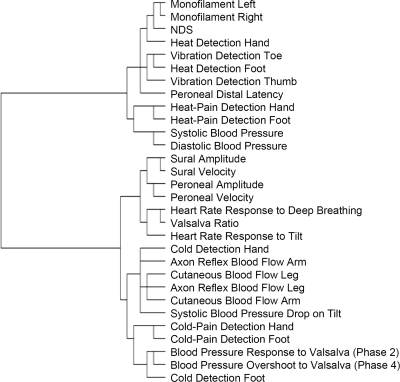
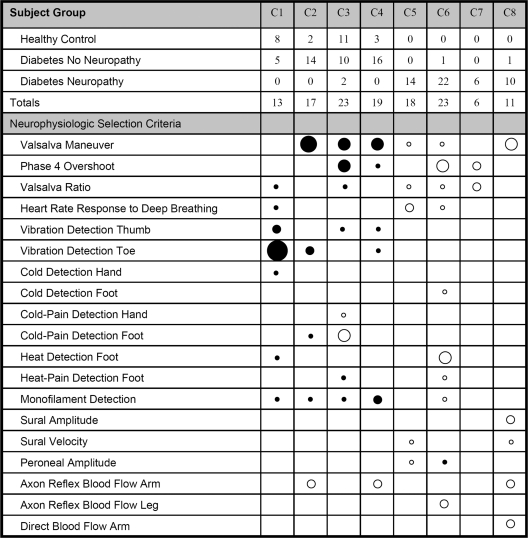
A two-step hierarchical cluster analysis demonstrates the relative proximities of each tested variable in the dendrogram of Fig. 1. The dendrogram identifies the tests that trend together; the most distal branch points reveal those tests that are most closely associated. When individual patients, de-identified from a diagnosis of neuropathy and diabetes, are assigned to clusters based solely on neurophysiological data, a total of eight clusters of subjects emerge. The numbers of control and diabetic subjects (with and without neuropathy) assigned to each cluster are shown in Fig. 2. Clusters 1 to 4 include all individuals (control and diabetic) without neuropathy, whereas clusters 5 to 8 include those with neuropathy. The contribution of each neurophysiological test toward cluster assignment is also shown in Fig. 2. The results indicate that relatively few tests are required to separate those individuals with neuropathy from those without.
Figure 1.
A dendrogram highlighting the association between tests using a two-step hierarchical cluster analysis. Tests that cluster more closely together, such as the monofilament of the left and right legs, reveal more similar test results. Many tests follow expected clustering, such as heat-pain detection in the hand and foot, sural amplitude and velocity, and systolic and diastolic blood pressures. Other tests that were expected to be more similar, such as vibration detection and nerve conduction studies, were not.
Figure 2.
This figure reports eight clusters (C1–C8) of individuals from the study. The numbers of healthy control and diabetic subjects with and without neuropathy are shown for each cluster. Clusters 1–4 are made up of healthy control subjects and subjects without neuropathy, whereas clusters 5–8 are made up of individuals with neuropathy. The tests that contributed to the formation of these clusters are listed in the lower portion of the table, with circle size demonstrating the relative weight of each test toward a particular cluster assignment: the larger the circle, the greater the weight. Black indicates a more normal (i.e., better) result, and white indicates a more abnormal (i.e., worse) result. For example, the large black circle on vibration detection at the toe indicates that a good result was the single most important factor in assigning individuals to cluster 1. Cluster 1 seems to be made up of individuals with entirely normal responses; clusters 2 and 4 are individuals with normal sensation and autonomic testing but some reduced vasomotor blood flow. Cluster 3 contains healthy individuals who have some decreased cold-pain detection. Cluster 5 indicates individuals with modest neuropathy across all neurophysiologic tests, while cluster 7 indicates those with autonomic neuropathy. Cluster 6 indicated significant neuropathy across all neurophysiologic tests with predominant “small nerve fiber” dysfunction, while cluster 8 indicates more “large nerve fiber” dysfunction.
CONCLUSIONS
We present the findings from a cross-sectional study of diabetic neuropathy and neurophysiology. In contrast to our hypothesis, our results indicate that typical measures of nerve fiber function do not necessarily correlate together and that traditional tests of large fiber neurophysiology (i.e., nerve conduction studies and vibration detection thresholds) do not have higher sensitivities and specificities than small fiber or autonomic tests compared with the NDS examination score (a large fiber–weighted examination score). Instead, our results suggest a more complex relationship between tests of autonomic, large, and small nerve fibers in diabetic neuropathy.
Our data also suggest that the use of a single quantifiable examination or neurophysiological measure does not adequately diagnose all presentations of diabetic neuropathy (5,20,21). To identify the specific tests that provide the greatest value in the differentiation of patients with neuropathy from those without neuropathy, we used cluster analysis, blinded to diabetes or neuropathy status, to group individuals with similar neurophysiological characteristics. Individual subjects were assigned to specific clusters by weighted calculations from each neurophysiological test. As outlined in Fig. 2, the actual number of neurophysiological variables that contribute to cluster formation is relatively small, and only a few tests are heavily weighted. The healthy subjects fall into the first four clusters, as do most of the diabetic individuals without clinical evidence of neuropathy. The last four clusters are made up predominantly of diabetic individuals that have clinical evidence of neuropathy. These data suggest that only a small number of neurophysiological tests are actually required to differentiate individuals with neuropathy from those without.
The dendrogram and cluster analysis did not support the traditional categorization of small, large, and autonomic nerve fibers by neurophysiological function testing. Correlation and cluster analysis suggested some unusual associations that we did not predict before study initiation. For example, as shown in the dendrogram of Fig. 1, both nerve conduction studies and vibration detection thresholds are considered measures of large, heavily myelinated nerve fibers; however, we found little association in the dendrogram between vibration detection and nerve conduction studies. We found a more distal branching on the dendrogram between nerve conduction studies and heat detection in the feet. These findings suggest a relationship that is more complex than a simple description of small, large, or autonomic neuropathy. Alternatively, these neurophysiological tests may not be specific for small, large, or autonomic nerve fiber subtypes. In the absence of pathological confirmation, we are unable to determine whether test variation is due to nerve dysfunction, demyelination, or axonal loss.
We did note that certain tests are more sensitive and specific for diagnosing the presence of neuropathy, whereas other tests seem to be better at identifying subjects without neuropathy. This observation is highlighted in Fig. 2, in which we demonstrate the relative importance of each specific test to cluster assignment. In cluster 1, a very low vibration detection threshold seems to be the single largest factor in identifying subjects without neuropathy. This result may be explained by the ability of subjects without neuropathy to quickly detect a stimulus using a method of limits approach (i.e., testing duration is brief). In subjects with neuropathy, the testing duration increases, as does response variability, because of the psychophysical nature of the method (22).
Some of the variability among techniques may also be explained by the psychophysical component of sensory tests. All measurements of small fiber sensory function require a subjective response from subjects and may be diminished in conditions of fatigue or illness. Other tests, such as laser Doppler flowmetry, are impartial measurements of small fiber function and provide results independent of subject attention. Theoretically, this gives laser Doppler flowmetry an advantage over sensory tests by providing a purely objective measurement of c-fiber function and the ability to detect early signs of neuropathy development.
We also noted a very interesting difference in baseline characteristics between groups: the total cholesterol and LDL cholesterol levels in patients with diabetes were lower than those in the healthy control subjects. Although these values reflect modern aggressive treatment of patients with diabetes, it is likely to have an impact on the natural history of the disease compared with an untreated state. However, aggressive treatment of all causative factors (smoking, blood pressure, lipids, and others) is now the standard of clinical care and will be important to consider in the expected outcomes of longitudinal studies of diabetes and neuropathy. In particular, the effects of lipid control on the development of small fiber neuropathy will be of interest (23–25).
In summary, the modest correlation coefficients seen among the different testing modalities suggest that these techniques measure different neurophysiological parameters and are therefore not interchangeable. In addition, the natural clustering of both patients and healthy control subjects into specific subgroups suggests that variations in the population will need to be taken into account when one is selecting subjects for specific studies.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke (grants K23-NS-50209 to C.H.G. and R01-NS-046710 to A.V.).
No potential conflicts of interest relevant to this article were reported.
C.H.G. research data, contributed to discussion, and wrote the manuscript. R.F. contributed to discussion and reviewed/edited the manuscript. A.V. researched data, contributed to discussion, and reviewed/edited the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Albers JW, Brown MB, Sima AA, Greene DA: Nerve conduction measures in mild diabetic neuropathy in the Early Diabetes Intervention Trial: the effects of age, sex, type of diabetes, disease duration, and anthropometric factors. Tolrestat Study Group for the Early Diabetes Intervention Trial. Neurology 1996;46:85–91 [DOI] [PubMed] [Google Scholar]
- 2.Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol 1995;38:869–880 [DOI] [PubMed] [Google Scholar]
- 3.Freeman R: Autonomic peripheral neuropathy. Lancet 2005;365:1259–1270 [DOI] [PubMed] [Google Scholar]
- 4.Hamdy O, Abou-Elenin K, LoGerfo FW, Horton ES, Veves A: Contribution of nerve-axon reflex-related vasodilation to the total skin vasodilation in diabetic patients with and without neuropathy. Diabetes Care 2001;24:344–349 [DOI] [PubMed] [Google Scholar]
- 5.Shy ME, Frohman EM, So YT, Arezzo JC, Cornblath DR, Giuliani MJ, Kincaid JC, Ochoa JL, Parry GJ, Weimer LH: Quantitative sensory testing: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2003;60:898–904 [DOI] [PubMed] [Google Scholar]
- 6.Vinik AI, Ziegler D: Diabetic cardiovascular autonomic neuropathy. Circulation 2007;115:387–397 [DOI] [PubMed] [Google Scholar]
- 7.Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A: Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 2000;23:606–611 [DOI] [PubMed] [Google Scholar]
- 8.Bril V, Ellison R, Ngo M, Bergstrom B, Raynard D, Gin H: Electrophysiological monitoring in clinical trials. Roche Neuropathy Study Group [see comments] Muscle Nerve 1998;21:1368–1373 [DOI] [PubMed] [Google Scholar]
- 9.Bril V, Kojic J, Ngo M, Clark K: Comparison of a neurothesiometer and vibration in measuring vibration perception thresholds and relationship to nerve conduction studies. Diabetes Care 1997;20:1360–1362 [DOI] [PubMed] [Google Scholar]
- 10.Caselli A, Spallone V, Marfia GA, Battista C, Pachatz C, Veves A, Uccioli L: Validation of the nerve axon reflex for the assessment of small nerve fibre dysfunction. J Neurol Neurosurg Psychiatry 2006;77:927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veves A, Uccioli L, Manes C, Van Acker K, Komninou H, Philippides P, Katsilambros N, De Leeuw I, Menzinger G, Boulton AJ: Comparison of risk factors for foot problems in diabetic patients attending teaching hospital outpatient clinics in four different European states. Diabet Med 1994;11:709–713 [DOI] [PubMed] [Google Scholar]
- 12.Veves A, Manes C, Murray HJ, Young MJ, Boulton AJ: Painful neuropathy and foot ulceration in diabetic patients. Diabetes Care 1993;16:1187–1189 [DOI] [PubMed] [Google Scholar]
- 13.Donaghue VM, Giurini JM, Rosenblum BI, Weissman PN, Veves A: Variability in function measurements of three sensory foot nerves in neuropathic diabetic patients. Diabetes Res Clin Pract 1995;29:37–42 [DOI] [PubMed] [Google Scholar]
- 14.Yarnitsky D: Quantitative sensory testing [see comments]. Muscle Nerve 1997;20:198–204 [DOI] [PubMed] [Google Scholar]
- 15.Hilz MJ, Stemper B, Axelrod FB, Kolodny EH, Neundörfer B: Quantitative thermal perception testing in adults. J Clin Neurophysiol 1999;16:462–471 [DOI] [PubMed] [Google Scholar]
- 16.Gibbons C, Freeman R: The evaluation of small fiber function—autonomic and quantitative sensory testing. Neurol Clin 2004;22:683–702, vii [DOI] [PubMed] [Google Scholar]
- 17.Low PA: Testing the autonomic nervous system. Semin Neurol 2003;23:407–421 [DOI] [PubMed] [Google Scholar]
- 18.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A: Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab 2009;94:2157–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veves A, Akbari CM, Primavera J, Donaghue VM, Zacharoulis D, Chrzan JS, DeGirolami U, LoGerfo FW, Freeman R: Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 1998;47:457–463 [DOI] [PubMed] [Google Scholar]
- 20.Dyck PJ, Litchy WJ, Daube JR, Harper CM, Dyck PJ, Davies J, O'Brien PC: Individual attributes versus composite scores of nerve conduction abnormality: sensitivity, reproducibility, and concordance with impairment. Muscle Nerve 2003;27:202–210 [DOI] [PubMed] [Google Scholar]
- 21.Kincaid JC, Price KL, Jimenez MC, Skljarevski V: Correlation of vibratory quantitative sensory testing and nerve conduction studies in patients with diabetes. Muscle Nerve 2007;36:821–827 [DOI] [PubMed] [Google Scholar]
- 22.Freeman R, Chase KP, Risk MR: Quantitative sensory testing cannot differentiate simulated sensory loss from sensory neuropathy. Neurology 2003;60:465–470 [DOI] [PubMed] [Google Scholar]
- 23.Gordon Smith A, Robinson Singleton J: Idiopathic neuropathy, prediabetes and the metabolic syndrome. J Neurol Sci 2006;242:9–14 [DOI] [PubMed] [Google Scholar]
- 24.Vincent AM, Hinder LM, Pop-Busui R, Feldman EL: Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst 2009;14:257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiggin TD, Sullivan KA, Pop-Busui R, Amato A, Sima AA, Feldman EL: Elevated triglycerides correlate with progression of diabetic neuropathy. Diabetes 2009;58:1634–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.