Cystic fibrosis–related diabetes (CFRD) is the most common comorbidity in people with cystic fibrosis (CF), occurring in ∼20% of adolescents and 40–50% of adults (1). While it shares features of type 1 and type 2 diabetes, CFRD is a distinct clinical entity. It is primarily caused by insulin insufficiency, although fluctuating levels of insulin resistance related to acute and chronic illness also play a role. The additional diagnosis of CFRD has a negative impact on pulmonary function and survival in CF, and this risk disproportionately affects women (2–4). In contrast to patients with other types of diabetes, there are no documented cases of death from atherosclerotic vascular disease in patients with CFRD, despite the fact that some now live into their sixth and seventh decades.
These guidelines are the result of a joint effort between the Cystic Fibrosis Foundation (CFF), the American Diabetes Association (ADA), and the Pediatric Endocrine Society (PES). They are intended for use by CF patients, their care partners, and health care professionals and include recommendations for screening, diagnosis, and medical management of CFRD. This report focuses on aspects of care unique to CFRD. A comprehensive summary of recommendations for all people with diabetes can be found in the ADA Standards of Medical Care, published annually in the January supplement to Diabetes Care (5).
METHODS
In 2009, CFF in collaboration with ADA and PES convened a committee of CF and diabetes experts to update clinical care guidelines for CFRD. Investigators at Johns Hopkins University conducted evidence reviews on relevant clinical questions identified by the guidelines committee. The reviews were provided to the committee to use in developing recommendations. Where possible, the evidence for each recommendation was considered and graded by the committee using the ADA (5) and the U.S. Preventive Services Task Force (USPSTF) (6) grading systems (Table 1). Recommendations from existing published guidelines were used when available and appropriate, and these are indicated as consensus statements. The committee also made consensus recommendations for topics not included in the evidence reviews or for which limited evidence was available in the literature. Recommendations will be updated as warranted by new evidence, and the guidelines will be reviewed 3 years after release date to determine if an update is needed. A summary of the committee's recommendations is presented in Table 2.
Table 1.
Evidence-grading system for clinical practice recommendations
| ADA classification system | |
|---|---|
| Level of evidence | Description |
| A | Clear evidence from well-conducted, generalizable, randomized, controlled trials that are adequately powered, including
|
| Compelling nonexperimental evidence, i.e., “all-or-none” rule developed by the Centre for Evidence-Based Medicine at Oxford | |
Supportive evidence from well-conducted, randomized, controlled trials that are adequately powered, including
|
|
| B | Supportive evidence from well-conducted cohort studies, including
|
| Supportive evidence from a well-conducted case-control study | |
| C | Supportive evidence from poorly controlled or uncontrolled studies, including
|
| Conflicting evidence with the weight of evidence supporting the recommendation | |
| E | Expert consensus or clinical experience |
| USPSTF recommendation classification system | ||||
|---|---|---|---|---|
| Estimate of effect | ||||
| Quality of evidence | Substantial | Moderate | Small | Zero/negative* |
| High | A | B | C | D |
| Moderate | B | B | C | D |
| Low | Insufficient (I) | |||
*A study with significant findings against something is given a grade of D.
Table 2.
Summary of recommendations for the clinical care of CFRD
Screening recommendations
|
Diagnosis recommendations
|
Management recommendations
|
Diabetes complications recommendations
|
SCREENING
CFRD is often clinically silent. In other populations, the primary consequences of unrecognized diabetes are macrovascular and microvascular disease. In CF, the nutritional and pulmonary consequences of diabetes are of greater concern. CFRD is associated with weight loss, protein catabolism, lung function decline, and increased mortality (2,3,7–17), and thus regular screening is warranted.
Screening tests for CFRD
Although hemoglobin A1C (A1C) may become the standard screening test for type 2 diabetes (5), the committee concluded that it is not sufficiently sensitive for diagnosis of CFRD and thus should not be used as a screening test. Eight studies were identified that assessed A1C as a screening test in this population (7,18–24). The authors of one prospective cohort study of 62 participants with CF and 107 healthy control subjects reported that A1C levels were higher in the CF group than among the control subjects, leading them to suggest that the use of A1C was appropriate (18). However, six studies (including two prospective cohort studies [7,21], two cross-sectional studies [19,20], one case-control study [23], and one case series [22[) with a total of 477 participants demonstrated low degrees of correlation between A1C and glucose tolerance status (7,19–23). Additionally, a cross-sectional study of 191 participants with CF demonstrated a low positive predictive value of the A1C test (24).
Use of A1C as a screening test for CFRD is not recommended. (ADA-B; USPSTF-D)
Fructosamine, urine glucose, and random glucose levels have low sensitivity in the CF population (20,23,25). Continuous glucose monitoring is not recommended as a screening tool because intermittent hyperglycemia detected in this fashion is not diagnostic for diabetes and there are no outcome data to determine its clinical significance. Fasting plasma glucose (FPG) identifies patients with CFRD with but not those without fasting hyperglycemia (FH), and thus this test will miss the diagnosis of diabetes in approximately half of CF patients (1). Self-monitoring of blood glucose (SMBG) with home meters is also not sufficiently accurate to screen for CFRD given that the International Organization for Standardization only requires that 95% of readings be within 20% of the actual glucose level (26).
Because of the poor performance of A1C and other tests, the oral glucose tolerance test (OGTT) is the screening test of choice for CFRD. Although it is an imperfect test due to the inherent variability of the test and the variability observed in individual CF patients over time, longitudinal studies demonstrate that a diabetes diagnosis by OGTT correlates with clinically important CF outcomes including the rate of lung function decline over the next 4 years (12), the risk of microvascular complications (27), and the risk of early death (1,2). In a multicenter, multinational study, the OGTT identified patients who benefited from insulin therapy (28).
The OGTT should be performed in the morning during a period of stable baseline health (at least 6 weeks since an acute exacerbation) using the World Health Organization protocol (5). Patients fast for at least 8 h (water is permitted) and should consume a minimum of 150 g (600 kcal) of carbohydrate per day for the preceding 3 days (generally not an issue because CF patients have high-calorie diets). The patient drinks a standard beverage containing 1.75 g/kg glucose (maximum 75 g) dissolved in water and sits or lies quietly for 2 h. Glucose levels are measured at baseline and 2 h. Unless the patient is experiencing classical symptoms of polyuria and polydipsia in the presence of a glucose level >200 mg/dl (11.1 mmol/l) or has two more diagnostic criteria for diabetes (such as both fasting and 2-h glucose elevation or a diabetes pattern on OGTT in the presence of an A1C level >6.5%), the test should be confirmed by repeat testing.
Screening for CFRD should be performed using the 2-h 75-g OGTT. (ADA-E; Consensus)
The age of screening for CFRD
Three studies with a total of 811 participants were identified that provided information about the appropriate age at which to start screening for CFRD (1,21,24). These studies—a retrospective cohort study (1), a prospective cohort study (21), and a cross-sectional study (24)—reported a significantly higher prevalence and incidence of CFRD beyond the first decade of life. Screening included both pancreatic sufficient and insufficient patients. The committee concluded that these findings suggest that annual screening for CFRD should start by age 10 years in all CF patients. Because clinical deterioration in nutritional and pulmonary status begins 6–24 months prior to a diagnosis of CFRD (29,30), early detection by annual screening is warranted.
Annual screening for CFRD should begin by age 10 years in all CF patients who do not have CFRD. (ADA-B; USPSTF-B)
Screening of CF patients during acute illness
CF patients experience frequent pulmonary exacerbations, some of which require treatment either in the hospital or at home with intravenous antibiotics. Treatment at times includes systemic glucocorticoids. In clinical experience, hyperglycemia that develops during acute illness occasionally resolves after a day or two of medical therapy, but usually lasts for at least 2–6 weeks. CF patients are frequently ill, and hyperglycemia returns with each subsequent bout of illness, often several times a year. Insulin deficiency and insulin resistance generally progress over time. Long-term microvascular (27) and pulmonary (1,2) outcomes correlate with duration of CFRD first diagnosed during acute illness, even with intervening periods of normal or impaired glucose tolerance (IGT).
During acute illness and/or a pulse of systemic glucocorticoid therapy, glucose levels should be monitored for at least the first 48 h, preferably fasting and 2 h postprandially. If glucose levels do not meet diagnostic criteria for CFRD, testing can be discontinued after 48 h. For patients receiving therapy at home, SMBG can be performed. However, SMBG levels are not sufficiently accurate to make a diagnosis of CFRD, and hyperglycemia should be confirmed by laboratory plasma glucose measurement.
CF patients with acute pulmonary exacerbation requiring intravenous antibiotics and/or systemic glucocorticoids should be screened for CFRD by monitoring fasting and 2-h postprandial plasma glucose levels for the first 48 h. If elevated blood glucose levels are found by SMBG, the results must be confirmed by a certified laboratory. (ADA-E; Consensus)
Screening of CF patients during continuous drip enteral feedings
Supplemental continuous drip feedings are commonly prescribed for malnourished CF patients. Although there are few data available specific to this situation, mid-feeding hyperglycemia may compromise efforts to gain weight. The Committee felt that glucose levels in the middle and immediately after the gastrostomy tube feeding should be measured in the hospital and at these same time points once a month at home using SMBG. SMBG levels are not sufficiently accurate to make a diagnosis of CFRD, and hyperglycemia detected by SMBG should be confirmed by laboratory plasma glucose measurement.
Screening for CFRD by measuring mid- and immediate postfeeding plasma glucose levels is recommended for CF patients on continuous enteral feedings, at the time of gastrostomy tube feeding initiation and then monthly at home. Elevated glucose levels detected by SMBG must be confirmed by a certified laboratory. (ADA-E; Consensus)
Screening CF patients who are pregnant or planning a pregnancy
Pregnancy is a state of marked insulin resistance, and many women with CF cannot produce the extra insulin required to meet this demand (31–33). In addition to the usual concerns about the effect of hyperglycemia on the fetus, diabetes can exacerbate the difficulties many women with CF have in achieving a positive protein balance and sufficient weight gain during pregnancy (32).
Women with CF not known to have CFRD who are contemplating pregnancy should be evaluated prior to conception to rule out preexisting CFRD or be tested immediately upon confirmation of the pregnancy if they have not had an OGTT in the previous 6 months. Because women with CF are at high risk for development of hyperglycemia during pregnancy (gestational diabetes mellitus), the 2-h 75-g OGTT should be performed at the end of both the first and second trimesters.
Women with CF who are planning a pregnancy or confirmed pregnant should be screened for preexisting CFRD with a 2-h 75-g fasting OGTT if they have not had a normal CFRD screen in the last 6 months. (ADA-E; Consensus)
Screening for gestational diabetes mellitus is recommended at both 12–16 weeks' and 24–28 weeks' gestation in pregnant women with CF not known to have CFRD, using a 2-h 75-g OGTT with blood glucose measures at 0, 1, and 2 h. (ADA-E; Consensus)
Screening for CFRD using a 2-h 75-g fasting OGTT is recommended 6–12 weeks after the end of the pregnancy in women with gestational diabetes mellitus (diabetes first diagnosed during pregnancy). (ADA-E; Consensus)
Screening CF patients undergoing transplantation
There is an almost universal requirement for insulin in the immediate critical care postoperative period in CF patients undergoing transplantation procedures, and many have long-term insulin requirements after transplantation (34–36). A diagnosis of CFRD prior to transplantation may increase complications of surgery and has a negative impact on survival, at least in the early postoperative period when infection, bleeding, and multiorgan failure are the most common causes of death (34,37). Aggressive management may have a positive impact on outcomes (35).
CF patients not known to have diabetes who are undergoing any transplantation procedure should be screened preoperatively by OGTT if they have not had CFRD screening in the last 6 months. Plasma glucose levels should be monitored closely in the perioperative critical care period and until hospital discharge. Screening guidelines for patients who do not meet diagnostic criteria for CFRD at the time of hospital discharge are the same as for other CF patients. (ADA-E; Consensus)
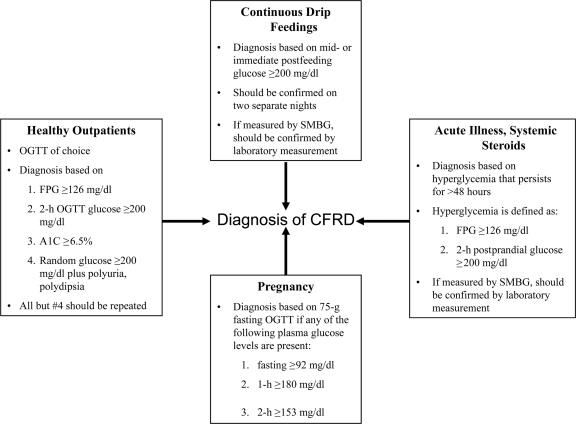
DIAGNOSIS (Fig. 1)
Figure 1.
Criteria for the diagnosis of CFRD under different conditions.
The spectrum of glucose tolerance abnormalities in CF
Diabetes is part of a continuum of glucose tolerance abnormalities defined by ADA (supplementary Table 1, available at http://care.diabetesjournals.org/cgi/content/full/dc10-1768/DC1). Few CF patients have truly “normal” glucose tolerance. Many patients with normal fasting and 2-h glucose levels have elevation in the middle of the OGTT (indeterminate glycemia [INDET]) or when assessed randomly or by continuous glucose monitoring. Impaired fasting glucose (IFG) (100–125 mg/dl [5.6–6.9 mmol/l[) may also be present (20,38). The clinical significance of IFG or INDET in CF is not known. In the general population, they are considered pre-diabetic conditions, associated with a high risk of future development of diabetes (39). In prepubertal children with CF both IGT and INDET are associated with early-onset CFRD (40).
Criteria for the diagnosis of CFRD in stable outpatients
ADA has established diagnostic criteria for diabetes that include specific fasting glucose levels, 2-h OGTT glucose levels (5), and A1C levels. They are based on the population risk of microvascular disease, and patients with CF are also at risk for these complications (27,41–43). The committee questioned whether the diagnostic thresholds should be lower for the CF population as CFRD is known to have a negative impact on CF pulmonary status (2,10,11), given that pulmonary disease is the chief morbidity in CFRD. Even less severe glucose tolerance abnormalities such as IGT are associated with lung function decline (12,17). However, sufficient outcome-based data are not available at present to determine whether more stringent diagnostic glucose thresholds more appropriately reflect risk for the CF population.
During a period of stable baseline health, the diagnosis of CFRD can be made in CF patients according to standard ADA criteria. Testing should be done on two separate days to rule out laboratory error unless there are unequivocal symptoms of hyperglycemia (polyuria and polydipsia); a positive FPG or A1C can be used as a confirmatory test, but if it is normal the OGTT should be performed or repeated. If the diagnosis of diabetes is not confirmed, the patient resumes routine annual testing. (ADA-E; Consensus)
2-h OGTT plasma glucose ≥200 mg/dl (11.1 mmol/l)
FPG ≥126 mg/dl (7.0 mmol/l)
A1C ≥6.5% (A1C <6.5% does not rule out CFRD because this value is often spuriously low in CF.)
Classical symptoms of diabetes (polyuria and polydipsia) in the presence of a casual glucose level ≥200 mg/dl (11.1 mmol/l)
Diagnosing CFRD during acute illness or continuous feedings
There are special situations when a diagnosis of CFRD must be considered in patients who are not in their baseline state of health. CF patients frequently first develop hyperglycemia during stressors such as acute illness or continuous enteral nutrition. Blood glucose levels may normalize when the stress is not present. In the past, this was called “intermittent CFRD” (44). Longitudinal outcome data have shown that CF morbidity and mortality are associated with CFRD first diagnosed in the acute illness setting when hyperglycemia has persisted beyond 48 h (1,2,27). Based on this experience, the committee developed the following recommendations.
The diagnosis of CFRD can be made in CF patients with acute illness (intravenous antibiotics in the hospital or at home, systemic glucocorticoid therapy) when FPG levels ≥126 mg/dl (7.0 mmol/l) or 2-h postprandial plasma glucose levels ≥200 mg/dl (11.1 mmol/l) persist for more than 48 h. (ADA-E; Consensus)
The diagnosis of CFRD can be made in CF patients on enteral continuous drip feedings when mid- or postfeeding plasma glucose levels exceed 200 mg/dl (11.1 mmol/l) on two separate days. (ADA-E; Consensus)
Gestational diabetes mellitus in CF
In the general population, the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study showed a continuous risk of adverse perinatal and maternal outcomes with increasing glycemia at 24–28 weeks' gestation (45), and a recent multicenter, randomized study has demonstrated that aggressive treatment of mild gestational diabetes mellitus improves outcomes (46).
Diagnosis of gestational diabetes mellitus should be made based on the recommendations of the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) (45) where diabetes is diagnosed based on 0-, 1-, and 2-h glucose levels with a 75-g OGTT if any one of the following is present:
FPG ≥92 mg/dl (5.1 mmol/l)
1-h plasma glucose ≥180 mg/dl (10.0 mmol/l)
2-h plasma glucose ≥153 mg/dl (8.5 mmol/l)
CF patients with gestational diabetes mellitus are not considered to have CFRD but require CFRD screening 6–12 weeks after the end of the pregnancy. (ADA-E; Consensus)
Differentiating CFRD with and without FH
The 1998 CFF CFRD Consensus Conference recommended that CFRD patients with and without FH (FH+ and FH-, respectively) be categorized separately because differences in their treatment needs were unknown (44). However, in a recent retrospective cohort study, 78 patients with CFRD FH- and 77 with CFRD FH+ were treated with insulin with similar positive effects on nutritional status and lung function (1). In addition, in a randomized controlled trial, insulin therapy reversed chronic weight loss in patients with CFRD FH- (28), suggesting that both groups of CFRD patients should receive insulin treatment and that there is no need to distinguish them diagnostically.
Distinguishing between CFRD with and without FH is not necessary. (ADA-B; USPSTF-D)
Date of onset of CFRD
Defining the date of onset of CFRD is important because long-term outcomes are related to disease duration. Glucose tolerance gradually worsens with age in CF as a result of steadily declining insulin production (1,47). At any point in time, however, an individual's glucose tolerance may acutely fluctuate depending on his or her general state of health.
The committee defined the onset of CFRD as the first time a patient meets diabetes diagnostic criteria. Longitudinal studies of patients whose date of diagnosis was considered to be either the first time they had a positive OGTT or the first time they had persistent hyperglycemia during acute illness have shown that duration of CFRD determined by these criteria correlates with clinically relevant outcomes including microvascular complications (27) and mortality (1,2). Hyperglycemia may resolve without treatment during periods of stable health, but insulin secretion remains insufficient to control glucose under stress, and hyperglycemia will recur.
Although in the general population critically ill patients who experience stress hyperglycemia are not given a diagnosis of diabetes, our recommendation differs for CF patients who develop hyperglycemia during acute exacerbations of their chronic illness. In CF, illness-associated hyperglycemia is a reflection of insulin insufficiency as well as resistance and is a recurrent event. Defining the disease by this criterion encourages early intervention to improve long-term outcomes.
The onset of CFRD should be defined as the date a person with CF first meets diagnostic criteria, even if hyperglycemia subsequently abates. (ADA-E; Consensus)
MANAGEMENT OF CFRD
The care team
As per ADA guidelines, CFRD should be managed by a multidisciplinary team of health professionals with expertise in CF and diabetes (5). The diabetes team should be intimately familiar with CFRD, recognizing differences between this and type 1 and type 2 diabetes pathophysiology and treatment. Good communication between diabetes and CF care providers is essential. Poor team communication and inadequate or conflicting information from health care providers have been identified as significant sources of stress for patients with CFRD (48).
Although there are few CF-specific data, it has been well established in the general diabetes population that patients must be given the educational tools and support they need to assume a central role in determining their treatment goals and implementing the management plan (5). Initial and ongoing diabetes self-management education (DSME) is an integral component of care. In addition to medical issues, the role of the patient-centered medical team is to encourage and support the patient and family. The treatment team should address psychosocial issues and recognize the risk of depression. Emotional well-being is strongly correlated with diabetes outcomes, and the additional diagnosis of diabetes can be a significant burden. There may also be financial concerns associated with this diagnosis.
Patients with CFRD should ideally be seen quarterly by a specialized multidisciplinary team with expertise in diabetes and CF. (ADA-E; Consensus)
Patients with CFRD should receive ongoing DSME from diabetes education programs that meet national standards for DSME. (ADA-E; Consensus)
Medical therapy
Patients with CFRD are insulin insufficient, and based on available data, insulin is the only recommended treatment. There is evidence that CF patients on insulin therapy who achieve glycemic control demonstrate improvement in weight, protein anabolism, pulmonary function, and survival. Ten studies (with a total of 783 participants) were identified that addressed insulin therapy in CFRD, including one randomized controlled trial (28), five before-after studies (49–53), one retrospective cohort study (1), one prospective cohort study (54), and two case-control studies (29,30). These studies reported improved outcomes associated with the use of insulin in patients with CFRD, including those without FH. Reported outcomes included improved lung function (five studies) (29,30,49,51,54), improved nutritional status (seven studies), (28,29,49–53), improved blood glucose/A1C control (two studies) (50,53), decreased pulmonary exacerbation rates (one study) (49), and decreased mortality (one study) (1).
There is little evidence regarding the superiority of specific insulin regimens in CFRD, and clinical judgment should be used to choose the best regimen for each patient. CFRD FH+ is usually treated with standard basal-bolus insulin regimens, including a combination of basal and rapid-acting insulin by multiple daily subcutaneous injections, or rapid-acting insulin by continuous subcutaneous infusion (insulin pump) (1,50,54,55). CFRD patients still have endogenous insulin secretion, and, except during acute illness, treatment is often similar to that of patients with type 1 diabetes in the honeymoon period. During acute illness or systemic glucocorticoid treatment, insulin requirements steeply rise, two- to fourfold. Once the illness resolves, it generally takes about 4–6 weeks for insulin requirements to gradually return to baseline. Careful monitoring for hypoglycemia is required during this period. Specific insulin treatment suggestions are presented in supplementary Table 2.
At the time of the last consensus conference (44), it was not clear whether CFRD patients without FH should receive insulin treatment. A recently completed trial demonstrated that treatment with premeal rapid-acting insulin was able to reverse chronic weight loss in this population, and thus insulin therapy is indicated (28). Whether basal insulin therapy alone (54) or basal-bolus insulin therapy would be as beneficial as premeal insulin alone in CFRD FH- remains to be determined.
The available data suggest that oral agents are not as effective as insulin in CFRD. Four studies (with a total of 153 participants) compared oral hypoglycemic therapy with insulin therapy in CFRD (14,28,56,57). These included one randomized controlled trial (28), one randomized controlled crossover trial (57), one prospective cohort study (56), and one retrospective cohort study (14). Oral hypoglycemic agents studied included sulfonylureas (e.g., glyburide), metformin, meglitinides (e.g., repaglinide), and thiazolidinediones. The two observational studies (14,56) reported no differences in lung function, nutritional status, or blood glucose/A1C control comparing those who received oral hypoglycemic agents with those who received insulin. However, two randomized studies suggested that oral hypoglycemic agents were not as effective as insulin in improving nutritional status (28), blood glucose/A1C control (28), and 2-h and 5-h insulin area under the curve (57). Potential CF-specific concerns associated with various noninsulin diabetes agents are presented in supplementary Table 3.
CF patients with CFRD should be treated with insulin therapy. (ADA-A; USPSTF-B)
Oral diabetes agents are not as effective as insulin in improving nutritional and metabolic outcomes in CFRD and are not recommended outside the context of clinical research trials. (ADA-A; USPSTF-D)
Management goals
ADA has established plasma glucose goals for people with diabetes (5). These are primarily based on the need to decrease the risk of microvascular complications and thus apply to CFRD with slight modifications (supplementary Table 4). Whether more stringent goals should be adopted for CF patients based on the relationship between hyperglycemia, nutrition, and pulmonary disease cannot be determined at present.
To safely achieve glucose goals, ADA recommends that all patients on insulin therapy perform SMBG at least three times daily (5). Continuous glucose monitoring has been validated in CF and may be useful for clinical management in some patients (58–60).
Patients with CFRD who are on insulin should perform SMBG at least three times a day. (ADA-E; Consensus)
Patients with CFRD should strive to attain plasma glucose goals as per the ADA recommendations for all people with diabetes, bearing in mind that higher or lower goals may be indicated for some patients and that individualization is important. (ADA-E; Consensus)
ADA considers A1C the primary target for glycemic control in type 1 and type 2 diabetes (5). Although A1C levels may be spuriously low in CF (7,20,21,23,27,59,61), they are generally higher in CF patients with CFRD compared with those with normal glucose tolerance or IGT, and elevated levels are associated with increased risk of microvascular complications (27). In one study of patients with >10-year duration CFRD, those with retinopathy and/or microalbuminuria had average A1C levels of 8.0% compared with 5.8% in CFRD patients with no eye or kidney changes, and 83% of those with microvascular complications had A1C levels ≥7.0% (27), consistent with data in the general diabetes population. For a given patient, the rise and fall in A1C may be a useful indicator of trends in glycemic control. Thus, regular monitoring of A1C is advised.
A1C measurement is recommended quarterly for patients with CFRD. (ADA-E; Consensus)
For most patients with CFRD, the A1C treatment goal is ≤7% to reduce the risk of microvascular complications, bearing in mind that higher or lower goals may be indicated for some patients and that individualization is important. (ADA-B; USPSTF-B)
Diet and exercise in CFRD
CF patients have nutrition requirements which are well established (62–64). Because adequate caloric intake to maintain BMI is critical to their health and survival, the additional diagnosis of CFRD does not alter usual CF dietary recommendations (Table 3). The goal is to achieve and maintain good nutritional status and normalize blood glucose levels.
Table 3.
Dietary recommendations for CFRD
| Nutrient | Type 1 and type 2 diabetes | CFRD |
|---|---|---|
| Calories | As needed for growth, maintenance, or reduction diets | 1.2–1.5 times DRI for age; individualized based on weight gain and growth |
| Carbohydrate | Individualized. Monitor carbohydrates to achieve glycemic control; choose from fruits, vegetables, whole grains and fiber-containing foods, legumes, and low-fat milk. Sugar alcohols and nonnutritive sweeteners are safe within U.S. Food and Drug Administration–established consumption guidelines. | Individualized. Carbohydrates should be monitored to achieve glycemic control. Artificial sweeteners should be used sparingly due to lower calorie content. |
| Fat | Limit saturated fat to <7% of total calories; intake of trans fat should be minimized; limit dietary cholesterol to <200 mg/day. Consume two or more servings per week of fish high in n-3 polyunsaturated fatty acids. | No restriction on type of fat. High fat necessary for weight maintenance. Aim for 35–40% total calories. |
| Protein | 15–20% of total calories; reduction to 0.8–1.0 g/kg with nephropathy | Approximately 1.5–2.0 times the DRI for age; no reduction for nephropathy |
| Sodium | <2,300 mg/day for blood pressure control | Liberal, high salt diet, especially in warm conditions and/or when exercising |
| Vitamins, minerals | No supplementation necessary unless deficiency noted | Routine supplementation with CF-specific multivitamins or a multivitamin and additional fat-soluble vitamins A, D, E, and K |
| Alcohol | If consumed, limit to a moderate amount; one drink per day for women and two or less drinks per day for men. | Consult with physician because of the higher prevalence of liver disease in CF and possible use of hepatotoxic drugs. |
| Special circumstances | ||
| Gestational diabetes mellitus | Restricted calories/carbohydrate for weight and blood glucose control | No calorie or carbohydrate restriction; adequate kcals for weight gain |
| IGT | Weight loss of 5–10% recommended; low-fat diet | No weight loss. Spread carbohydrates throughout the day; consume nutrient-dense beverages. |
DRI, daily recommended intake.
CF patients require a very high-calorie diet that is usually 120–150% of the daily recommended intake for age because they have both increased resting energy expenditure and increased loss of calories through malabsorption. Thus, calories should almost never be restricted. The need for high caloric intake, however, does not replace well-established principles of good nutrition and a healthy, well-balanced diet. A BMI ≥50th percentile for ages 2–20 years and for adults a BMI ≥22 kg/m2 for female subjects and ≥23 kg/m2 for male subjects is the goal for all persons with CF (64). The use of carbohydrate counting and insulin-to-carbohydrate ratios in conjunction with the usual CF diet to guide insulin therapy can help to optimize glycemic control.
CFF evidence–based guidelines for nutritional management are recommended for patients with CFRD. (ADA-E; Consensus)
Exercise is beneficial and is known to play a vital role in overall health. Most CF patients including those with severe pulmonary disease (<40% predicted FEV1) are capable of engaging in strength and aerobic exercise activities (65).
Patients with CFRD should be advised to do moderate aerobic exercise for at least 150 min per week. (ADA-E; Consensus)
COMPLICATIONS
Acute complications of CFRD
Acute complications of CFRD include hypoglycemia and, rarely, diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemic state. Because DKA is so uncommon (66), patients are not routinely taught to measure ketones and any CF patient with DKA should be screened for diabetes autoantibodies to rule out type 1 diabetes.
Hypoglycemia that is not severe (i.e., not requiring assistance from another individual) is common even in CF patients without CFRD. It occurs both in the fasting state, where it may reflect malnutrition and/or increased energy needs due to inflammation and infection, and postprandially, where it is related to delayed and disordered insulin secretion (67). Insulin-induced hypoglycemia can occur in CFRD as in any patient on insulin therapy, although severe hypoglycemia may be less common in CF (68). While CF patients do not have a good glucagon response to hypoglycemia (69), they have a brisk catecholamine response and normal hypoglycemia awareness. Hypoglycemia education including the use of glucagon is important for patients and their families. Regular SMBG, especially during unusual activity, diet changes, or illness is the best protection against insulin-induced hypoglycemia (5). Patients should be counseled regarding the hypoglycemic effects of alcohol and the risks of driving or operating machinery while hypoglycemic. They should be encouraged to exercise; however, they should be counseled to check their glucose level before vigorous physical activity and to potentially consume extra carbohydrate or alter their insulin dose, depending on the glucose level and the intensity and duration of the planned exercise.
Education about the symptoms, prevention, and treatment of hypoglycemia, including the use of glucagon, is recommended for patients with CFRD and their care partners. (ADA-E; Consensus)
Chronic complications of CFRD
Microvascular disease does not typically become clinically apparent in CFRD until patients have had the disease for at least 5 years and have developed FH (27,41,70). Tight glycemic control and treatment of microalbuminuria with ACE inhibitors or angiotensin receptor blockers combined with optimal control of hypertension delay progression of diabetic renal disease in the general diabetes population (5). They are assumed to also be beneficial in CFRD although there are no specific data in this population. ACE inhibitors are associated with development of cough in ∼10% of subjects, and this can occur months after drug initiation—a side effect with special significance in CF as increased cough is among the symptoms of a pulmonary exacerbation (71). Cough may also occur with angiotensin receptor blockers but is less frequent (∼1%).
Early diabetic nephropathy is characterized by microalbuminuria (a spot urine ACR of 30–299 μg/mg creatinine) (5). Macroalbuminuria (≥300 μg/mg creatinine) indicates clinically significant nephropathy that is progressing toward renal failure. A patient must demonstrate two out of three abnormal tests within a 3- to 6-month period to receive a diagnosis. Renal failure due solely to diabetes is uncommon in CF, but microalbuminuria has been reported to occur in 4–21% of individuals with CFRD (27,41,70). Recent strenuous exercise, fever, hypertension, congestive heart failure, infection, menstruation, and orthostatic proteinuria can result in a positive screen. It is therefore important to exclude other causes before concluding that microalbuminuria is CFRD related.
Diabetic retinopathy is seen in ∼10–23% of patients with CFRD and is seldom severe, although isolated severe cases have been reported (27,41,43,70,72). As in all persons with diabetes, dilated retinal exams are necessary in patients with CFRD to evaluate for the presence of retinopathy and the need for treatment.
Annual neurologic assessment and foot evaluation are recommended for the general diabetes population (5). Current data suggest that the severity of this microvascular complication may be less in CFRD (27). Gastroparesis is common in CF patients with and without CFRD, and the role that CFRD plays in aggravating this condition can be difficult to determine (27). Gastroparesis may make good glycemic control difficult to achieve.
Hypertension is not uncommon in adult CF patients, particularly after transplantation (41). Although atherosclerotic vascular disease has not been described in CF, hypertension is a known risk factor for diabetic kidney disease. As for all persons with diabetes, the recommended systolic and diastolic blood pressure goals are ≤130 mmHg and ≤80 mmHg, respectively, or <90th percentile for age and sex for pediatric patients (5). Hyperlipidemia is rare in CF but may occur, especially after transplantation or in pancreatic-sufficient individuals. While cholesterol levels are typically quite low, isolated triglyceride elevation has been noted. Because CF patients are at low risk for atherosclerotic cardiovascular disease, it is not clear that lipid elevation requires treatment in this population, and there are no data regarding the efficacy or safety of medical therapy for CF dyslipidemia. CFRD is not an autoimmune disease; thus, there is no increased risk of other autoimmune endocrinopathies.
Patients with CFRD should have their blood pressure measured at every routine diabetes visit as per ADA guidelines. Patients found to have systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥80 mmHg or >90th percentile for age and sex for pediatric patients should have repeat measurement on a separate day to confirm a diagnosis of hypertension. (ADA-E; Consensus)
Annual monitoring for microvascular complications of diabetes is recommended using ADA guidelines, beginning 5 years after the diagnosis of CFRD or, if the exact time of diagnosis is not known, at the time that fasting hyperglycemia is first diagnosed. (ADA-E; Consensus)
Patients with CFRD diagnosed with hypertension or microvascular complications should receive treatment as recommended by ADA for all people with diabetes, except that there is no restriction of sodium and, in general, no protein restriction. (ADA-E; Consensus)
An annual lipid profile is recommended for patients with CFRD and pancreatic exocrine sufficiency or if any of the following risk factors are present: obesity, family history of coronary artery disease, or immunosuppressive therapy following transplantation. (ADA-E; Consensus)
FUTURE RESEARCH CONSIDERATIONS
The CFRD Guidelines Committee identified the following as the most pressing research questions in CFRD: 1) Do nondiabetic CF patients with abnormal glucose tolerance benefit from diabetes therapy and, if so, what method of treatment has the greatest impact on nutritional and pulmonary status? 2) What are the obstacles to OGTT screening of the CF population and how can they best be overcome? 3) What are the mechanisms by which CFRD impacts pulmonary function and survival in CF? 4) Should target goals for glucose and/or A1C in CFRD differ from ADA target goals? 5) How can we assess and improve patient acceptance of the diagnosis of CFRD to improve diabetes self-management and psychosocial well-being?
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
APPENDIX
The CFRD Guidelines Committee: Paula Alexander, LCSW, CGC, CT (East Tennessee Children's Hospital, Knoxville, Tennessee); Robert J. Beall, PhD (CFF, Bethesda, Maryland); Dorothy Becker, MD (Children's Hospital of Pittsburgh, Pittsburgh, Pennsylvania); Carol Brunzell RD, LD, CDE (University of Minnesota Medical Center, Fairview, Minneapolis, Minnesota); Preston W. Campbell, III, MD (CFF, Bethesda, Maryland); Samuel J. Casella, MD (Children's Hospital at Dartmouth, Lebanon, New Hampshire); Melissa Chin, BA (CFF, Bethesda, Maryland); Richard C. Cohen, MD (Kaiser Permanente, Portland, Oregon) (Chair, Screening Subcommittee); Joan Finnegan Brooks, BA (Patient-Focused Market Research, Boston, Massachusetts); Cynthia George, MSN, FNP, BC (CFF, Bethesda, Maryland); Peter A. Gottlieb, MD (Barbara Davis Center, University of Colorado at Denver, Aurora, Colorado); Leslie Hazle, MS, RN, CPN (CFF, Bethesda, Maryland); Marcia Katz, MD (Baylor College of Medicine, Houston, Texas) (Chair, Diagnosis Subcommittee); M. Sue Kirkman, MD (ADA, Alexandria, Virginia); Bruce C. Marshall, MD (CFF, Bethesda, Maryland); Catherine McKeon, PhD (National Institutes of Health, Bethesda, Maryland); Antoinette Moran, MD (University of Minnesota, Minneapolis, Minnesota) (Committee Co-Chair); Gary Onady, MD, PhD (Wright State University Boonshoft School of Medicine, Dayton, Ohio) (Chair, Management Subcommittee); Karen A. Robinson, PhD (Johns Hopkins University, Baltimore, Maryland); Theresa Rodgers, RN, MSN, CRNP-AC/PC (University of Alabama, Birmingham, Irondale, Alabama); Kathryn A. Sabadosa, MPH (The Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, New Hampshire); Terri Schindler, MS, RD (Rainbow Babies and Children's Hospital, Cleveland, Ohio); Bonnie Slovis, MD (Vanderbilt University Medical Center, Nashville, Tennessee) (Committee Co-Chair); Arlene Stecenko, MD (Emory University School of Medicine, Atlanta, Georgia) (Chair, Complications Subcommittee); Mary E. Wood, RN, MS, CDE, BC-ADM (Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire); David Young, PharmD (Intermountain Cystic Fibrosis Adult Center, Salt Lake City, Utah).
Footnotes
Reviewed by the Professional Practice Committee, July 2010; accepted after revisions by the Executive Committee of the American Diabetes Association Board of Directors, September 2010; open for public comments on the Cystic Fibrosis Foundation Web site, July 2010; and endorsed by the Board of Directors of the Pediatric Endocrine Society, September 2010.
See accompanying articles, pp. 2660, 2677, 2716.
References
- 1.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W: Cystic fibrosis–related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009;32:1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milla CE, Billings J, Moran A: Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care 2005;28:2141–2144 [DOI] [PubMed] [Google Scholar]
- 3.Miller RJ, Tildesley HD, Wilcox PG, Zhang H, Kreisman SH: Sex disparities in effects of cystic fibrosis–related diabetes on clinical outcomes: a matched study. Can Respir J 2008;15:291–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sims EJ, Green MW, Mehta A: Decreased lung function in female but not male subjects with established cystic fibrosis-related diabetes. Diabetes Care 2005;28:1581–1587 [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association Clinical Practice Recommendations—2010. Diabetes Care 2010;33(Suppl. 1):S1–S100 [PubMed] [Google Scholar]
- 6.Sawaya GF, Guirguis-Blake J, LeFevre M, Harris R, Petitti D: Update on the methods of the U.S. Preventive Services Task Force: estimating certainty and magnitude of net benefit. Ann Intern Med 2007 2007;147:871–875 [DOI] [PubMed] [Google Scholar]
- 7.Bismuth E, Laborde K, Taupin P, Velho G, Ribault V, Jennane F, Grasset E, Sermet I, de Blic J, Lenoir G, Robert JJ: Glucose tolerance and insulin secretion, morbidity, and death in patients with cystic fibrosis. J Pediatr 2008;152:540–545 [DOI] [PubMed] [Google Scholar]
- 8.Cawood TJ, McKenna MJ, Gallagher CG, Smith D, Chung WY, Gibney J, O'Shea D: Cystic fibrosis related diabetes in adults. Irish Med J 2006;99:83–86 [PubMed] [Google Scholar]
- 9.Finkelstein SM, Wielinski CL, Elliott GR, Warwick WJ, Barbosa J, Wu SC, Klein DJ: Diabetes mellitus associated with cystic fibrosis. J Pediatr 1988;112:373–377 [DOI] [PubMed] [Google Scholar]
- 10.Koch C, Rainisio M, Madessani U, Harms HK, Hodson ME, Mastella G, McKenzie SG, Navarro J, Strandvik B: Investigators of the European Epidemiologic Registry of Cystic Fibrosis Presence of cystic fibrosis-related diabetes mellitus is tightly liked to poor lung function in patients with cystic fibrosis: data from the European Epidemiologic Registry of Cystic Fibrosis. Pediatr Pulmonol 2001;32:343–350 [DOI] [PubMed] [Google Scholar]
- 11.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ: Epidemiology of cystic fibrosis-related diabetes. J Pediatr 2005;146:681–687 [DOI] [PubMed] [Google Scholar]
- 12.Milla CE, Warwick WJ, Moran A: Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med 2000;162:891–895 [DOI] [PubMed] [Google Scholar]
- 13.Peraldo M, Fasulo A, Chiappini E, Milio C, Marianelli L: Evaluation of glucose tolerance and insulin secretion in cystic fibrosis patients. Horm Res 1998;49:65–71 [DOI] [PubMed] [Google Scholar]
- 14.Rosenecker J, Höfler R, Steinkamp G, Eichler I, Smaczny C, Ballmann M, Posselt HG, Bargon J, von der Hardt H: Diabetes mellitus in patients with cystic fibrosis: the impact of diabetes mellitus on pulmonary function and clinical outcome. Eur J Med Res 2001;6:345–350 [PubMed] [Google Scholar]
- 15.Schaedel C, de Monestrol I, Hjelte L, Johannesson M, Kornfält R, Lindblad A, Strandvik B, Wahlgren L, Holmberg L: Predictors of deterioration of lung function in cystic fibrosis. Pediatr Pulmonol 2002;33:483–491 [DOI] [PubMed] [Google Scholar]
- 16.White H, Pollard K, Etherington C, Clifton I, Morton AM, Owen D, Conway SP, Peckham DG: Nutritional decline in cystic fibrosis-related diabetes: the effect of intensive nutritional intervention. J Cyst Fibros 2009;8:179–185 [DOI] [PubMed] [Google Scholar]
- 17.Tofé S, Moreno JC, Máiz L, Alonso M, Escobar H, Barrio R: Insulin secretion abnormalities and clinical deterioration related to impaired glucose tolerance in cystic fibrosis. Eur J Endocrinol 2005;152:241–247 [DOI] [PubMed] [Google Scholar]
- 18.Hunkert F, Lietz T, Stach B, Kiess W: Potential impact of HbA1c determination on clinical decision making in patients with cystic fibrosis-related diabetes (Letter). Diabetes Care 1999;22:1008–1010 [DOI] [PubMed] [Google Scholar]
- 19.Franzese D, Valerio G, Buono P, Spagnuolo MI, Sepe A, Mozzillo E, De Simone I, Raia V: Continuous glucose monitoring system in the screening of early glucose derangements in children and adolescents with cystic fibrosis. J Pediatr Endocrinol Metab 2008;21:109–116 [DOI] [PubMed] [Google Scholar]
- 20.Elder DA, Wooldridge JL, Dolan LM, D'Alessio DA: Glucose tolerance, insulin secretion, and insulin sensitivity in children and adolescents with cystic fibrosis and no prior history of diabetes. J Pediatr 2007;151:653–658 [DOI] [PubMed] [Google Scholar]
- 21.Solomon MP, Wilson DC, Corey M, Kalnins D, Zielenski J, Tsui LC, Pencharz P, Durie P, Sweezey NB: Glucose intolerance in children with cystic fibrosis. J Pediatr 2003;142:128–132 [DOI] [PubMed] [Google Scholar]
- 22.Holl RW, Buck C, Babka C, Wolff A, Thon A: HbA1c is not recommended as a screening test for diabetes in cystic fibrosis. Diabetes Care 2000;23:126. [DOI] [PubMed] [Google Scholar]
- 23.Godbout A, Hammana I, Potvin S, Mainville D, Rakel A, Berthiaume Y, Chiasson JL, Coderre L, Rabasa-Lhoret R: No relationship between mean plasma glucose and glycated haemoglobin in patients with cystic fibrosis-related diabetes. Diabetes Metab 2008;34:568–573 [DOI] [PubMed] [Google Scholar]
- 24.Lanng S, Hansen A, Thorsteinsson B, Nerup J, Koch C: Glucose tolerance in patients with cystic fibrosis: a five year prospective study. BMJ 1995;311:655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yung B, Kemp M, Hooper J, Hodson ME: Diagnosis of cystic fibrosis-related diabetes: a selective approach in performing the oral glucose tolerance test based on a combination of clinical and biochemical criteria. Thorax 1999;54:40–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Organization for Standardization Requirements for in vitro blood glucose monitoring systems for self-testing in managing diabetes mellitus. ISO/TC 212/WG 3. Draft International Standard ISO/DIS 15197; Geneva, ISO, 2001 [Google Scholar]
- 27.Schwarzenberg SJ, Thomas W, Olsen TW, Grover T, Walk D, Milla C, Moran A: Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007;30:1056–1061 [DOI] [PubMed] [Google Scholar]
- 28.Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, Tullis E, Liou TG, Allen H: Cystic Fibrosis Related Diabetes Therapy Study Group Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: results of the Cystic Fibrosis Related Diabetes Therapy trial. Diabetes Care 2009;32:1783–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolon MA, Benali K, Munck A, Navarro J, Clement A, Tubiana-Rufi N, Czernichow P, Polak M: Cystic fibrosis-related diabetes mellitus: clinical impact of prediabetes and effects of insulin therapy. Acta Paediatr 2001;90:860–867 [PubMed] [Google Scholar]
- 30.Lanng S, Thorsteinsson B, Nerup J, Koch C: Diabetes mellitus in cystic fibrosis: effect of insulin therapy on lung function and infections. Acta Paediatrica 1994;83:849–853 [DOI] [PubMed] [Google Scholar]
- 31.Goss CH, Rubenfeld GD, Otto K, Aitken ML: The effect of pregnancy on survival in women with cystic fibrosis. Chest 2003;124:1460–1468 [DOI] [PubMed] [Google Scholar]
- 32.Hardin DS, Rice J, Cohen RC, Ellis KJ, Nick JA: The metabolic effects of pregnancy in cystic fibrosis. Obstet Gynecol 2005;106:367–375 [DOI] [PubMed] [Google Scholar]
- 33.McMullen AH, Pasta DJ, Frederick PD, Konstan MW, Morgan WJ, Schechter MS, Wagener JS: Impact of pregnancy on women with cystic fibrosis. Chest 2006;129:706–711 [DOI] [PubMed] [Google Scholar]
- 34.Bradbury RA, Shirkhedkar D, Glanveill AR, Campbell LV: Prior diabetes mellitus is associated with increased morbidity in cystic fibrosis patients undergoing bilateral lung transplantation: an “orphan” area? A retrospective case-control study. Intern Med J 2009;39:384–388 [DOI] [PubMed] [Google Scholar]
- 35.Hadjiliadis D, Madill J, Chaparro C, Tsang A, Waddell TK, Singer LG, Hutcheon MA, Keshavjee S, Elizabeth Tullis D: Incidence and prevalence of diabetes mellitus in patients with cystic fibrosis undergoing lung transplantation before and after lung transplantation. Clin Transplant 2005;19:773–778 [DOI] [PubMed] [Google Scholar]
- 36.Mekeel KL, Langham MR, Jr, Gonzalez-Perralta R, Reed A, Hemming AW: Combined en bloc liver pancreas transplantation for children with CF. Liver Transpl 2007;13:406–409 [DOI] [PubMed] [Google Scholar]
- 37.Madden BP, Hodson ME, Tsang V, Radley-Smith R, Khaghani A, Yacoub MY: Intermediate-term results of heart-lung transplantation for cystic fibrosis. Lancet 1992;339:1583–1587 [DOI] [PubMed] [Google Scholar]
- 38.Mueller-Brandes C, Holl RW, Nastoll M, Ballmann M: New criteria for impaired fasting glucose and screening for diabetes in cystic fibrosis. Eur Respir J 2005;25:715–717 [DOI] [PubMed] [Google Scholar]
- 39.Sosenko JM, Palmer MP, Rafkin-Mervis L, Krishcher JP, Cuthbertson D, Mahon J, Greenbaum CJ, Cowie CC, Skyler JS:; Diabetes Prevention Trial-Type 1 Study Group Incident dysglycemia and progression to type 1 diabetes among participants in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:1603–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ode KL, Frohnert B, Laguna T, Phillips J, Holmes B, Regelmann W, Thomas W, Moran AM: Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes. February2010[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen HU, Lanng S, Pressler T, Laugesen CS, Mathiesen ER: Cystic fibrosis-related diabetes: the presence of microvascular diabetes complications. Diabetes Care 2006;29:2660–2663 [DOI] [PubMed] [Google Scholar]
- 42.Lanng S, Thorsteinsson B, Lund-Andersen C, Nerup J, Schiøtz PO, Koch C: Diabetes mellitus in Danish cystic fibrosis patients: prevalence and late diabetic complications. Acta Paediatr 1994;83:72–77 [DOI] [PubMed] [Google Scholar]
- 43.Yung B, Landers A, Mathalone B, Gyi KM, Hodson ME: Diabetic retinopathy in adult patients with cystic fibrosis-related diabetes. Respir Med 1998;92:871–872 [DOI] [PubMed] [Google Scholar]
- 44.Moran A, Hardin D, Rodman D, Allen HF, Beall RJ, Borowitz D, Brunzell C, Campbell PW, Chesrown SE, Duchow C, Fink RJ, FitzSimmons SC, Hamilton N, Hirsch I, Howenstine MS, Klein DJ, Madhun Z, Pencharz PB, Quittner AL, Robbins MK, Schindler T, Schissel K, Schwarzenberg SJ, Stallings VA, Tullis DE, Zipf WB: Diagnosis, screening, and management of cystic fibrosis related diabetes mellitus: a consensus conference report. Diabetes Res Clin Pract 1999;45:61–73 [DOI] [PubMed] [Google Scholar]
- 45.HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA: Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 46.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM, Jr, Sciscione A, Catalano P, Harper M, Saade G, Lain KY, Sorokin Y, Peaceman AM, Tolosa JE, Anderson GB: Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lombardo F, De Luca F, Rosano M, Sferlazzas C, Lucanto C, Arrigo T, Messina MF, Crisafulli G, Wasniewska M, Valenzise M, Cucinotta D: Natural history of glucose tolerance, beta-cell function and peripheral insulin sensitivity in cystic fibrosis patients with fasting euglycemia. Eur J Endocrinol 2003;149:53–59 [DOI] [PubMed] [Google Scholar]
- 48.Collins S, Reynolds F: How do adults with cystic fibrosis cope following a diagnosis of diabetes? J Adv Nurs 2008;64:478–487 [DOI] [PubMed] [Google Scholar]
- 49.Mozzillo E, Franzese A, Valerio G, Sepe A, De Simone I, Mazzarella G, Ferri P, Raia V: One-year glargine treatment can improve the course of lung disease in children and adolescents with cystic fibrosis and early glucose derangements. Pediatric Diabetes 2009;10:162–167 [DOI] [PubMed] [Google Scholar]
- 50.Hardin DS, Rice J, Rice M, Rosenblatt R: Use of the insulin pump to treat cystic fibrosis-related diabetes. J Cyst Fibros 2009;8:174–178 [DOI] [PubMed] [Google Scholar]
- 51.Mohan K, Israel KL, Miller H, Grainger R, Ledson MJ, Walshaw MJ: Long-term effect of insulin treatment in cystic fibrosis-related diabetes. Respiration 2008;76:181–186 [DOI] [PubMed] [Google Scholar]
- 52.Rafii M, Chapman K, Stewart C, Kelly E, Hanna A, Wilson DC, Tullis E, Pencharz PB: Changes in response to insulin and the effects of varying glucose tolerance on whole-body protein metabolism in patients with cystic fibrosis. Am J Clin Nutr 2005;81:421–426 [DOI] [PubMed] [Google Scholar]
- 53.Hayes DR, Sheehan JP, Ulchaker MM, Rebar JM: Mangement dilemmas in the individual with cystic fibrosis and diabetes. J Am Diet Assoc 1994;94:78–80 [DOI] [PubMed] [Google Scholar]
- 54.Franzese A, Spagnuolo MI, Sepe A, Valerio G, Mozzillo E, Raia V: Can glargine reduce the number of lung infections in patients with cystic fibrosis-related diabetes? Diabetes Care 2005;28:2333. [DOI] [PubMed] [Google Scholar]
- 55.Grover P, Thomas W, Moran A: Glargine versus NPH insulin in cystic fibrosis related diabetes. J Cyst Fibros 2008;7:134–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Onady GM, Langdon LJ: Insulin versus oral agents in the management of cystic fibrosis related diabetes: a case based study. BMC Endocr Disord 2006;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moran A, Phillips J, Milla C: Insulin and glucose excursion following premeal insulin lispro or repaglinide in cystic fibrosis-related diabetes. Diabetes Care 2001;24:1706–1710 [DOI] [PubMed] [Google Scholar]
- 58.Dobson L, Sheldon CD, Hattersley AT: Validation of interstitial fluid in continuous glucose monitoring in cystic fibrosis (Letter). Diabetes Care 2003;26:1940–1941 [DOI] [PubMed] [Google Scholar]
- 59.Moreau F, Weiller MA, Rosner V, Weiss L, Hasselmann M, Pinget M, Kessler R, Kessler L: Continuous glucose monitoring in cystic fibrosis patients according to the glucose tolerance. Horm Metab Res 2008;40:502–506 [DOI] [PubMed] [Google Scholar]
- 60.Jefferies C, Solomon M, Perlman K, Sweezey N, Daneman D: Continuous glucose monitoring in adolescents with cystic fibrosis. J Pediatr 2005;147:396–398 [DOI] [PubMed] [Google Scholar]
- 61.Mansour KM: Investigation of screening methods for impaired glucose control in children with cystic fibrosis. TSMJ 2000;1:7–11 [Google Scholar]
- 62.Borowitz D, Baker RD, Stallings V: Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 2002;35:246–259 [DOI] [PubMed] [Google Scholar]
- 63.Brunzell C, Schwarzenberg SJ: CFRD and abnormal glucose tolerance: overview and medical nutrition therapy. Diabetes Spectr 2002;15:124–127 [Google Scholar]
- 64.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H: Clinical Practice Guidelines on Growth and Nutrition Subcommittee, Ad Hoc Working Group, for the Clinical Practice Guidelines on Growth and Nutrition Subcommittee Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systemic review. J Am Diet Assoc 2008;108:832–839 [DOI] [PubMed] [Google Scholar]
- 65.Flume PA, Robinson KA, O'Sullivan BP, Finder JD, Vender RL, Willey-Courand DB, White TB, Marshall BC: Clinical Practice Guidelines for Pulmonary Therapies Committee Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care 2009;54:522–537 [PubMed] [Google Scholar]
- 66.Swartz LM, Laffel LM: A teenage girl with cystic fibrosis-related diabetes, diabetic ketoacidosis, and cerebral edema. Pediatr Diabetes 2008;9:426–430 [DOI] [PubMed] [Google Scholar]
- 67.Battezzati A, Battezzati PM, Costantini D, Seia M, Zazzeron L, Russo MC, Daccò V, Bertoli S, Crosignani A, Colombo C: Spontaneous hypoglycemia in patients with cystic fibrosis. Eur J Endocrinol 2007;156:369–376 [DOI] [PubMed] [Google Scholar]
- 68.Tierney S, Webb K, Jones A, Dodd M, McKenna D, Rowe R, Whitehouse J, Deaton C: Living with cystic fibrosis-related diabetes or type 1 diabetes mellitus: a comparative study exploring health-related quality of life and patients' reported experiences of hypoglycaemia. Chronic Illn 2008;4:278–288 [DOI] [PubMed] [Google Scholar]
- 69.Moran A, Diem P, Klein DJ, Levitt MD, Robertson RP: Pancreatic endocrine function in cystic fibrosis. J Pediatr 1991;118:715–723 [DOI] [PubMed] [Google Scholar]
- 70.van den Berg JM, Morton AM, Kok SW, Pijl H, Conway SP, Heijerman HG: Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J Cyst Fibros 2008;7:515–519 [DOI] [PubMed] [Google Scholar]
- 71.Dykewicz MS: Cough and angioedema from angiotensin-converting enzyme inhibitors: new insights into mechanisms and management. Curr Opin Allergy Clin Immunol 2004;4:267–270 [DOI] [PubMed] [Google Scholar]
- 72.Sullivan MM, Denning CR: Diabetic microangiopathy in patients with cystic fibrosis. Pediatrics 1989;84:642–646 [PubMed] [Google Scholar]