Abstract
Earlier studies identified serglycin proteoglycan and its heparin chains to be important for storage and activity of mast cell proteases. However, the importance of serglycin for secretion and activity of mast cell proteases in response to parasite infection has been poorly investigated. To address this issue, we studied the effects on mast cell proteases in serglycin-deficient and wild type mice after peritoneal infection with the obligate intracellular parasite Toxoplasma gondii. In line with previous results, we found severely reduced levels of cell-bound mast cell proteases in both noninfected and infected serglycin-deficient mice. However, serglycin-deficient mice secreted mast cell proteases at wild type levels at the site of infection, and enzymatic activities associated with mast cell proteases were equally up-regulated in wild type and serglycin-deficient mice 48 h after infection. In both wild type and serglycin-deficient mice, parasite infection resulted in highly increased extracellular levels of glycosaminoglycans, including hyaluronan and chondroitin sulfate A, suggesting a role of these substances in the general defense mechanism. In contrast, heparan sulfate/heparin was almost undetectable in serglycin-deficient mice, and in wild type mice, it was mainly confined to the cellular fraction and was not increased upon infection. Furthermore, the heparan sulfate/heparin population was less sulfated in serglycin-deficient than in wild type mice indicative for the absence of heparin, which supports that heparin production is dependent on the serglycin core protein. Together, our results suggest that serglycin proteoglycan is dispensable for normal secretion and activity of mast cell proteases in response to peritoneal infection with T. gondii.
Keywords: Chondroitin Sulfate; Glycosaminoglycan; Heparan Sulfate; Heparin; Mast Cell; Parasite; CPA, Carboxypeptidase; Serglycin; mMCP-4; mMCP-6
Introduction
Serglycin (SG)3 is an intracellular proteoglycan (PG) predominantly expressed by hematopoietic cells. Previous studies in the serglycin-deficient (SG−/−) mouse strain (1) have demonstrated that the SGPGs are essential for correct storage of some bioactive substances in secretory granules in a variety of immune cells, e.g. granzyme B in cytotoxic T-cells (2), elastase in neutrophils (3), as well as platelet factor 4, β-thromboglobulin, and platelet-derived growth factor in platelets (4). In addition, SGPGs have been implicated in the extracellular transport of the cytotoxic complex comprising granzyme B and perforin (5), and in the regulation of cytokine function (6).
In mast cells (MCs), the negatively charged glycosaminoglycan (GAG) chains associated with SG are either heparin, a highly sulfated form of the heparan sulfate (HS) type of chains, or chondroitin sulfate (CS) (7). In murine connective tissue type MCs, where SG carries heparin (8, 9), serotonin and histamine were found to be highly dependent on SG for proper storage (10). Also the MC proteases, i.e. the chymase mMCP-4 (mouse mast cell protease), the elastase mMCP-5, the tryptase mMCP-6, and the mast cell carboxypeptidase A (MC-CPA) have shown strong dependence on SGPGs, i.e. the negatively charged GAGs (in particular heparin) attached, for proper granular storage (1, 8, 9, 11–14) and activation (15–19).
MC proteases are implicated in a variety of processes, e.g. in the degradation of toxins (20, 21), in the innate immune response to infection (22–25), and in the regulation of allergic airway inflammation (26). Moreover, MC proteases possibly provide a soluble link between the innate and adaptive immunity in vivo, as demonstrated by the reduced adaptive response of mMCP-4-deficient mice in experimental arthritis (27) and by the inability of mMCP-6 deficient mice to recruit eosinophils to skeletal muscle tissue in response to infection with Trichinella spiralis larvae (28). We recently reported that SG deficiency results in several age-dependent alterations in the lymphoid system of naïve mice, possibly as a result of the impaired storage of potent inflammatory mediators within secretory granules (29). However, the importance of SGPGs for regulation of MC proteases in different inflammatory reactions is only partly understood. Especially the functional interplay between SGPGs and the MC proteases upon infection has not been studied in detail. To gain further insight into this issue, we studied the dependence of protease release and activity in the SG-deficient mice in response to the obligate intracellular parasite Toxoplasma gondii.
Intraperitoneal infection induced MC recruitment and release of substantial levels of active MC proteases, i.e. mMCP-4 with chymotrypsin-like activity, mMCP-5 with elastase-like activity, mMCP-6 with trypsin-like activity, and MC-CPA with zinc-dependent exoproteolytic activity, also in SG−/− mice. This suggests that although SG is important for the correct storage of MC proteases in naïve resting MCs, it is not necessary for protease release and activity upon infection. In addition, a massive release of GAGs at the site of infection was found, and we speculate that the observed functional recovery of MC proteases in the SG−/− mice could be achieved by the increased levels of extracellular GAGs that might substitute for the absence of SGPGs.
EXPERIMENTAL PROCEDURES
Mice
Congenic SG-deficient (SG−/−) and wild type (SG+/+) C57BL/6 mice (1, 29) were bred and maintained at the National Veterinary Institute (SVA; Uppsala, Sweden). Experiments in this study were approved by the local ethics committee and performed on age- and sex-matched mice (6–10 weeks).
Parasites and Challenge Experiments
The virulent RH strain of T. gondii was used for all experimental infections and for preparation of STag (soluble T. gondii antigen). Parasites were propagated in Vero cells as described previously (30), and tachyzoites were harvested 3 days after inoculation when the Vero cell monolayer was destroyed. For preparation of STag, tachyzoites were passed through Percoll (31) to remove cell debris, and the water-soluble fraction was collected after repeated freeze-thawing, sonication, and centrifugation at 3500 × g for 20 min to remove insoluble material. For inoculation of mice, tachyzoites were suspended in PBS. To study cellular influx, proinflammatory cytokine responses, and MC responses during the first 48 h, groups of SG+/+ and SG−/− mice (n = 3–5) were injected intraperitoneally (i.p.) with 106 tachyzoites in 0.5 ml of PBS, whereas in experiments lasting 8 days, 104 tachyzoites were given. Noninfected control mice of both genotypes received 0.5 ml of PBS, and at 12 h, 24 h, 48 h and 8 d post-infection, mice were killed by terminal anesthesia. In Fig. 1, results obtained from two or more experiments were pooled giving accumulated results from at least six animals per group and time point. Representative results from 1 to 4 animals of each genotype are shown in Figs. 2 to 7 and Table 1.
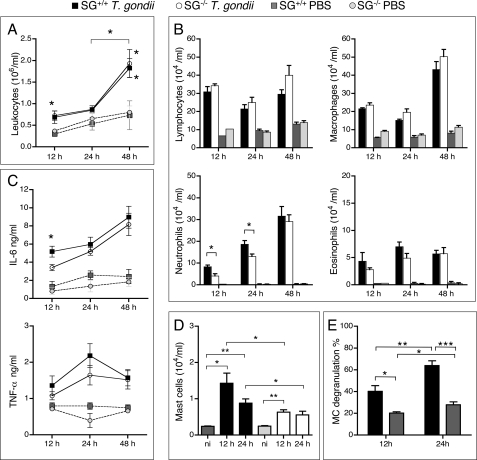
FIGURE 1.
Accumulation of leukocytes in T. gondii-infected SG+/+ and SG−/− mice. Peritoneal exudate cells were collected 12, 24, and 48 h after intraperitoneal inoculation of 106 RH strain tachyzoites or PBS and analyzed as described under “Experimental Procedures.” A, leukocyte recruitment into exudate fluids after infection of SG+/+ and SG−/− mice. B, differential counting of peritoneal leukocytes on cytospin slides stained with May-Grünwald Giemsa. C, levels of the proinflammatory cytokines IL-6 and TNF-α in peritoneal exudates were determined by ELISA. D, MCs were analyzed by counting CD117+ cells by flow cytometry. Samples from noninfected (ni) mice were used as controls. E, the proportion of MCs that were degranulated in infected and naïve SG+/+ mice were determined. Results are expressed as means ± S.E. Pooled results from at least two independent experiments are shown with n = 7, 10, and 12 for SG−/− mice, and n = 6, 10, and 11 for SG+/+ mice at 12, 24, and 48 h, respectively. *, p ≤ 0.05; **, p < 0.001; ***, p < 0.0001.
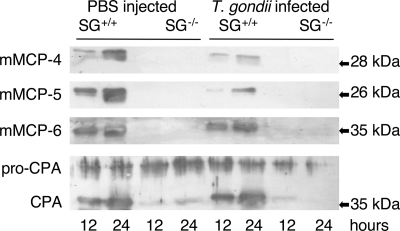
FIGURE 2.
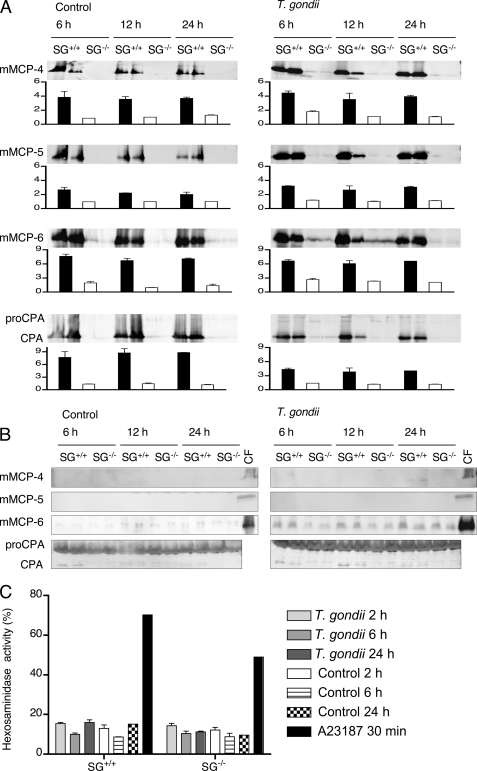
Defective storage of mast cell proteases in SG-deficient animals. Peritoneal cells from SG+/+ and SG−/− mice collected at 12 and 24 h after infection with T. gondii (T. gondii infected) were assayed by Western blots for the content of mMCP-4, mMCP-5, mMCP-6, and MC-CPA. PBS buffer was injected into control mice, which were analyzed in the same manner (PBS injected). A blot showing representative results from two time points per group from one of three independent experiments is shown.
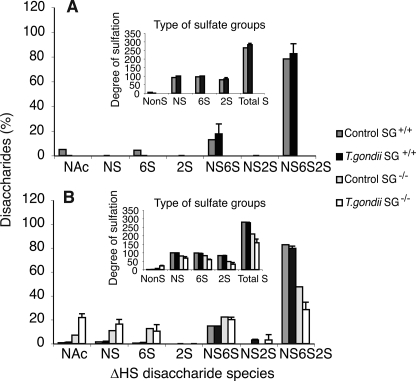
FIGURE 7.
HS/Heparin disaccharide analysis of peritoneal lavage fluids and cells from naïve and T. gondii infected mice. The HS/heparin pool of GAGs isolated (Fig. 5) was characterized for disaccharide components by extensive heparin lyase digestion as described under “Experimental Procedures” and analyzed by reversed phase ion pairing chromatography and fluorescent detection. Disaccharides were quantified against standard disaccharides and different species plotted as percent of total disaccharides recovered for the lavage (A) and cell fraction (B) of the low salt lavage. The disaccharide species created by lyase treatment are indicated: ΔHexA-GlcNAc (NAc), ΔHexA-GlcNS (NS), ΔHexA-GlcNAc6S (6S), ΔHexA2S-GlcNAc (2S), ΔHexA-GlcNS6S (NS6S), ΔHexA2S-GlcNS (NS2S), and ΔHexA2S-GlcNS6S (NS6S2S), where ΔHexA indicates a 4,5-unsaturated hexuronic acid created by action of the lyase, and S indicates a sulfate group in the indicated position of the respective sugar. In the insets, the overall number of nonsulfated (NonS), N-sulfated (NS), 6-O-sulfated (6S), and 2-O-sulfated (2S) disaccharides per 100 disaccharides and the overall degree of sulfation (TotalS) are indicated.
TABLE 1.
Protease activities in peritoneal exudates
Cell-free peritoneal exudates collected at indicated time points after intraperitoneal infection with T. gondii and from noninfected animals (n = 3) were assayed for chymotrypsin-, trypsin-, and CPA-like activities as described under “Experimental Procedures.”
| Time point/Mice | Chymotrypsin-like activity (substrate S-2586)a | Trypsin-like activity (substrate S-2288)a | Carboxypeptidase A-like activity (substrate M-2245)a |
|---|---|---|---|
| Noninfected | |||
| SG+/+ | 0.2 ± 0.1 | 13.6 ± 3.2 | ND |
| SG−/− | NDb | 1.0 ± 0.1 | ND |
| 12 h | |||
| SG+/+ | 0.8 ± 0.1 | 13.8 ± 0.5 | ND |
| SG−/− | 0.7 ± 0.5 | 21.2 ± 1.6 | ND |
| 24 h | |||
| SG+/+ | 0.4 ± 0.1 | 4.9 ± 1.1 | ND |
| SG−/− | 0.7 ± 0.2 | 15.6 ± 4.9 | ND |
| 48 h | |||
| SG+/+ | 0.9 ± 0.4 | 26.4 ± 7.1 | −3.4 ± 1.7 |
| SG−/− | 1.2 ± 0.4 | 31.0 ± 1.5 | −2.9 ± 0.8 |
| Day 8 | |||
| SG+/+ | 4.4 ± 0.9 | 32.7 ± 0.70 | −2.7 ± 0.8 |
| SG−/− | 7.6 ± 3.8 | 31.3 ± 0.30 | −5.0 ± 0.5 |
a Activities are expressed as mean change in absorbance (Δmilli OD/(min × ml of cell-free exudates) ± S.E.). Note that hydrolysis of the CPA substrate results in decreased absorbance.
b ND, not detected.
Differential Peritoneal Cell Counting
Peritoneal exudates were collected by peritoneal washing at 12 h, 24 h, 48 h, and 8 d with 5 ml of ice-cold PBS (pH 7.4). Volumes of recovered fluids and cell counts were recorded. After centrifugation and removal of the lavage fluid (which was kept for analysis of proteases and cytokines), the cells were resuspended in buffer (25 mm Tris, pH 7.4, 120 mm NaCl, 1 mm EDTA, and 0.05% BSA) and collected onto object glasses by cytospin (700 rpm for 5 min). May-Grünwald/Giemsa stained slides were mounted using VectaMount H-5000 (Vector Laboratories, Burlingame, CA). The number of lymphocytes, macrophages, neutrophils, eosinophils as well as the fraction of degranulated MCs was calculated by differential counting of at least 300 cells at a minimum of five different fields of each slide. Results are expressed as cells/ml or percent cells ± S.E.
Cytokine Analyses by ELISA
The cytokine levels of IL-6 and TNF-α were measured in cell-free peritoneal exudates by capture ELISA development kits (Peprotech EC) according to the manufacturer's instructions. Triplicates of each sample were measured at 405 nm in 96-well plates on a Titertek Multiscan spectrophotometer (Flow Laboratories).
Flow Cytometry
Peritoneal cells (0.5 × 106) were stained with a PE-conjugated CD117-specific antibody or with an isotype-matched control antibody (Immunotools, Friesoythe, Germany) as described (29) and counted on a Beckman Coulter flow cytometer.
Peritoneal Mast Cell Culture
Peritoneal cell-derived MCs (PCMCs) were generated as described (32), and cultured at 106 cells/ml in DMEM-Glutamax-1 (Invitrogen, GIBCO), 10% heat-inactivated fetal calf serum (Biotech Line AS), 8–10% of kit-ligand conditioned medium from CHO-KL cells (a kind gift of Odile Malbec and Marc Daeron), 50 μm β-mercaptoethanol, 1× minimum essential medium nonessential amino acids, and 50 μg/ml G418. In the SG+/+ cultures, >99% of the non-adherent cells were identified as MCs after 3 weeks based on metachromatic staining with toluidine blue. The SG-deficient PCMCs did not stain with toluidine blue but were considered as MCs based on morphological similarities with the SG−/− bone marrow-derived MCs described previously (1, 12). For stimulation experiments, 4-to-9-week-old nonadherent PCMC cultures were washed 2× in PBS. Triplicate cultures seeded in serum-free Tyrode's buffer, pH 7.4 (137 mm NaCl, 2.68 mm KCl, 0.42 mm NaH2PO4, 1.0 mm MgCl2, 2.0 CaCl, 11.9 mm NaHCO3, 5 mm glucose, 0.35% BSA) at 106 cells/ml were stimulated for 6 h, 12 h, 24 h, and 48 h with live T. gondii tachyzoites in a 1:1 parasite to cell ratio or with STag at a concentration of 20 μg/ml. Supernatants were collected for measurement of enzymatic activities and detection of proteases by Western blot analysis as described below. Stimulated and nonstimulated PCMCs were treated with TRIzol reagent (Invitrogen), and the resulting protein extracts were subjected to Western blotting. As a control for viability, cells were counted by Trypan blue exclusion (Sigma Aldrich).
Western Blot Analysis
Protein concentration of extracts from 106 PCMCs or 5 × 106 peritoneal exudate cells, concentrated cell-free peritoneal exudates, and cell-free supernatants from the in vitro-cultured PCMCs were determined by Bradford's protein assay (Bio-Rad). Ten μg of protein from each fraction were mixed with 3× SDS-PAGE sample buffer containing 5% β-mercaptoethanol, run on a 12% SDS-PAGE gel, and blotted to nitrocellulose membranes. The membranes were incubated with antisera against mMCP-4, MCP-5, mMCP-6, or MC-CPA (a kind gift from Lars Hellman and Gunnar Pejler) as described (1). The mean relative signal intensity of the bands appearing in the Western blots was determined using NIH ImageJ software. The results, expressed as mean relative density values minus background, are displayed in inset graphs.
Degranulation Assay
PCMCs (106) seeded in triplicates were left unstimulated or were stimulated for 2, 6, and 24 h with tachyzoites (in a 1:1 ratio) to induce degranulation. Stimulation with the calcium ionophore A23187 (2 μm, Sigma Aldrich) for 30 min was used as a positive control of MC degranulation. The level of degranulation was analyzed by measuring β-hexosaminidase release as described previously (12).
Analysis of Protease Activity in Peritoneal Exudates and PCMC Supernatants
From the control mice and the infected mice at least three cell-free peritoneal exudates per time point and genotype were analyzed. From the in vitro infection, three SG+/+ and SG−/− cell-free PCMC supernatants were collected. The samples were mixed with PBS, 2 m NaCl, 0.5% Triton X-100, and analyzed in triplicates. Twenty μl of a 1.8 mm solution of chromogenic substrate (in H2O) for either chymotrypsin-like proteases (S-2586), trypsin-like proteases (S-2288) (both from Chromogenix), or CPA (M-2245) (from Bachem) were added to 100 μl of the samples. As a negative control, 20 μl of the different substrates were added to 100 μl H2O. The absorbance was monitored at 405 nm with a Titertek Multiscan spectrophotometer (Flow Laboratories), and the reaction velocities were determined.
Isolation of Glycosaminoglycans from Peritoneal Exudates
Peritoneal cells and exudates collected by peritoneal lavage with a physiological salt solution (PBS, 0.15 m NaCl), followed by a high salt lavage (PBS, 1.5 m NaCl) were analyzed for GAG content. Isolation of GAGs was done according to the miniscale method described by Ledin et al. (33) with the exception that DEAE anion exchange columns were eluted with 3 m NaCl (to guarantee heparin release from these columns) followed by desalting on PD-5 columns (GE Healthcare). Separation of isolated HS and CS disaccharides after heparin lyase and chondroitinase treatment, respectively, was performed on a Phenomenex Luna 5 u column as described (33). Resulting peaks were quantified against known amounts of standard ΔHS and ΔCS disaccharides.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA). Significant differences between SG−/− and SG+/+ mice were determined using Mann-Whitney nonparametric test and p values ≤ 0.05 were considered significant. Results are presented as mean ± S.E. and calculated p values indicated as: not significant (ns) p > 0.05, significant *, ≤0.05, **, <0.001, ***, <0.0001.
RESULTS
Inflammatory Response in T. gondii Infected SG+/+ and SG−/− Mice
In line with previous studies (34, 35), intraperitoneal inoculation with T. gondii induced a significant leukocyte recruitment to the site of infection, and this was seen in both SG+/+ and SG−/− mice (Fig. 1A). Infected mice of both genotypes displayed an increased number of peritoneal lymphocytes, macrophages, neutrophils, and eosinophils, in comparison with naïve mice (Fig. 1B). However, neutrophil recruitment was significantly delayed in SG−/− mice (Fig. 1B), suggesting that attraction of these cells is, at least initially, dependent on the presence of SG. Furthermore, the levels of proinflammatory cytokines IL-6 and TNF-α in peritoneal exudates were increased in response to infection in both genotypes, although the level of IL-6 was significantly lower at the early time point in infected SG−/− mice as compared with infected SG+/+ mice (Fig. 1C).
It has been demonstrated that MCs are among the cell types recruited upon T. gondii infection (36), but the role of SGPGs in the recruitment and activation of MCs is not known. To address this issue, we compared numbers of MCs in SG+/+ versus SG−/− mice at different time points post-infection. Because SG−/− MCs are difficult to identify by conventional histochemistry, due to poor staining properties with cationic dyes, the number of cells expressing the MC surface receptor CD117 (c-kit) was analyzed. As expected from a previous study (29) noninfected SG−/− and SG+/+ mice had equal numbers of CD117-positive cells in the peritoneum (Fig. 1D). Twelve hours post-infection, the numbers of peritoneal CD117+ cells were significantly increased in both SG+/+ and SG−/− mice as compared with noninfected controls (Fig. 1D), thus supporting the notion that mast cells are recruited in response to T. gondii infection (36). However, the lower number of MCs in infected SG−/− mice relative to that in infected SG+/+ mice (Fig. 1D) suggests that MC recruitment in response to infection is slightly impaired in SG−/− animals. Notably, in SG+/+ mice, the fraction of degranulated MCs was significantly increased at 12 and 24 h after infection (Fig. 1E), indicating that MCs are activated in response to T. gondii infection in vivo.
Peritoneal Infection with T. gondii Induces Secretion of MC Proteases in Both SG+/+ and SG−/− Mice
The parasite-induced increase in MC numbers and in MC degranulation implicates a role for MC mediators in the host response to infection. MC-specific proteases constitute a major part of the released MC products, and we next examined the content of MC proteases in peritoneal cells and exudates collected from infected animals at various time points. In agreement with the previously reported dependence on SG for storage of MC proteases (1, 12, 37), SG−/− cells contained undetectable or minute amounts of mMCP-4, mMCP-5, mMCP-6, and mature MC-CPA, whereas large amounts were found in peritoneal cells from SG+/+ mice (Fig. 2). The slightly lower amounts of intracellular MC proteases in the cellular fraction from infected versus noninfected SG+/+ cells may reflect the release of proteases upon degranulation or could be due to normal individual variation.
Because T. gondii infection resulted in an increased MC degranulation (Fig. 1E), we next analyzed the extracellular levels of MC-specific proteases. Cell-free exudates from naïve noninfected animals of both genotypes had undetectable levels of mMCP-4 and mMCP-5 (Fig. 3, A and B), whereas mMCP-6 and the pro-form of MC-CPA were detected at low levels (Fig. 3, C and D). Twelve hours after T. gondii infection, mMCP-4 and mMCP-5 were only detectable in SG+/+ mice. At later time points, however, the expression of mMCP-5 was variable but seen in both genotypes, and the expression of mMCP-4 was increasing with time, resulting in substantial amounts of mMCP-4 in both SG+/+ and SG−/− mice by day 8 (Fig. 3, A and B).
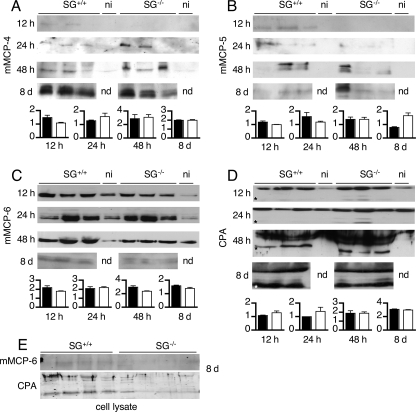
FIGURE 3.
Peritoneal challenge with T. gondii leads to MC protease secretion in SG-deficient animals. Cell-free peritoneal lavage fluid was collected from T. gondii-infected and noninfected (ni) animals at 12, 24, and 48 h and 8 days post-infection. The lavage fluids were concentrated, and equal amounts of protein were separated by SDS-PAGE and transferred to membranes. Western blots were developed with antibodies against the respective enzymes, and for each time point, the mean signal intensity minus background for the proteases is given as an inset graph as described under “Experimental Procedures.” Black bars represent SG+/+, and open bars represent SG−/−. Representative blots for mMCP-4 (A), mMCP-5 (B), mMCP-6 (C), and MC-CPA (D) from infected (n = 3) and noninfected (n = 1) SG+/+ and SG−/− mice are shown. E, Western blots for mMCP-6 and MC-CPA of cellular lysates 8 days post-infection. Asterisks indicate the expected position of the active form of MC-CPA. nd, not determined.
The extracellular levels of mMCP-6 were increased upon infection at early time points (12, 24, and 48 h) and without any obvious differences between SG+/+ and SG−/− mice. However, during late infection (day 8), the levels of mMCP-6 had decreased in both genotypes, possibly indicating SG-independent degradation of mMCP-6 (Fig. 3C). Analysis of the MC-CPA levels in peritoneal exudates showed that already 12 h after infection, the pro-form of MC-CPA was slightly increased, and the active form of MC-CPA was detected, even in SG−/− mice (Fig. 3D). The levels of MC-CPA continued to increase and at day 8, the active form of MC-CPA was present in similar and large amounts in both SG+/+ and SG−/− mice (Fig. 3D). Finally, when assessing day 8 cellular fractions, SG+/+ mice showed detectable levels of mMCP-6 and MC-CPA, whereas SG−/− mice displayed less protein (Fig. 3E).
Together, our results demonstrate that SG-deficient animals can efficiently secrete MC proteases in response to parasite challenge despite a severely reduced ability to store these enzymes within secretory granules. This strongly suggests that SG is not required for MC activation and release of MC proteases upon parasite infection in vivo. Moreover, it implies that under these conditions MCs can directly produce and release high amounts of MC proteases even in the absence of prestored proteases.
SG-independent Increase of Peritoneal Protease Activity upon Infection with T. gondii
We next assessed whether the T. gondii-induced release of MC proteases was accompanied by an increased extracellular enzyme activity. Chromogenic substrates were used to detect chymotrypsin-like (S-2586), trypsin-like (S-2288), and CPA-like (M-2245) activities in peritoneal exudates from naïve and T. gondii-infected SG+/+ and SG−/− mice. In naïve exudates, a trypsin-like activity was found in both genotypes (Table 1), supporting the detection of mMCP-6 on Western blot (Fig. 3C). Chymotrypsin-like activity was found in naïve SG+/+ exudates but was absent in the corresponding SG−/− exudates, whereas CPA-like activity was below the detection limit in all naïve exudates (Table 1). After parasite infection, the enzymatic activity against all tested substrates increased with time without significantly reduced activity in SG−/− compared with SG+/+ mice (Table 1). Chymotrypsin- and trypsin-like activities were detected already at 12 h at low levels and continued to increase up to day 8 (Table 1). In contrast, CPA-like activity was only detectable at later time points, 48 h and 8 days, when substantial amounts of the active form of MC-CPA appeared on Western blots (Fig. 3D and Table 1).
Although we cannot rule out that other proteases may have contributed to the measured proteolytic activities, the unaltered activity in SG−/− mice suggest that SG does not influence the total enzymatic activity in peritoneal exudates of infected mice. Thus, it is unlikely that the extracellular activity of individual MC proteases is severely affected by SG deficiency.
SG-dependent Storage of MC Proteases in Peritoneal Cell-derived MCs
To study the direct effects of T. gondii on MC activation, peritoneal MCs were prepared from naïve SG−/− and SG+/+ mice and cultivated in vitro as peritoneal cell-derived MCs (PCMCs) (32). PCMCs were stimulated with live parasites, and levels of cell-associated and secreted proteases were analyzed 6, 12, and 24 h after infection (Fig. 4). Nonstimulated control cultures of SG+/+ PCMCs expressed high levels of cell associated MC-proteases, whereas the expression of these proteases in nonstimulated SG−/− PCMCs were considerably lower or even undetectable (Fig. 4A). Challenge with live parasite did not substantially affect the intracellular levels of MC-specific proteases in SG+/+ or SG−/− PCMCs (Fig. 4A), and only small amounts of secreted mMCP-6 and MC-CPA were found without any clear difference between stimulated and nonstimulated cultures. Active MC-CPA was only detected in supernatants from SG+/+ and not from SG−/− cultures (Fig. 4B).
FIGURE 4.
In vitro stimulation of peritoneal derived mast cells with live T. gondii. A, SG+/+ and SG−/− PCMCs (106 cells) were stimulated with 106 live T. gondii tachyzoites for the indicated time periods. Nonstimulated (control) and stimulated cells were then analyzed by Western blotting for the indicated MC proteases. Western blots were developed with antibodies against the respective enzymes, and for each time point, the mean signal intensity minus background for the proteases is given as an inset graph as described under “Experimental Procedures.” Black bars represent SG+/+, and open bars represent SG−/−. In B, representative Western blots of supernatants from T. gondii-stimulated or control PCMCs are shown. The two bands shown for each time point and genotype represent two independent stimulations performed in separate wells. Cell fractions (CF) of SG+/+ PCMCs were included as positive controls (C). As a control of degranulation, β-hexosaminidase release was measured from PCMCs after stimulation with T. gondii for 2, 6, and 24 h. The calcium ionophore A23187 was included as a positive control for degranulation. Results are presented as means of three animals of each genotype ± S.E.
To assess the effect of SG on MC degranulation, SG−/− and SG+/+ PCMCs were stimulated by T. gondii or a calcium ionophore and the release of β-hexosaminidase was measured at different time points (Fig. 4C). The calcium ionophore A23187 induced a pronounced degranulation of both SG−/− and SG+/+ PCMCs, whereas no significant MC degranulation was found after stimulation with T. gondii, even after an extended period of time (24 h) (Fig. 4C). These results suggest that SG−/− and SG+/+ PCMCs indeed have a similar capacity to degranulate, but they are unresponsive to stimulation by the parasite in vitro. As this was in contrast with the in vivo situation where strong MC activation was observed, we hypothesized that processed parasite, which is soluble T. gondii antigen (STag), could be a stronger activator than live parasites. However, the levels of cell-associated and secreted proteases were not substantially affected by stimulation by STag (supplemental Fig. 1). Furthermore, STag-stimulated SG−/− PCMCs did not convert chromogenic substrates as efficiently as SG+/+ PCMCs (supplemental Table 1).
Massive Release of Hyaluronan and Chondroitin Sulfate after Challenge with T. gondii
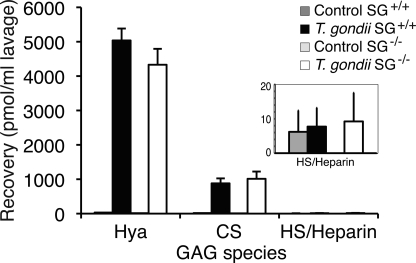
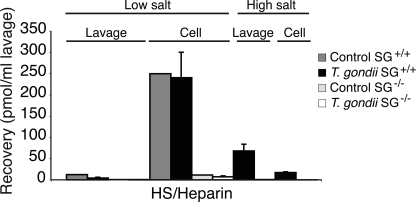
The in vivo challenge with T. gondii clearly induced strong MC activation and release of MC proteases, also in the absence of SG (Figs. 1 and 3). Furthermore, these presumably SG-carried GAG chain-dependent proteases exhibited normal levels of activity in SG-deficient animals, suggesting that heparin is not needed for normal MC protease activity in vivo (Table 1). Possibly, the apparently normal protease activity could depend on other GAGs sources present in the extracellular matrix or expressed by other cells that substitute with heparin-like or CS GAGs and support the secreted proteases after the challenge with T. gondii. To this end, we isolated GAGs from the peritoneal lavage (38) and characterized their composition (Figs. 5 and supplemental Fig. 2; see Fig. 7). Infected SG−/− and SG+/+ mice were subjected to peritoneal lavage 48 h after challenge because protease expression and activity were up-regulated in both genotypes at this time point. T. gondii-infected animals of both genotypes released massive amounts of hyaluronan and chondroitin sulfate A or CS-A into the peritoneal cavity (Fig. 5). Di-O-sulfated CS disaccharides accounted for ∼1% of the released CS in the lavage. Also, in the CS pool of the cellular fraction, small but significant amounts of di-4,6-O-sulfated disaccharides were found (supplemental Fig. 2). The HS/heparin content in the cell-free peritoneal lavage of challenged mice was minimal for both genotypes (Fig. 5, inset). This finding was unexpected, as previous studies had suggested heparin/HS to be the main type of GAGs involved in MC protease activation and function (11, 15, 16, 39). Therefore, we analyzed whether peritoneal heparin/HS was confined to the cellular pool or readsorbed immediately by the surrounding tissue when secreted, thus explaining the low levels of HS/heparin obtained from the extracellular fraction. Indeed, the cellular pool of the peritoneal lavage contained substantial amounts of HS/heparin in both challenged and naïve wild type animals, whereas the HS/heparin amounts were ∼10 times lower in SG−/− mice (Fig. 6).
FIGURE 5.
GAG recovery in peritoneal lavage of naïve and T. gondii challenged mice. GAGs were isolated from the peritoneal lavage from naïve and challenged animals (48 h post-infection) and subjected to disaccharide analysis as described under “Experimental Procedures.” The level of recovered hyaluronan (Hya), chondroitin sulfate, and heparan sulfate/heparin is indicated. In the inset, the levels of HS/heparin are expanded (n = 3 for infected animals and n = 2 for naïve animals).
FIGURE 6.
HS/heparin recovery in peritoneal lavage of naïve and T. gondii challenged mice. Challenged and naïve animals (48 h post-infection) were flushed with phosphate-buffered saline at physiological concentration (low salt) and followed with a high saline buffer (1.5 m NaCl) (high salt). GAGs were then isolated from lavage and cell fractions, respectively, and analyzed for HS/heparin content as described under “Experimental Procedures” (n = 3 for infected animals and n = 1 for naïve animals).
Next, we performed an experiment with two consecutive peritoneal lavages, the first with physiological saline and the second with a higher salt concentration meant to extract cell surface adsorbed heparin/HS from the peritoneal cavity. This resulted in recovery of additional amounts of heparin/HS from the SG+/+ mice but not from the SG−/− animals (Fig. 6). Taken together, when comparing the GAG content in the low and high salt lavage with that in the cellular fraction, it is evident that most HS/heparin remains cell associated after T. gondii challenge (Fig. 6). Moreover, the almost complete absence of HS/heparin in all fractions obtained from SG−/− mice suggests that SG is the major HS/heparin-containing proteoglycan in the peritoneum and that HS/heparin is not essential for the activity of MC proteases in vivo.
SG Is Needed for Generation of Heparin in Peritoneum
The recovered heparin/HS chains were analyzed for their disaccharide composition. Both challenged and naïve SG+/+ animals had mainly heparin both in the low salt lavage (Fig. 7A) and the cell fraction (Fig. 7B), with >80% of disaccharides being of the trisulfated type ΔHexA2S-GlcNS6S characteristic for heparin. Similar results were obtained when analyzing GAGs released by high salt lavage from SG+/+ mice (data not shown). In contrast, the GAG chains isolated from cells of SG−/− animals contained both nonsulfated and monosulfated disaccharide species in addition to di- and trisulfated disaccharides (Fig. 7B). The overall degree of sulfation in these chains (Fig. 7B, inset) was thus typical for HS chains and was lower than for heparin from SG+/+ animals.
DISCUSSION
Here, we sought to evaluate the in vivo role of SGPGs and their GAG chains by analyzing the levels and activities of SG-dependent MC proteases during parasite infection. In the in vivo situation, the GAGs of SGPGs are either heparin chains as in connective tissue type MCs or CS chains, sulfated to various degrees, in most other SG-expressing cell types (14). Previous work identified a crucial role for the GAGs attached to SG in granular retention and to affect activity of MC proteases (reviewed in Refs. 14 and 19). For example, MC-CPA is considered to be highly dependent on heparin for proper activation, i.e. for processing of pro-MC-CPA to mature enzyme (11, 18), and mMCP-6 relies on heparin for proper tetramerization (40). Furthermore, the biological functions of mMCP-4, mMCP-6, and CPA have been suggested to be tightly linked to the presence of heparin (reviewed in Refs. 13 and 41). Deletion of either the NDST (N-deacetylase/N-sulfotransferase 2) enzyme involved in heparin biosynthesis (8, 9) or the SG core protein (1) showed that SGPGs indeed are essential for MC homeostasis in resting tissues because naïve NDST2−/− and SG−/− mice had undetectable levels of MC proteases.
Considering previous findings, the up-regulated secretion of active MC-proteases in SG−/− mice during infection was unexpected, and the results strongly suggest that MC-specific proteases can be synthesized and released as active enzymes by a SGPG-independent mechanism (Fig. 3 and Table 1). This could possibly mean that once activated by infection the MCs secrete their de novo synthesized proteases independently of SGPGs and by a granule-independent route. However, it cannot be excluded that other types of GAG chains are compensating for the loss of heparin in SG-deficient animals.
The extracellular matrix contains large amounts of GAGs that potentially could be released after infection. In addition, sentinel cells and infiltrating leukocytes express cell surface glypicans and syndecans, PGs that may be proteolytically shed into the extracellular compartment. Interestingly, it has been suggested that released CS could be inhibitory for infectious agents as shown by Le Doux et al. (42). On the other hand, CS could also act as a target for microbial adhesion to the extracellular matrix (43). We therefore analyzed the content of soluble GAGs in peritoneal exudates from SG+/+ and SG−/− animals upon parasite challenge. T. gondii infection indeed resulted in enhanced peritoneal levels of hyaluronan and CS, whereas the release of HS/heparin was minimal. Despite lacking SG, the animals mount a similar CS response as the WT animals. No significant difference could be seen concerning structural features or relative amounts of hyaluronan and CS between SG+/+ and SG−/− mice, suggesting that the level, production, and release of these GAGs in response to infection are independent of SG, arguing against SGPGs as the major source for extracellular CS GAGs 48 h post-infection. The massively elevated levels of hyaluronan and CS suggest a role of these GAGs in the host defense against the parasite. Even though the released CS is considerably less sulfated than heparin and thus a principally weaker binder for protein ligands, the large amounts and the presence of di-O-sulfated disaccharide building blocks may support protease activity by stabilization of the secreted enzymes in the absence of SGPGs.
Alternatively, heparin or HS chains present on other PGs than SG may provide a compensatory mechanism for the lack of SG-associated heparin. In SG+/+ mice, the majority of the heparin/HS GAG pool was found in the cell fraction after lavage with PBS, consistent with MC association. Subsequent lavage with a high salt buffer released additional heparin originating from remaining cells or from the surrounding tissue to which heparin could have been adsorbed after degranulation. In contrast, no HS/heparin was found in the PBS lavage or the high salt lavage collected from SG−/− mice. However, the cellular fraction from SG−/− mice contained HS of a relatively high sulfation degree with a high proportion of the trisulfated disaccharide ΔHexA2S-GlcNS6S and an average of 1.5 sulfate groups/disaccharide. The HS sulfation levels found in the peritoneal cavity of the SG−/− mice after infection are actually much higher than the levels found in HS of most other mouse tissues (38). These findings indicate that SG−/− MCs, or other cell types, can produce HS with a high level of sulfation. Although the cellular fraction from SG−/− mice contains HS structures that may assist in MC protease activation, the low levels of total HS in SG−/− mice argues against this mechanism as the sole explanation for the recovered function of MC proteases in infected SG−/− mice.
In line with previous studies (36), we found rapid recruitment and degranulation of MCs after challenge with T. gondii. However, as neither live parasites nor soluble antigen induced degranulation of PCMC in vitro, it seems likely that early <24 h degranulation in vivo is not directly induced by the parasite but rather through substances, possibly complement or chemokines, released by other cells in response to the infection. Interestingly, early MC recruitment seem to depend on SG because we see a reduction in MC numbers in SG-deficient animals at 12 and 24 h. Possibly, the recruitment of MCs after infection could be partly dependent on release of proteases that induce tissue remodeling and/or activation of chemokines and thereby facilitate cell migration.
Both tryptase and chymase have been reported to contribute to neutrophil accumulation during inflammation in arthritic joints and the skin (44, 45). We found a significantly delayed neutrophil infiltration and IL-6 production at early time-points in the SG−/− mice (Fig. 1). These findings may reflect a general defect in the local early inflammatory response in SG−/− mice that could be due to the presumed lower amounts of immediately released (<12 h) prestored mediators from MCs and other immune cells.
In contrast to the in vivo situation, we found that the SG deficiency affected MC protease secretion and activity in infected PCMC cultures. Only minute chymase and tryptase activities were detected, and an impairment in CPA-like activity was seen in SG−/− PCMCs. However, this may simply reflect that the PCMCs did not degranulate in contact with either live parasite or STag. Alternatively, the discrepancies between the in vivo and in vitro findings imply that other cell types or molecular effectors such as secreted or cell surface exposed GAGs possibly would account for regulatory mechanisms of the MC proteases upon their release after MC stimulation. Together, this suggests that SG mainly is required in cis for storage in MC granules both in vivo and in vitro, whereas production per se, activation, and secretion would not be drastically affected by the absence of the heparin chains on SG in vivo, at least under conditions of massive MC activation.
Collectively, our results indicate that although SG−/− MCs have a storage defect and are not recruited to the same extent as SG+/+ MCs during infection, SG−/− MCs can produce and secrete normal amounts of active MCs proteases when they are activated by an ongoing infection. This unexpected finding, which partly may be explained by the released GAGs in the peritoneal cavity of T. gondii-infected mice, merits further studies on the role of SG and other PGs in MC protease biology.
Supplementary Material
Acknowledgments
We thank the animal facility at the National Veterinary Institute; E. B. Jakubek for culturing parasites; and O. Malbec and M. Daeron for the CHO-KL cells.
This work was supported by FORMAS (to M. Å.), the Göran Gustafsson Foundation (to M. Å.), the Medical Research Council (to M. Å.), the Ivar and Elsa Sandberg Foundation (to A. L.), Polysackaridforskning AB (to D. S.), and the Vårdal Foundation (to S. W.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1 and 2.
- SG
- serglycin
- CS
- chondroitin sulfate
- GAG
- glycosaminoglycan
- HS
- heparan sulfate
- MC
- mast cell
- MC-CPA
- MC carboxypeptidase A
- PCMC
- peritoneal cell-derived MC
- PG
- proteoglycan
- STag
- soluble T. gondii antigen.
REFERENCES
- 1.Abrink M., Grujic M., Pejler G. (2004) J. Biol. Chem. 279, 40897–40905 [DOI] [PubMed] [Google Scholar]
- 2.Grujic M., Braga T., Lukinius A., Eloranta M. L., Knight S. D., Pejler G., Abrink M. (2005) J. Biol. Chem. 280, 33411–33418 [DOI] [PubMed] [Google Scholar]
- 3.Niemann C. U., Abrink M., Pejler G., Fischer R. L., Christensen E. I., Knight S. D., Borregaard N. (2007) Blood 109, 4478–4486 [DOI] [PubMed] [Google Scholar]
- 4.Woulfe D. S., Lilliendahl J. K., August S., Rauova L., Kowalska M. A., Abrink M., Pejler G., White J. G., Schick B. P. (2008) Blood 111, 3458–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raja S. M., Metkar S. S., Höning S., Wang B., Russin W. A., Pipalia N. H., Menaa C., Belting M., Cao X., Dressel R., Froelich C. J. (2005) J. Biol. Chem. 280, 20752–20761 [DOI] [PubMed] [Google Scholar]
- 6.Muramatsu T., Muramatsu H. (2008) Proteomics 8, 3350–3359 [DOI] [PubMed] [Google Scholar]
- 7.Tantravahi R. V., Stevens R. L., Austen K. F., Weis J. H. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 9207–9210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries D. E., Wong G. W., Friend D. S., Gurish M. F., Qiu W. T., Huang C., Sharpe A. H., Stevens R. L. (1999) Nature 400, 769–772 [DOI] [PubMed] [Google Scholar]
- 9.Forsberg E., Pejler G., Ringvall M., Lunderius C., Tomasini-Johansson B., Kusche-Gullberg M., Eriksson I., Ledin J., Hellman L., Kjellén L. (1999) Nature 400, 773–776 [DOI] [PubMed] [Google Scholar]
- 10.Ringvall M., Rönnberg E., Wernersson S., Duelli A., Henningsson F., Abrink M., García-Faroldi G., Fajardo I., Pejler G. (2008) J. Allergy Clin. Immunol. 121, 1020–1026 [DOI] [PubMed] [Google Scholar]
- 11.Henningsson F., Ledin J., Lunderius C., Wilén M., Hellman L., Pejler G. (2002) Biol. Chem. 383, 793–801 [DOI] [PubMed] [Google Scholar]
- 12.Henningsson F., Hergeth S., Cortelius R., Abrink M., Pejler G. (2006) Febs J. 273, 4901–4912 [DOI] [PubMed] [Google Scholar]
- 13.Pejler G., Abrink M., Ringvall M., Wernersson S. (2007) Adv. Immunol. 95, 167–255 [DOI] [PubMed] [Google Scholar]
- 14.Pejler G., Abrink M., Wernersson S. (2009) Biofactors 35, 61–68 [DOI] [PubMed] [Google Scholar]
- 15.Pejler G., Maccarana M. (1994) J. Biol. Chem. 269, 14451–14456 [PubMed] [Google Scholar]
- 16.Hallgren J., Spillmann D., Pejler G. (2001) J. Biol. Chem. 276, 42774–42781 [DOI] [PubMed] [Google Scholar]
- 17.Henningsson F., Wolters P., Chapman H. A., Caughey G. H., Pejler G. (2003) Biol. Chem. 384, 1527–1531 [DOI] [PubMed] [Google Scholar]
- 18.Henningsson F., Yamamoto K., Saftig P., Reinheckel T., Peters C., Knight S. D., Pejler G. (2005) J. Cell Sci. 118(Pt 9), 2035–2042 [DOI] [PubMed] [Google Scholar]
- 19.Kolset S. O., Tveit H. (2008) Cell Mol. Life Sci. 65, 1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurer M., Wedemeyer J., Metz M., Piliponsky A. M., Weller K., Chatterjea D., Clouthier D. E., Yanagisawa M. M., Tsai M., Galli S. J. (2004) Nature 432, 512–516 [DOI] [PubMed] [Google Scholar]
- 21.Metz M., Piliponsky A. M., Chen C. C., Lammel V., Abrink M., Pejler G., Tsai M., Galli S. J. (2006) Science 313, 526–530 [DOI] [PubMed] [Google Scholar]
- 22.Knight P. A., Wright S. H., Lawrence C. E., Paterson Y. Y., Miller H. R. (2000) J. Exp. Med. 192, 1849–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawrence C. E., Paterson Y. Y., Wright S. H., Knight P. A., Miller H. R. (2004) Gastroenterology 127, 155–165 [DOI] [PubMed] [Google Scholar]
- 24.Thakurdas S. M., Melicoff E., Sansores-Garcia L., Moreira D. C., Petrova Y., Stevens R. L., Adachi R. (2007) J. Biol. Chem. 282, 20809–20815 [DOI] [PubMed] [Google Scholar]
- 25.Orinska Z., Maurer M., Mirghomizadeh F., Bulanova E., Metz M., Nashkevich N., Schiemann F., Schulmistrat J., Budagian V., Giron-Michel J., Brandt E., Paus R., Bulfone-Paus S. (2007) Nat. Med. 13, 927–934 [DOI] [PubMed] [Google Scholar]
- 26.Waern I., Jonasson S., Hjoberg J., Bucht A., Abrink M., Pejler G., Wernersson S. (2009) J. Immunol. 183, 6369–6376 [DOI] [PubMed] [Google Scholar]
- 27.Magnusson S. E., Pejler G., Kleinau S., Abrink M. (2009) Faseb J. 23, 875–882 [DOI] [PubMed] [Google Scholar]
- 28.Shin K., Watts G. F., Oettgen H. C., Friend D. S., Pemberton A. D., Gurish M. F., Lee D. M. (2008) J. Immunol. 180, 4885–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wernersson S., Braga T., Sawesi O., Waern I., Nilsson K. E., Pejler G., Abrink M. (2008) J. Leukoc Biol. [DOI] [PubMed] [Google Scholar]
- 30.Lundén A., Marks J., Maley S. W., Innes E. A. (1998) Parasite Immunol. 20, 519–526 [DOI] [PubMed] [Google Scholar]
- 31.Björkman C., Lundén A. (1998) Int. J. Parasitol. 28, 187–193 [DOI] [PubMed] [Google Scholar]
- 32.Malbec O., Roget K., Schiffer C., Iannascoli B., Dumas A. R., Arock M., Daëron M. (2007) J. Immunol. 178, 6465–6475 [DOI] [PubMed] [Google Scholar]
- 33.Ledin J., Staatz W., Li J. P., Götte M., Selleck S., Kjellén L., Spillmann D. (2004) J. Biol. Chem. 279, 42732–42741 [DOI] [PubMed] [Google Scholar]
- 34.Sayles P. C., Johnson L. L. (1996) Nat. Immun. 15, 249–258 [PubMed] [Google Scholar]
- 35.Bliss S. K., Zhang Y., Denkers E. Y. (1999) J. Immunol. 163, 2081–2088 [PubMed] [Google Scholar]
- 36.S. Ferreira G. L., Mineo J. R., Oliveira J. G., V. Ferro E. A., Souza M. A., D. Santos A. A. (2004) Microbes Infect. 6, 172–181 [DOI] [PubMed] [Google Scholar]
- 37.Braga T., Grujic M., Lukinius A., Hellman L., Abrink M., Pejler G. (2007) Biochem. J. 403, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledin J., Ringvall M., Thuveson M., Eriksson I., Wilén M., Kusche-Gullberg M., Forsberg E., Kjellén L. (2006) J. Biol. Chem. 281, 35727–35734 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz L. B., Riedel C., Caulfield J. P., Wasserman S. I., Austen K. F. (1981) J. Immunol. 126, 2071–2078 [PubMed] [Google Scholar]
- 40.Hallgren J., Estrada S., Karlson U., Alving K., Pejler G. (2001) Biochemistry 40, 7342–7349 [DOI] [PubMed] [Google Scholar]
- 41.Stevens R. L., Adachi R. (2007) Immunol. Rev. 217, 155–167 [DOI] [PubMed] [Google Scholar]
- 42.Le Doux J. M., Morgan J. R., Snow R. G., Yarmush M. L. (1996) J. Virol. 70, 6468–6473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srinoulprasert Y., Kongtawelert P., Chaiyaroj S. C. (2006) Microb. Pathog. 40, 126–132 [DOI] [PubMed] [Google Scholar]
- 44.Shin K., Nigrovic P. A., Crish J., Boilard E., McNeil H. P., Larabee K. S., Adachi R., Gurish M. F., Gobezie R., Stevens R. L., Lee D. M. (2009) J. Immunol. 182, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He S., Walls A. F. (1998) Br. J. Pharmacol. 125, 1491–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.