Abstract
The γ-secretase protein complex executes the intramembrane proteolysis of amyloid precursor protein (APP), which releases Alzheimer disease β-amyloid peptide. In addition to APP, γ-secretase also cleaves several other type I membrane protein substrates including Notch1 and N-cadherin. γ-Secretase is made of four integral transmembrane protein subunits: presenilin (PS), nicastrin, APH1, and PEN2. Multiple lines of evidence indicate that a heteromer of PS-derived N- and C-terminal fragments functions as the catalytic subunit of γ-secretase. Only limited information is available on the domains within each subunit involved in the recognition and recruitment of diverse substrates and the transfer of substrates to the catalytic site. Here, we performed mutagenesis of two domains of PS1, namely the first luminal loop domain (LL1) and the second transmembrane domain (TM2), and analyzed PS1 endoproteolysis as well as the catalytic activities of PS1 toward APP, Notch, and N-cadherin. Our results show that distinct residues within LL1 and TM2 domains as well as the length of the LL1 domain are critical for PS1 endoproteolysis, but not for PS1 complex formation with nicastrin, APH1, and PEN2. Furthermore, our experimental PS1 mutants formed γ-secretase complexes with distinct catalytic properties toward the three substrates examined in this study; however, the mutations did not affect PS1 interaction with the substrates. We conclude that the N-terminal LL1 and TM2 domains are critical for PS1 endoproteolysis and the coordination between the putative substrate-docking site and the catalytic core of the γ-secretase.
Keywords: Intramembrane Proteolysis, Notch Receptor, Presenilin, Protein-Protein Interactions, Secretases, Alzheimer Disease, Cadherin, Endoproteolysis, Gamma-Secretase
Introduction
Cerebral deposition of β-amyloid (Aβ)2 peptides in senile plaques is a pathological hallmark of Alzheimer's disease. Aβ is generated by sequential proteolytic processing of amyloid precursor protein (APP) by β- and γ-secretases (1). γ-Secretase is a multiprotein complex made of four integral subunits namely presenilin, nicastrin, APH1, and PEN2 (2). These core components form subcomplexes early in the secretory pathway and cooperatively exit the endoplasmic reticulum (ER), and the fully assembled γ-secretase complex is then trafficked to post-Golgi compartments (reviewed in Refs. 3–5). Stability and subcellular trafficking of the remaining three subunits is severely impaired in the absence of any one of the four γ-secretase subunits. Maturation and ER exit of nicastrin is impaired in cells lacking presenilin 1 (PS1), and PS1 deficiency causes destabilization and altered intracellular trafficking of PEN2 and APH1 (6–8). During γ-secretase assembly, the highly unstable PS1 holoprotein undergoes endoproteolysis within the large cytoplasmic loop domain between TM6 and TM7 to generate N-terminal (NTF) and C-terminal fragments (CTF), which remain as stable heterodimers (9, 10). This endoproteolytic event is highly regulated because it occurs only when PS1 holoprotein successfully assembles with the other three subunits (11–13).
Multiple lines of evidence indicate that PS is the catalytic subunit of the γ-secretase complex. First, familial early onset AD-linked mutations in PSEN1 or PSEN2 alter γ-secretase cleavage of APP in a manner that increases the abundance of the 42-residue Aβ peptides (Aβ42) relative to that of the 40-residue peptides (Aβ40) (14–16). Second, mutating either of two conserved aspartate residues within the PS transmembrane domain (TM) 6 or 7 abrogates Aβ production (17). Third, active site-directed transition state analog inhibitors of γ-secretase could be directly cross-linked to PS1-derived NTF/CTF heterodimers (18, 19). Fourth, photoaffinity probes designed to mimic APP specifically bound to PS1 NTF/CTF interface (20). Finally, although details regarding the γ-secretase structure are only beginning to emerge, data from single-particle analysis by electron microscopy and substituted cysteine accessibility method suggest the existence of a water-accessible catalytic pore in the proximity of PS1 TM6, TM7, and TM9 within the γ-secretase complex (21–25).
The aspartyl protease activity of γ-secretase mediates regulated intramembrane proteolysis of several type I membrane proteins in addition to APP, including APP homologs, Notch receptor, ErB4, DCC (deleted in colorectal cancer), low density lipoprotein receptor protein, CD44, syndecan 3, N- and E-cadherin, etc. (reviewed in Ref. 26). Without exception, γ-secretase cleavage is preceded by ectodomain shedding of these proteins by ADAM (a disintegrin and metalloprotease) proteases or β-secretase. Although the exact intramembrane cleavage site(s) in each substrate have not been mapped, many substrates appear to undergo γ-secretase-dependent proteolysis at least at two distinct sites, termed γ- and ϵ-sites. For example, γ-secretase cleavage of the APP C-terminal fragment at the γ- and ϵ-sites generates Aβ and the APP intracellular domain (AICD), respectively. Whereas Aβ is released into the extracellular milieu, AICD forms a transcriptionally active complex with the nuclear adaptor protein Fe65 and the histone acetyltransferase Tip60 (27). In the case of Notch, γ-secretase-mediated ϵ-cleavage results in the release of Notch intracellular domain (NICD) (28), which translocates into the nucleus to mediate Notch signaling by transcriptional activation. Intracellular domains released from substrates such as syndecan 3 and E-cadherin do not have an obvious role in nuclear signaling and are likely destined for degradation (26).
Previously we reported that experimental deletion of TM1, TM2, and the intervening loop (Val82–Tyr154) results in the loss of PS1 endoproteolysis and impaired γ-secretase activity (29). More than 25% of PSEN1 mutations (47 of the 178 gene mutations) are found within the sequences that encode this stretch of 73 amino acids (aa); these mutations are responsible for 24 of the ∼106 FAD-linked aa changes in PS1. In this study, we have characterized the luminal loop 1 (LL1) and TM2 domains of PS1 using insertion, deletion, and aa substitution. Our results show that both the length and specific residues of LL1 and residues near the C terminus of TM2 are important for PS1 endoproteolysis. However, there is no correlation between PS1 endoproteolysis and the ability of experimental mutants to assemble into γ-secretase complexes or bind to three γ-secretase substrates (APP, Notch, and N-cadherin). The characterization of substrate processing reveals interesting differences in the extent to which each substrate is processed, revealing the critical involvement of PS1 residues within the LL1 and TM2 domains in substrate catalysis or the coordination between the substrate docking site and the catalytic site.
EXPERIMENTAL PROCEDURES
Plasmids and Retrovirus-mediated Gene Expression
The cDNA fragments encoding PS1 mutants were generated as Asp718 and PflMI fragments by two-step PCR mutagenesis and cloned by exchanging the corresponding segment in human WT PS1 expression plasmid. The cDNA encoding PS1 TM4-NF to YI mutant (a gift from Dr. Seong-Hun Kim, University of Florida) was used as template to generate TM2-aa6/15-TM4YI. The resulting cDNAs were subcloned into pMXs retroviral vector (generously provided by Dr. Toshio Kitamura, University of Tokyo, Tokyo, Japan). Similarly, cDNAs encoding APPSwe, NotchΔEMV-6myc (a gift from Dr. Raphael Kopan, Washington University) and C99–6myc (a gift from Dr. Alison Goate, Washington University) were subcloned into pMXs vector.
PS1−/−/PS2−/− mouse embryonic fibroblasts (MEF) were a gift from Drs. Wim Annaert and Bart De Strooper (KULeuven and Flanders Interuniversitary Institute for Biotechnology, Belgium). MEF and 293T-derived Plat-E retroviral packaging cells (30) were maintained in DMEM supplemented with 10% fetal bovine serum, 2 mm glutamine, and 1% penicillin-streptomycin. For retroviral gene expression, Plat-E cells were transfected with pMXs plasmids, and supernatants containing retroviruses collected between 24 and 48 h after transfection were used to infect PS1−/−/PS2−/− MEF in the presence of 10 μg/ml polybrene. Stably transduced PS1−/−/PS2−/− MEF pools were selected in 5 μg/ml puromycin for 2 weeks and used as stable pools. For transient expression, cells infected with APP, C99, and Notch retroviruses were lysed for 48 h after infection.
Protein Analyses
To prepare detergent lysates, the cells were lysed in a buffer containing 150 mm NaCl, 50 mm Tris-HCl (pH 7.4), 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 5 mm EDTA, 0.25% SDS, 0.25 mm phenylmethylsulfonyl fluoride, and protease inhibitor mixture (1:200; Sigma) and briefly sonicated. For co-immunoprecipitation analyses, the cells were lysed in CHAPSO buffer (1% CHAPSO, 150 mm NaCl, 50 mm HEPES (pH 7.4), 2 mm EDTA, and protease inhibitor mixture) at 4 °C for 30 min. The lysates were clarified by centrifugation at 10,000 rpm for 10 min at 4 °C, and aliquots of the supernatants (corresponding to 500 μg of protein) were adjusted to 500 μl with CHAPSO buffer and precleared by incubating with protein A beads at 4 °C for 2 h. Three μl of αPS1Loop or PS1NT antiserum was added to each sample and incubated overnight at 4 °C with gentle mixing. Immune complexes were bound by incubating with 40 μl of protein A-agarose beads for 2 h at 4 °C, and washed twice with 700 μl of CHAPSO buffer. Bound proteins were eluted in Laemmli buffer and analyzed by immunoblotting along with an aliquot of the input lysate. Rabbit polyclonal antibodies against PS1 (αPS1Loop and PS1NT) and PEN2 (PNT2), APH1aL (A1tag), and APP (CTM1) have been described (31, 32). Antibodies against Myc epitope (9E10; ATCC), N-cadherin (BD Transduction Laboratories), and nicastrin (Santa Cruz) were purchased. GRP78 antibody was generated in guinea pig against the synthetic peptide QPIISKLYGSGGPPPTGEEDTSEKDEL. The blots were developed using protein A-HRP (Sigma) followed by chemiluminescence detection or using IRDye800-conjugated secondary antibodies followed by Odyssey infrared imaging (LI-COR).
Aβ ELISA
Fresh medium was added to cells 24 h after infection with APPSwe retrovirus, and conditioned media were collected at 48 h. The levels of secreted Aβ40 and Aβ42 were quantified using sandwich ELISAs as described previously (33). Briefly, 96-well plates were coated with the appropriate capture mAb (B113 for Aβ40 and A387 for Aβ42). Following incubation with culture supernatants, the plates were washed, and Aβ40 or Aβ42 was detected with alkaline phosphatase-conjugated mAb B436 (which reacts with the N-terminal region of Aβ) and CSPD-Sapphire II Luminescence Substrate (Applied Biosystems). Each sample was assayed in duplicate using appropriate dilution of the conditioned medium so that the relative luminescent units were in the linear range of the standards included on each plate. Synthetic Aβ40 and Aβ42 peptides were diluted in culture medium to generate a standard curve.
RESULTS
The Amino Acid Sequence as Well as the Length of the PS1 LL1 Domain Contributes to Endoproteolysis
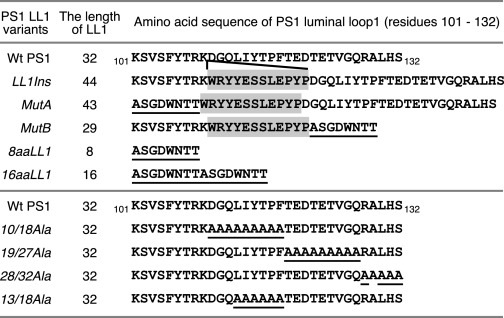
Previously, we and others reported that the segment encompassing the first two TMs of PS1 is important for both PS1 endoproteolysis and γ-secretase activity (29, 34). To further characterize the role of the luminal loop connecting the first two TMs (LL1; corresponding to Lys101–Ser132) for PS1 function, we generated a series of mutants within the LL1 domain including an insertion mutant, deletion mutants, and alanine substitutions (Fig. 1). Each mutant was stably expressed in PS1−/−/PS2−/− MEF to examine PS1 endoproteolysis and γ-secretase activity. LL1Ins is a mutant where we inserted 12 unrelated aa between Lys109 and Asp110 to disrupt the contiguity of the LL1 sequence. This sequence modification did not affect PS1 endoproteolysis, which suggests that the LL1 domain can be interrupted at this position (Fig. 2A). We then replaced the sequences on either side of the insertion in the LL1Ins mutant. In MutA, the first 9 aa of LL1 (Lys101–Lys109) was replaced with 8 aa (corresponding to residues 400–407 of PS1); in MutB, the last 23 aa of LL1 (Asp110–Ser132) were replaced with the same 8 aa as in MutA. The presence of the LL1Ins sequence aids in keeping the length of the LL1 in the mutants close to that of the LL1 of WT PS1. Cells stably expressing MutA and MutB still generated NTF and CTF, although the steady-state levels were somewhat lower in MutA and markedly lower in MutB relative to cells expressing WT PS1 or LL1Ins (Fig. 2A and supplemental Fig. S1). When the entire LL1 was replaced with the 8-aa sequence, endoproteolysis of the resulting 8aaLL1 mutant was also markedly impaired. This defect was only partially restored when the length of LL1 was increased to 16 aa (Fig. 2A). Despite similar or even higher levels of holoPS1 expression, lower levels of NTF and CTF accumulation are readily apparent when MutA, MutB, 8aaLL1, and 16aaLL1 are compared with LL1Ins.
FIGURE 1.
The amino acid sequences of PS1 LL1 variants. The sequence of the luminal loop connecting TM domains 1 and 2 is depicted along with the sequences of the LL1 mutants. The aa substitutions are underlined. The shaded letters represent a 12-aa insert. The length of the luminal loop domain (number of aa) is indicated for each construct.
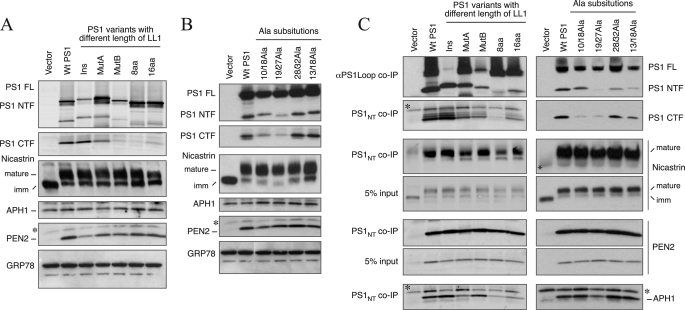
FIGURE 2.
Characterization of γ-secretase complex formation by PS1 LL1 variants. A and B, PS1−/−/PS2−/− MEF were stably transduced with recombinant retroviruses expressing the indicated mutants, and the cell lysates were subject to immunoblotting using antibodies against PS1 NTF, PS1 CTF, nicastrin, APH1aL, and PEN2. The levels of GRP78 were analyzed as a loading control. Ins, LL1Ins mutant; imm, immature. C, co-immunoprecipitation (co-IP) analysis of γ-secretase subunits. Stable PS1−/−/PS2−/− MEF pools expressing PS1 LL1 variants were lysed in a buffer containing 1% CHAPSO and immunoprecipitated with αPS1Loop or PS1NT antibody. An aliquot of the input lysate was also analyzed in parallel. The blots were sequentially probed with antibodies against each γ-secretase subunit (PS1NT, αPS1Loop, nicastrin, APH1aL, and PEN2). The asterisks indicate nonspecific reactivity.
Next, we designed additional mutants to determine the subdomains in LL1 that are important for PS1 processing. Because the levels of NTF and CTF were markedly lower in MutB cells as compared with Wt or MutA cells, we focused our attention on the residues (aa 10–32 of LL1) that were deleted in this mutant. We first subdivided this region into three segments: aa 10–18, 19–27, and 28–32, and replaced all of the aa within each segment with alanine. As shown in Fig. 2B, PS1 endoproteolysis was impaired to different extents in the 10/18Ala and 19/27Ala mutants but not in the 28/32Ala mutant. The last mutant we made in this region is 13/18Ala, which is endoproteolytically processed with efficiency similar to that of WT PS1. Together, these studies show that the length of the LL1 domain and specific residues within this region (110DGQ and 119TEDTETVGQ of human PS1) are important for PS1 endoproteolysis.
As previously reported (35), nicastrin fails to undergo maturation by complex glycosylation and remains as core-glycosylated immature nicastrin in PS1−/−/PS2−/− MEF (Fig. 2A). Furthermore, the steady-state levels of PEN2 are markedly diminished in PS1−/−/PS2−/− MEF. Stable expression of WT PS1 in PS1−/−/PS2−/− cells results in nicastrin maturation and restores PEN2 levels. Similarly, stable expression of each PS1 mutant described above restored the deficiency in nicastrin maturation and increased the steady-state levels of PEN2, comparable with that found in cells expressing WT PS1 (Fig. 2, A and B). Interestingly, the rescue of nicastrin maturation and PEN2 levels did not correlate with the lower steady-state levels of endoproteolyzed PS1 derivatives in cells expressing MutB, 8aaLL1, 16aaLL1, 10/18Ala, and 19/27Ala (Fig. 2, A and B). Consistent with previous reports (36–38), the steady-state level of APH1aL was not markedly altered by the loss of PS expression or stable expression of WT or mutant PS1 in PS1−/−/PS2−/− MEF (Fig. 2A).
To test our prediction that rescue of nicastrin maturation and steady-state PEN2 levels are indications of their successful assembly into γ-secretase complex, we lysed cells in 1% CHAPSO and co-immunoprecipitated PS1 LL1 mutant polypeptides and bound proteins using PS1NT and PS1Loop antibodies. PS1Loop or PS1NT immunoprecipitates and aliquots of the input lysates were analyzed by immunoblots. Regardless of whether the protein underwent endoproteolysis or not, PS1 mutants formed complexes with endogenous nicastrin, PEN2, and APH1 (Fig. 2C). These results are in agreement with the findings that sequences within the TM4 domain of PS1 bind to PEN2, and PS1 CTF binds to NCT (39–41). Together, these results indicate that the reduced ability of certain PS1 mutants to generate NTF and CTF by endoproteolysis does not influence their successful association with other γ-secretase subunits to form a protein complex.
Luminal Loop 1 of PS1 Is Essential for γ-Secretase Activity
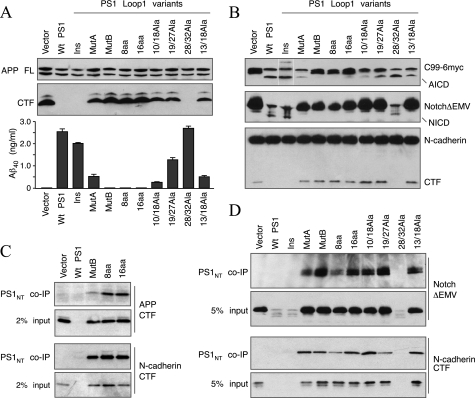
Having shown that PS1 LL1 mutants form complexes with other γ-secretase subunits, we turned our attention to γ-secretase processing of substrates. We initially examined intramembranous proteolysis of APP with two approaches. First, we transiently expressed human APP695 harboring the “Swedish” double mutation (APPSwe) in stable PS1−/−/PS2−/− pools expressing PS1 LL1 variants described above. We performed Western blots to confirm comparable expression of full-length APP and to determine the levels of APP CTFs in the cell lysates and measured the levels of secreted Aβ40 in the conditioned medium by ELISA. PS1−/−/PS2−/− cells accumulate high levels of APP CTFs and fail to secrete detectible levels of Aβ40. Restoration of γ-secretase activity in PS1−/−/PS2−/− cells by stable expression of WT PS1 results in processing of CTFs, which fall below the level of detection, and secretion of Aβ40 (Fig. 3A). Insertion of 12 aa within LL1 did not affect γ-secretase processing of APP. However, there was a marked loss of γ-secretase processing of APP when the residues on either side of the insertion (MutA and MutB) or the entire LL1 (8aaLL1 and 16aaLL1) were replaced. We considered the possibility that the presence of the residues NTT in these four mutants might have resulted in N-glycosylation within the LL1 region, contributing to a loss of γ-secretase activity. However, treatment of MEF with tunicamycin, an inhibitor of N-glycosylation, did not alter the electrophoretic migration of these PS1 mutants but clearly altered that of the N-glycosylated transmembrane protein CD147 by generating a faster migrating nonglycosylated form (supplemental Fig. S1B). Alanine substitution mutants with the exception of 28/32Ala exhibited varying degrees of partial loss of γ-secretase processing of APP (Fig. 3A).
FIGURE 3.
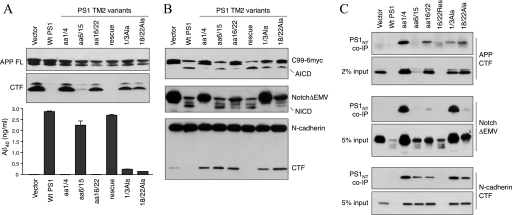
γ-Secretase processing of different substrates by PS1 LL1 mutants. A, stable PS1−/−/PS2−/− MEF pools expressing PS1 LL1 variants were transiently infected with APPSwe retrovirus. The cell lysates were analyzed by immunoblotting with antibody CTM1 (raised against the APP C terminus). The levels of secreted Aβ40 peptides in the conditioned media were quantified by ELISA. Ins, LL1Ins mutant; imm, immature. B, stable PS1−/−/PS2−/− MEF pools expressing PS1 LL1 variants were transiently infected with a retrovirus expressing APP C99–6Myc (top panel) or NotchΔEMV-6myc (middle panel). The lysates were analyzed by immunoblotting with mAb 9E10 or N-cadherin antibody. C, co-immunoprecipitation (co-IP) of γ-secretase and APP CTF. Stable PS1−/−/PS2−/− MEF pools expressing PS1 LL1 variants were transiently infected with APPSwe, and the lysates were analyzed by co-immunoprecipitation with PS1NT antibody and blotted APP antibody. D, co-immunoprecipitation of γ-secretase with NotchΔEMV-6myc and N-cadherin CTF. Lysates of NotchΔEMV-6myc infected or uninfected stable PS1−/−/PS2−/− MEF pools were analyzed by co-immunoprecipitation with PS1NT antibody and blotted with mAb 9E10 and N-cadherin antibody.
In the second approach, we transiently expressed APP C99–6myc and assessed the levels AICD generated by γ-secretase cleavage of C99 between Leu49 and Val50, which is referred to as the ϵ-cleavage (42). There have been contrasting reports on the correlation between Aβ production (γ-cleavage) and AICD generation (ϵ-cleavage) (43–46). Therefore, we felt it is important to determine how LL1 mutations influenced ϵ-cleavage activity. As expected, PS1−/−/PS2−/− MEF fail to generate AICD, but γ-secretase cleavage of C99–6myc releases readily detectible levels of AICD in stably infected PS1−/−/PS2−/− MEF expressing WT PS1 (Fig. 3B, top panel). Similarly, AICD was efficiently generated by the cleavage of C99–6myc in PS1−/−/PS2−/− MEF expressing LL1Ins and 28/32Ala. Little or no AICD-6myc was detected in PS1−/−/PS2−/− MEF stably expressing MutB, 8aaLL1, 16aaLL1, and 10/18Ala, and lower levels were detected in cells expressing MutA, 19/27Ala, and 13/18Ala (Fig. 3B, top panel). These results indicate that sequences within the LL1 domain have a similar influence on γ- and ϵ-cleavage of APP. Nevertheless, we note the inconsistency between the steady-state levels of PS1 derivatives generated by endoproteolysis and γ- or ϵ-cleavage of APP CTFs in cells expressing few of the LL1 mutants. For instance, MutB and 16aaLL1 are endoproteolytically processed to some extent and form PS1 complexes (Fig. 2C), but in cells stably expressing these mutants, APP CTF and C99–6myc are not at all cleaved (Fig. 3, A and B, top panel).
Next, we turned to processing of Notch1, which is one of the well studied substrates of γ-secretase. Intramembranous γ-secretase cleavage of Notch releases NICD (47), which is analogous to AICD release by ϵ-cleavage of APP. We transiently expressed NotchΔEMV-6myc in stably transduced PS1−/−/PS2−/− MEF expressing PS1 LL1 variants and analyzed NICD production by immunoblotting the cell lysates with mAb 9E10. As expected, PS1−/−/PS2−/− MEF failed to process NotchΔEMV-6myc, but the cells stably expressing WT PS1 generated readily detectible levels of NICD, with a concomitant reduction in the levels of NotchΔEMV-6myc precursor (Fig. 3B, middle panel). Interestingly, with the exception of LL1Ins and 28/32Ala, all other LL1 mutants we examined failed to generate NICD and accumulated NotchΔEMV-6myc precursor at levels comparable with that found in PS1−/−/PS2−/− vector MEF lacking γ-secretase activity (Fig. 3B, middle panel).
Because in the studies described above, we transiently overexpressed APP or Notch substrates, it is possible that the results might not accurately reflect the efficiency of γ-secretase processing of endogenous substrates. To address this issue, we examined γ-secretase processing of endogenous N-cadherin in MEF. N-cadherin is cleaved by metalloprotease to generate a membrane-bound 40-kDa N-cadherin CTF, which is further cleaved by the γ-secretase to release a cytoplasmic fragment (48). A C-terminal antibody detects endogenous full-length N-cadherin and the 40-kDa CTF in PS1−/−/PS2−/− vector MEF lacking γ-secretase activity, whereas the 40-kDa CTF is not detectible in PS1−/−/PS2−/− MEF stably expressing WT PS1 (Fig. 3B, bottom panel). Thus, the accumulation of this CTF is an indication of the lack of γ-secretase processing of N-cadherin. Immunoblots show the loss of γ-secretase processing of N-cadherin in all LL1 mutants with the exception of LL1Ins and 28/32Ala (Fig. 3B, bottom panel). It is interesting to note that PS1 LL1 variants MutA, 10/18Ala, 19/27Ala, and 13/18Ala generated reduced levels of Aβ40 and AICD but completely lost the ability to process Notch and N-cadherin. This finding indicates that PS1 LL1 sequences alerted in these mutants may contribute either to the recruitment of substrates to the γ-secretase complex, their transfer to the catalytic site, or substrate proteolysis.
Three of the mutants examined in this study: MutB, 8aaLL1, and 16aaLL1, exhibited a total loss of their ability to process APP CTF, C99–6myc, NotchΔEMV-6myc, or N-Cardherin (Fig. 3, A and B). We wanted to determine whether this defect is due to the inability of mutant LL1 containing γ-secretase complexes to recruit these substrates for proteolytic processing. To this end, we expressed APPSwe in PS1−/−/PS2−/− MEF stably expressing MutB, 8aaLL1, or 16aaLL1 and co-immunoprecipitated γ-secretase complexes with PS1NT antibody. Immunoblot analysis of bound proteins revealed co-immunoprecipitation of APP CTF with mutant PS1 polypeptides (Fig. 3C). Similarly, we performed co-immunoprecipitation experiments to determine whether PS1 LL1mutants were able to bind to NotchΔEMV-6myc and endogenous N-cadherin CTF substrates. These experiments clearly show that both NotchΔEMV-6myc and N-cadherin CTF were able to bind to PS1 LL1 mutants (Fig. 3D). In cells expressing mutants with the least catalytic activity, the complex of enzyme-substrate lingers around longer (because whatever substrate is bound to PS1 is not processed), thus enabling us to get a better co-immunoprecipitation signal. Alternatively, these data can be interpreted to mean that certain mutants that bind the substrate tightly (enabling us to get a better co-immunoprecipitation signal) are unable to process the substrate. These results indicate that MutB, 8aaLL1, and 16aaLL1 complexes are able to successfully recognize and associate with these substrates but are either defective in substrate proteolysis or unable to transfer the substrates to the catalytic site following their initial recruitment into the γ-secretase complex.
Residues within TM2 Are Important for PS1 Endoproteolysis
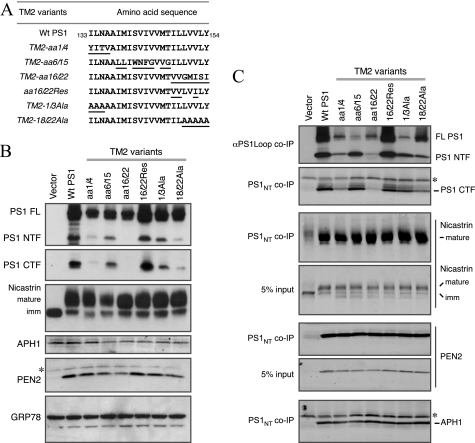
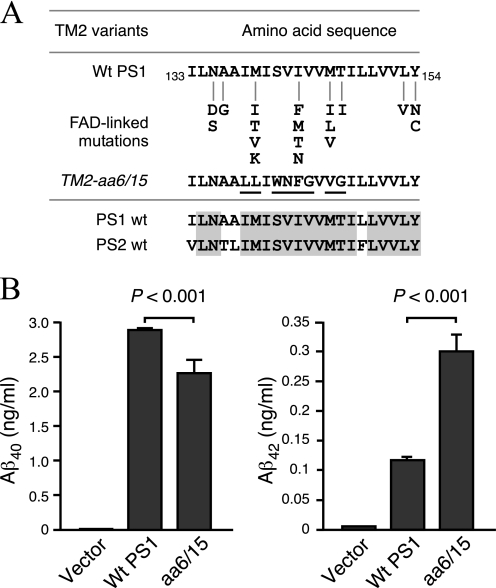
At least 21 FAD-linked PSEN1 mutations (which alter 8 residues) have been identified within the putative TM2 domain of PS1, leading to the suggestion that TM2 may contain sequences that are critical for γ-secretase cleavage site modulation of Aβ40 and Aβ42 production. To investigate whether domains within TM2 have a critical role in PS1 endoproteolysis as well as processing of different γ-secretase substrates, we generated a set of PS1 TM2 variants. To maintain the hydrophobic property of the TM2 domain, we divided the predicted TM2 domain (Ile133–Tyr154) into three regions and exchanged them individually with the corresponding sequence from TM4 of PS1 (aa 195–213). To generate TM2-aa1/4, we replaced the first 4 aa (133ILNA) with the first 4 aa of PS1 TM4 (195YITV). Similarly, in mutant TM2-aa6/15 aa 6–15 of TM2 (138IMISVIVVMT) were replaced with aa 6–15 of TM4 (200LLIWNFGVVG). In mutant TM2-aa16/22, the last 7 aa of TM2 (148ILLVVLY) were replaced with the last 7 aa of TM4 (207VVGMISI) (Fig. 4A).
FIGURE 4.
PS1 endoproteolysis and the formation of γ-secretase complex in PS1 TM2 variants. A, the aa sequence of the PS1 TM2 domain is depicted along with the corresponding sequence of each PS1 TM2 mutant. The aa substitutions are underlined. B, lysates of PS1−/−/PS2−/− MEF stably transduced with retroviruses expressing WT PS1 or the indicated TM2 mutants were analyzed by immunoblotting with antibodies against PS1, nicastrin, APH1aL, and PEN2. The levels of GRP78 were analyzed as a loading control. C, co-immunoprecipitation (co-IP) analysis of γ-secretase subunits was performed essentially as described under the legends to Fig. 2. The asterisks indicate nonspecific reactivity.
When stably expressed in PS1−/−/PS2−/− MEF, TM2-aa6/15 was endoproteolytically processed to levels comparable with that of WT PS1, whereas the levels of PS1 derivatives were markedly reduced in cells expressing TM2-aa1/4 and TM2-aa16/22 mutants. To further narrow down the critical residues within the TM2 residues 16–22 by assaying for the rescue of PS1 endoproteolysis, we generated an additional mutant termed TM2-aa16/22Res where four of the seven residues mutated in TM2-aa16/22 were restored to the WT sequence. This change allowed the full recovery of PS1 endoproteolysis into NTF and CTF (Fig. 4B). Finally, to rule out the possibility that the observed defects in PS1 endoproteolysis are entirely due to the TM4 sequences inserted into TM2 domain, we generated two alanine mutants, TM2-1/3Ala and TM2-18/22Ala, where the first 3 and last 4 residues were replaced with alanine. We found that TM2-1/3Ala was endoproteolytically processed, but processing of TM2-18/22Ala was markedly impaired (Fig. 4B). Western blot analysis of lysates showed that despite the differences in the steady-state levels of PS1 NTF and CTF generated from the TM2 mutants, stable expression of each mutant restored nicastrin maturation and PEN2 protein stability, suggesting that each mutant was able to interact with these subunits. Co-immunoprecipitation experiments with PS1NT antibody verified that each PS1 TM2 mutant was able to associate with endogenous mature nicastrin, PEN2, and APH1 to form γ-secretase complexes (Fig. 4C). We generated one additional construct, TM2-aa6/15-TM4YI where we mutated the TM4 residues NF to YI in TM2-aa6/15 to ensure that the presence of TM4 residues within TM2 did not confound our data interpretation. As reported previously (39, 40), this mutation was sufficient to disrupt PS1 interaction with PEN2 (supplemental Fig. S4), formally establishing that association between TM2-aa6/15 and PEN2 was still mediated through the critical NF motif in TM4. These results indicate that the C-terminal end of the TM2 domain contains critical residues that are important for PS1 endoproteolysis but not for γ-secretase complex assembly.
Subdomains in TM2 Differentially Affect the Processing of APP, Notch, and N-cadherin
We performed a series of experiments to assess the contribution of TM2 to γ-secretase processing of substrates including full-length APP, APP C99–6myc, NotchΔEMV-6myc, and N-cadherin. Analysis of APP processing following transient expression of full-length APPSwe showed a marked loss of γ-secretase activity toward processing of APP in the TM2 mutants aa1/4, aa16/22, 1/3Ala, and 18/22Ala, evidenced by the accumulation of APP CTFs and reduced secretion of Aβ40 peptides (Fig. 5A). Processing of APP C99–6myc was also severely impaired in TM2 mutants aa1/4 and aa16/22 but not in 1/3Ala and 18/22Ala (Fig. 5B). Co-immunoprecipitation analysis showed association between aa1/4, aa16/22, 1/3Ala, and 18/22Ala with APP CTFs, albeit to varying levels (Fig. 5C). Aβ40 secretion and AICD production in TM2 mutants aa6/15 and aa16/22Res were comparable with that of WT PS1. Although cells expressing the mutants 1/3Ala and 18/22Ala secreted significantly lower levels of Aβ40 relative to WT PS1 cells, AICD production in these cells were only slightly lower comparable with that of WT PS1, indicating subtle differences in the efficiency of APP processing at γ- and ϵ-sites by TM2 mutants 1/3Ala and 18/22Ala (Fig. 5, A and B).
FIGURE 5.
γ-Secretase processing of different substrates by PS1 TM2 variants. A, stable PS1−/−/PS2−/− MEF pools expressing PS1 TM2 variants were transiently infected with APPSwe retrovirus. The cell lysates were analyzed by immunoblotting with antibody CTM1. The levels of secreted Aβ40 peptides in the conditioned media were quantified by ELISA. B, stable PS1−/−/PS2−/− MEF pools expressing PS1 TM2 variants were transiently infected with a retrovirus expressing APP C99–6Myc (top panel) or NotchΔEMV-6myc (middle panel). The lysates were analyzed by immunoblotting with mAb 9E10. The blots were reprobed with N-cadherin antibody (bottom panel). C, co-immunoprecipitation (co-IP) of γ-secretase and substrates. Stable PS1−/−/PS2−/− MEF pools expressing PS1 TM2 variants were transiently infected with retrovirus expressing APPSwe or NotchΔEMV-6myc. The lysates were analyzed by co-immunoprecipitation with PS1NT antibody and immunoblotted with APP, 9E10, or N-cadherin antibodies.
At first glance, the data from the middle region of TM2 does not appear very exciting because in mutant TM2-aa6/15 (TM2 residues 6–15 138IMISVIVVMT replaced with TM4 residues 6–15 200LLIWNFGVVG), PS1 undergoes endoproteolysis, and APP is processed to generate Aβ. However, 12 familial AD-linked mutations are located within TM2 residues 6–15, including two substitutions I143F and M146V that are present in mutant TM2-aa6/15 (Fig. 6A). This prompted us to examine the generation of Aβ42 in this particular mutant. PS1−/−/PS2−/− MEF stably expressing TM2-aa6/15 secreted 2.5-fold higher levels of Aβ42 as compared with cells expressing WT PS1 (0.2935 ± 0.029 versus 0.113 ± 0.005 ng/ml Aβ42, respectively). Moreover, stable TM2-aa6/15 MEF secreted significantly lower levels of Aβ40 relative to WT PS1 cells such that the ratio of Aβ42/Aβ40 secreted by cells expressing mutant TM2-aa6/15 is 3.3-fold higher than the cells expressing WT PS1 (0.13 ± 0.004 versus 0.039 ± 0.002, respectively) (Fig. 6B).
FIGURE 6.
Increase of Aβ42 generation by substitutions within TM2. A, the sequence of TM2 domain of WT PS1, WT PS2, and TM2 aa6/15 variant are shown. FAD-linked mutations found within TM2 domain are indicated. Substitutions in aa6/15 variant are underlined. B, stable PS1−/−/PS2−/− MEF pools expressing PS1 TM2 variants were transiently infected with a retrovirus expressing APPSwe. Conditioned medium was collected 48 h after infection, and the levels of secreted Aβ40 and Aβ42 peptides were measured by ELISA.
Examination of NotchΔEMV-6myc processing revealed NICD production in PS1−/−/PS2−/− MEF stably expressing the mutants aa6/15, aa16/22, aa16/22Res, and 18/22Ala (Fig. 5B). Concomitantly, NotchΔEMV-6myc levels were markedly reduced in these cells as compared with PS1−/−/PS2−/− harboring empty vector. Cells expressing the mutants aa1/4 and 1/3Ala accumulated high levels of NotchΔEMV-6myc and were impaired in NICD production (Fig. 5B). Both of these mutants can be co-immunoprecipitated with NotchΔEMV-6myc, indicating successful interaction of Notch with PS1 complexes (Fig. 5C). There were two surprising findings: first, cells expressing the 1/3AlaTM2 mutant showed no evidence of NICD production, although they produced considerable levels of AICD; second, cells expressing TM2-aa16/22 showed the opposite scenario where NICD was produced to some extent, but AICD production was markedly impaired. Analysis of N-cadherin processing revealed the unexpected finding that with the exception of TM2-aa16/22Res, all other mutants accumulated N-cadherin CTF, indicative of a loss of γ-secretase processing of N-cadherin (Fig. 5B). Notably, cells stably expressing TM2-aa6/15 accumulated high levels of uncleaved N-cadherin CTF, despite efficient Aβ40 and Aβ42 secretion as well as Notch processing (Fig. 5B). Successful co-immunoprecipitation of N-cadherin CTF with PS1 indicates that impaired processing cannot be attributed to a lack of substrate recognition (Fig. 5C). Together, analysis of TM2 mutants reveals a spectrum of phenotypes with reference to PS1 endoproteolysis as well as processing of different substrates at γ- and ϵ-sites.
Mutations within the LL1 and TM2 Domains Do Not Affect Subcellular Localization of PS1
By confocal microscopy we previously reported that endogenous PS1 localizes to multiple organelles including the ER, ER/Golgi intermediate compartments, Golgi apparatus, TGN-derived vesicles, and endosomes (49). This steady-state localization represents staining of nascent PS1 in the ER and PS1-derived NTF/CTF heterodimers that have successfully assembled with nicastrin, APH1, and PEN2 and exited the ER. However, it is known that when PS1 is overexpressed, unassembled PS1 is mainly retained in the ER as a holoprotein. We conducted double immunofluorescence staining experiments to visualize the subcellular localization of WT PS1 and PS1 variants stably overexpressed in PS1−/−/PS2−/− MEF. As expected, overexpressed WT PS1 predominantly showed a reticular staining pattern consistent with localization in the ER but also shows co-localization with the Golgi marker GM130 (supplemental Fig. S2). We did not observe marked differences in the reticular staining pattern or co-localization with GM130 in cells overexpressing LL1 or TM2 mutants (supplemental Fig. S2). Co-localization of WT and mutant PS1 with GM130 in the Golgi is consistent with the results of our co-immunoprecipitation experiments, which demonstrated that the LL1 and TM2 mutants indeed assemble with other subunits to form γ-secretase complexes, which normally exit the ER upon assembly.
Finally, we performed sucrose density gradient analysis to determine the ability of PS1 variants generated in this study to partition into detergent-resistant membrane domains. Previously we reported that all four integral subunits of γ-secretase associate with detergent-resistant membranes, indicating that they are present in membrane microdomains rich in cholesterol and sphingolipids, termed lipid rafts (31, 49). Therefore we asked whether mutations within LL1 and TM2 domains affect detergent-resistant membrane association of PS1. In addition to full-length PS1 and PS1 NTF, we examined the levels of endogenous nicastrin as a measure of γ-secretase complex distribution in detergent-resistant membranes. We found that with the exception of 8aaLL1 and 16aaLL1 mutants, NTFs derived from PS1 variants partitioned into detergent-resistant fractions, albeit at lower efficiency when compared with NTFs derived from WT PS1 (supplemental Fig. S3). Analysis of nicastrin revealed that in stable cells overexpressing PS1 variants that are processed, the levels of mature nicastrin in detergent-resistant fractions parallel that of PS1 NTFs (supplemental Fig. S3). Like WT PS1 holoprotein, the majority of PS1 holoprotein of each mutant remained in detergent-soluble nonraft fractions, although variable levels of certain mutant PS1 holoprotein were detectable in detergent-resistant raft fractions as is the case in 10/18Ala, aa1/4, aa16/22, and 18/22Ala. Thus, detergent-resistant lipid raft association of PS1 variants tested in this study largely reflects their ability to assemble with other γ-secretase subunits and their endoproteolysis status.
DISCUSSION
γ-Secretase mediates the intramembrane proteolysis of a diverse set of type I membrane substrates that undergo ectodomain shedding (50). Extensive research is beginning to reveal much needed insights to understand how γ-secretase recruits and proteolyses its substrates. It is established that the highly conserved multipass membrane proteins PS1 and PS2 function as the catalytic subunits of γ-secretase (18, 51). γ-Secretase substrates are thought to first interact with a putative docking site in PS1 before they are presented to the active site (52). These results and other evidence indicate that at the minimum, cleavage of a substrate by γ-secretase involves three sequential steps: 1) binding of the substrate to the docking site; 2) transfer of the substrate from the docking site to the catalytic site; and 3) proteolysis of the substrate. Although several studies have focused on the aspartate residues that are critical for PS1 catalytic activity (17), little is known about the sequences within PS1 or other γ-secretase subunits that meditate substrate docking and transfer to the catalytic pocket. In this study, by introducing mutations in LL1 and TM2, we identified residues that are critical for PS1 processing and/or γ-secretase activity toward three substrates, APP, Notch, and N-cadherin (Table 1). Although mutant PS1 polypeptides were able to bind all three substrates, interesting differences in the extent of their processing at γ- and ϵ-sites suggest that the region encompassing LL1 and TM2 of PS1 plays important role(s) in substrate catalysis and/or coordinating the transfer of substrates from the initial putative docking site to the catalytic pocket.
TABLE 1.
Summary of PS1 LL1 and TM2 experimental mutant characterization
The extent of PS1 endoproteolysis, Aβ secretion, and processing of C99-6myc, NotchΔEMV-6myc, and N-cadherin CTF are indicated. ++, robust activity comparable with that of WT PS1. +, reduced activity relative to WT PS1. +/−, only weak activity. −, no activity.
| PS1 processing | Aβ generation | AICD generation | Notch processing | N-Cadherin processing | |
|---|---|---|---|---|---|
| PS1-wt | ++ | ++ | ++ | ++ | ++ |
| LL1Ins | ++ | ++ | ++ | ++ | ++ |
| MutA | ++ | + | + | − | − |
| MutB | + | − | − | − | − |
| 8aaLL1 | − | − | − | − | − |
| 16aaLL1 | + | − | − | − | − |
| LL1-10/18Ala | + | + | + | − | − |
| LL1-13/18Ala | ++ | + | + | − | − |
| LL1-19/27Ala | + | + | + | − | − |
| LL1-28/32Ala | ++ | ++ | ++ | ++ | ++ |
| TM2-1/3Ala | ++ | + | + | − | − |
| TM2-aa1/4 | +/− | − | − | − | − |
| TM2-aa6/15 | ++ | ++ | ++ | ++ | − |
| TM2-aa16/22 | − | − | − | + | − |
| TM2-18/22Ala | + | + | + | + | − |
| TM2-aa16/22Res | ++ | ++ | ++ | ++ | ++ |
Residues within LL1 and TM2 of PS1 Contribute to Endoproteolytic Processing
Previously, two reports showed that deletion of the region encompassing the first two TM domains of PS1 or PS2 impairs endoproteolytic processing without affecting the stability of PS1 or PS2 (29, 34). In the present study, we have used deletion and substitution mutagenesis to characterize the critical sequences within the LL1 and TM2 segments. We find that PS1 endoproteolysis is severely impaired in four LL1 mutants (MutBLL1, 8aaLL1, 16aaLL1, and 19/22AlaLL1) and three TM2 mutants (TM2-aa1/4, TM2-aa16/22, and 18/22AlaTM2) (Figs. 2 and 4). Close examination of the data revealed that the sequences in the middle of the LL1 segment (119TEDTETVGQ) and the residues at either ends of TM2 (133ILNA and 150LVVLY) contribute to PS1 endoproteolysis. Mutations that altered these residues resulted in marked reduction in the levels of PS1 NTF and CTF. Culturing PS1−/−/PS2−/− MEF stably expressing these mutants in the presence of proteasome inhibitors (lactacystin and MG262) had no effect on the steady-state levels of the levels of PS1 NTF and CTF (not shown), indicating that PS1 endoproteolysis is impaired in these mutants.
LL1 and TM2 of PS1 Are Unlikely to Be Involved in the Formation of γ-Secretase Complex or Recruitment of Substrates
How γ-secretase complex is assembled and recruits its substrates remains intriguing. The details regarding the sequence of events in the assembly of the four integral subunits into mature γ-secretase complexes are not fully understood at the present time. The following subcomplexes have been identified or suggested to exist: nicastrin-APH1, PS1-PEN2, PS1-nicastrin-APH1, and PS1-APH1-PEN2 (53). Thus, exactly how γ-secretase components assemble into functional complexes remains controversial. Nevertheless, it is widely accepted that PS1 is the catalytic subunit of γ-secretase and that the other components function in stabilizing, trafficking, or scaffolding of the γ-secretase complex. Accumulating evidence also suggests that PS1 itself is responsible for the initial substrate docking and selection (20, 54–56). Although nicastrin has been suggested to function as the receptor that facilitated the initial recognition of γ-secretase substrate, a consensus has not emerged (55–57).
Each of the LL1 and TM2 mutants generated in this study was able to form γ-secretase complexes (Figs. 2C and 4C) and recruit the three substrates tested (Figs. 3, C and D, and 5C). These findings are in agreement with the published findings that the C terminus of PS1 binds to the nicastrin-APH1 subcomplex (41, 58); TM4 of PS1 binds to PEN2 (39, 40); and the TM1 and C terminus of PS1 interacts with APP (59). Because the TM1, TM4, and C terminus of PS1 are intact in each of the PS1 mutants we generated, we did not expect that the formation of γ-secretase complex or APP recruitment to the γ-secretase complex will be perturbed. Our data also suggest that LL1 and TM2 of PS1 are not involved in the initial recruitment of Notch or N-cadherin to PS1 complexes. In several of our PS1 mutants (namely MutBLL1, 8aaLL1, 16aaLL1, 10/18AlaLL1, 19/27AlaLL1, TM2-aa1/4, TM2-aa16/22, TM2-18/22Ala, and TM2-aa6/15-TM4YI), PS1 endoproteolysis was severely impaired (judging from the lower steady-state levels of PS1 NTF and CTF generated from the holoprotein), yet the formation of γ-secretase complex was not markedly affected (Figs. 2C and 4C and supplemental Fig. S4B). Together, these observations are consistent with previous conclusions that PS1 endoproteolysis and nicastrin maturation can occur after the initial assembly of γ-secretase complex within the ER (60, 61). Moreover, as illustrated by MutBLL1 and 16aaLL1, successful association of PS1 with other γ-secretase subunits and recruitment of substrates is still not a predictor for the constitution of active γ-secretase capable of substrate proteolysis.
LL1 and TM2 of PS1 Contribute to Catalysis or Transfer of Docked Substrates to the Catalytic Site
In our experimental mutants, the extent of APP cleavage at γ- and ϵ-sites showed a high degree of similarity, whereas a comparison of APP, Notch, and N-cadherin revealed interesting differences in their relative cleavage efficiencies. In each of the mutants examined in this study, it is evident that successful generation of Aβ40 (an indication of γ-cleavage of APP) is accompanied by the generation of AICD (an indication of ϵ-cleavage of APP). However, when one compares the processing of the three γ-secretase substrates (Figs. 3B and 5B), different patterns can be observed: APP is cleaved but not Notch or N-cadherin (10/18AlaLL1, 19/27AlaLL1, 1/3AlaTM2), APP and Notch are cleaved but not N-cadherin (TM2-aa6/15 and 18/22AlaTM2), and Notch is cleaved but not APP or N-cadherin (TM2-aa16/22). Collectively, these data suggest that sequences within LL1 and TM2 of PS1 play important role(s) in catalysis of different substrates or the transfer of docked substrates to the catalytic site but have little effect on the relative efficiency of cleavage at γ- or ϵ-sites within the same substrate (at least in the case of APP).
In the past few years, several domains of PS1 have been extensively studied. In one study, TM6 and TM7 of PS1 were shown to contribute to the formation of a hydrophilic pore where the proteolysis of the substrates occurs and to contribute to enzyme specificity toward different substrates (23). The C-terminal PAL motif and TM9 were defined to be part of the hydrophilic pore in another study (24). Recently, the large hydrophilic loop (the loop between TM6 and 7) was shown to be important for APP but not Notch processing (62). Our study focused on lumen loop 1 (the loop between TM1 and 2) and TM2 and revealed that the LL1and TM2 region plays an important role in enzyme specificity toward different substrates but not to the distinction between γ- or ϵ-cleavage within APP. Our study contributes to the ongoing efforts of understanding how γ-secretase selects, recruits, and acts on its substrates. The results of our study show that LL1 and TM2 of PS1 are critical for PS1 endoproteolysis and for substrate catalysis and/or coordinating the transfer of docked substrates to the catalytic site of γ-secretase for proteolysis. However, this domain of PS1 has little influence on γ-secretase assembly, initial recruitment of the substrates, or directing the cleavage at γ- or ϵ-site on APP.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants AG021495 (to G. T.), AG019070 (to G. T.), and NS055223 (to A. T. P.). This work was also supported by grants from the Alzheimer's Association (to G. T. and K. S. V.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental text and Figs. S1–S4.
- Aβ
- β-amyloid
- aa
- amino acid(s)
- APP
- amyloid precursor protein
- CTF
- C-terminal fragment
- LL1
- luminal loop domain 1
- NTF
- N-terminal fragment
- PS
- presenilin(s)
- TM
- transmembrane domain;
- ER
- endoplasmic reticulum
- AICD
- APP intracellular domain
- NICD
- Notch intracellular domain
- MEF
- mouse embryonic fibroblast(s)
- CHAPSO
- 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propanesulfonic acid.
REFERENCES
- 1.Thinakaran G., Koo E. H. (2008) J. Biol. Chem. 283, 29615–29619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwatsubo T. (2004) Curr. Opin. Neurobiol. 14, 379–383 [DOI] [PubMed] [Google Scholar]
- 3.Iwatsubo T. (2004) Mol. Psychiatry 9, 8–10 [DOI] [PubMed] [Google Scholar]
- 4.Vetrivel K. S., Thinakaran G. (2006) Neurology 66 (2 Supp1 1), S69–S73 [DOI] [PubMed] [Google Scholar]
- 5.Steiner H. (2008) Curr. Alzheimer Res. 5, 147–157 [DOI] [PubMed] [Google Scholar]
- 6.Leem J. Y., Vijayan S., Han P., Cai D., Machura M., Lopes K. O., Veselits M. L., Xu H., Thinakaran G. (2002) J. Biol. Chem. 277, 19236–19240 [DOI] [PubMed] [Google Scholar]
- 7.Wang H., Luo W. J., Zhang Y. W., Li Y. M., Thinakaran G., Greengard P., Xu H. (2004) J. Biol. Chem. 279, 40560–40566 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y. W., Luo W. J., Wang H., Lin P., Vetrivel K. S., Liao F., Li F., Wong P. C., Farquhar M. G., Thinakaran G., Xu H. (2005) J. Biol. Chem. 280, 17020–17026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thinakaran G., Borchelt D. R., Lee M. K., Slunt H. H., Spitzer L., Kim G., Ratovitsky T., Davenport F., Nordstedt C., Seeger M., Hardy J., Levey A. I., Gandy S. E., Jenkins N. A., Copeland N. G., Price D. L., Sisodia S. S. (1996) Neuron 17, 181–190 [DOI] [PubMed] [Google Scholar]
- 10.Thinakaran G., Regard J. B., Bouton C. M., Harris C. L., Price D. L., Borchelt D. R., Sisodia S. S. (1998) Neurobiol. Dis. 4, 438–453 [DOI] [PubMed] [Google Scholar]
- 11.Thinakaran G., Harris C. L., Ratovitski T., Davenport F., Slunt H. H., Price D. L., Borchelt D. R., Sisodia S. S. (1997) J. Biol. Chem. 272, 28415–28422 [DOI] [PubMed] [Google Scholar]
- 12.Kim S. H., Ikeuchi T., Yu C., Sisodia S. S. (2003) J. Biol. Chem. 278, 33992–34002 [DOI] [PubMed] [Google Scholar]
- 13.Kimberly W. T., LaVoie M. J., Ostaszewski B. L., Ye W., Wolfe M. S., Selkoe D. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 6382–6387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W., Larson E., Levy-Lahad E., Viitanen M., Peskind E., Poorkaj P., Schellenberg G., Tanzi R., Wasco W., Lannfelt L., Selkoe D., Younkin S. (1996) Nat. Med. 2, 864–870 [DOI] [PubMed] [Google Scholar]
- 15.Borchelt D. R., Thinakaran G., Eckman C. B., Lee M. K., Davenport F., Ratovitsky T., Prada C. M., Kim G., Seekins S., Yager D., Slunt H. H., Wang R., Seeger M., Levey A. I., Gandy S. E., Copeland N. G., Jenkins N. A., Price D. L., Younkin S. G., Sisodia S. S. (1996) Neuron 17, 1005–1013 [DOI] [PubMed] [Google Scholar]
- 16.Citron M., Westaway D., Xia W., Carlson G., Diehl T., Levesque G., Johnson-Wood K., Lee M., Seubert P., Davis A., Kholodenko D., Motter R., Sherrington R., Perry B., Yao H., Strome R., Lieberburg I., Rommens J., Kim S., Schenk D., Fraser P., St George Hyslop P., Selkoe D. J. (1997) Nat. Med. 3, 67–72 [DOI] [PubMed] [Google Scholar]
- 17.Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. (1999) Nature 398, 513–517 [DOI] [PubMed] [Google Scholar]
- 18.Li Y. M., Xu M., Lai M. T., Huang Q., Castro J. L., DiMuzio-Mower J., Harrison T., Lellis C., Nadin A., Neduvelil J. G., Register R. B., Sardana M. K., Shearman M. S., Smith A. L., Shi X. P., Yin K. C., Shafer J. A., Gardell S. J. (2000) Nature 405, 689–694 [DOI] [PubMed] [Google Scholar]
- 19.Esler W. P., Kimberly W. T., Ostaszewski B. L., Diehl T. S., Moore C. L., Tsai J. Y., Rahmati T., Xia W., Selkoe D. J., Wolfe M. S. (2000) Nat. Cell Biol. 2, 428–434 [DOI] [PubMed] [Google Scholar]
- 20.Kornilova A. Y., Bihel F., Das C., Wolfe M. S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3230–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazarov V. K., Fraering P. C., Ye W., Wolfe M. S., Selkoe D. J., Li H. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 6889–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato C., Morohashi Y., Tomita T., Iwatsubo T. (2006) J. Neurosci. 26, 12081–12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolia A., Chávez-Gutiérrez L., De Strooper B. (2006) J. Biol. Chem. 281, 27633–27642 [DOI] [PubMed] [Google Scholar]
- 24.Sato C., Takagi S., Tomita T., Iwatsubo T. (2008) J. Neurosci. 28, 6264–6271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolia A., Horré K., De Strooper B. (2008) J. Biol. Chem. 283, 19793–19803 [DOI] [PubMed] [Google Scholar]
- 26.Kopan R., Ilagan M. X. (2004) Nat. Rev. Mol. Cell Biol. 5, 499–504 [DOI] [PubMed] [Google Scholar]
- 27.Cao X., Südhof T. C. (2001) Science 293, 115–120 [DOI] [PubMed] [Google Scholar]
- 28.De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J. S., Schroeter E. H., Schrijvers V., Wolfe M. S., Ray W. J., Goate A., Kopan R. (1999) Nature 398, 518–522 [DOI] [PubMed] [Google Scholar]
- 29.Leem J. Y., Saura C. A., Pietrzik C., Christianson J., Wanamaker C., King L. T., Veselits M. L., Tomita T., Gasparini L., Iwatsubo T., Xu H., Green W. N., Koo E. H., Thinakaran G. (2002) Neurobiol. Dis. 11, 64–82 [DOI] [PubMed] [Google Scholar]
- 30.Morita S., Kojima T., Kitamura T. (2000) Gene Ther. 7, 1063–1066 [DOI] [PubMed] [Google Scholar]
- 31.Vetrivel K. S., Cheng H., Kim S. H., Chen Y., Barnes N. Y., Parent A. T., Sisodia S. S., Thinakaran G. (2005) J. Biol. Chem. 280, 25892–25900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng H., Vetrivel K. S., Drisdel R. C., Meckler X., Gong P., Leem J. Y., Li T., Carter M., Chen Y., Nguyen P., Iwatsubo T., Tomita T., Wong P. C., Green W. N., Kounnas M. Z., Thinakaran G. (2009) J. Biol. Chem. 284, 1373–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vetrivel K. S., Gong P., Bowen J. W., Cheng H., Chen Y., Carter M., Nguyen P. D., Placanica L., Wieland F. T., Li Y. M., Kounnas M. Z., Thinakaran G. (2007) Mol. Neurodegener. 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cervantes S., Saura C. A., Pomares E., Gonzàlez-Duarte R., Marfany G. (2004) J. Biol. Chem. 279, 36519–36529 [DOI] [PubMed] [Google Scholar]
- 35.Herreman A., Van Gassen G., Bentahir M., Nyabi O., Craessaerts K., Mueller U., Annaert W., De Strooper B. (2003) J. Cell Sci. 116, 1127–1136 [DOI] [PubMed] [Google Scholar]
- 36.Nyabi O., Bentahir M., Horré K., Herreman A., Gottardi-Littell N., Van Broeckhoven C., Merchiers P., Spittaels K., Annaert W., De Strooper B. (2003) J. Biol. Chem. 278, 43430–43436 [DOI] [PubMed] [Google Scholar]
- 37.Gu Y., Chen F., Sanjo N., Kawarai T., Hasegawa H., Duthie M., Li W., Ruan X., Luthra A., Mount H. T., Tandon A., Fraser P. E., St George-Hyslop P. (2003) J. Biol. Chem. 278, 7374–7380 [DOI] [PubMed] [Google Scholar]
- 38.Shirotani K., Edbauer D., Kostka M., Steiner H., Haass C. (2004) J. Neurochem. 89, 1520–1527 [DOI] [PubMed] [Google Scholar]
- 39.Watanabe N., Tomita T., Sato C., Kitamura T., Morohashi Y., Iwatsubo T. (2005) J. Biol. Chem. 280, 41967–41975 [DOI] [PubMed] [Google Scholar]
- 40.Kim S. H., Sisodia S. S. (2005) J. Biol. Chem. 280, 41953–41966 [DOI] [PubMed] [Google Scholar]
- 41.Kaether C., Capell A., Edbauer D., Winkler E., Novak B., Steiner H., Haass C. (2004) EMBO J. 23, 4738–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weidemann A., Eggert S., Reinhard F. B., Vogel M., Paliga K., Baier G., Masters C. L., Beyreuther K., Evin G. (2002) Biochemistry 41, 2825–2835 [DOI] [PubMed] [Google Scholar]
- 43.Moehlmann T., Winkler E., Xia X., Edbauer D., Murrell J., Capell A., Kaether C., Zheng H., Ghetti B., Haass C., Steiner H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 8025–8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hecimovic S., Wang J., Dolios G., Martinez M., Wang R., Goate A. M. (2004) Neurobiol. Dis. 17, 205–218 [DOI] [PubMed] [Google Scholar]
- 45.Tesco G., Ginestroni A., Hiltunen M., Kim M., Dolios G., Hyman B. T., Wang R., Berezovska O., Tanzi R. E. (2005) J. Neurochem. 95, 446–456 [DOI] [PubMed] [Google Scholar]
- 46.Kakuda N., Funamoto S., Yagishita S., Takami M., Osawa S., Dohmae N., Ihara Y. (2006) J. Biol. Chem. 281, 14776–14786 [DOI] [PubMed] [Google Scholar]
- 47.Schroeter E. H., Kisslinger J. A., Kopan R. (1998) Nature 393, 382–386 [DOI] [PubMed] [Google Scholar]
- 48.Marambaud P., Wen P. H., Dutt A., Shioi J., Takashima A., Siman R., Robakis N. K. (2003) Cell 114, 635–645 [DOI] [PubMed] [Google Scholar]
- 49.Vetrivel K. S., Cheng H., Lin W., Sakurai T., Li T., Nukina N., Wong P. C., Xu H., Thinakaran G. (2004) J. Biol. Chem. 279, 44945–44954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolfe M. S., Kopan R. (2004) Science 305, 1119–1123 [DOI] [PubMed] [Google Scholar]
- 51.Wolfe M. S., De Los Angeles J., Miller D. D., Xia W., Selkoe D. J. (1999) Biochemistry 38, 11223–11230 [DOI] [PubMed] [Google Scholar]
- 52.Wolfe M. S. (2006) Biochemistry 45, 7931–7939 [DOI] [PubMed] [Google Scholar]
- 53.Spasic D., Annaert W. (2008) J. Cell Sci. 121, 413–420 [DOI] [PubMed] [Google Scholar]
- 54.Yamasaki A., Eimer S., Okochi M., Smialowska A., Kaether C., Baumeister R., Haass C., Steiner H. (2006) J. Neurosci. 26, 3821–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Futai E., Yagishita S., Ishiura S. (2009) J. Biol. Chem. 284, 13013–13022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao G., Liu Z., Ilagan M. X., Kopan R. (2010) J. Neurosci. 30, 1648–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah S., Lee S. F., Tabuchi K., Hao Y. H., Yu C., LaPlant Q., Ball H., Dann C. E., 3rd, Südhof T., Yu G. (2005) Cell 122, 435–447 [DOI] [PubMed] [Google Scholar]
- 58.Bergman A., Hansson E. M., Pursglove S. E., Farmery M. R., Lannfelt L., Lendahl U., Lundkvist J., Näslund J. (2004) J. Biol. Chem. 279, 16744–16753 [DOI] [PubMed] [Google Scholar]
- 59.Annaert W. G., Esselens C., Baert V., Boeve C., Snellings G., Cupers P., Craessaerts K., De Strooper B. (2001) Neuron 32, 579–589 [DOI] [PubMed] [Google Scholar]
- 60.Capell A., Beher D., Prokop S., Steiner H., Kaether C., Shearman M. S., Haass C. (2005) J. Biol. Chem. 280, 6471–6478 [DOI] [PubMed] [Google Scholar]
- 61.Kim J., Kleizen B., Choy R., Thinakaran G., Sisodia S. S., Schekman R. W. (2007) J. Cell Biol. 179, 951–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wanngren J., Frånberg J., Svensson A. I., Laudon H., Olsson F., Winblad B., Liu F., Näslund J., Lundkvist J., Karlström H. (2010) J. Biol. Chem. 285, 8527–8536 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.