Abstract
Nicotinic acid adenine dinucleotide phosphate (NAADP) is the most potent Ca2+-mobilizing intracellular messenger and is linked to a variety of stimuli and cell surface receptors. However, the enzyme responsible for endogenous NAADP synthesis in vivo is unknown, and it has been proposed that another enzyme differing from ADP-ribosyl cyclase family members may exist. The ecto-enzyme CD38, involved in many functions as diverse as cell proliferation and social behavior, represents an important alternative. In pancreatic acinar cells, the hormone cholecystokinin (CCK) stimulates NAADP production evoking Ca2+ signals by discharging acidic Ca2+ stores and leading to digestive enzyme secretion. From cells derived from CD38−/− mice, we provide the first physiological evidence that CD38 is required for endogenous NAADP generation in response to CCK stimulation. Furthermore, CD38 expression in CD38-deficient pancreatic AR42J cells remodels Ca2+-signaling pathways in these cells by restoring Ca2+ mobilization from lysosomes during CCK-induced Ca2+ signaling. In agreement with an intracellular site for messenger synthesis, we found that CD38 is expressed in endosomes. These CD38-containing vesicles, likely of endosomal origin, appear to be proximal to lysosomes but not co-localized with them. We propose that CD38 is an NAADP synthase required for coupling receptor activation to NAADP-mediated Ca2+ release from lysosomal stores in pancreatic acinar cells.
Keywords: Calcium Intracellular Release, Enzymes, Lysosomes, Pancreas, Signal Transduction, CD38, NAADP
Introduction
The Ca2+-mobilizing messenger nicotinic acid adenine dinucleotide phosphate (NAADP)5 is the most potent of the three established Ca2+-mobilizing messengers. NAADP was first shown to evoke Ca2+ release from intracellular stores in sea urchin eggs (1). It has been proposed that NAADP initially releases Ca2+ from lysosomal related organelles (2–6), which may then be amplified by Ca2+-induced Ca2+ release mechanisms in the endoplasmic reticulum (ER) (7). The recent demonstration that NAADP mobilizes Ca2+ from endolysosomal stores by targeting two-pore channels (1, 8, 9) has intensified interest in this messenger and its signaling pathways (10, 11). Two-pore channel-mediated Ca2+ release may be coupled to further Ca2+ release by inositol trisphosphate (IP3) receptors or ryanodine receptors (1, 8). To assign NAADP as a second messenger, it was important to show that endogenous levels of NAADP could be controlled by external stimuli, and now NAADP has been shown to be linked to various stimuli and cell surface receptors (12, 13). Dramatic increases in NAADP occur during sea urchin egg fertilization due to production in sperm upon contacting egg jelly (14). Increases in NAADP levels have been also reported in pancreatic β-cells after glucose (15) and glucagon-like peptide-1 stimulation (17), in pancreatic acinar cells after CCK stimulation (16), in pulmonary smooth muscle following exposure to endothelin-1 (3), in response to histamine in human myometrial cells (18), and glutamate in neurones (19). However, the enzymes responsible for endogenous NAADP synthesis in vivo remain to be clearly identified (20), although members of the ADP-ribosyl cyclase family are possibly involved because the purified CD38 and Aplysia ADP-ribosyl cyclases have been shown to be capable of NAADP synthesis in vitro (21–23). CD38 is a widely expressed mammalian ecto-enzyme CD38, involved in many functions as diverse as cell proliferation and social behavior (22, 23). From in vitro experiments, NAADP and the related Ca2+-mobilizing messenger, cyclic ADP-ribose (cADPR) can be synthesized by CD38 using alternate substrates and with different pH dependences (24). NAADP synthesis occurs by base exchange of the nicotinamide moiety of NADP with nicotinic acid with a pH optimum of between 4 and 5. However, surprisingly, only one study has linked the in vivo production of endogenous NAADP to any member of the ADP-ribosyl cyclase family (25) and a physiological role for CD38 as an NAADP synthase has been ruled out by some (17, 18).
In mammalian and human pancreatic acinar cells, cholecystokinin (CCK) and acetylcholine (ACh) are two important secretagogues that induce specific Ca2+ signals and stimulate digestive enzyme and fluid secretion (26). However, these agonists appear to be coupled to different combinations of Ca2+-mobilizing messengers to exert their effects (27). All three major Ca2+-mobilizing messengers, IP3, cADPR, and NAADP have been shown to play a role in stimulus-secretion coupling in these cells (27), but in mouse pancreatic acinar cells, the high affinity CCKA receptor, activated by low physiological picomolar CCK concentrations (28), appears to be coupled to NAADP signaling (7, 16, 29, 30). In addition, these low concentrations of CCK, like ACh, stimulates cADPR synthesis (16), but IP3 production only is linked to muscarinic and low affinity CCK receptors (28). In accordance with this, CCK mobilizes Ca2+ from both acidic and ER stores, whereas ACh mobilizes Ca2+ from the ER alone (4, 6, 31).
Using pancreatic acinar cells derived from CD38−/− mice, we provide the first physiological evidence for the requirement of CD38 in CCK-mediated Ca2+ signaling by NAADP. In pancreatic AR42J cells, which lack CD38, we found that expression of this new member of the Ca2+ signaling tool kit (32), was sufficient to remodel Ca2+-signaling pathways in these cells, so that now CCK receptor activation was linked to NAADP-mediated Ca2+ mobilization from lysosomes. Furthermore, in agreement with our previous work, our data suggest that CCK may enhance the endocytosis of plasma membrane CD38 into endosomal vesicles, thus creating an intracellular site for messenger synthesis (4, 33). These results reveal that CD38 is an NAADP synthase in mammalian cells coupling receptor activation to NAADP-dependent Ca2+ release from lysosomal stores.
MATERIALS AND METHODS
Pancreatic Acinar Cell Preparation
Male C57BL6 mice (CD38 sufficient) were purchased from CER Janvier and male C57BL/6 CD38−/− mice, which have a deletion of exons 2 and 3 and consequently exhibit no residual enzymatic activity in vitro (34) were obtained from the laboratory of F. Lund (Trudeau Institute). For genotyping, genomic DNA was isolated from mice tails using the Nucleospin tissue kit (Macherey-Nagel). Exon 2 was amplified by PCR, and products were analyzed by agarose gel electrophoresis. CD38−/− mice were bred in our animal house and used at 8–26 weeks of age for all experiments. Cells were prepared as described previously (27).
Cell Preparation and Culture
The rat pancreatic acinar cell line AR42J was cultured in 40% of HAM-F12 and DMEM containing 4.5 g liter−1 glucose and 2 mm glutamine, supplemented with 20% (v/v) fetal bovine serum and 1 mm sodium pyruvate.
Calcium Imaging in Pancreatic Acinar Cells
Cells were loaded with Fluo4-AM (5 μm) and stimulated at room temperature with CCK at 5 and 50 pm. Imaging are performed with a conventional microscope (Leica microscope, CCD camera) or using a confocal microscope (Leica SP2 RS).
Calcium and Lysosomes Imaging in Rat Pancreatic Acinar Cells and AR42J Cells
For imaging studies, cells were incubated in culture medium with Fura-2/AM (2 μm final and incubated 25 min at 37 °C). Lysosomes are stained 5 min at room temperature with 200 nm LysoTracker Red.
CD38 Base Exchange Activity
Cells were homogenized and incubated at pH 4.5 with 200 μm NADP and 14 mm nicotinic acid. Reactions were stopped with 10% (v/v) perchloric acid (4 °C). Samples were then neutralized with 2.5 m K2CO3 and analyzed on a Poros HQ column (Applied Biosystems) using HPLC (Beckman) coupled to a UV detector.
Acinar Cell Stimulation for Endogenous NAADP Measurements
Pancreatic acinar cells were prepared as above but with protease inhibitor mixture and rinsed in extracellular buffer under agitation during 30 min to regenerate calcium stores. Suspensions were stimulated with 50 pm CCK. Reactions were stopped with perchloric acid (20%) at an appropriate time, sonicated, and placed on ice for 30 min. Then, the homogenates were centrifuged (11,000 × g, 1 min), supernatants were neutralized with 1 mm K2CO3 and centrifuged for 10 min as before. Supernatants were kept for binding analyses (storage at −80 °C).
Binding Methods
[32P]NAADP was synthesized as described previously (35); sea urchin microsomes were prepared as described (16, 36). NAADP binding reactions were initiated by adding microsomes to a mixture of 32[P]NAADP and cells extractions during 30 min as described (16).
Construction of CD38 in pEGFP-N1
Total RNA was extract from rat pancreatic acinar cells with Tri Reagent (Sigma) using a standard protocol. mRNA was reverse transcribed into cDNA using a poly(A) primer. The PCR product is digested with AgeI and NotI at the appropriate digestion sites, purified, and ligated into the mammalian expression vector pEGFP-N1 (Clontech, Palo Alto, CA), which contains EGFP downstream of its multiple cloning sites.
Immunocytochemistry and Confocal Microscopy
Immunostaining was performed on cells fixed with paraformaldehyde. After blocking with 3% (w/v) bovine serum albumin, cells were probed with monoclonal anti-LAMP1 (1:200; Sigma), mouse anti-GFP (1:200; Santa Cruz Biotechnology), rabbit anti-amylase (1:500; Sigma), rabbit anti-EEA1 (early endosomal antigen 1; 1:200, Abcam), and rabbit anti-a1 (1:500, kind gift of Dr Morel). CD38 immunostaining was performed after methanol fixation with monoclonal anti-CD38 (1:50, Sigma). Alexa Fluor 568, Alexa Fluor 488 anti-mouse IgG, and Alexa 568 anti-rabbit IgG (1:1000; Molecular Probes) were used for visualization. All imaging was performed on a Leica TCS SP2 confocal spectrophotometer using a Leica DMIRE2 inverted microscope (63× water immersion objective).
Analysis and Statistics
Data represent means ± S.E. of at least four independent experiments in each case. Statistical analysis was performed using the paired Student's t test. p values are summarized as not significant (not significant, >0.05), significant (*, <0.05), or highly significant (***, <0.001).
RESULTS
Agonist-evoked Ca2+ Mobilization Is Impaired in CD38−/− Cells
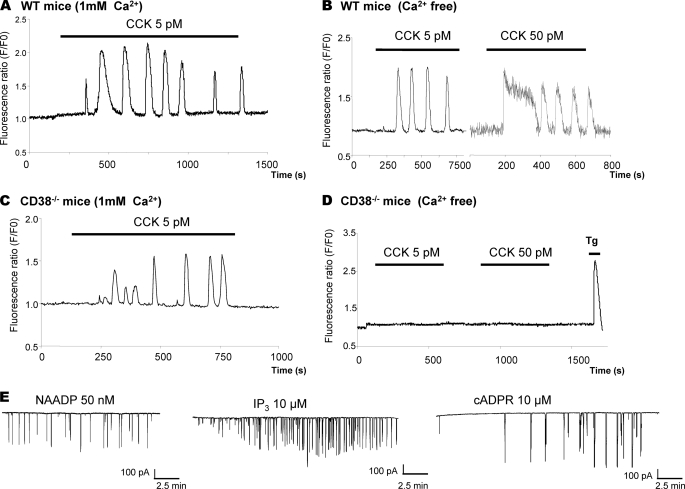
To elucidate the role of CD38 in agonist-evoked intracellular Ca2+ signaling, we used video imaging and confocal microscopy. Pancreatic acinar cells isolated from CD38−/− mice were found having normal secretory pole features and were not distinguishable from wild type cells. As agonist-evoked Ca2+ signals mostly originate from intracellular stores, we have performed experiments in the presence and absence of extracellular Ca2+. In wild type cells, CCK evokes typical oscillatory Ca2+ responses (Fig. 1A), which persist in the absence of extracellular Ca2+ (Fig. 1B). In the case of cells derived from CD38−/− mice, in the presence of extracellular Ca2+, the number of cells responding to CCK (Fig. 1C) was similar to the wild type. However, in the absence of Ca2+ in the external solution, CD38−/− cells no longer responded to CCK (Fig. 1D). The CD38−/− cells appear to have remodeled their Ca2+-signaling mechanisms so that agonist-evoked Ca2+ responses may largely depend on Ca2+ entry to maintain functional agonist-secretion coupling. A detailed quantitative analysis of CCK-evoked Ca2+ spiking is summarized in supplemental Table S1. We also investigated whether the inability of the agonists to evoke Ca2+ mobilization in cells from CD38−/− mice was due to loss of responsiveness to intracellular messengers. We used the patch clamp technique in whole cell configuration to perfuse cells with the messengers and to record the Ca2+-dependent currents as an index of cytosolic Ca2+ changes. As shown in Fig. 1E, NAADP, IP3, and cADPR all elicited repetitive short-lasting Ca2+ spikes in CD38−/− pancreatic acinar cells and were equivalent to those evoked by these messengers in pancreatic acinar cells extracted from wild type mice (7, 27). These data indicate that NAADP, IP3, and cADPR receptors are present and functional in CD38−/− cells, and lack of responsiveness to messengers is not a factor in the changes in sensitivity to agonist-evoked Ca2+ mobilization from intracellular stores in these cells.
FIGURE 1.
Ca2+ signaling patterns evoked by CCK in pancreatic acinar cells extracted from wild type and CD38−/− mice. Ca2+ oscillations were observed in wild type cells after 5 or 50 pm CCK stimulation (n = 40 and 18, respectively) in the presence (A) and absence (B) of Ca2+ in the extracellular solution (n = 27 and 22, respectively). C, cytosolic Ca2+ signals evoked by CCK at 5 pm in CD38−/− cells (n = 37) in the presence of extracellular Ca2+. D, no Ca2+ responses were observed in cells extracted from CD38−/− mice following stimulation with 5 or 50 pm CCK in Ca2+-free external solution, although the SERCA inhibitor, thapsigargin (1 μm; Tg) still evokes Ca2+ release (n = 87 and 86, respectively). E, whole cell configuration of the patch clamp technique recordings of the Ca2+-dependent Cl− current in CD38−/− acinar cells, which was used as an index of the cytosolic Ca2+ elevation. The Ca2+-sensitive current evoked by NAADP (50 nm) or by IP3 at 15 μm or cADPR at 10 μm when present, respectively, in the intracellular pipette solution (n = 4, 3, and 3, respectively). The messenger-evoked Ca2+ spikes are similar to those observed in pancreatic acinar cells derived from wild type mice (7, 27).
Agonist-evoked NAADP Synthesis in CD38−/− Cells
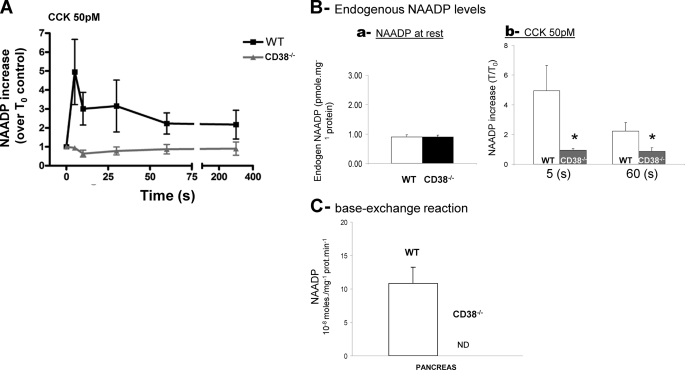
To identify the enzyme involved in CCK-evoked NAADP synthesis, we examined whether CD38 was required for the synthesis of endogenous NAADP in response to agonist using a standard competitive binding assay (16, 37). In wild type pancreatic acinar cells stimulated with CCK at 50 pm, endogenous NAADP levels were increased ∼4-fold more than basal levels. However, in CD38−/− cells, the transient increase in NAADP synthesis in response to CCK stimulation was completely abolished, indicating that CD38 is essential for NAADP synthesis in response to CCK (Fig. 2A). Surprisingly, in unstimulated CD38−/− cells, endogenous levels of NAADP were detected at similar levels to those measured in wild type pancreatic acinar cells (Fig. 2B). These data suggest that in addition to CD38, another NAADP synthase may exist that is unmasked in CD38−/− cells. It is unlikely that this activity is related to the base-exchange mechanism for NAADP synthesis as proposed for CD38 (21), because such activity was not detected in homogenates prepared from CD38−/− cells, although robust NAADP synthesis was evident in wild type cell preparations (Fig. 2C).
FIGURE 2.
NAADP synthesis in pancreatic extracts and membranes from wild type and CD38−/− mice. A, NAADP synthesis in pancreatic extracts from wild type and CD38−/− mice. Endogenous NAADP synthesis increases after CCK (50 pm) stimulation in wild type cells, but not in pancreatic acinar cells from CD38−/− mice (n = six separate experiments). B, histograms showing endogenous levels of NAADP in cells derived from wild type mice (white bar) and from CD38−/− mice (black bar) under resting conditions (a), in response to CCK stimulation at 50 pm at 5 and 60 s (b). C, base-exchange activity and NAADP synthesis after exogenous addition of NADP (200 μm) and nicotinic acid (7 mm) in pancreatic extracts from wild type (white) and CD38−/− mice (black). Base-exchange activity for NAADP production was not detectable (ND) in CD38−/− mice (n = 3). *, statistical significance test.
CD38 Localizes to Endosomal Structures Concentrated at Secretory Pole
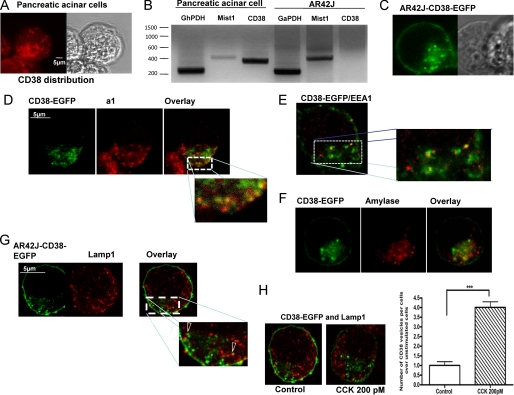
We investigated the localization of CD38 in primary pancreatic acinar cells by immunofluorescence and confocal microscopy (Fig. 3A) and by expression of a fluorescently tagged rat EGFP-CD38 construct in the rat AR42J pancreatic cell line, which does not endogenously express this protein (Fig. 3B).
FIGURE 3.
Subcellular distribution of CD38 in pancreatic acinar and AR42J cells. A, confocal microscopy reveals endogenous CD38 distribution in primary pancreatic acinar cells. B, CD38 is not present at the mRNA levels in AR42J cells, whereas CD38 mRNA was detected in normal pancreatic acinar cells. Mist 1 is a basic helix-loop-helix transcription factor that is highly expressed in the adult pancreas (B). C, transiently expressed CD38-EGFP construct in AR42J cells. D, co-immunostaining CD38 and a1 subunit of the vacuolar V-H+-ATPase, an endolysosomal marker. E, shown is a co-localization of CD38-containing vesicles with the early endosomal marker, EEA1. F, shown is a co-immunostaining of CD38-containing vesicles with amylase containing granules at the secretory pole (G) lysosomes, marked with LAMP1 staining appose CD38-containing vesicles but do not co-localize. H, application of CCK (200 pm for 30 s) sharply increases the number of CD38-expressing vesicles in AR42J-CD38 cells. However, even after CCK stimulation, co-immunostaining of CD38 and Lamp1 indicates that CD38-containing vesicles are distinct from lysosomes.
CD38-EGFP appears to be mostly targeted to the plasma membrane but also has a prominent distribution in vesicular structures in the secretory pole (Fig. 3C) similar to the staining we observed with an anti-CD38 antibody in primary pancreatic acinar cells (Fig. 3A). Overlap of the immunostaining of CD38 with the a1 subunit of the vaculoar H+-ATPase (Fig. 3D) suggested its presence in acidic vesicles of the endolysosomal pathway. Co-localization of EEA1 with CD38-EGFP revealed that CD38 is present in early endosomes (Fig. 3E). In contrast, CD38-containing vesicles do not co-localize with amylase secretory granules (Fig. 3F), and lysosomal Lamp1 staining showed that although CD38-containing vesicles and lysosomes are in close proximity, they also do not co-localize (Fig. 3G).
Importantly, we found that in unstimulated cells, CD38 is already present in preformed endosomes, and therefore, the acidic environment and the substrate accumulation required for NAADP synthesis could be achieved in these vesicles as suggested for cADPR synthesis in sea urchin eggs (33). Finally, in a previous study, we have implicated endocytosis in the maintenance of Ca2+ oscillations evoked by physiological CCK concentrations in pancreatic acinar cells (4). We have also previously reported that CCK from 5 to 500 pm stimulates NAADP synthesis in these cells (16). In agreement with these previous findings, we observed that CCK (200 pm) stimulation promoted endocytosis of CD38 so that this enzyme translocates inside the cell, where it is better placed to synthesize intracellular messengers (Fig. 3H). Thus, the endosomal localization of CD38 could, in principle, bring the synthesis of NAADP in close proximity to lysosomes, the target for this Ca2+-mobilizing messenger.
CD38 Expression Is Required for Agonist-mediated NAADP Signaling in AR42J and Pancreatic Acinar Cells
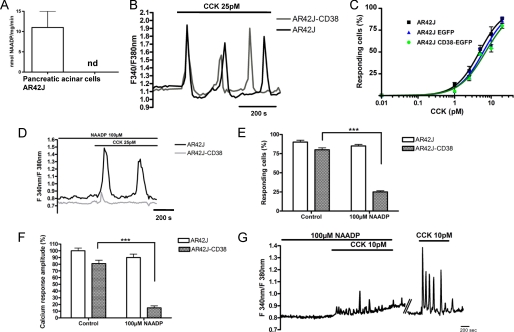
We next investigated CCK-evoked Ca2+ signaling in AR42J cells and those expressing EGFP-CD38 to compare responses with cells from the CD38−/− and wild type mice. Homogenates from AR42J cells, like those from CD38−/− cells, also showed an inability to synthesize NAADP by base-exchange as seen for wild type primary cells (Fig. 4A). At a physiological concentration of CCK (25 pm), oscillatory Ca2+ responses of similar amplitude and frequency were observed in both AR42J and AR42J-CD38 cells (Fig. 4B), and the concentration-response response curves superimpose regardless of CD38 expression (Fig. 4C).
FIGURE 4.
Restoration of CCK-evoked NAADP-dependent Ca2+ signaling by CD38 expression in the pancreatic cell line AR42J. A, HPLC measurement of NAADP synthesis by the base-exchange reaction in primary pancreatic acinar cells and AR42J extracts (n = 3). B, CCK-evoked Ca2+ responses of similar amplitude and frequency in AR42J cells and in AR42J-CD38 at 25 pm. C, concentration-response curve of CCK-induced Ca2+ responses in AR42J, AR42J CD38-EGFP, and AR42J EGFP-transfected cells. CCK induces a comparable effect in control and transfected cells (n = three separate experiments). D, a high desensitizing concentration of NAADP (100 μm) blocks the CCK response (25 pm) in AR42J-CD38 (n = 34) but not in AR42J cells (n = 65), summarized in E and F. G, incubation of primary pancreatic acinar cells with a high concentration of NAADP (100 μm) blocks the Ca2+-signaling response to 10 pm CCK application (n = three separate experiments). The effect is reversed by washout of NAADP from the extracellular medium.
We next compared the pharmacological properties of CCK-evoked Ca2+ signaling in AR42J cells and those expressing CD38 in presence of 1 mm Ca2+ in the extracellular solution. To investigate whether CCK-evoked Ca2+ response involved the NAADP pathway in the pancreatic AR42J cells, we first used the propensity of NAADP receptors to inactivate to high concentrations of NAADP to dissect the contribution of the NAADP signaling pathway to the agonist response (7). Remarkably, incubation of cells with a high desensitizing concentration of NAADP (100 μm for 30 min) essentially blocked the CCK response (25 pm) in EGFP-CD38 expressing cells, as also observed in primary wild type cells (Fig. 4G), while having little effect on the control AR42J cells (Fig. 4, D–F). Our findings suggest that in AR42J cells, the high affinity CCK receptor is not linked to the NAADP signaling pathway, and that CD38 expression alone is sufficient to switch the CCK receptor coupling mechanism in AR42J cells to one similar to that observed in wild type primary acinar cells (Fig. 4F) (7).
CD38 Is Required for Agonist-mediated Ca2+ Mobilization from Lysosomes
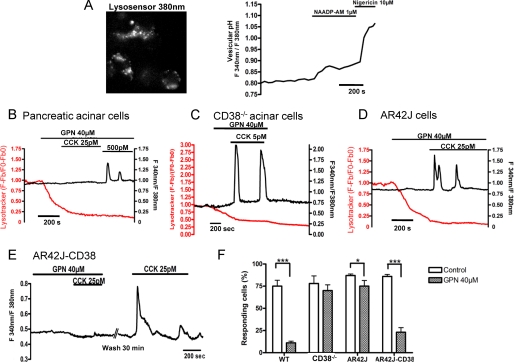
We subsequently examined which Ca2+ stores were recruited during CCK stimulation in both primary and AR42J cells. As we and others have previously shown, in many cells including pancreatic acinar cells, NAADP releases Ca2+ from acidic stores, likely endolysosomal related organelles (2, 4, 6), by activating two pore channels (1). In concert with Ca2+ release from lysosomes, NAADP has also been shown to cause an increase in lumenal pH of these organelles (5). Using a membrane-permeant analog of NAADP, NAADP-AM, we found that this compound also caused an increase in lumenal pH in AR42J cells, an effect also mimicked by the protonophore, nigericin (Fig. 5A). These findings are consistent with NAADP targeting acidic Ca2+ stores in pancreatic acinar cells.
FIGURE 5.
Recruitment of lysosomal Ca2+ stores during CCK signaling requires expression of CD38. A, AR42J cells were incubated for 30 min with the LysoSensor DND-160 (2 μm) at 37 °C. The dye was excited at 380 nm (emission, 510 nm), and a punctuate staining resembling lysosomes was observed in AR42J cells. Fluorescence intensity of lysosensor DND-160 excited at 380 nm is inversely proportional to the intravesicular pH, whereas the dye fluorescence is proportional to its concentration at 340 nm (emission, 510 nm). The ratio 340/380 nm of emitted fluorescence reflects pH changes in lysosomes. The cell-permeant form of NAADP, NAADP-AM (1 μm), and nigericin (10 μm) successively induce a pH increase in the lumen of AR42J lysosomes. B, application of GPN decreased the lysotracker staining in primary pancreatic acinar cells. Cells were stimulated by CCK at 25 pm. GPN application drastically reduced Ca2+ responses to CCK 25 pm (n = 35). C, in pancreatic acinar cells extracted from CD38−/− mice, GPN application decreases the LysoTracker staining but has no effect on CCK-evoked Ca2+ response at 5–25 pm (n = 98). D, in AR42J cells, GPN application decreased LysoTracker staining but has no effect on CCK-evoked Ca2+ response at 5–25 pm (n = 61). E, in AR42J-CD38 cells treated with GPN, 25 pm CCK failed to evoke Ca2+ responses, indicating that lysosomes are now necessary for CCK-evoked Ca2+ signaling (n = 28). F, Summary of GPN (40 μm) effects on the responsiveness of CCK-evoked Ca2+ signals in wild type and CD38−/− pancreatic acinar cells and AR42J-EGFP and AR42J cells. *, **, statistical tests.
Lysosomes from primary pancreatic acinar cells were stained by LysoTracker Red. Application of the lysosomotropic agent, Gly-Phe-β-naphthylamide (GPN) decreased the lysotracker staining indicating the specific loss of lysosome integrity as shown previously (4, 6, 14). Upon completion, lysosome disruption was attained, primary pancreatic acinar cells were stimulated by CCK at 25 pm. GPN application drastically reduced the Ca2+ responses to 25 pm CCK (Fig. 5B) because 31 of 35 cells failed to respond to CCK, whereas cells responded to a higher, IP3-producing (26) concentration of CCK of 500 pm, indicating that the ER Ca2+ stores were not depleted by GPN application, as we have shown previously (4). In contrast, GPN application did not affect the CCK-evoked Ca2+ response in CD38−/− cells, indicating that lysosomes are not targeted by agonists in cells lacking CD38 (Fig. 5C).
We then investigated the effect of lysosome disruption in pancreatic AR42J cells. As in primary pancreatic acinar cells, lysosomes display a punctuate staining when cells were labeled with LysoTracker Red. GPN application at 40 μm strongly reduced the staining (supplemental Fig S1B). In contrast to the effects of GPN on CCK-evoked Ca2+ signals in wild type primary pancreatic acinar cells, in AR42J cells, despite the loss of lysosome integrity, the vast majority of cells still responded to CCK stimulation at 25 pm (Fig. 5D). We then investigated whether the expression of CD38 was able to restore the recruitment of lysosome Ca2+ stores. Pancreatic AR42J-CD38 cells were treated with GPN, and then cells were stimulated with CCK at 25 pm. In the presence of GPN, CCK failed to elicit any Ca2+ responses (Fig. 5E), indicating that functional lysosomes are necessary for CCK-evoked Ca2+ signaling in AR42J-CD38 cells. Removal of GPN allowed the restoration of CCK-evoked Ca2+ responses after 30 min as observed previously for NAADP-induced Ca2+ release in sea urchin eggs (2) as lysosomal structures rapidly reform. Taken together, the abolition of CCK responses by GPN (as summarized in Fig. 5F) or by NAADP receptor desensitization (Fig. 4, D and E) in CD38 expressing cells and their insensitivity to these protocols in the absence of CD38, is highly suggestive that CD38 is required to link receptor activation to Ca2+ mobilization from lysosomes mediated by NAADP.
CD38 Is Required for Agonist-induced cADPR Synthesis and Ca2+ Mobilization from Ryanodine-sensitive Stores
In addition to NAADP synthesis, CD38 has been established as playing a major role in cADPR synthesis in mammalian cells (23). In pancreatic acinar cells, ACh, like CCK, stimulates increases in cADPR levels (16, 38), but NAADP has been found not to play a significant role in ACh-mediated Ca2+ signaling (30). We investigated whether CCK stimulates cADPR synthesis in CD38−/− cells. Under basal conditions, we found that endogenous levels of cADPR are slightly lower in cells from CD38−/− mice than in cells prepared from wild type mice (0.108 ± 0.019 pmol/mg protein versus 0.153 ± 0.011 pmol/mg protein, respectively; n = 10, n = 13, p < 0.05, supplemental Fig S2A). We examined cADPR levels after 5 min of stimulation, the time for which cADPR content peaks in these cells (16). The levels of cADPR in cells from wild type mice increased from 0.153 ± 0.011 pmol/mg protein (n = 13; p < 0.05) to 0.215 ± 0.018 (n = 10; p < 0.05) pmol/mg protein in cells stimulated by CCK (50 pm) (supplemental Fig. S2A). In contrast, in cells from CD38−/− mice, no significant increases in endogenous cADPR content could be detected after CCK stimulation (0.108 ± 0.019 pmol/mg protein, n = 10, versus 0.084 ± 0.022 pmol/mg protein, n = 6) (supplemental Fig. S2A). Our study clearly shows that in pancreatic acinar cells, CCK requires CD38 to increase cADPR levels as previously reported for ACh (38).
Because the major mechanism for cADPR-evoked Ca2+ release involves activation of ryanodine receptors (39), including in pancreatic acinar cells (40), we examined the effect of blocking ryanodine receptors with ryanodine in AR42J-CD38 and AR42J cells. Ryanodine at a high, inhibiting concentration had little effect on CCK-evoked Ca2+ oscillations in AR42J cells but effectively abolished oscillations in AR42J-CD38 cells (supplemental Figs. S2B and S2C).
The expression of CD38 clearly increases the sensitivity of CCK responses to ryanodine as also observed in wild type primary pancreatic acinar cells (28). This would be consistent with the hypothesis that by catalyzing cADPR production, expression of CD38 also confers coupling of receptor activation to Ca2+ mobilization from ryanodine-sensitive ER stores.
DISCUSSION
In agreement with the hypothesis of NAADP acting as a second messenger, recent studies have reported that a dramatic increase in NAADP occurs in response to external stimuli such as sea urchin egg fertilization (14, 41), in pancreatic β-cells after glucose and glucagon-like peptide-1 stimulation, in pancreatic acinar cells after CCK stimulation, and in smooth muscle cells following exposure to endothelin-1 (3, 15–17), and more recently, in response to histamine in human myometrial cells (18). However, to date, the enzyme responsible for the endogenous synthesis of NAADP is controversial.
At least from in vitro experiments, NAADP and cADPR are the products of multifunctional enzymes belonging to the ADP-ribosyl cyclase family. Several of these enzymes have been purified and cloned, such as the ecto-enzymes CD38 and CD157, and the Aplysia cyclase. Two classes of reactions may be catalyzed by the cyclases: cyclization and base exchange (42). These reactions depend on the pH; the base-exchange reaction occurs preferentially at acidic pH, producing NAADP from β-NADP and nicotinic acid. To our knowledge, there are no data yet indicating that CD157 could synthesize NAADP.
Although these cyclases could in principle form NAADP in vitro, only in NK1 cells have agonist-stimulation of endogenous NAADP levels been linked to CD38 expression (25). In fact, the opposite has been reported also. A recent study from Soares et al. (18) using CD38−/− mice have actually demonstrated that histamine increases endogenous levels of NAADP is independent of CD38 and does not requires base-exchange reaction at acidic pH myometrial cells (18). This later study clearly points to an additional enzyme responsible for the NAADP synthesis in cells. Even more recently, a study reached a similar conclusion that endogenous NAADP synthesis in response to glucagon-like peptide-1 is largely CD38-independent (17). However, remodeling in CD38−/− mice could have occurred, making it difficult to really assess the physiological role of CD38. The new NAADP synthesis pathway observed in these studies may as well result from remodeling of the NAADP signaling pathway in CD38−/− mice. Interestingly, they recorded a slower time course of NAADP synthesis, which peaks at 30 s compared with 5 s in our work (Fig. 2B). As reported in previous studies, some of our data on CD38−/− mice indicates that a NAADP synthase different from CD38 also exist in the pancreas. This new enzyme may have also different kinetics parameters, as it does not appear to use the base-exchange reaction and therefore shows some similarity with Ref. 18. However, it does not appear to be regulated by CCK in our cell model system.
To investigate the role of CD38 in agonist-evoked Ca2+ signaling in pancreatic acinar cells, we not only took advantage of CD38−/− mice but also the lack of CD38 expression in AR42J cells and the functional consequences of its re-expression. Our study shows that expression of CD38 is required for both cADPR and NAADP synthesis and signaling in a single cell type, the pancreatic acinar cell. Synthesis of NAADP in response to CCK is a fast process and takes less than 30 s to reach its peak, whereas cADPR production is slower and peaks later at ∼2 min (16). In pancreatic acinar cells, the hormone CCK is the major physiologic secretagogue, which stimulates fluid, lysosomal, and digestive enzyme secretion in a Ca2+-dependent manner (26). Our study using CD38−/− mice shows that possible CD38-independent pathways linked to NAADP production are not linked to CCK receptor activation. One possibility might be that this new pathway could be regulated by acetylcholine, the other main secretagogue in the pancreatic acinar cells. In support of this hypothesis, a previous study has shown that cADPR synthesis through CD38 is also controlled by ACh (38). This intriguing hypothesis will deserve future experimentation to clarify how CD38−/− mice remodel when several agonists in a same target cell use CD38 for either cADPR synthesis or both cADPR/NAADP synthesis according the agonist used. Finally, our present study provides the first evidence that CD38 functions as a cellular NAADP synthase and is required to couple receptor activation to mobilization of Ca2+ from NAADP-sensitive lysosomal Ca2+ stores.
In contrast to cytosolic Aplysia cyclase (42, 43), the catalytic domain of CD38 is exposed at the surface of the cells; therefore, enzymatic activity may produce messengers extracellularly. Paradoxically, the known activity of this messenger is intracellular, and the way they reach the intracellular compartment or whether NAADP could be produced in vivo is very controversial. However, in our study, CD38 is localized both at the plasma membrane and intracellularly. Additional signals or processes may be required to couple receptor activation at the plasma membrane to endosomes. Additionally, the fact that acidic condition is required and that the substrate nicotinic acid is mostly not free in the cytosol suggest more that an intravesicular accumulation of the substrates could exist. Alternatively, endocytosed agonist-receptor complexes might directly interact with CD38 within vesicular membranes that could switch Ca2+-signaling pathways by sequestering activated receptors away from plasma membrane-associated phospholipase C and hence IP3 generation.
In the wild type pancreatic acinar cells, CCK produces NAADP, which acts as an universal Ca2+ trigger, and this very small but very localized primary Ca2+ release is then amplified by the neighboring IP3 and ryanodine receptors to generate cytosolic Ca2+ oscillations. In cells devoid of CD38, receptors appear to couple to cADPR and NAADP-independent Ca2+-signaling mechanisms, including Ca2+ influx pathways, which may play the role of sensitizing the IP3 and ryanodine receptors. Similarly, expression of CD38 appears not to drastically change the overall Ca2+ responses, which are in agreement with a trigger role of NAADP because the vast majority of the cytosolic Ca2+ is released from IP3-sensitive stores as we have observed previously (44). The multiplicity of receptor-linked Ca2+-signaling mechanisms and their profound ability to remodel and select different components of the Ca2+ signaling tool kit depending on availability attest to the great flexibility and plasticity of this most ubiquitous of signal transduction pathways to preserve information processing and cellular responsiveness in health and disease (32). However, the concept emerging from our work is that despite the extraordinary plasticity of the Ca2+-signaling mechanisms, specific organelle recruitments such as lysosomes may have an absolute requirement for a specific transduction pathway and may be relevant to diseases linked to disturbances in Ca2+ homeostasis.
Supplementary Material
Acknowledgment
We thank M. Ziegler (University of Bergen) for the generous gift of NAD+ kinase.
This work was supported by an ATIP program grant from the CNRS, by the Association pour la Recherche sur le Cancer (ARC), Fondation de la Recherche Médicale (FRM), and the Association Française contre les Myopathies (AFM) (to J.-M. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Materials and Methods,” Table 1, Figs. S1 and S2, and additional references.
- NAADP
- nicotinic acid adenine dinucleotide phosphate
- CCK
- cholecystokinin
- ACh
- acetylcholine
- GPN
- Gly-Phe-β-naphthylamide
- cADPR
- cyclic ADP-ribose.
REFERENCES
- 1.Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K. T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C. N., Parrington J., Ma J., Evans A. M., Galione A., Zhu M. X. (2009) Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. (2002) Cell 111, 703–708 [DOI] [PubMed] [Google Scholar]
- 3.Kinnear N. P., Boittin F. X., Thomas J. M., Galione A., Evans A. M. (2004) J. Biol. Chem. 279, 54319–54326 [DOI] [PubMed] [Google Scholar]
- 4.Menteyne A., Burdakov A., Charpentier G., Petersen O. H., Cancela J. M. (2006) Curr. Biol. 16, 1931–1937 [DOI] [PubMed] [Google Scholar]
- 5.Morgan A. J., Galione A. (2007) J. Biol. Chem. 282, 37730–37737 [DOI] [PubMed] [Google Scholar]
- 6.Yamasaki M., Masgrau R., Morgan A. J., Churchill G. C., Patel S., Ashcroft S. J., Galione A. (2004) J. Biol. Chem. 279, 7234–7240 [DOI] [PubMed] [Google Scholar]
- 7.Cancela J. M., Churchill G. C., Galione A. (1999) Nature 398, 74–76 [DOI] [PubMed] [Google Scholar]
- 8.Brailoiu E., Churamani D., Cai X., Schrlau M. G., Brailoiu G. C., Gao X., Hooper R., Boulware M. J., Dun N. J., Marchant J. S., Patel S. (2009) J. Cell Biol. 186, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zong X., Schieder M., Cuny H., Fenske S., Gruner C., Rötzer K., Griesbeck O., Harz H., Biel M., Wahl-Schott C. (2009) Pflugers Arch. 458, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galione A., Evans A. M., Ma J., Parrington J., Arredouani A., Cheng X., Zhu M. X. (2009) Pflugers Arch. 458, 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guse A. H. (2009) Curr. Biol. 19, R521–523 [DOI] [PubMed] [Google Scholar]
- 12.Galione A. (2006) Biochem. Soc. Trans. 34, 922–926 [DOI] [PubMed] [Google Scholar]
- 13.Lee H. C. (2003) Curr. Biol. 13, R186–188 [DOI] [PubMed] [Google Scholar]
- 14.Churchill G. C., O'Neill J. S., Masgrau R., Patel S., Thomas J. M., Genazzani A. A., Galione A. (2003) Curr. Biol. 13, 125–128 [DOI] [PubMed] [Google Scholar]
- 15.Masgrau R., Churchill G. C., Morgan A. J., Ashcroft S. J., Galione A. (2003) Curr. Biol. 13, 247–251 [DOI] [PubMed] [Google Scholar]
- 16.Yamasaki M., Thomas J. M., Churchill G. C., Garnham C., Lewis A. M., Cancela J. M., Patel S., Galione A. (2005) Curr. Biol. 15, 874–878 [DOI] [PubMed] [Google Scholar]
- 17.Kim B. J., Park K. H., Yim C. Y., Takasawa S., Okamoto H., Im M. J., Kim U. H. (2008) Diabetes 57, 868–878 [DOI] [PubMed] [Google Scholar]
- 18.Soares S., Thompson M., White T., Isbell A., Yamasaki M., Prakash Y., Lund F. E., Galione A., Chini E. N. (2007) Am. J. Physiol. Cell Physiol. 292, C227–239 [DOI] [PubMed] [Google Scholar]
- 19.Pandey V., Chuang C. C., Lewis A. M., Aley P. K., Brailoiu E., Dun N. J., Churchill G. C., Patel S. (2009) Biochem. J. 422, 503–512 [DOI] [PubMed] [Google Scholar]
- 20.Palade P. (2007) Am. J. Physiol. Cell Physiol. 292, C4–7 [DOI] [PubMed] [Google Scholar]
- 21.Aarhus R., Graeff R. M., Dickey D. M., Walseth T. F., Lee H. C. (1995) J. Biol. Chem. 270, 30327–30333 [DOI] [PubMed] [Google Scholar]
- 22.Lee H. C. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 317–345 [DOI] [PubMed] [Google Scholar]
- 23.Malavasi F., Deaglio S., Funaro A., Ferrero E., Horenstein A. L., Ortolan E., Vaisitti T., Aydin S. (2008) Physiol. Rev. 88, 841–886 [DOI] [PubMed] [Google Scholar]
- 24.Lee H. C., Aarhus R. (1995) J. Biol. Chem. 270, 2152–2157 [DOI] [PubMed] [Google Scholar]
- 25.Rah S. Y., Mushtaq M., Nam T. S., Kim S. H., Kim U. H. (2010) J. Biol. Chem. 285, 21877–21887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen O. H., Tepikin A. V. (2008) Annu. Rev. Physiol. 70, 273–299 [DOI] [PubMed] [Google Scholar]
- 27.Cancela J. M., Van Coppenolle F., Galione A., Tepikin A. V., Petersen O. H. (2002) EMBO J. 21, 909–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancela J. M. (2001) Annu. Rev. Physiol. 63, 99–117 [DOI] [PubMed] [Google Scholar]
- 29.Burdakov D., Galione A. (2000) Curr. Biol. 10, 993–996 [DOI] [PubMed] [Google Scholar]
- 30.Cancela J. M., Gerasimenko O. V., Gerasimenko J. V., Tepikin A. V., Petersen O. H. (2000) EMBO J. 19, 2549–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerasimenko J. V., Sherwood M., Tepikin A. V., Petersen O. H., Gerasimenko O. V. (2006) J. Cell Sci. 119, 226–238 [DOI] [PubMed] [Google Scholar]
- 32.Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 33.Davis L. C., Morgan A. J., Ruas M., Wong J. L., Graeff R. M., Poustka A. J., Lee H. C., Wessel G. M., Parrington J., Galione A. (2008) Curr. Biol. 18, 1612–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Partida-Sánchez S., Cockayne D. A., Monard S., Jacobson E. L., Oppenheimer N., Garvy B., Kusser K., Goodrich S., Howard M., Harmsen A., Randall T. D., Lund F. E. (2001) Nat. Med. 7, 1209–1216 [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki M., Churchill G. C., Galione A. (2005) FEBS J. 272, 4598–4606 [DOI] [PubMed] [Google Scholar]
- 36.Aarhus R., Dickey D. M., Graeff R. M., Gee K. R., Walseth T. F., Lee H. C. (1996) J. Biol. Chem. 271, 8513–8516 [DOI] [PubMed] [Google Scholar]
- 37.Lewis A. M., Masgrau R., Vasudevan S. R., Yamasaki M., O'Neill J. S., Garnham C., James K., Macdonald A., Ziegler M., Galione A., Churchill G. C. (2007) Anal. Biochem. 371, 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukushi Y., Kato I., Takasawa S., Sasaki T., Ong B. H., Sato M., Ohsaga A., Sato K., Shirato K., Okamoto H., Maruyama Y. (2001) J. Biol. Chem. 276, 649–655 [DOI] [PubMed] [Google Scholar]
- 39.Galione A., Lee H. C., Busa W. B. (1991) Science 253, 1143–1146 [DOI] [PubMed] [Google Scholar]
- 40.Thorn P., Gerasimenko O., Petersen O. H. (1994) EMBO J. 13, 2038–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasudevan S. R., Galione A., Churchill G. C. (2008) Biochem. J. 411, 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H. C. (2005) J. Biol. Chem. 280, 33693–33696 [DOI] [PubMed] [Google Scholar]
- 43.Bezin S., Charpentier G., Lee H. C., Baux G., Fossier P., Cancela J. M. (2008) J. Biol. Chem. 283, 27859–27870 [DOI] [PubMed] [Google Scholar]
- 44.Cancela J. M., Petersen O. H. (2002) Diabetes 51, S349–357 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.