Abstract
Apolipoprotein E (apoE) ϵ4 and hyperhomocysteinemia are risk factors for Alzheimer disease (AD). The dimerization of apoE3 by disulfide bonds between cysteine residues enhances apoE3 function to generate HDL. Because homocysteine (Hcy) harbors a thiol group, we examined whether Hcy interferes with the dimerization of apoE3 and thereby impairs apoE3 function. We found that Hcy inhibits the dimerization of apoE3 and reduces apoE3-mediated HDL generation to a level similar to that by apoE4, whereas Hcy does not affect apoE4 function. Western blot analysis of cerebrospinal fluid showed that the ratio of apoE3 dimers was significantly lower in the samples from the patients with hyperhomocysteinemia than in those that from control subjects. Hyperhomocysteinemia induced by subcutaneous injection of Hcy to apoE3 knock-in mice decreased the level of the apoE3 dimer in the brain homogenate. Because apoE-HDL plays a role in amyloid β-protein clearance, these results suggest that two different risk factors, apoE4 and hyperhomocysteinemia, may share a common mechanism that accelerates the pathogenesis of AD in terms of reduced HDL generation.
Keywords: Apolipoproteins, Cholesterol, HDL, Homocysteine, Lipid, Neurochemistry, Neuron, Apolipoprotein E, ApoE Knock-in Mouse, Astrocyte
Introduction
It has been shown that the possession of the apolipoprotein E (apoE) ϵ4 allele is a major risk factor for Alzheimer disease (AD)2 (1). In the central nervous system, apoE is one of the major lipid acceptors to remove cholesterol from cells and generate HDL particles. Previous studies have shown that apoE isoforms do not affect apoE binding to ABCA1, that apoE-mediated ABCA1-dependent cholesterol efflux is not affected by apoE isoforms in fibroblasts (2), and that there is no apoE-isoform-dependence on apoE-mediated lipid efflux from mouse astrocytes (3). Other lines of evidence have shown that apoE induces lipid efflux from macrophages and neural cells in an isoform-dependent manner; apoE3 induces a greater lipid efflux than apoE4 (4–9). It has been shown that two major factors cause this apoE-isoform-dependent generation of HDL. Namely, intramolecular domain interaction occurring in apoE4 attenuates apoE4 ability to generate HDL and intermolecular dimerization by disulfide bonds between cysteines in apoE3 enhances apoE3 ability to generate HDL in neural cells (10).
Recent studies have shown other functions of apoE as well, including an intracellular function of apoE (11, 12) and a function of apoE in clearance and degradation of Aβ. It has been demonstrated that apoE isoforms differentially regulate Aβ clearance from the brain (13), and that an increased level of lipidated apoE, namely, apoE-HDL, stimulates Aβ degradation (14). These lines of evidence suggest that the lower ability of apoE4 than apoE3 to generate HDL would result in an enhanced Aβ deposition in the brain owing to the lower Aβ degradation/clearance from the brain. Similarly, apoE-isoform-dependent HDL generation results in a lower HDL-cholesterol level in serum in those who possess apoE ϵ4 allele, which is a risk factor for atherosclerosis (15) and cerebral infarction (16).
In light of these findings, it is interesting to note that similar to the apoE ϵ4 allele, hyperhomocysteinemia is a risk factor not only for atherosclerosis (17), cerebral infarction (18), and vascular dementia (19), but also for AD (20–23), and that homocysteine (Hcy) level has been reported to increase in the cerebrospinal fluid (CSF) of patients with AD compared with that of control subjects (24). Hcy is generated from the metabolism of methionine, the sulfur-containing amino acid. Previous studies have shown that Hcy generates oxidative stress, leading to cell damage (25), impairs blood-brain barrier function (26), and increases brain Aβ levels (27, 28). However, the molecular mechanism underlying hyperhomocysteinemia-mediated AD development has not yet been fully understood. Because Hcy is a molecule harboring a thiol, it is possible that the thiol of Hcy associates with the thiol of cysteine residues in apoE3, and this disulfide bonds interferes with apoE3 dimerization. Because the dimerization of apoE3 enhances its ability to generate HDL (7, 10), Hcy bound to cysteine residues of apoE3 deteriorates apoE3 HDL generation. In this study, we found that Hcy interferes with apoE3 dimerization by forming disulfide bonds with cysteine residues of apoE3 and impairs apoE3 ability to generate HDL to a level similar to that of apoE4. This is also the case for human CSF obtained from patients with hyperhomocysteinemia and for brain of apoE3 knock-in mice subcutaneously injected with Hcy. These results suggest that two different risk factors for AD, namely, apoE4 and hyperhomocysteinemia, may share a common mechanism; that is, apoE4 has a lower ability to generate HDL than apoE3 and the Hcy modification of apoE3 impairs the ability of apoE3 to generate HDL to a level similar to that of apoE4.
EXPERIMENTAL PROCEDURES
Animals
ApoE knock-out mice (B6.129P2-Apoetm1Unc/J) were purchased from The Jackson Laboratory (Bar Harbor, Maine). Mice expressing human apoE3 in place of mouse apoE (apoE3 knock-in mice) (29) were kindly provided by the Mitsubishi Kagaku Institute of Life Sciences.
Cell Culture
All experiments were performed in compliance with existing laws and institutional guidelines. Highly astrocyte- and neuron-rich cultures were prepared in accordance with a method described previously (30). The astrocyte-rich cultures were maintained in DMEM containing 10% FBS until use.
ApoE Preparation and Hcy Treatment
Five hundred micrograms of apoE3 or apoE4, purchased from Wako (Osaka, Japan), was dissolved in 10 mm Tris-HCl buffer (pH 8.0) containing 6 m urea to obtain 1 ml of an apoE-containing solution, dialyzed against PBS overnight at 4 °C, and stocked in aliquots at −80 °C as described previously (10). Hcy was dissolved in 10 mm Tris-HCl buffer (pH 8.0) to make a stock solution at a concentration of 100 mm, and the Hcy stock solution was divided into aliquots and kept at −80 °C until use. For Hcy treatment, 7.5 μl of 100 mm Hcy was added to 500 μl of an apoE-containing solution. The apoE solutions with or without Hcy were incubated overnight at room temperature. The samples were then dialyzed against PBS overnight at 4 °C. The protein concentration of each sample was determined using a BCA protein assay kit (Pierce) and used for experiments to determine lipid efflux. For dithiothreitol (DTT) treatment, 5 mm DTT was incubated with an apoE stock solution overnight at room temperature. The samples were then dialyzed against PBS overnight at 4 °C. The protein concentration of each sample was determined using a BCA protein assay kit and used for experiments to determine lipid efflux.
Determination of Levels of Cholesterol and Phosphatidylcholine (PC) Released from Astrocytes Labeled with [14C]Acetate
Astrocytes plated in 12-well dishes were cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin solution for 72 h. The cultures were then treated with 37 kBq/ml [14C]acetate (Moravek Biochemicals, Inc., Brea, CA) for 48 h. The astrocytes were washed in DMEM twice and treated with apoE in DMEM. The culture medium was quickly removed, and the astrocytes were dried at room temperature, and the levels of cholesterol and PC released were determined as described previously (7). The levels of [14C]cholesterol and [14C]PC efflux were calculated using the following formula: % efflux = media × 100/(media + cell).
Reverse-phase High Performance Liquid Chromatography and Mass Spectrometry
A synthetic peptide, LGADMEDVCGR or LGADMEDVC(Hcy)GR, or recombinant apoE3 was dissolved in PBS, to a concentration of 1 mm or 15 μm, respectively. Synthetic peptide LGADMEDVCGR or ApoE3 was mixed with or without 10-fold molar concentration of Hcy at 4 °C for 1 day using a vortex mixer. ApoE3 incubated with or without Hcy was digested with trypsin (1 μg/ml; Trypsin Gold, Promega) at an enzyme/substrate ratio of 1:100 (w/w) at 37 °C overnight. Incubated synthetic peptides or apoE3 tryptic peptides were separated by reverse-phase HPLC (model 1100 Series; Agilent Technology, Waldbronn, Germany) on a C18 column (2 × 30 mm; Cadenza CD-C18, Imtakts, Kyoto, Japan) with a linear gradient of 0–64% acetonitrile in 0.1% TFA for 64 min at a flow rate of 0.2 ml/min. The fractionated peptides were subjected to mass spectrometry (AXIMA-CFR, Shimadzu, Kyoto, Japan). Mass spectrometric analysis was performed by MALDI-TOF MS. Samples were prepared by mixing with α-cyano-4-hydroxycinnamic acid as a matrix.
Sampling of Human Plasma and CSF
Human plasma and CSF were obtained from patients in Fukushimura Hospital (Toyohashi, Japan). The plasma and CSF were frozen immediately in liquid nitrogen at lumbar tap and then stored at −80 °C until use. Experiments using human CSF were performed after obtaining informed consent from the patients' guardians for diagnosis and research for biochemical, molecular biological, and genomic analysis.
Determination of Levels of Hcy in Plasma and CSF
The Hcy concentrations in human plasma and CSF were determined by HPLC as demonstrated previously (24). The apoE genotype was also determined and the samples from apoEϵ3/3 patients were used for this study.
Western Blot Analysis
For the determination of apoE3 dimers, the conditioned media or human CSF were dissolved in a sampling buffer consisting of 100 mm Tris-HCl (pH 7.4), 10% glycerol, 4% SDS, and 0.01% bromphenol blue and analyzed by 12.5% Tris/Tricine SDS-PAGE under nonreducing conditions. Blots were probed for 4 h at room temperature with a goat anti-apoE polyclonal antibody, AB947 (1: 2,000; Chemicon, Temecula, CA). Band detection was carried out using an ECL kit (GE Healthcare). The signals corresponding to apoE of each sample in the immunoblot membrane were quantified by densitometry with NIH ImageJ software, with varying concentrations of recombinant apoE protein (Wako, Tokyo, Japan) as standards. Standard signals were demonstrated to be linear in the range of apoE protein amounts from 0 to 2 μg per lane. The apoE concentrations in the conditioned culture media within this range were used for analysis.
Chemically Induced Hyperhomocysteinemia
Mice expressing human apoE3 in place of mouse apoE (apoE3 knock-in mice) (29) at 40–42 weeks of age were subcutaneously injected with Hcy. PBS (100 μl) containing Hcy at a concentration of 13 μmol/μl (0.6 μmol/g body weight) was injected into the mice twice a day (in the morning and evening) for 6 days. For control mice, 100 μl of PBS was injected. In the morning of the seventh day, the animals were deeply anesthetized with isofluorane. Through an incision of the skin covering the occipital bone and the cervical dorsum, the atlanto-occipital membrane was exposed and incised under an operating microscope. The animals were perfused transcardially with PBS, and the brains were removed. Peripheral blood (0.5–1.0 ml) was collected from the caudal vena cava immediately before the perfusion. For the preparation of brain samples, the brain hemispheres from a PBS- or Hcy-injected apoE3 mouse were homogenized in 800 μl of PBS containing a protease inhibitor mixture (Roche Applied Science, Mannheim, Germany), and then centrifuged at 13,000 × g at 4 °C for 15 min. The supernatant was then used for Western blot analysis.
Statistical Analyses
StatView computer software (Windows) was used for statistical analysis. The statistical significance of differences between samples was evaluated by multiple pairwise comparisons among the sets of data using analysis of variance and the Bonferroni t test.
RESULTS
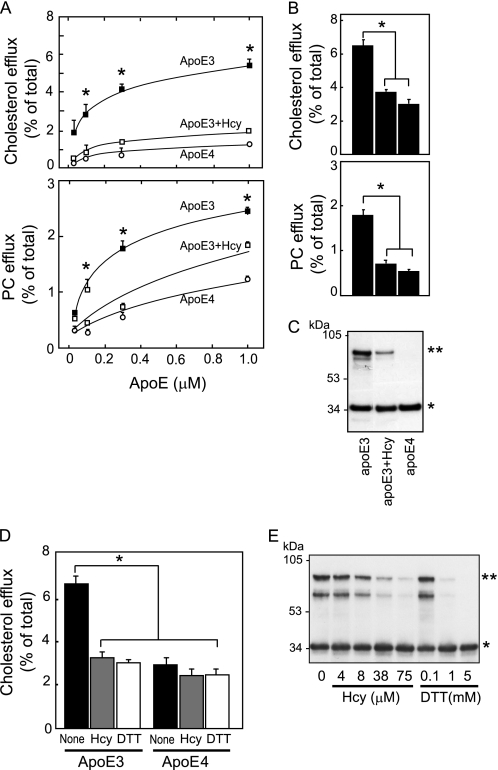
We examined the cholesterol and PC efflux from cultured astrocytes induced by apoE3, apoE3 pretreated with Hcy, and apoE4 24 h after the commencement of treatment of apoEs at various concentrations (Fig. 1A). The levels of cholesterol and PC efflux induced by apoE3 were higher than those induced by apoE3 preincubated with Hcy or apoE4 at 0.1, 0.3, and 1.0 μm. Because our previous study showed that the dimer formation of apoE3 enhances apoE3 ability to release lipids (6, 10), we determined the levels of cholesterol and PC efflux and also the assembly state of apoE3 and apoE4. The levels of cholesterol and PC efflux induced by apoE3 were significantly higher than those induced by apoE4 and apoE3 pretreated with Hcy 24 h after the commencement of treatment (Fig. 1B). A reduced level of lipid efflux was accompanied by a reduced level of apoE3 dimers in apoE3 samples pretreated with Hcy (Fig. 1C, asterisks). ApoE4 does not form dimers owing to a lack of cysteine. In these experiments, we confirmed that Hcy at concentrations used in our study was not toxic (see supplemental Fig. 1).
FIGURE 1.
Hcy impairs apoE3 function to generate HDL in cultured astrocytes. A, the cultured astrocytes labeled with [14C]acetate were exposed to apoE3 (black squares), apoE3+Hcy (red squares), and apoE4 (yellow circles) at 0.05, 0.01, 0.3, and 1.0 μm for 24 h. The lipids released into the media, and the lipids retained in the cells were determined. Data are means ± S.E. of four samples. *, p < 0.001 versus apoE3+Hcy and apoE4 at each dose point. The basal levels of cholesterol and PC efflux in the absence of apoEs are 1.0 ± 0.1 (%) and 0.4 ± 0.1 (%), respectively. Three independent experiments showed similar results. B, the percentages of released cholesterol and PC levels with respect to the total levels were calculated. Data are means ± S.E. of four samples. *, p < 0.001. Three independent experiments showed similar results. C, Western blot analysis of the samples of apoE3, apoE3+Hcy, and apoE4 was performed under nonreducing conditions. D, each culture was exposed to apoE3, apoE3+Hcy, apoE3+DTT, apoE4, apoE4+Hcy, and apoE4+DTT at an apoE concentration of 0.3 μm for 24 h. The percentages of released cholesterol levels with respect to the total levels were calculated. Data are means ± S.E. of four samples. *, p < 0.001. Three independent experiments showed similar results. E, Hcy at various concentrations of 4, 8, 38, and 75 μm and DTT at 0.1, 1, and 5 mm were added to the apoE3 solution, and the apoE3 solution was incubated for 24 h at 4 °C. Each solution was then dialyzed using a cassette dialyzer in PBS for 15 h at 4 °C. The apoE3-containing solutions were then analyzed by Western blot analysis under nonreducing conditions using the anti-apoE antibody (AB947). * and **, apoE3 monomers and dimers, respectively.
These results suggest the possibility that Hcy inhibits the dimer formation of apoE3 and this may be responsible for the reduced level of lipid efflux induced by apoE3 pretreated with Hcy, because our previous studies showed that apoE serves as a lipid acceptor in an isoform-dependent manner; apoE3 induces greater HDL generation than apoE4 (6, 7, 9). It is possible to assume that the thiol in Hcy can form disulfide bonds with the thiol of cysteine residues in apoE3. To examine whether the inhibition of thiol-disulfide bonds in apoE3 dimers affects apoE3-mediated cholesterol efflux, apoE3 or apoE4 was preincubated with a thiol-reducing agent, DTT, and then dialyzed against PBS and used for the experiment to determine cholesterol efflux. The levels of cholesterol released by apoE3 were significantly greater than those released by apoE3 pretreated with Hcy or DTT at 24 h after the commencement of treatment (Fig. 1D). The level of cholesterol efflux induced by apoE4 was significantly lower than that induced by apoE3, and Hcy or DTT pretreatment did not affect apoE4-induced cholesterol release (Fig. 1D). A reduced level of lipid efflux was accompanied by a reduced level of apoE3 dimers in apoE3 samples pretreated with Hcy or DTT (Fig. 1D). The effects of Hcy and DTT on apoE3 dimer formation are shown in Fig. 1E. The levels of apoE3 dimers (Fig. 1E, asterisks) in apoE3 samples pretreated with Hcy or DTT decreased in a Hcy- or DTT-dose-dependent manner (Fig. 1E). These lines of evidence suggest that the lower level of HDL generated by Hcy-bound apoE3 than by intact apoE3 results in an earlier Aβ deposition and inferior synaptic plasticity, causing earlier AD development.
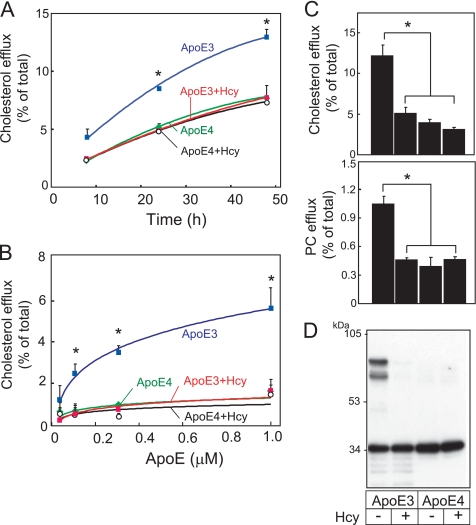
We next determined whether these are also the cases for neurons. We have examined the cholesterol efflux from cultured neurons induced by apoE3, apoE3 pretreated with Hcy, apoE4, and apoE4 pretreated with Hcy at varying hours after the commencement of treatment of apoEs at 0.3 μm (Fig. 2A). The levels of cholesterol efflux induced by apoE3 were greater than those induced by apoE3 preincubated with Hcy, apoE4, or apoE4 preincubated with Hcy at 24 and 48 h. We also determined the levels of cholesterol efflux 24 h after the commencement of treatment of apoEs at 0.1, 0.3, and 1.0 μm (Fig. 2B). The levels of cholesterol efflux induced by apoE3 were greater than those induced by apoE3 preincubated with Hcy, apoE4, or apoE4 preincubated with Hcy at apoE concentrations of 0.3 and 1.0 μm. Next, we determined the levels of cholesterol and PC efflux and also the assembly state of apoE3 and apoE4. The levels of cholesterol and PC released by apoE3 were significantly greater than those released by apoE4 and by apoE3 and apoE4 pretreated with Hcy 24 h after the commencement of treatment (Fig. 2C). A reduced level of lipid efflux was accompanied by a reduced level of apoE3 dimers in apoE3 samples pretreated with Hcy (Fig. 2D). ApoE4 does not form dimers, owing to a lack of cysteine and Hcy pretreatment dis not affect the apoE4 assembly state.
FIGURE 2.
Impairment of apoE3 function by Hcy in cultured neurons. A, the cultured neurons labeled with [14C]acetate were exposed to apoE3 (blue squares), apoE3+Hcy (red squares), apoE4 (green circles), and apoE4+Hcy (open circles) for various times at 0.3 μm apoE. The lipids released into the media and the lipids retained in the cells were determined. Data are means ± S.E. of four samples. *, p < 0.001 versus apoE3+Hcy, apoE4, and apoE4+Hcy at each dose point. B, each culture was exposed to apoE3 (blue squares), apoE3+Hcy (red squares), apoE4 (green circles), and apoE4+Hcy (open circles) at 0.05, 0.01, 0.3, and 1.0 μm for 24 h. The percentages of released cholesterol and PC levels with respect to the total levels were calculated. Data are means ± S.E. of four samples. *, p < 0.001. Three independent experiments showed similar results. C, the levels of cholesterol and PC efflux were determined in neuron cultures treated with apoE3, apoE3+Hcy, apoE4, and apoE4+Hcy 24 h after the commencement of treatment of apoEs at 0.3 μm. The percentages of released cholesterol and PC levels over the total levels were calculated. Data are means ± S.E. of four samples. *, p < 0.0001. Three independent experiments showed similar results. D, Western blot analysis of the samples of apoE3, apoE3+Hcy, apoE4, and apoE4+Hcy was performed under nonreducing conditions.
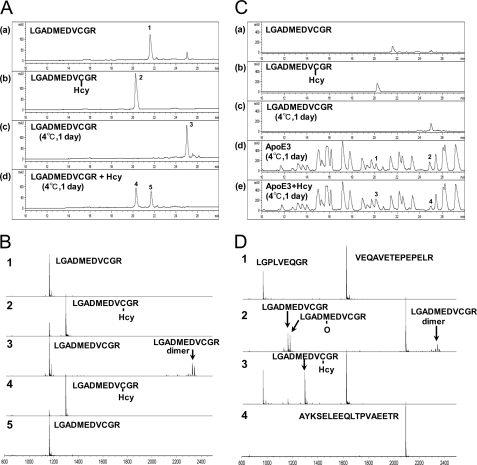
Regarding the underlying molecular mechanism, we determined whether Hcy and apoE3 form disulfide bonds. We performed reverse-phase HPLC and MS of apoE3-derived peptides, LGADMEDVCGR (residues 104–114) and the Hcy-bound form of LGADMEDVCGR, namely LGADMEDVC(Hcy)GR. The HPLC profiles of the synthetic peptides LGADMEDVCGR and LGADMEDVC(Hcy)GR are shown in Fig. 3A, panels a and b, respectively. We also analyzed LGADMEDVCGR incubated with (Fig. 3A, panel d) or without Hcy (Fig. 3A, panel c) for 24 h at 4 °C. The LGADMEDVCGR peptides incubated with Hcy (peaks 4 and 5) eluted at positions corresponding to those of LGADMEDVCGR (peak 1) and LGADMEDVC(Hcy)GR (peak 2), respectively. Peaks 1–5 shown in Fig. 3A were analyzed by MALDI-TOF MS. A signal in peak 4 corresponds to the same molecular mass of LGADMEDVC(Hcy)GR (Fig. 3B, 4) as peak 2 (Fig. 3B, 2). A signal in peak 5 corresponds to the same molecular mass of LGADMEDVCGR (Fig. 3B, 5) as peak 1 (Fig. 3B, 1). Signals in peak 3 correspond to LGADMEDVCGR and LGADMEDVCGR dimers (Fig. 3B, 3). Some of the dimers dissociate into monomers by laser irradiation during MS. These data also show that the LGADMEDVCGR peptides tend to form dimers by disulfide bonds in an environment susceptible to oxidation; however, in the presence of Hcy, Hcy inhibited the dimer formation by direct interaction with the thiol of Cys residues.
FIGURE 3.
Reverse-phase HPLC profiles and MS of apoE3-derived peptides. A, LGADMEDVCGR peptides (panel a), LGADMEDVC(Hcy)GR peptides (panel b), and LGADMEDVCGR peptides incubated at 4 °C for 1 day with (panel d) or without (panel c) Hcy were subjected to HPLC. B, peaks 1–5 as shown in Fig. 3A were subjected to MALDI-TOF MS using α-cyano-4-hydroxycinnamic acid as a matrix. C, the tryptic peptides of intact recombinant apoE3 incubated at 4 °C for 1 day with or without Hcy were analyzed by HPLC. Elution profiles of LGADMEDVCGR (panel a), LGADMEDVC(Hcy)GR (panel b), and LGADMEDVCGR peptides incubated at 4 °C for 1 day (panel c), and tryptic peptides of incubated apoE3 with (panel e) or without (panel d) Hcy are shown. The elution conditions were the same as those described in A. D, peaks 1 and 3, which correspond to LGADMEDVC(Hcy)GR in C, panel b, and peaks 2 and 4, which correspond to LGADMEDVCGR dimers in Fig. 3C, panel c, were subjected to MS.
Next, we analyzed the interaction between intact apoE3 and Hcy. The solution containing intact apoE3 was incubated with or without Hcy at 4 °C for 1 day. ApoE3 in the solution was digested with trypsin, and the tryptic peptides of intact apoE3 incubated with (Fig. 3C, panel e) or without (Fig. 3C, panel d) Hcy were analyzed by HPLC. The level of peak 3 (Fig. 3C, panel e), which has the same elution time as LGADMEDVC(Hcy)GR (Fig. 3C, panel b), increased compared with that of peak 1 (Fig. 3C, panel d), whereas that of peak 4 (Fig. 3C, panel e), which has the same elution time as LGADMEDVCGR (Fig. 3C, panel c), decreased compared with that of peak 2 (Fig. 3C, panel d).
Furthermore, peaks 1–4, shown in Fig. 3C, were also analyzed by MS (Fig. 3D). The signals in peak 1 correspond to LGPLVEQGR (residues 181–189) and VEQAVETEPEPELR (residues 2–15) (Fig. 3D, peak 1). The signals in peak 3, which has the same elution time as peak 1, correspond to LGADMEDVC(Hcy)GR, indicating that intact apoE3 binds to Hcy in addition to LGPLVEQGR and VEQAVETEPEPELR (Fig. 3D, peak 3). The signals in peak 2, which has the same elution time as peak 4, correspond to LGADMEDVCGR and LGADMEDVCGR dimer in addition to AYKSELEEQLTPVAEETR (residues 73–90) (Fig. 3D, peak 2).
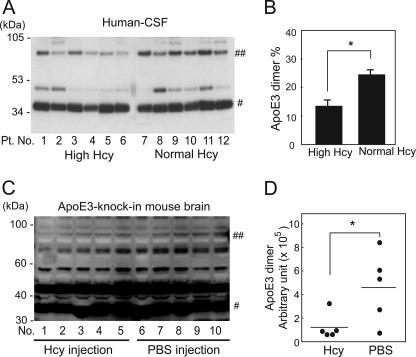
Next, we examined whether the ratio of the apoE3 dimer is lower in the human subjects with hyperhomocysteinemia than in human subjects with normal Hcy. The CSF from human apoEϵ3/3 carriers with normal plasma Hcy and hyperhomocysteinemia were analyzed. The profiles of patients are shown in Table 1. The Hcy concentrations in the plasma and CSF from the patients with hyperhomocysteinemia were higher (mean ± S.E., 232.58 ± 191.91 μm for plasma and 20.17 ± 12.30 nm for CSF, Table 1) than those from the patients with normal plasma Hcy (4.07 ± 0.86 μm for plasma and 0.45 ± 0.16 nm for CSF, Table 1). The results of Western blot analysis of these samples under nonreducing conditions are shown in Fig. 4A. The band signals of apoE3 dimers and monomers were scanned by densitometry, and the ratio of the levels of dimers with respect to the level of total apoE3 was calculated. The ratio of the levels of apoE3 dimers with respect to the level of total apoE3 is significantly lower in those who have hyperhomocysteinemia (Fig. 4B). The CSF samples from the human subjects with hyperhomocysteinemia contain a higher level of Hcy (mean ± S.E. = 20.17 ± 12.30 nm, Table 1) compared with those from the human subjects with normal plasma Hcy (0.45 ± 0.16 nm, Table 1), suggesting that a larger amount of Hcy binds apoE3 molecules via disulfide bonds and inhibits apoE3 dimerization. The correlation between the level of Hcy and the ratio of apoE3 dimer is shown in supplemental Fig. 2. Dimer ratios tended to negatively correlate with Hcy level in CSF and serum samples, although it does not reach statistical significance (supplemental Fig. 1, A and B). This tendency for a negative correlation becomes stronger when the separately distributed data (a, b, or c) are removed. The serum lipid profiles of the patients and the correlations of the lipid profiles and the ratio of apoE3 dimer are also shown in supplemental Table 1 and supplemental Fig. 3.
TABLE 1.
The profiles of patients examined
Patient Nos. 1–6 were diagnosed as hyperhomocysteinemia and Nos. 7 to 12 were diagnosed as normal plasma Hcy. VD, vascular dementia; NPH, normal pressure hydrocephalus; SD-NFT, senile dementia of neurofibrillary tangle type; DLB, dementia with Lewy body disease; CVD, cerebrovascular disease; PSP, progressive supranuclear palsy.
| No. | Age | Sex | Diagnosis | Serum Hcy | CSF Hcy |
|---|---|---|---|---|---|
| μm | nm | ||||
| 1 | 89 | F | CVD | 50.4 | 0.90 |
| 2 | 75 | F | CVD | 1192 | 61.8 |
| 3 | 96 | F | AD | 45.5 | 1.14 |
| 4 | 89 | F | DLB | 43.8 | 56.2 |
| 5 | 71 | M | PSP | 25.8 | 0.40 |
| 6 | 91 | F | CVD | 38.0 | 0.6 |
| 7 | 81 | M | VD | 3.7 | 1.23 |
| 8 | 86 | F | AD | 5.0 | 0.43 |
| 9 | 83 | F | AD | 1.4 | 0.32 |
| 10 | 95 | F | SD-NFT | 5.7 | 0.32 |
| 11 | 84 | M | NPH | 6.7 | 0.20 |
| 12 | 79 | M | DLB | 1.9 | 0.22 |
FIGURE 4.
Assembly state of apoE3 in the CSF from human subjects with normal plasma Hcy and hyperhomocysteinemia. A, CSF from human subjects with hyperhomocysteinemia or normal plasma Hcy was mixed with an equal amount of sampling buffer consisting of 100 mm Tris-HCl (pH 7.4), 10% glycerol, 4% SDS, and 0.01% bromphenol blue, and analyzed using 12.5% Tris/Tricine SDS-PAGE under nonreducing conditions. The proteins transferred to the membrane were subjected to Western blot analysis using the anti-apoE antibody. # and ##, apoE3 monomers and dimers, respectively. Pt. No., patient number. B, the ratio of signal intensities of apoE dimers with respect to total apoE (monomers plus dimers) in each sample was determined by densitometry and calculation. The values are means ± S.E. of six CSF samples from human subjects with high and normal plasma Hcy. *, p < 0.003. C, Western blot analysis of brain homogenate prepared from apoE3 knock-in mice subcutaneously injected with Hcy. Numbers 1–5 are the samples from mice treated with Hcy, and numbers 6–10 are those from mice treated with PBS. # and ##, the apoE3 monomers and dimers, respectively. D, quantification of signals representing the apoE3 dimer in D is determined by densitometry. The values are means ± S.E. of five brain homogenate samples. *, p < 0.005.
We next examined whether hyperhomocysteinemia inhibits apoE3 dimer formation in the brains of mice expressing human apoE3 without expressing rodent endogenous apoE. ApoE3 knock-in mice were injected with 100 μl of 0.6 μm Hcy subcutaneously twice a day for 6 days. The mice were then sacrificed, brains and serum were isolated, and the level of the apoE3 dimer in brain samples was determined by Western blot analysis under nonreducing conditions. The level of the apoE3 dimer in each sample that was loaded with equal amounts of brain homogenate protein is shown in Fig. 4, C and D. The Hcy level in the serum from the Hcy-injected mice is significantly higher than those from PBS-injected mice (mean ± S.E. for Hcy-treated samples is 10.26 ± 0.47 μm and that for control is 4.98 ± 0.48 μm; p < 0.0001). The level of the apoE3 dimer is significantly lower in Hcy-injected mouse brains than in control brains (Fig. 4D). Although the ratio of the apoE3 dimer in the mouse brain was very low compared with that of human CSF, these results show that a higher level of serum Hcy resulted in the attenuation of dimer formation of apoE3 in the brain.
DISCUSSION
Our previous studies have shown that intramolecular interaction (i.e. domain interaction) and intermolecular interaction (i.e. dimerization) determine the apoE isoform-dependent ability to generate HDL (7, 10). Because Hcy is a molecule harboring a thiol, we hypothesized that the thiol of Hcy associates with the thiol of cysteine residues in apoE3, and the formation of this disulfide bonds interferes with apoE3 dimerization. In the present study, we found that Hcy binds to cysteine residues of apoE3, thereby interfering with apoE3 dimerization and impairing the ability of apoE3 to generate HDL to a level similar to that of apoE4. These in vitro results are supported by those of the analysis of human CSF samples from patients with hyperhomocysteinemia and normal serum homocysteine, showing that the ratio of the levels of apoE3 dimers with respect to the level of total apoE3 in CSF samples from patients with hyperhomocysteinemia is significantly lower than that from normal controls. In addition, the subcutaneous injection of Hcy into apoE3 knock-in mice resulted in a reduced level of the apoE3 dimer in the brain homogenate, suggesting that hyperhomocysteinemia decreases the level of apoE3 dimer in CSF or the brain.
To determine the effect of Hcy bound to apoE3, we preincubated Hcy and apoE3 at relatively high concentrations. Under these conditions, ∼66% of apoE3 binds to Hcy, whereas the level of Hcy bound to apoE4 was not detected (Table 2). One may question whether the inhibitory effect of Hcy on apoE3 dimerization and apoE3-mediated lipid efflux were observed when lower concentrations of Hcy similar to those in serum or CSF were used in the presence of comparable concentrations of apoE3 in culture. Hcy at lower concentrations did not inhibit apoE3 dimerization nor attenuate apoE3-mediated lipid efflux. This may be because it takes a longer time to form disulfide bonds between Hcy and apoE3 at physiological concentrations in culture. However, this is not the case for in vivo conditions, including those in human CSF and the mouse brain. Although the precise mechanism underlying this discrepancy cannot be provided by the current study, the disulfide bonds between apoE3 and Hcy molecules occurs in vivo at concentrations lower than those used in in vitro experiments. In support of this notion, previous studies have shown that >70% of Hcy in plasma forms disulfide-bonded to cysteine residues of proteins including transthyretin (31, 32), suggesting that Hcy at single digit μm concentrations forms disulfide bonds to cysteine residues in vivo.
TABLE 2.
Association of Hcy with apoE3 and apoE4
To determine the association of Hcy with apoE, 7.5 μl of 100 mm Hcy was added to 500 μl of apoE-containing solution at 14.7 mm apoE. The apoE3 and apoE4 containing solutions with or without Hcy, or Hcy solution without apoE were incubated overnight at room temperature. The solutions were then dialyzed against PBS overnight at room temperature, and the level of Hcy in each solution was determined. UD, under detectable level.
| Hcy (n = 8) | apoE3 (n = 3) | apoE4 (n = 3) | apoE3 + Hcy (n = 8) | apoE4 + Hcy (n = 8) | |
|---|---|---|---|---|---|
| Hcy (μm) | UD | UD | UD | 5.75 ± 0.25 | UD |
Previous studies have shown other biological and pathological effects of Hcy; namely, Hcy induces neuronal damage (33, 34), compromises blood-brain barrier integrity (26), modulates Aβ toxicity (35), and modulates Aβ generation (27, 28). However, the molecular mechanism(s) by which Hcy directly interacts with the molecule(s) in these studies are not fully understood. In this study, we showed that a high level of Hcy impairs apoE3 function to a similar level of apoE4 by preventing/breaking the disulfide bonds, thereby leading to a decreased HDL generation. Because apoE-HDL plays a role in Aβ clearance(13,14), the lines of evidence suggest that two different risk factors for AD, apoE4 and hyperhomocysteinemia, may share a common mechanism; that is, apoE4 has a lower ability to generate HDL than apoE3 and the Hcy-induced modification of apoE3 impairs the ability of apoE3 to generate HDL to a level similar to that of apoE4. Our observations in the present study also provide new insight into concerning the apoE genotype-dependent treatment of hyperhomocysteinemia, especially for reducing the risk of AD.
Supplementary Material
This work was supported by Ministry of Health, Labor and Welfare of Japan Comprehensive Research on Aging and Health Grant H20-007, the Program for Promotion of Fundamental Studies in Health of the National Institute of Biomedical Innovation (NIBIO), and Grant-in-aid for Scientific Research on Priority Areas 20023040 (Research on Pathomechanisms of Brain Disorders) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, Table 1, and Figs. 1–3.
- AD
- Alzheimer disease
- DTT
- dithiothreitol
- PC
- phosphatidylcholine
- CSF
- cerebrospinal fluid
- Hcy
- homocysteine.
REFERENCES
- 1.Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krimbou L., Denis M., Haidar B., Carrier M., Marcil M., Genest J., Jr. (2004) J. Lipid Res. 45, 839–848 [DOI] [PubMed] [Google Scholar]
- 3.DeMattos R. B., Brendza R. P., Heuser J. E., Kierson M., Cirrito J. R., Fryer J., Sullivan P. M., Fagan A. M., Han X., Holtzman D. M. (2001) Neurochem. Int. 39, 415–425 [DOI] [PubMed] [Google Scholar]
- 4.Hara M., Matsushima T., Satoh H., Iso-o N., Noto H., Togo M., Kimura S., Hashimoto Y., Tsukamoto K. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 269–274 [DOI] [PubMed] [Google Scholar]
- 5.Altenburg M., Johnson L., Wilder J., Maeda N. (2007) J. Biol. Chem. 282, 7817–7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michikawa M., Fan Q. W., Isobe I., Yanagisawa K. (2000) J. Neurochem. 74, 1008–1016 [DOI] [PubMed] [Google Scholar]
- 7.Gong J. S., Kobayashi M., Hayashi H., Zou K., Sawamura N., Fujita S. C., Yanagisawa K., Michikawa M. (2002) J. Biol. Chem. 277, 29919–29926 [DOI] [PubMed] [Google Scholar]
- 8.Xu Q., Brecht W. J., Weisgraber K. H., Mahley R. W., Huang Y. (2004) J. Biol. Chem. 279, 25511–25516 [DOI] [PubMed] [Google Scholar]
- 9.Gong J. S., Morita S. Y., Kobayashi M., Handa T., Fujita S. C., Yanagisawa K., Michikawa M. (2007) Mol. Neurodegener. 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minagawa H., Gong J. S., Jung C. G., Watanabe A., Lund-Katz S., Phillips M. C., Saito H., Michikawa M. (2009) J. Neurosci. Res. 87, 2498–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y., Liu X. Q., Wyss-Coray T., Brecht W. J., Sanan D. A., Mahley R. W. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 8838–8843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T., Watanabe A., Fujino T., Hosono T., Michikawa M. (2009) Mol. Neurodegener. 4, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deane R., Sagare A., Hamm K., Parisi M., Lane S., Finn M. B., Holtzman D. M., Zlokovic B. V. (2008) J. Clin. Invest. 118, 4002–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Q., Lee C. Y., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T. M., Collins J. L., Richardson J. C., Smith J. D., Comery T. A., Riddell D., Holtzman D. M., Tontonoz P., Landreth G. E. (2008) Neuron 58, 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolovou G. D., Anagnostopoulou K. K. (2007) Ageing Res. Rev. 6, 94–108 [DOI] [PubMed] [Google Scholar]
- 16.McCarron M. O., Delong D., Alberts M. J. (1999) Neurology 53, 1308–1311 [DOI] [PubMed] [Google Scholar]
- 17.Guthikonda S., Haynes W. G. (1999) Curr. Opin. Cardiol. 14, 283–291 [DOI] [PubMed] [Google Scholar]
- 18.Kim N. K., Choi B. O., Jung W. S., Choi Y. J., Choi K. G. (2003) Neurology 61, 1595–1599 [DOI] [PubMed] [Google Scholar]
- 19.Perry I. J. (1999) J. Cardiovasc. Risk 6, 235–240 [DOI] [PubMed] [Google Scholar]
- 20.Seshadri S., Beiser A., Selhub J., Jacques P. F., Rosenberg I. H., D'Agostino R. B., Wilson P. W., Wolf P. A. (2002) N. Engl. J. Med. 346, 476–483 [DOI] [PubMed] [Google Scholar]
- 21.Luchsinger J. A., Tang M. X., Shea S., Miller J., Green R., Mayeux R. (2004) Neurology 62, 1972–1976 [DOI] [PubMed] [Google Scholar]
- 22.Ravaglia G., Forti P., Maioli F., Martelli M., Servadei L., Brunetti N., Porcellini E., Licastro F. (2005) Am. J. Clin. Nutr. 82, 636–643 [DOI] [PubMed] [Google Scholar]
- 23.Van Dam F., Van Gool W. A. (2009) Arch. Gerontol. Geriatr. 48, 425–430 [DOI] [PubMed] [Google Scholar]
- 24.Isobe C., Murata T., Sato C., Terayama Y. (2005) Life Sci. 77, 1836–1843 [DOI] [PubMed] [Google Scholar]
- 25.Starkebaum G., Harlan J. M. (1986) J. Clin. Invest. 77, 1370–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamath A. F., Chauhan A. K., Kisucka J., Dole V. S., Loscalzo J., Handy D. E., Wagner D. D. (2006) Blood 107, 591–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacheco-Quinto J., Rodriguez de Turco E. B., DeRosa S., Howard A., Cruz-Sanchez F., Sambamurti K., Refolo L., Petanceska S., Pappolla M. A. (2006) Neurobiol. Dis. 22, 651–656 [DOI] [PubMed] [Google Scholar]
- 28.Zhang C. E., Wei W., Liu Y. H., Peng J. H., Tian Q., Liu G. P., Zhang Y., Wang J. Z. (2009) Am. J. Pathol. 174, 1481–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamanaka H., Katoh-Fukui Y., Suzuki K., Kobayashi M., Suzuki R., Motegi Y., Nakahara Y., Takeshita A., Kawai M., Ishiguro K., Yokoyama M., Fujita S. C. (2000) Hum. Mol. Genet. 9, 353–361 [DOI] [PubMed] [Google Scholar]
- 30.Michikawa M., Gong J. S., Fan Q. W., Sawamura N., Yanagisawa K. (2001) J. Neurosci. 21, 7226–7235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim A., Sengupta S., McComb M. E., Théberge R., Wilson W. G., Costello C. E., Jacobsen D. W. (2003) J. Biol. Chem. 278, 49707–49713 [DOI] [PubMed] [Google Scholar]
- 32.Sass J. O., Nakanishi T., Sato T., Sperl W., Shimizu A. (2003) Biochem. Biophys. Res. Commun. 310, 242–246 [DOI] [PubMed] [Google Scholar]
- 33.Kruman II, Kumaravel T. S., Lohani A., Pedersen W. A., Cutler R. G., Kruman Y., Haughey N., Lee J., Evans M., Mattson M. P. (2002) J. Neurosci. 22, 1752–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maler J. M., Seifert W., Hüther G., Wiltfang J., Rüther E., Kornhuber J., Bleich S. (2003) Neurosci. Lett. 347, 85–88 [DOI] [PubMed] [Google Scholar]
- 35.White A. R., Huang X., Jobling M. F., Barrow C. J., Beyreuther K., Masters C. L., Bush A. I., Cappai R. (2001) J. Neurochem. 76, 1509–1520 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.