Abstract
Integrins are postulated to undergo structural rearrangement from a low affinity bent conformer to a high affinity extended conformer upon activation. However, some reports have shown that a bent conformer is capable of binding a ligand, whereas another report has shown that integrin extension does not absolutely lead to activation. To clarify whether integrin affinity is indeed regulated by the so-called switchblade-like movement, we have engineered a series of mutant αIIbβ3 integrins that are constrained specifically in either a bent or an extended conformation. These mutant αIIbβ3 integrins were expressed in mammalian cells, and fibrinogen binding to these cells was examined. The bent integrins were created through the introduction of artificial disulfide bridges in the β-head/β-tail interface. Cells expressing bent integrins all failed to bind fibrinogen unless pretreated with DTT to disrupt the disulfide bridges. The extended integrins were created by introducing N-glycosylation sites in amino acid residues located close to the α-genu, where the integrin legs fold backward. Among these mutants, activation was maximized in one integrin with an N-glycosylation site located behind the α-genu. This extension-induced activation was completely blocked when the swing-out of the hybrid domain was prevented. These results suggest that the bent and extended conformers represent low affinity and high affinity conformers, respectively, and that extension-induced activation depends on the swing-out of the hybrid domain. Taken together, these results are consistent with the current hypothesis that integrin affinity is regulated by the switchblade-like movement of the integrin legs.
Keywords: Adhesion, Cell Surface Receptor, Integrin, Platelet, Signal Transduction, Activation, Structural Change, Structure-Function Relationship
Introduction
Integrin-mediated bidirectional signaling is closely associated with the structural rearrangement of integrin itself. During inside-out signaling, talin has been shown to bind to the β cytoplasmic tail and to disrupt the endogenous interaction between the α and β cytoplasmic tails (1, 2). The dissociation of the two tails induces a structural rearrangement of the extracellular domains increasing the affinity to the ligand. During outside-in signaling, ligand binding in turn induces the structural rearrangement of the extracellular domains. This structural change propagates through the plasma membrane to separate the cytoplasmic tails, providing binding sites for numerous cytoplasmic proteins (3). Thus, the two structures flanking the plasma membrane affect each other. This structural rearrangement can be detected using a group of monoclonal antibodies (mAbs) that bind preferentially to the ligand-bound form (4). These anti-LIBS2 (ligand-induced binding site) mAbs have been used not only to investigate the activation status of a specific integrin but also to activate it (5). However, without information on the actual three-dimensional structure, it is impossible to determine the specific conformation recognized by each anti-LIBS mAb.
The first observation of the actual three-dimensional structure of integrin was made using conventional electron microscopy (EM) studies of αIIbβ3 purified from platelets (6, 7). Although this modality had a relatively low resolution, αIIbβ3 was shown to consist of a globular head with two short legs extending outward. A crystal structure analysis of the extracellular domains of αVβ3 integrin (PDB 1M1X) revealed that the α-chain consists of the N-terminal β-propeller domain followed by the thigh, calf-1, and calf-2 domains, whereas the β-chain consists of the plexin-semaphorin-integrin domain, βA domain, hybrid domain, four EGF domains, and βT domain (8). The β-propeller and βA domains non-covalently associate with each other to form the globular head that was observed in the EM images. In contrast, the thigh, calf-1, and calf-2 domains of the α-chain and the plexin-semaphorin-integrin, EGF, and βT domains of the β-chain form the two leg-like regions, respectively. Thus, the crystal structure is consistent with the conventional EM image described above. However, a striking difference in the orientation of the head was noted. In the crystal structure, the two legs were folded backward, with a 135° angle between the thigh and the calf-1 domains, unlike the straight legs observed using conventional EM. Consequently, the head region pointed downward, facing the plasma membrane. The discrepancies between these two structures were reconciled using high resolution EM images of the extracellular domains of recombinant αVβ3 integrin (9). This modality revealed that αVβ3 could adopt multiple distinct structures, including the bent and extended conformers observed in the crystal structure analysis and the conventional EM study, respectively. Because Mn2+ and a ligand peptide significantly increased the number of extended forms, the extended form was suggested to represent a high affinity state, whereas the bent form was thought to represent a low affinity state. Thus, the transition from one conformer to another (or the so-called switchblade-like movement) might regulate the affinity of integrin to its ligand. Aside from this movement, substantial structural rearrangement has been observed in the head region (10). A crystal structure analysis of the αIIbβ3 head region when the molecule forms a complex with ligand mimetics revealed that the β-hybrid domain swings outward upon ligand binding (11). This movement is accompanied by the rearrangement of the ligand- and/or cation-binding loops in the βA domain, thereby regulating ligand binding (11).
However, contradictory reports suggest that integrin extension is not an essential event for ligand binding. Cryo-electron microscopic observations of αIIbβ3 purified from activated platelets revealed that this molecule adopts a rather compact structure, unlike the extended conformer (12). The crystal structure of αVβ3 complexed with a small peptide ligand revealed that the bent conformer was capable of binding a ligand (13). In this experiment, αVβ3 was understandably unable to undergo gross structural rearrangement upon ligand binding because of the constraints of the crystal lattice. However, a single particle analysis of recombinant αVβ3 complexed with a fibronectin fragment has shown that αVβ3 can bind macromolecular ligands while in a bent conformation in the presence of Mn2+ (14, 15). This evidence suggests that the bent conformer is capable of binding both small ligands and macromolecular ligands without requiring substantial structural rearrangements.
In this study, we examined the relationship between the three-dimensional structure of integrin and its ligand affinity. Our findings provide evidence that the extended conformer represents a highly activated state, whereas the bent conformer represents a low affinity state. These results are consistent with the view that the ligand binding activity of integrin can be regulated allosterically through the switchblade-like movement of the legs of integrin, centering on the genu region.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Normal mouse IgG was purchased from Sigma-Aldrich. Anti-αIIb mAb PL98DF6 (16) was a generous gift from Drs. J. Ylänne (University of Oulu, Finland) and I. Virtanen (University of Helsinki, Finland). Anti-β3 mAbs anti-LIBS2 and anti-LIBS6 (17) were generous gifts from Dr. Mark H. Ginsberg (University of California, San Diego, La Jolla, CA). Anti-αIIbβ3 complex-specific ligand-mimetic mAb OP-G2 (18) was a kind gift from Dr. Yoshiaki Tomiyama (University of Osaka, Japan). Anti-αIIbβ3 complex-specific function-blocking mAb A2A9 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-αIIbβ3 complex-specific activating mAb PT25-2 and non-functional anti-β3 mAb VNR5-2 have previously been characterized (19). Anti-β3 mAb SZ21 was purchased from Beckman Coulter. Anti-FLAG mAb M2 was purchased from Sigma-Aldrich. R-Phycoerythrin-conjugated goat anti-mouse polyclonal antibody was purchased from BIOSOURCE (Camarillo, CA). Synthetic peptide Gly-Arg-Gly-Asp-Ser (GRGDS) was purchased from Peptide Research Institute (Osaka, Japan). Fluorescein-isothiocyanate (FITC) was purchased from Sigma-Aldrich. Human fibrinogen (Fbg) was purchased from Enzyme Research Laboratories (South Bend, IN). The tobacco etching virus (TEV) protease TurboTEV protease and peptide N-glycosidase F were purchased from Accelagen (San Diego, CA) and New England Biolabs (Ipswich, MA), respectively.
Construction of Mutant αIIb and β3 cDNA Clones
The full-length cDNAs for the integrin αIIb and β3 subunits, generous gifts from Dr. Joseph C. Loftus (Mayo Clinic, Scottsdale, AZ), were cloned into the mammalian expression vector pBJ-1, kindly provided by Dr. Mark Davis (University of California, San Francisco). The cDNAs for the β3 mutants V332C, S367C, G382C, S551C, T564C, S674C, S367C/S551C, G382C/T564C, V332C/S674C, V332N, S674N/K676T, V332N/S674N/K676T, and V359C and the cDNAs for the αIIb mutants T478N, D589N/H591T, Q595N/R597T, Q595A/R597T, Q595W/R597T, Q595D/R597T, Q595N, R597T, Q595N/R597A, Q595N/R597S, D319C, and Q595N/R597T/D319C were created using the Transformer site-directed mutagenesis kit (BD Biosciences). The cDNAs for β3 del 671–676 (del-CD) and β3 FLAG 671–676 (FLAG-CD) were also created using site-directed mutagenesis and 5′-GATTCCAGTACTATTCCATCCTGTATGTG-3′ and 5′-CAGATTCCAGTACTATTCCATCCTGTATG-3′ as mutagenic primers, respectively. The TEV protease recognition site Glu-Asn-Leu-Tyr-Phe-Gln-Gly was introduced to amino acid residues 475–481 of the β3 chain (475TEV) using 5′-GTGTGAGTGCTCAGAGAACCTCTATTTCCAAGGCCAGCAGGACGAATGCAGC-3′ as a mutagenic primer. Two additional mutations, C473S and C503S, were introduced in 475TEV using site-directed mutagenesis (475TEVCS). The S367C/S551C, G382C/T564C, or V332C/S674C double mutations were introduced in 475TEVCS utilizing common restriction sites. To create the cDNA for the FLAG-αIIb450 fragment, a FLAG tag sequence was inserted at the 5′ terminus of wild-type αIIb cDNA followed by a BstEII restriction site using 5′-GCTGCCCCTCCAGCCTGGGCCGACTACAAGGACGACGATGACAAGGGGTCACCATTGAACCTGGACCCAGTGCAGC-3′ as a mutagenic primer (FLAG-αIIb). Next, the BstEII restriction site was introduced at the 5′ side of Ala-450 using 5′-CCAGGTGGCTGGGTCACCAGCTCAGCCAGTG-3′ as a mutagenic primer (αIIbBstE450). Then, the cDNA for the FLAG-αIIb450 fragment was created by replacing the 5′-terminal BstEII/BglII fragment of FLAG-αIIb with the same fragment from αIIbBstE450. Finally, the cDNA for the FLAG-αIIb450 fragment carrying the T478N, D589N/H591T, Q595N/R597T, or R597T mutation was created by combining these mutations using common restriction sites.
Cell Culture and Transfection
Chinese hamster ovary (CHO)-K1 cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal calf serum (HyClone, Logan, UT), 1% penicillin and streptomycin (Invitrogen), and 1% non-essential amino acids (Sigma-Aldrich) and maintained at 37 °C in a humidified incubator supplemented with 5% CO2. Fifty micrograms of αIIb cDNA construct was co-transfected with 50 μg of β3 cDNA construct into CHO-K1 cells using electroporation. After 48 h, the cells were detached and used for further experiments.
Flow Cytometry
Cells were detached with phosphate-buffered saline (PBS) containing 3.5 mm EDTA. After washing, the cells were incubated with 10 μg/ml mAb in modified HEPES-Tyrode's buffer (5 mm HEPES, 5 mm glucose, 0.2 mg/ml bovine serum albumin, 1× Tyrode's solution) supplemented with 1 mm CaCl2 and 1 mm MgCl2 for 30 min at 4 °C. In some experiments, 1 mm GRGDS peptide was included together with the mAbs. After washing, the cells were incubated with the R-Phycoerythrin-conjugated F(ab′)2 fragment of goat anti-mouse IgG for 30 min at 4 °C. After washing, the cells were resuspended in HEPES-buffered saline (10 mm HEPES, 150 mm NaCl (pH 7.4)) containing 1 mm CaCl2 and 1 mm MgCl2. Fluorescence was measured using a FACSCalibur (BD Biosciences). To compare the binding of conformation-dependent mAbs among cells expressing different αIIbβ3 mutants, each mAb binding was normalized by the expression of αIIbβ3 on the cell surface. This relative mAb binding was calculated by dividing the mean fluorescent intensity obtained for each mAb by the mean fluorescent intensity obtained for the non-conformation-dependent anti-β3 mAb SZ21 or the anti-αIIbβ3 complex-specific mAb A2A9.
Fibrinogen Binding Assay
FITC labeling of human Fbg was performed as described previously (20). Briefly, after adjusting the pH of human Fbg at 1 mg/ml in PBS to 8.5 using 5% Na2CO3, 1/100 volume of 10 mg/ml FITC in dimethyl sulfoxide (DMSO) was added and incubated at room temperature for 10 min. FITC-labeled Fbg was separated from free FITC on a PD-10 column (Amersham Biosciences, Uppsala, Sweden) equilibrated with HEPES-buffered saline. The concentration and fluorescence-to-protein ratio of FITC-labeled Fbg were calculated as described previously. The typical concentration and fluorescence-to-protein ratio were 3.4 mg/ml and 5.0–6.0, respectively. Forty-eight hours after transfection, the cells were detached and washed once with HEPES-Tyrode's buffer. The αIIbβ3-transfected cells were incubated with non-functional anti-αIIb mAb PL98DF6 followed by incubation with the ribulose-phosphate 3-epimerase-conjugated F(ab′)2 fragment of goat anti-mouse IgG. In some experiments, cells were treated with dithiothreitol (DTT) prior to incubation with the mAbs, as described previously (19). After washing, the cells were incubated with 340 μg/ml FITC-labeled Fbg with or without 1 mm GRGDS peptide in HEPES-Tyrode's buffer containing 1 mm CaCl2 and 1 mm MgCl2 or 1 mm MnCl2 for 2 h at 4 °C. In some experiments, the mAb PT25-2 was included at a concentration of 10 μg/ml to activate αIIbβ3. After washing, fluorescence was measured using a FACSCalibur. The mean Fbg binding (FL1) to cell populations expressing high levels of αIIb (FL2 > 500) was calculated. Background binding in the presence of 1 mm GRGDS peptide was subtracted to obtain the specific binding.
Immunoprecipitation
Biotin labeling of the cell surface protein was done using Sulfo-NHS-Biotin (Thermo Scientific) following the manufacturer's instructions. Cells were lysed in 1 ml of lysis buffer (100 mm n-octylglucopyranoside, 20 mm N-ethyl maleimide, 1 mm PMSF, 25 mm Tris-HCl, and 150 mm NaCl, pH 7.4). After removing the insoluble material by centrifugation, the supernatant was used for further analysis. Two hundred microliters of cell lysate was precleared by adding 1 μg of mouse IgG, together with 20 μl of protein G-agarose beads. After centrifugation, the supernatant was recovered and further incubated with 1 μg of PL98DF6 or VNR5-2, together with 20 μl of protein G-agarose beads overnight at 4 °C. Then, the supernatant was discarded, and the remaining protein G-agarose beads were washed three times with washing buffer (25 mm Tris-HCl, 150 mm NaCl, 0.01% Triton X-100 (pH 8.0)). The protein G-agarose beads were resuspended in 10 μl of washing buffer. The TEV protease digestion of the immunoprecipitates was performed by adding 1 μl of TurboTEV to the suspension with or without 1 mm DTT followed by incubation for 3 h at 30 °C. The peptide N-glycosidase F digestion of the immunoprecipitates was done according to the manufacturer's instructions except that the DTT was excluded from the denaturation buffer. After digestion, the samples were subjected to 7.5 or 10% SDS-PAGE, transferred to a polyvinylidene difluoride membrane, probed with horseradish peroxidase-conjugated avidin, and detected using chemiluminescence with West Pico chemiluminescent substrate (Thermo Scientific).
RESULTS
Bent Conformer of αIIbβ3 Represents a Low Affinity Form
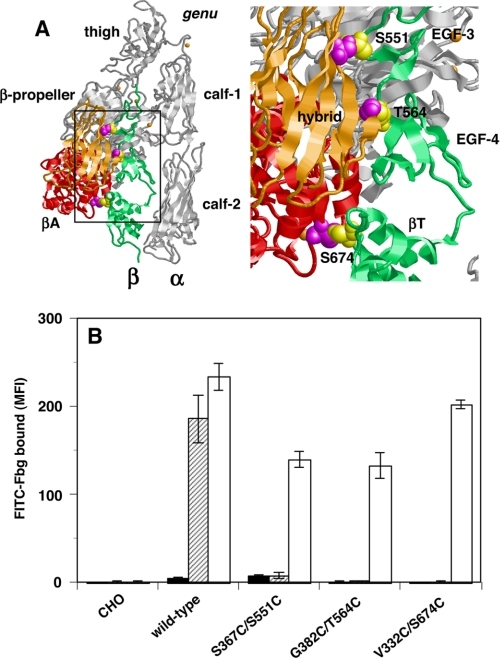
In the αVβ3 crystal structure, in addition to the α-head and β-head, the β-head and β-tail domains create a large interface that keeps αVβ3 in a bent conformation (8, 13). To constrain αIIbβ3 in this bent conformation, artificial disulfide bridges were introduced at different locations in the β-head/β-tail interface. As shown in Fig. 1A, the amino acid residues Ser-367 and Gly-382 in the hybrid domain and Val-332 in the βA domain are localized close to Ser-551 in the EGF-3 domain, Thr-564 in the EGF-4 domain, and Ser-674 in the βT domain, respectively. If these residues are simultaneously mutated to Cys, a disulfide bride is expected to form. Thus, the resulting S367C/S551C, G382C/T564C, and V332C/S674C double mutations are expected to stabilize the hybrid/EGF-3, the hybrid/EGF-4, and the βA/βT interfaces, respectively. In either case, these mutations should prevent the β3 chain from adopting an extended conformation. These mutants were expressed in CHO cells, and FITC-labeled Fbg binding to these cells was examined using FACS. As reported previously, the wild-type αIIbβ3 expressed in CHO cells is in a low affinity state and requires activation by the anti-αIIbβ3 mAb PT25-2 to bind Fbg in the presence of 1 mm Ca2+/1 mm Mg2+ (20). Cells expressing single Cys mutations, such as S367C, G382C, V332C, S551C, T564C, or S674C, bound Fbg in the presence of PT25-2, albeit slightly less than that observed in wild-type cells (data not shown). By contrast, cells expressing double Cys mutations were completely unable to bind Fbg unless they were pretreated with DTT to disrupt the disulfide bridges (Fig. 1B). These artificially introduced disulfides did not affect PT25-2 binding to αIIbβ3 (supplemental Fig. S1). These results suggest that the blocking effect of the double mutation is actually caused by disulfide bridge formation between the mutated residues, rather than a local effect of the mutation itself.
FIGURE 1.
Effect of β-head/β-tail interface stabilization on the αIIbβ3-Fbg interaction. A, crystal structure of the αVβ3 integrin. The entire αV chain is shown as the gray ribbon. The βA and hybrid domains that compose the head region of the β3 chain are shown as red and orange ribbons, respectively. The EGF-3, EGF-4, and βT domains that compose the β-tail of the β3 chain are shown as the green ribbon. The β-head/β-tail interface shown in the rectangle is magnified in the right-hand panel. Amino acid residues Ser-367 and Gly-382 in the hybrid domain and Val-332 in the βA domain are shown as magenta spacefill. These residues are closely located to Ser-551 in the EGF-3 domain, Thr-564 in the EGF-4 domain, and Ser-674 in the βT domain, respectively, which are shown as yellow spacefill. Only the residues in the β-tail are labeled. To constrain αIIbβ3 in its bent conformation, these four residue couples were simultaneously mutated to Cys to facilitate disulfide bridge formation. B, Fbg binding to cells expressing αIIbβ3 constrained in the bent conformation in the presence of 1 mm Ca2+/1 mm Mg2+ and control antibody (solid column), in the presence of 1 mm Ca2+/1 mm Mg2+ and PT25-2 (hatched column), and in cells pretreated with DTT in the presence of 1 mm Ca2+/1 mm Mg2+ (open column) is shown. MFI, mean fluorescent intensity. Error bars indicate S.E.
To examine whether these artificially introduced disulfide bridges stabilize the β-head/β-tail interface, we introduced a TEV protease recognition site between the head and tail regions of the β3 chain (21). This mutation (475TEV) was designed to separate the N-terminal head region (amino acids 1–480) from the C-terminal tail region (amino acids 481–762) of the β3 chain upon TEV protease digestion. Because these two regions are connected by a disulfide bridge formed by Cys-473 and Cys-503, these residues were mutated to Ser (475TEVCS) to facilitate separation upon digestion. Then, we introduced S367C/S551C, G382C/T564C, and V332C/S674C double mutations in 475TEVCS. These mutant β3 chains were expressed together with wild-type αIIb in CHO cells. The surface-expressed β3 was immunoprecipitated with anti-β3 mAb and analyzed using SDS-PAGE. All the mutant β3 chains co-precipitated with αIIb, as did wild-type β3, with the exception that a slight difference in the electrophoretic mobility of the mutant β3 chains was noted (supplemental Fig. S2A). When digested with TEV protease under non-reducing conditions, only 475TEVCS generated a 70-kDa band in place of a 98-kDa intact β3 chain (supplemental Fig. S2B). However, all but the wild-type β3 generated 73- and 43-kDa bands in place of a 116-kDa band when digested with TEV protease under reducing conditions (supplemental Fig. S2C). The results indicate that 475TEVCS is indeed cleaved into a 73-kDa N-terminal head region and a 43-kDa C-terminal tail region by TEV protease, as expected. Because these two regions are still connected by a disulfide bridge in 475TEVCS 367/551, 475TEVCS 382/564, and 475TEVCS 332/674 as well as in 475TEV, the two fragments could not separate from each other under non-reducing conditions, although β3 was already cleaved by the TEV protease treatment. However, under reducing conditions, these mutant β3 molecules readily separated into two fragments. These results prove that in S367C/S551C, G382C/T564C, and V332C/S674C mutants, the disulfide bridge actually ligates the head and the tail regions of the β3 chain, thereby stabilizing the β-head/β-tail interface.
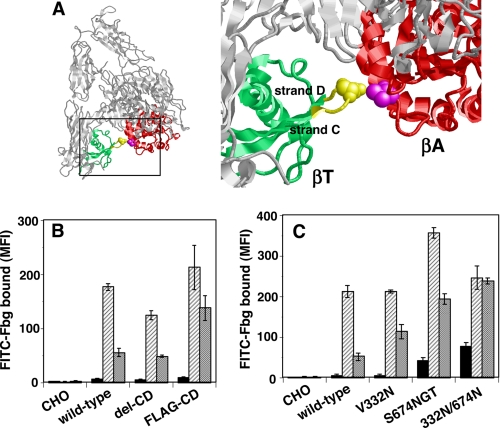
βA/βT Interface Interaction Is Not Sufficient to Act as a Deadbolt to Maintain Integrin in a Low Affinity State
Xiong et al. (8) initially reported that the βA/βT interface interaction might act as a deadbolt to keep integrin in a low affinity state by preventing the movement of the α7 helix of βA, which is associated with ligand binding. Indeed, stabilizing this interface with a disulfide bridge (V332C/S674C) completely inhibited ligand binding (Fig. 1B). To further examine this hypothesis, amino acid residues 671–676 of β3, composing most of the CD loop (Fig. 2A) that participates in βA/βT interface formation, was either deleted (del-CD) or replaced with an irrelevant FLAG tag sequence (FLAG-CD). When expressed in CHO cells, the del-CD or FLAG-CD mutant did not bind Fbg unless activated by PT25-2 in the presence of Ca2+/Mg2+. One mm Mn2+ did not significantly increase Fbg binding in del-CD, as it did in wild-type cells. However, cells expressing the FLAG-CD mutant bound ∼2.5 times as much Fbg as cells expressing wild-type αIIbβ3 (Fig. 2B). These results suggest that although the endogenous βA/CD loop interface interaction alone does not play a critical role in constraining αIIbβ3 in a low affinity state, alterations in its interactions might affect the activation status. The FLAG tag sequence (DYKDDDDK) consists of 8 amino acid residues when compared with the 6 residues in the wild-type sequence. This may imply that physical separation of the two domains can induce activation. To induce complete separation, we attempted to insert a bulky spacer between the βA and the βT domains. This would not only disrupt the βA/βT interface interaction completely but would also affect the hybrid/βT interface formation and slightly extend the integrin. For this purpose, we created an N-linked glycosylation site (NX(T/S) motif) at Val-332 and/or Ser-674 in the interface (Fig. 2A). When the 332VLS3 sequence in the βA was mutated to 332NLS (V332N), it did not have any effect on Fbg binding in the presence of Ca2+/Mg2+. However, cells expressing the V332N mutant bound approximately twice as much Fbg as cells expressing the wild-type in the presence of Mn2+. When the 674SGK sequence was mutated to 674NGT (S674N/K676T), slight but consistent Fbg binding was observed in the presence of Ca2+/Mg2+ without any activators. The addition of PT25-2 or Mn2+ induced more robust Fbg binding than the wild type. Combining these two mutations (V332N/S674N/K676T) had a synergistic effect on constitutive binding, although it did not further increase binding in the presence of Mn2+ (Fig. 2C). These results suggest that the more the βA and βT domains are separated, the stronger the activation of αIIbβ3. In other words, integrin extension by itself may induce activation.
FIGURE 2.
Role of the βA/CD loop interaction in αIIbβ3 activation. A, the βA and βT domains are shown as red and green ribbons, respectively. The βA/CD loop interface shown in the rectangle is magnified on the right-hand side. Amino acid residues 671–676 in the β3 domain that contain the CD loop in the βT domain are shown in yellow. Val-332 in the βA domain and Ser-674 in the CD loop are shown as magenta and yellow spacefill, respectively. B, the CD loop sequences were either deleted (del-CD) or replaced with an irrelevant FLAG tag sequence (FLAG-CD). MFI, mean fluorescent intensity. C, bulky N-glycan-binding sites were introduced either at Val-332 (V332N) or at Ser-674 (S674N/K676T), or in combination (332N/674N). Fbg binding to the cells in the presence of 1 mm Ca2+/1 mm Mg2+ and a control antibody and in the presence of 1 mm Ca2+/1 mm Mg2+ and PT25-2 is shown as the solid and hatched columns, respectively. Fbg binding in the presence of 1 mm Mn2+ with control antibody is shown as the gray column. Error bars indicate S.E.
Extended Conformer of αIIbβ3 Represents a Highly Activated Form
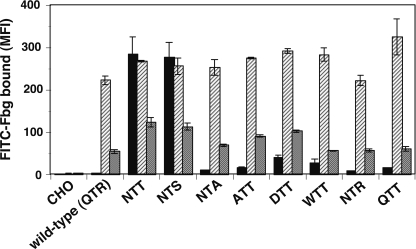
The βA domain provides a part of the ligand-binding site. Therefore, any direct change imposed on the βA domain might affect ligand binding. To induce integrin extension without directly affecting the ligand-binding domains, we introduced N-linked glycosylation sites in αIIb amino acid residues Asp-589, Gln-595, and Thr-478, which are located in the proximity of the α-genu region where the integrin folds backwards in the bent conformation. These residues are all located in the thigh domain (Fig. 3A). We have previously shown that swapping the entire thigh domain between αIIb and αV did not have a significant impact on the αIIbβ3-Fbg interaction (22). Among these residues, Gln-595 is located immediately behind the α-genu. When 595QTR4 was mutated to 595NTT (Q595N/R597T), robust Fbg binding was observed in the presence of Ca2+/Mg2+, and the addition of PT25-2 did not significantly increase the binding. When the 589DTH sequence located distal to the α-genu region was mutated to 589NTT (D589N/H591T), it induced moderate Fbg binding, and PT25-2 significantly increased the binding. In contrast, when 478TKT, which is located above the α-genu region, was mutated to 478NKT (T478N), it did not affect Fbg binding at all (Fig. 3B). It is possible that these mutations affect ligand binding by directly altering the local structure of the αIIbβ3, regardless of the actual N-glycan binding. To rule out these possibilities, the 595QTR sequence was mutated. Mutating 595QTR to ATT, DTT, WTT, NTR, QTT, or NTA did not induce significant activation. However, mutating 595QTR to NTS induced activation comparable with that induced by NTT (Fig. 4). These results suggest that the introduction of virtually any sequence other than the NX(T/S) sequence fails to induce constitutive activation. Thus, these results indicate that the activating effect of these mutations actually depends on the attachment of a bulky N-glycan to these sites.
FIGURE 3.
Effect of integrin extension on ligand binding. A, the three-dimensional structure of the αV chain is shown as semitransparent blue spacefill with its backbone as a blue ribbon. The β3 chain is shown as a gray ribbon. Structures around α-genu in the rectangle are magnified below. The positions of the amino acid residues Thr-466, Pro-583, and Gln-589, which are homologous to Thr-478, Asp-589, and Gln-595 in αIIb, are shown as cyan spacefill and labeled as such. Note that Gln-595 is located immediately behind the genu. B, an N-glycosylation site was introduced at Thr-478, Asp-589, and Gln-595 in the αIIb chain or at Ser-674 in the β3 chain. The resulting αIIbβ3 mutants T478N, D589N/H591T, Q595N/R597T, and S674N/K676T were expressed in CHO cells. Fbg binding to cells in the presence of 1 mm Ca2+/1 mm Mg2+ and control antibody and in the presence of 1 mm Ca2+/1 mm Mg2+ and PT25-2 is shown as the solid column and hatched columns, respectively. Fbg binding in the presence of 1 mm Mn2+ with control antibody is shown as the gray column. MFI, mean fluorescent intensity. Error bars indicate S.E.
FIGURE 4.
Constitutive activation depends on the binding of N-glycan in the Q595N/R597T mutant. Three amino acid residues starting from Gln-595 in the αIIb chain were mutated to various sequences. Fbg binding to the cells in the presence of 1 mm Ca2+/1 mm Mg2+ with a control antibody (solid column) or with PT25-2 (hatched column) and in the presence of 1 mm Mn2+ with a control antibody (gray column) was examined. MFI, mean fluorescent intensity. Error bars indicate S.E.
To confirm whether N-glycans are actually attached to the intended sites in these mutants, we next compared their molecular sizes using SDS-PAGE. If an extra N-glycan is indeed attached to these mutants, their molecular size should be larger than that of the wild-type. When surface-expressed αIIbβ3 was labeled with biotin and immunoprecipitated with anti-αIIb mAb, the β3 chain was always co-precipitated from the cells expressing wild-type or mutant αIIbβ3. However, the size of the mutant αIIb chain that carries an extra N-glycan-binding site (T478N, D589N/H591T, Q595N/R597T) was not remarkably different from that of the wild-type (supplemental Fig. S3A). As the size of the N-glycan was relatively small when compared with the entire αIIb chain, it was difficult to discriminate such small differences in molecular weight using SDS-PAGE. To circumvent this problem, the αIIb leg region encompassing amino acid residues 450–1008 was generated using a FLAG tag sequence on its N terminus. This fragment was surface-expressed and migrated as an 89-kDa band on SDS-PAGE when immunoprecipitated with PL98DF6. In contrast, a similar fragment carrying an extra N-glycan binding site migrated as a 91-kDa band, which is slightly larger than that of the wild type. The fragment that was not supposed to attach N-glycan (R597T) migrated as fast as the wild type (supplemental Fig. S3B). Similar results were obtained when anti-FLAG M2 was used instead of PL98DF6 (data not shown). This difference in apparent molecular weight was completely lost when the fragments were digested with peptide N-glycosidase F as all the fragments migrated as 69-kDa bands (supplemental Fig. S3C). These results clearly indicate that the mutant αIIb that carries an extra NX(T/S) motif indeed binds N-glycan to these sites.
αIIbβ3 Activation Induced by Integrin Extension Depends on the Swing-out of the Hybrid Domain
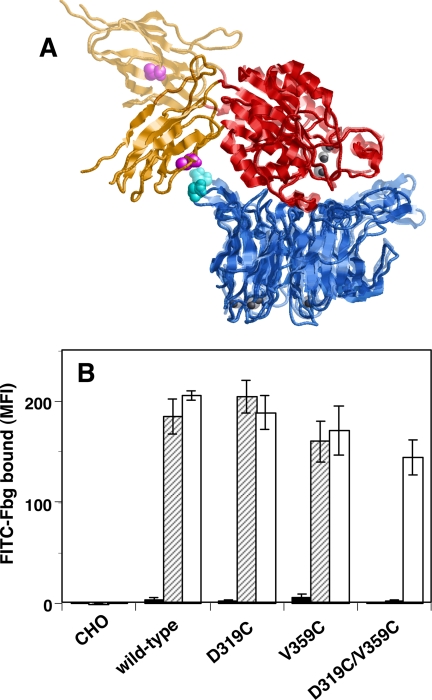
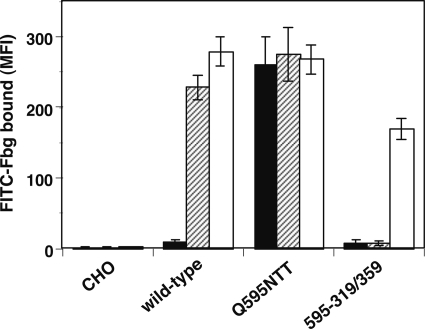
Takagi et al. (10) and Luo et al. (23) have reported that the outward swing of the hybrid domain is the most critical step in integrin activation. To examine whether this step is truly required for activation, the swing-out of the hybrid domain was prevented by covalently ligating the β-propeller and the hybrid domains with a disulfide bridge. The amino acid residues Asp-319 of the αIIb β-propeller and Val-359 of the β3 hybrid domain are physically close in the closed head (swing-in) conformation, whereas they are separated in the open head (swing-out) conformation (Fig. 5A). If these residues are simultaneously mutated to Cys, a disulfide bridge will be formed between these domains, thereby fixing the angle between the βA and hybrid regions in the closed head conformation. The 2-3 loop in blade 5 of the αIIb propeller, where Asp-319 is located, was previously shown not to participate in ligand binding (24). As shown in Fig. 5B, single D319C or V359C mutation did not significantly affect Fbg binding. However, D319C/V359C double mutation completely abolished the Fbg binding induced by PT25-2 unless the cells were pretreated with DTT. The binding of PT25-2 was unaffected by disulfide formation (supplemental Fig. S1). Next, we examined the binding of an activation-independent ligand-mimetic mAb, OP-G2. OP-G2 has an RGD-related RYD sequence in the CDR3 and binds αIIbβ3 in almost the same fashion as Fbg, although it does not require integrin activation for binding (25). Unlike the V332C/S674C mutation, which keeps integrin in a bent conformation, the D319C/V359C mutation did not affect OP-G2 binding (Fig. 6, A and B). To examine the effect on the conformational change induced by ligand binding, the binding of anti-LIBS mAb was examined. The binding of anti-LIBS2 and anti-LIBS6 increased significantly in the presence of RGD peptide in cells expressing wild-type αIIbβ3 as well as in cells expressing the single Cys mutation V332C or S674C. However, cells expressing V332C/S674C bound significantly less anti-LIBS mAb than cells expressing wild-type αIIbβ3, and these cells did not respond to RGD peptide (Fig. 6, C and E). In contrast, cells expressing D319C/V359C showed a basal binding comparable with that of the wild type or single Cys mutants, although the response to RGD peptide was slightly attenuated (Fig. 6, D and F). These results indicate that the swing-out of the hybrid domain is only required for high affinity ligand binding and that the prevention of the swing-out does not completely inhibit the conformational change associated with ligand binding. To examine whether the swing-out of the hybrid domain is required for the activation induced by integrin extension, we combined Q595N/R597T with D319C/V359C and examined its effect on Fbg binding. The resulting 595-319/359 mutant was expected to adopt an extended with a closed head conformation. The activating effect of the Q595N/R597T mutation was completely suppressed by the D319C/V359C mutation. PT25-2 was ineffective, unless the cells were pretreated with DTT (Fig. 7). These results suggest that integrin extension must be accompanied by the swing-out of the hybrid domain for it to induce activation.
FIGURE 5.
Swing-out of the hybrid domain is required for integrin activation. A, crystal structure of the αVβ3 head domains derived from the bent conformation complexed with RGD peptide is overlaid on the crystal structure of the αIIbβ3 head domains complexed with the ligand, which is shown as a semitransparent ribbon. In both parts of this graphic, the β-propeller domain in the α-chain is shown as a blue ribbon. The βA and the hybrid domains in the β3 chain are shown as red and orange ribbons, respectively. Note that Asp-319 in αIIb (Asp-306 in αV), shown as cyan spacefill, and Val-359 in β3, shown as magenta spacefill, are close to each other in the closed head (as in αVβ3) but are separated in the open head (as in αIIbβ3). B, to investigate the role of the swing movement of the hybrid domain in integrin activation, Asp-319 in αIIb and/or Val-359 in β3 were mutated to Cys to facilitate disulfide bridge formation between the two residues. Fbg binding to cells in the presence of 1 mm Ca2+/1 mm Mg2+ is shown. The solid column represents binding in the presence of control antibody, and the hatched column represents binding in the presence of PT25-2. The open column represents binding to cells pretreated with DTT. MFI, mean fluorescent intensity. Error bars indicate S.E.
FIGURE 6.
mAb binding to cells expressing αIIbβ3 constrained in the bent conformation or in the closed head. The binding of the ligand-mimetic mAb OP-G2 (A and B), anti-LIBS mAbs anti-LIBS2 (C and D), and anti-LIBS6 (E and F) to cells expressing αIIbβ3 constrained in the bent conformation (A, C, and E) or in the closed head (B, D, and F) was examined. Values in the y axis represent normalized mAb binding. The open column represents mAb binding in the presence of 1 mm Ca2+/1 mm Mg2+. The hatched column represents binding in the presence of 1 mm GRGDS peptide under the same cation conditions. Error bars indicate S.E.
FIGURE 7.
Swing-out of the hybrid domain is required for activation induced by integrin extension. The effect of the swing-out of the hybrid domain on extension-induced integrin activation was examined. To constrain αIIbβ3 in the extended conformation with a closed head, the Q595N/R597T and D319C/V359C mutations were combined (595-319/359). Fbg binding to the cells in the presence of 1 mm Ca2+/1 mm Mg2+ and the control antibody (solid column) or PT25-2 (hatched column) and Fbg binding to cells pretreated with DTT (open column) is shown. MFI, mean fluorescent intensity. Error bars indicate S.E.
DISCUSSION
By characterizing the recombinant αIIbβ3 integrin expressed in CHO cells, we established that αIIbβ3 constrained in its bent conformation represents a low affinity form, whereas αIIbβ3 constrained in its extended conformation represents a high affinity form. This constitutive activation depends on the swing-out of the hybrid domain because the prevention of this swing-out completely inhibited ligand binding regardless of the bent/extended state.
Integrin domains make large interdomain interfaces between the α-head and the β-head, the α-tail and the β-tail, and the β-head and the β-tail. Among these interfaces, the β-head/β-tail interface is presumed to play a central role in keeping integrin in its bent conformation because this is the only interaction that directly connects the head with the tail region in the bent conformer but not in the extended conformer. This interface is maintained by multiple interdomain interactions. The βA and the hybrid domains in the β-head make contact with the C-terminal βT domain. The hybrid domain also makes contact with the EGF-3 and EGF-4 domains. We attempted to stabilize this interface by introducing artificial disulfide bridges between these domains. As previously reported, stabilizing the βA/βT interface (V332C/S674C) completely blocked Fbg binding (9). Likewise, stabilizing the hybrid/EGF-3 (S367C/S551C) or hybrid/EGF-4 (G382C/T564C) interface completely abolished Fbg binding. Regardless of the positions of the disulfide bridges that were introduced, stabilizing these interfaces prevented integrin from adopting the extended conformation. However, the S367C/S551C and G382C/T564C mutations not only prevented integrin extension but also restricted the relative movements of the hybrid and β-tail domains. For this reason, it might be premature to conclude that integrin extension is essential for activation. However, the fact that ligating the α-head with the β-tail or limiting αIIb extension using intrachain disulfide bridges that do not directly restrict hybrid/β-tail movement also prevented activation (9, 26) suggests that the completely bent conformer observed in the crystal structure represents the low affinity form rather than the high affinity form. These results also indicate that the β-head/β-tail interface must be disrupted all the way up to the linker region for the integrin to be activated.
The fact that the ligand-mimetic non-activation-dependent mAb OP-G2 did not bind to cells expressing the V332C/S674C mutant suggests that this mutant does not support low affinity ligand binding. In addition, anti-LIBS mAb binding to this particular mutant indicates that V332C/S674C is unable to undergo structural rearrangement in the presence of the RGD peptide. Taken together, these results suggest that the V332C/S674C mutant is not capable of binding either macromolecular ligands or ligands as small as the RGD peptide. Because the V332C/S674C mutation ligates the α7 helix in βA with the CD loop in βT, the possible downward movement of the α7 helix required for ligand binding in the integrin A domains would be inhibited. Thus, the effect of the V332C/S674C mutation is a combination of both β-head/β-tail stabilization and the inhibition of α7 helix movement. In agreement with these findings, the S367C/S551C mutation, which does not restrict α7 helix movement, only partially blocked OP-G2 binding (data not shown).
The contribution of each interface interaction in maintaining integrin in the bent conformation has not been clarified. It has been reported that replacing the β2 CD loop sequence with the homologous β3 sequence or inserting a N-glycan-binding site in the CD loop in αMβ2 integrin induced robust ligand binding (27). However, the fact that the deletion of the CD loop of the βT domain failed to activate αIIbβ3 in our experiment strongly argues against the deadbolt theory, in which an endogenous βA/βT interface interaction plays a critical role in maintaining integrin in its low affinity state. In addition, the insertion of a bulky N-glycan at the interface only slightly activated αIIbβ3 in the presence of Ca2+/Mg2+. These results suggest that hybrid/EGF-3, hybrid/EGF-4, and hybrid/βT interface interactions, rather than the βA/βT interface alone, play important roles in maintaining integrin in a low affinity state. In agreement with these conclusions, a computer-assisted approach has identified key interactions in the hybrid/β-tail interface in β3 integrin (28). Although the disruption of the hybrid/βT or the hybrid/EGF-3 interaction alone only produced weak activation, disrupting multiple interactions at the same time induced significant activation. Thus, the hybrid/β-tail interface seems to be maintained by a group of several key interactions that individually are not sufficiently strong to do so. The apparently distinct role of the βA/βT interface interaction in regulating activation in β2 and β3 integrins suggests that the contributions of each interdomain interaction to maintaining integrin in a low affinity state may differ among different integrin subfamilies.
A high resolution electron microscopic analysis of recombinant αVβ3 extracellular domains revealed that ligand-bound αVβ3 preferentially adopts an extended, rather than a bent, conformation (9). Based on these observations, it has been tentatively concluded that the extended conformer represents a high affinity form. The current study provides direct evidence that the highly extended conformer indeed has a higher ligand affinity than the completely bent conformer. Among the three mutants that showed constitutive Fbg binding in this study, β3S674N/K676T showed the lowest, αIIbQ595N/R597T showed the highest, and αIIbD589N/H591T showed an intermediate binding affinity. These results suggest that the degree of extension may be correlated with the extent of activation. These results also indicate that integrins are capable of assuming a wide range of affinity states depending on the degree of extension. Recently, it has been shown that integrin extension may not necessarily be accompanied by activation based on discrepancies between the expression of an extension-reporting epitope for KIM127 and an activation-reporting epitope for mAb24 on αLβ2 under flow conditions (29). It is possible that αA domain-containing integrin may require an additional step to achieve activation, unlike integrins without αA domains. It would be interesting to examine whether the introduction of a neoglycan that induces αL extension activates αLβ2.
Our results apparently contradict a report that αVβ3 is capable of binding fibronectin while in a bent conformation (14). However, it is not possible to tell to what degree integrin must extend to enable substantial ligand binding based on our experiments. The fact that αIIbβ3 can exist in a wide range of affinity states depending on the degree of the extension implies that as long as it is not completely bent, ligand binding could be observed to a varying extent. In other words, relaxation of the β-head/β-tail interface interaction, but not complete extension, may be sufficient for ligand binding to occur, especially in the presence of Mn2+. As shown in Fig. 3B, Mn2+ seems to lessen the requirement for integrin extension for Fbg binding. Mn2+ activation alone has consistently been reported not to be accompanied by integrin extension (30). A recent report by Blue et al. (26) has provided a plausible explanation for the discrepancies in ligand binding observed under different cation conditions. Limiting αIIb extension using intrachain disulfides did not block Fbg binding in the presence of Mn2+, although binding was blocked in the presence of Ca2+/Mg2+. In contrast, limiting β-head/β-tail movement using S367C/S551C, G382C/T564C, and V332C/S674C double mutations blocked Fbg binding significantly in the presence of Mn2+ as well as in the presence of Ca2+/Mg2+ (data not shown). Taken together, these results may imply that it is not integrin extension per se, but the relative β-head/β-tail movement (e.g. the swing-out of the hybrid domain), that is essential for activation in the presence of Mn2+. These results may explain why ligand binding was observed for the bent conformer in some studies in which Mn2+ was utilized to induce ligand binding (13, 14). Further study is required to determine the differences in the structural requirements for activation under different cation conditions.
Springer and co-workers (10, 23) have shown that ligand binding induces swing-out of the hybrid domain and that this change induces strong activation by itself, regardless of the bent/extend conformation. Our results show that the swing-out of the hybrid domain is essential for activation and that extension-induced activation absolutely depends on this change. These results indicate that the affinity state of the extended conformer is controlled by the swing-out of the hybrid domain and that to down-regulate activation, integrin does not necessarily need to go back to its original bent conformation but that this can rather be accomplished by the swing-in of the hybrid domain. Interestingly, constraining the integrin head in a closed state did not prevent OP-G2 binding at all (Fig. 6B). Unlike PAC-1, which binds αIIbβ3 in an activation-dependent fashion, OP-G2 is less dependent on integrin activation (18). This difference indicates that the swing-out of the hybrid domain is required only for high affinity ligand binding but not for low affinity ligand binding. However, we are not sure at this point whether the swing-out of the hybrid domain alone is sufficient for high affinity ligand binding, as reported by Springer and co-workers (23). Our experiments using recombinant αIIbβ3 expressed on the CHO cell surface have shown that the swing-out of the hybrid domain only induced moderate activation. To induce full activation, integrin extension was required.5 It is possible that the proximity of the integrin head domains to the plasma membrane in the bent conformation may limit the access of macromolecular ligands. Experiments utilizing cell-free binding studies should help to clarify these discrepancies. Interestingly, anti-LIBS mAb binding was still observed in the closed head mutant (D319C/V359C) in the presence of RGD peptide. Because ligand binding induces the outward swing of the hybrid domain and this movement would probably disrupt the β-head/β-tail interface, it is reasonable to assume that this swing-out triggers the structural transition from a bent to an extended conformation in outside-in signaling. However, our result suggests the possibility that a structural change in addition to the swing-out may trigger the conformational change upon ligand binding. A recent report also suggests that integrin affinity may be regulated independently from the swing-out of the hybrid domain based on the expression of anti-LIBS epitope located in the hybrid domain (31). Because we do not know the specific conformation to which each of the anti-LIBS mAbs binds, further analysis is needed to address this issue.
Then, what triggers the structural transition from the bent to the extended conformation during inside-out signaling? Numerous studies have suggested the importance of integrin cytoplasmic tails in regulating integrin activation. It has been shown that integrin cytoplasmic tails undergo structural rearrangement upon ligand binding (32). It was subsequently shown that the two cytoplasmic tails separate from each other upon ligand binding (3). On the other hand, the deletion of the entire α or β cytoplasmic tail at the membrane-proximal sites induced significant activation (33). NMR studies on recombinant αIIbβ3 cytoplasmic tails have shown that talin binding to the β3 cytoplasmic tail disrupts the endogenous interaction between the α and β cytoplasmic tails (2). Because talin binding to the β3 cytoplasmic tail activates αIIbβ3, it was concluded that the separation of the two cytoplasmic tails somehow induces structural rearrangement of the integrin extracellular domains (1). We have previously shown that stabilizing the α-tail/β-tail interface with artificial disulfide bridges completely abolished the activation induced by cytoplasmic tail deletion (22). Based on these observations, we hypothesized that the separation of the two extracellular tails following the cytoplasmic tail dissociation induces structural rearrangement from the bent to the extended conformation. Indeed, the separation of the α-tail/β-tail interface induced robust activation.6 The α-tail/β-tail interface and the β-head/β-tail interface are located next to each other, flanking the β-tail. Because the β-head/β-tail interface, and not the α-tail/β-tail interface, maintains integrin in its bent conformation, it is reasonable to assume that the separation of one interface destabilizes the other. Further elucidation of the role of these interface interactions in integrin affinity regulation will facilitate understanding of integrin-mediated bidirectional signaling.
Supplementary Material
Acknowledgments
We thank Dr. Joseph C. Loftus for providing the αIIb, αV, and β3 cDNAs and Drs. Mark H. Ginsberg, Jari Ylänne, Ismo Virtanen, and Yoshiaki Tomiyama for providing the anti-αIIbβ3 mAbs.
This work was supported in part by Labor Sciences research grants (Research on Public Essential Drugs and Medical Devices) from the Ministry of Health, Labour and Welfare (to T. K. and M. H.), a grant from the Fukuzawa Memorial Foundation (to T. K.), and a grant-in-aid for scientific research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology (to S. A.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
332VLS and 674SGK designate wild-type β3 sequences. The three letters following the number represent the amino acid residues starting from the position the number represents.
478TKT, 589DTH, and 595QTR designate wild-type αIIb sequences. The three letters following the number represent the amino acid residues starting from the position the number represents.
T. Kamata, M. Handa, S. Ito, Y. Sato, T. Ohtani, Y. Kawai, Y. Ikeda, and S. Aiso, unpublished data.
T. Kamata, M. Handa, Y. Kawai, Y. Ikeda, and S. Aiso, manuscript in preparation.
- LIBS
- ligand-induced binding site
- Fbg
- fibrinogen
- TEV
- tobacco etching virus.
REFERENCES
- 1.Calderwood D. A., Zent R., Grant R., Rees D. J., Hynes R. O., Ginsberg M. H. (1999) J. Biol. Chem. 274, 28071–28074 [DOI] [PubMed] [Google Scholar]
- 2.Vinogradova O., Velyvis A., Velyviene A., Hu B., Haas T., Plow E., Qin J. (2002) Cell 110, 587–597 [DOI] [PubMed] [Google Scholar]
- 3.Kim M., Carman C. V., Springer T. A. (2003) Science 301, 1720–1725 [DOI] [PubMed] [Google Scholar]
- 4.Frelinger A. L., 3rd, Cohen I., Plow E. F., Smith M. A., Roberts J., Lam S. C., Ginsberg M. H. (1990) J. Biol. Chem. 265, 6346–6352 [PubMed] [Google Scholar]
- 5.Du X. P., Plow E. F., Frelinger A. L., 3rd, O'Toole T. E., Loftus J. C., Ginsberg M. H. (1991) Cell 65, 409–416 [DOI] [PubMed] [Google Scholar]
- 6.Carrell N. A., Fitzgerald L. A., Steiner B., Erickson H. P., Phillips D. R. (1985) J. Biol. Chem. 260, 1743–1749 [PubMed] [Google Scholar]
- 7.Weisel J. W., Nagaswami C., Vilaire G., Bennett J. S. (1992) J. Biol. Chem. 267, 16637–16643 [PubMed] [Google Scholar]
- 8.Xiong J. P., Stehle T., Diefenbach B., Zhang R., Dunker R., Scott D. L., Joachimiak A., Goodman S. L., Arnaout M. A. (2001) Science 294, 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takagi J., Petre B. M., Walz T., Springer T. A. (2002) Cell 110, 599–611 [DOI] [PubMed] [Google Scholar]
- 10.Takagi J., Strokovich K., Springer T. A., Walz T. (2003) EMBO J. 22, 4607–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao T., Takagi J., Coller B. S., Wang J. H., Springer T. A. (2004) Nature 432, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adair B. D., Yeager M. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14059–14064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong J. P., Stehle T., Zhang R., Joachimiak A., Frech M., Goodman S. L., Arnaout M. A. (2002) Science 296, 151–155 [DOI] [PubMed] [Google Scholar]
- 14.Adair B. D., Xiong J. P., Maddock C., Goodman S. L., Arnaout M. A., Yeager M. (2005) J. Cell Biol. 168, 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adair B. D., Yeager M. (2007) Methods Enzymol. 426, 337–373 [DOI] [PubMed] [Google Scholar]
- 16.Ylänne J., Hormia M., Järvinen M., Vartio T., Virtanen I. (1988) Blood 72, 1478–1486 [PubMed] [Google Scholar]
- 17.Frelinger A. L., 3rd, Du X. P., Plow E. F., Ginsberg M. H. (1991) J. Biol. Chem. 266, 17106–17111 [PubMed] [Google Scholar]
- 18.Tomiyama Y., Tsubakio T., Piotrowicz R. S., Kurata Y., Loftus J. C., Kunicki T. J. (1992) Blood 79, 2303–2312 [PubMed] [Google Scholar]
- 19.Tokuhira M., Handa M., Kamata T., Oda A., Katayama M., Tomiyama Y., Murata M., Kawai Y., Watanabe K., Ikeda Y. (1996) Thromb. Haemost. 76, 1038–1046 [PubMed] [Google Scholar]
- 20.Kamata T., Irie A., Tokuhira M., Takada Y. (1996) J. Biol. Chem. 271, 18610–18615 [DOI] [PubMed] [Google Scholar]
- 21.Zhu J., Luo B. H., Xiao T., Zhang C., Nishida N., Springer T. A. (2008) Mol. Cell 32, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamata T., Handa M., Sato Y., Ikeda Y., Aiso S. (2005) J. Biol. Chem. 280, 24775–24783 [DOI] [PubMed] [Google Scholar]
- 23.Luo B. H., Springer T. A., Takagi J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 2403–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamata T., Tieu K. K., Irie A., Springer T. A., Takada Y. (2001) J. Biol. Chem. 276, 44275–44283 [DOI] [PubMed] [Google Scholar]
- 25.Tomiyama Y., Brojer E., Ruggeri Z. M., Shattil S. J., Smiltneck J., Gorski J., Kumar A., Kieber-Emmons T., Kunicki T. J. (1992) J. Biol. Chem. 267, 18085–18092 [PubMed] [Google Scholar]
- 26.Blue R., Li J., Steinberger J., Murcia M., Filizola M., Coller B. S. (2010) J. Biol. Chem. 285, 17604–17613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta V., Gylling A., Alonso J. L., Sugimori T., Ianakiev P., Xiong J. P., Arnaout M. A. (2007) Blood 109, 3513–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto A., Kamata T., Takagi J., Iwasaki K., Yura K. (2008) Biophys. J. 95, 2895–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuwano Y., Spelten O., Zhang H., Ley K., Zarbock A. (2010) Blood 116, 617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye F., Liu J., Winkler H., Taylor K. A. (2008) J. Mol. Biol. 378, 976–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chigaev A., Waller A., Amit O., Halip L., Bologa C. G., Sklar L. A. (2009) J. Biol. Chem. 284, 14337–14346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leisner T. M., Wencel-Drake J. D., Wang W., Lam S. C. (1999) J. Biol. Chem. 274, 12945–12949 [DOI] [PubMed] [Google Scholar]
- 33.Hughes P. E., O'Toole T. E., Ylänne J., Shattil S. J., Ginsberg M. H. (1995) J. Biol. Chem. 270, 12411–12417 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.