Abstract
Previously, we have shown that lack of expression of triadins in skeletal muscle cells results in significant increase of myoplasmic resting free Ca2+ ([Ca2+]rest), suggesting a role for triadins in modulating global intracellular Ca2+ homeostasis. To understand this mechanism, we study here how triadin alters [Ca2+]rest, Ca2+ release, and Ca2+ entry pathways using a combination of Ca2+ microelectrodes, channels reconstituted in bilayer lipid membranes (BLM), Ca2+, and Mn2+ imaging analyses of myotubes and RyR1 channels obtained from triadin-null mice. Unlike WT cells, triadin-null myotubes had chronically elevated [Ca2+]rest that was sensitive to inhibition with ryanodine, suggesting that triadin-null cells have increased basal RyR1 activity. Consistently, BLM studies indicate that, unlike WT-RyR1, triadin-null channels more frequently display atypical gating behavior with multiple and stable subconductance states. Accordingly, pulldown analysis and fluorescent FKBP12 binding studies in triadin-null muscles revealed a significant impairment of the FKBP12/RyR1 interaction. Mn2+ quench rates under resting conditions indicate that triadin-null cells also have higher Ca2+ entry rates and lower sarcoplasmic reticulum Ca2+ load than WT cells. Overexpression of FKBP12.6 reverted the null phenotype, reducing resting Ca2+ entry, recovering sarcoplasmic reticulum Ca2+ content levels, and restoring near normal [Ca2+]rest. Exogenous FKBP12.6 also reduced the RyR1 channel Po but did not rescue subconductance behavior. In contrast, FKBP12 neither reduced Po nor recovered multiple subconductance gating. These data suggest that elevated [Ca2+]rest in triadin-null myotubes is primarily driven by dysregulated RyR1 channel activity that results in part from impaired FKBP12/RyR1 functional interactions and a secondary increased Ca2+ entry at rest.
Keywords: Calcium Channels, Calcium Imaging, Endoplasmic Reticulum (ER), Sarcoplasmic Reticulum, Skeletal Muscle, Calcium Imaging, Lipid Bilayer, Myotubes, Resting Calcium, Ryanodine Receptor
Introduction
In skeletal muscle, where control of cytosolic Ca2+ concentration is key to muscle contraction, the overall Ca2+ homeostasis is preserved by a concerted action of several ionic channels and transporters within the plasma membrane and the sarcoplasmic reticulum (SR).3 These proteins, including plasma membrane Ca2+ ATPase, Na+/Ca2+ exchanger, dihydropyridine receptor, ryanodine receptor (RyRs), and the sarcoendoplasmic reticulum Ca2+ ATPase, among others, interact functionally and physically with each other and with a plethora of regulatory proteins that ultimately modulate their activity, hence, the resting intracellular Ca2+ levels.
In muscle cells where SR Ca2+ stores represent the main intracellular sources of Ca2+ release, RyR1 seems positioned to play a key role in regulating myoplasmic [Ca2+]rest. An effective example of this can be found in dyspedic cells that lack expression of all isoforms of RyRs (1), where we have recently demonstrated that RyR1 expression was sufficient to remodel the Ca2+ regulatory machinery in a way that ultimately resulted in a significant shift in steady-state Ca2+ fluxes contributing to [Ca2+]rest (2). Alterations of RyR1 activity, like those conferred by malignant hyperthermia mutations, also resulted in long term changes in [Ca2+]rest (3, 4), raising the possibility that indirect modulation of RyR1 by its protein binding partners may be important for homeostatic regulation of [Ca2+]rest. Therefore, finding and understanding the role of RyR1 regulatory proteins could be instrumental in understanding the mechanisms that control long term Ca2+ homeostasis in muscle.
Among the endogenous regulators, the RyR1/triadin interactions are particularly intriguing, as its role in skeletal muscle function remains elusive (5). Although originally thought to be the link between RyR1 and DHPR (6–8) and a key modulator of EC coupling (9–11), it appears evident now that triadin acts primarily as a negative regulator of RyR1 activity (12–15). Although the mechanism by which triadin regulate RyRs remains unclear, there is support for the hypothesis that triadin is involved in facilitating the cross-communication between calsequestrin (CSQ) and the RyRs (16–18). In a recent study aimed at unmasking the role of triadin in EC coupling, we found that the lack of triadin expression in skeletal myotubes and adult fibers caused a significantly elevated [Ca2+]rest with no obvious changes in RyR1 expression level or activity (14), suggesting a role for triadin in regulating resting Ca2+ homeostasis.
The present study addresses the role of triadin in regulating [Ca2+]rest by comparing Ca2+ entry and Ca2+ release pathways that regulate global Ca2+ homeostasis in WT and triadin-null myotubes. Using a combination of Ca2+ and Mn2+ imaging studies, planar lipid bilayer analysis, and Ca2+-sensitive microelectrodes, we identify a new role for triadin, that of regulating the FKBP12/RyR1 interaction. We also present evidence that support the hypothesis that elevated [Ca2+]rest observed in triadin-null myotubes is the combined effect of at least two interlinked pathways: (i) an augmented basal SR Ca2+ release as the result of enhanced RyR1 channel activity induced by a deficient functional FKBP12/RyR1 interaction and (ii) an increased resting sarcolemmal Ca2+ entry mediated in part by store-operated Ca2+ entry activity. A model of resting Ca2+ regulation by RyRs modulators is discussed. Part of this work has been published previously in abstract form (19).
EXPERIMENTAL PROCEDURES
Materials
FK506 binding proteins (FKBPs) for fluorescent FKBP12 (F-FKBP12) binding studies were expressed in E. coli BL21(DE3)pLysS and purified as described previously (20). Recombinant FKBP12.6 for BLM studies was expressed in Escherichia coli BL21 and purified using StrepTrapTM HP column (GE Healthcare), and FKBP12 was obtained from Sigma. bastadin-5 (B5) was extracted and purified from Lanthella basta as described previously (21). All other reagents were analytical grade and purchased from Sigma.
Cell Culturing, Ca2+, and Mn2+ Imaging
Primary myoblasts from triadin-null mice were isolated and differentiated as described previously (14). Ca2+ imaging was performed 5 days after differentiation in myotubes loaded with 2 μm Fluo-4-AM (Molecular Probes). Myotubes were imaged in imaging buffer (125 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1.2 mm MgSO4, 6 mm glucose, and 25 mm Hepes/Tris, pH 7.4, with 2 mm Ca2+ or not added Ca2+ plus 0.5 mm Cd2+ and 0.1 mm La3+) at 490–500 nm with a Stanford Photonics 12-bit digital intensified CCD using a DG4 multiwavelength light source, and the data were displayed and analyzed using QED imaging software (QED Software, Pittsburgh, PA). Ca2+ entry rates were estimated by the rate of dye quench by Mn2+ entry in myotubes loaded with 5 μm Fura-2-AM. Cells were exited at the isosbestic wavelength for Fura-2 (360 nm), and fluorescence emission at 510 nm was then captured from regions of interest within each myotube at 6 frames per second.
SR Ca2+ Content
SR Ca2+ content of cultured myotubes was estimated from the peak amplitude of the Ca2+ transient induced by 40 mm caffeine stimulation. For this experiment, the cells were loaded with 5 μm Fura-4F, a ratiometric low affinity (Kd = 770 nm) Ca2+ dye, to avoid both the distortion associated with differences in dye loading between cells and dye saturation at high Ca2+ concentrations. Fluorescent emission at 510 was captured from regions of interest within each myotube at 2 frames per second and expressed as ratio of signal collected at an alternating 340/380 nm excitation wavelength.
Membrane Vesicle Preparation and Immunoblotting
Crude membrane homogenates from primary myotubes and lower limb muscle were prepared as described previously (14). The heavy SR fraction from muscle homogenates was isolated by discontinuous sucrose gradient (10 to 38 to 45% (w/v)) in 20 mm Hepes, pH 7.4, 0.5 m NaCl, 1 mm EDTA. The isolated fraction was then resuspended in 10 mm imidazole, pH 7.4, 0.3 m sucrose, and frozen in liquid N2. Proteins from crude membrane homogenates were separated in SDS-PAGE (22) and transferred to a PVDF membrane. Expression of specific proteins was tested by incubation of immunoblots with poly- or monoclonal antibodies against the following: RyR1 (34C, Developmental Studies Hybridoma Bank, University of Iowa), calsequestrin, FKBP12/12.6, and DHPR α1S (MA3-913, PA1-901, and MA3-927, respectively, from Thermo Fisher Scientific), junctin (1E6, a gift from Dr. L. Jones), and GAPDH (FL-335 from Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were then incubated with either anti-mouse or anti-rabbit horseradish peroxidase-conjugated goat, secondary antibody, and developed with SuperSignal ultrachemoluminescent substrate (Pierce), and the intensity of the signal was collected using a Kodak Imaging Station 4000MM PRO.
Resting Free Ca2+ Measurements
Determination of myoplasmic resting free Ca2+ concentrations of myotubes was performed with double-barreled Ca2+-selective microelectrodes assembled with ETH129 resin as described previously (23).
Immunoprecipitation
Co-immunoprecipitation assays were performed according to the methods of Lee et al. (24, 25). Briefly, 300 μg of crude membrane homogenate were solubilized 45 min in 10 mm Tris/HCl, pH 7.4, 150 mm NaCl, 5 mm EDTA, 1 mm Na3VO4, 1% Triton X-100, and 1% digitonin, supplemented with protease inhibitors. The lysate was then cleared from insoluble particles by centrifugation at 12,000 × g for 20 min. The cleared lysate was then incubated with 50 μl of anti-RyRs monoclonal antibody (34C) overnight at 4 °C, followed by an additional incubation with Protein G-conjugated magnetic beads (Dynabead®, Invitrogene) for 4 h. Protein-bead complexes were washed three times with solubilization buffer and then processed for SDS-PAGE and Western blot as described above. Band identification and densitometry of the immunoprecipitate were performed using Kodak MI Software.
F-FKBP12 Binding Measurements
The steady-state binding of a F-FKBP12 to RyR1 channels in membranes prepared from WT and triadin-null muscle was measured essentially as described previously (20). Briefly, a single-cysteine FKBP12 (T14C/C22A FKBP12) was stoichiometrically labeled with fluorescein maleimide (Invitrogen). Crude membrane homogenates (0.4 mg/ml) were incubated for 90 min in media containing 150 mm KCl, 20 mm K-PIPES (pH 7.0), 5 mm Glutathione, 1 mm EGTA, and 3 to 250 nm F-FKBP. For determinations of nonspecific binding, media were supplemented with 10 μm FK506. Membrane-bound F-FKBP12 was separated by centrifugation, and fluorescence of solubilized pellets was determined from the integrated fluorescence intensity from 510 to 610 nm, acquired using a Gemini EM microplate fluorometer (Molecular Devices, Sunnyvale, CA) with excitation at 488 nm and a 495 nm emission long pass filter. F-FKBP12 binding was expressed as function of the relative amount of RyR1 measure by binding of [3H]ryanodine.
[3H]Ryanodine Binding Assay
Specific binding of [3H]ryanodine to high affinity sites on muscle crude homogenates was determined by incubating 100 μg of protein with 5 nm [3H]ryanodine at 37 °C for 90 min as described previously (14).
Overexpression of FKBP12.6
A five-plasmid lentiviral expression system was used to produce lentiviral particles by transient transfection of helper HEK 293 cultured cells (26). The transfer plasmid vector was engineered to contain the rabbit FKBP12.6 gene driven by the cytomegalovirus promoter and a hygromycin-B resistance cassette. Primary myoblast from WT and triadin-null muscle were infected with a lentiviral suspension at multiplicity of infection of 1.0 in the presence of protamine sulfate followed by selection with 20 μg/ml of hygromycin-B 2 days after transduction. Permanently transduced cells were expanded and differentiated in the presence of hygromycin-B.
Measurement of RyR1 Single Channel Activity in Planar Lipid Bilayer
Bilayers were formed by a mixture of phosphatidylethanolamine:phosphatidylserine:phosphatidylcholine (5:3:2 (w/w), Avanti Polar Lipids, Inc) at a final concentration of 30 mg/ml in decane. Recording was performed in 20 mm Hepes/Tris, pH 7.4, supplemented with 500 mm CsCl, 1 or 50 μm free Ca2+ with or without 2 mm ATP (cis chamber) and 50 mm CsCl, 100 μm or 1 mm Ca2+ (trans chamber). Ca2+ concentrations were adjusted with EGTA according to calculation with Bound & Determined software (27). Single channel gating was monitored and recorded at a holding potential of −40 mV (applied to the trans side). SR vesicles, FKBP12.6, or FKBP12 proteins were added to the cis chamber. The amplified current signals were filtered at 1 kHz with a Low-Pass Bessel Filter 8 Pole (Warner Instrument, CT), digitized and acquired at a sampling rate of 10 kHz (Digidata 1320A, Axon-Molecular Devices, Union City, CA). All channels were recorded for at least 2 min under each specific condition. The channel open probability (Po) for all gating levels, mean open and mean closed dwell times (to and tc,respectively) were calculated using Clampfit, pClamp software (version 9.0) without further filtration (Axon-Molecular Devices).
Data Analysis
Statistical significant differences among the data were evaluated using Student's t test analysis (unpaired t test with Welch's correction, GraphPad Software, San Diego, Ca). Unless otherwise is indicated, data are presented as mean ± S.D.
RESULTS
Resting Free Ca2+ in Primary Myotubes
As previously reported (14) under resting conditions, primary culture of triadin-null myotubes had significantly higher myoplasmic [Ca2+]rest than was observed in WT myotubes (Table 1 and Fig. 1). To identify the mechanism by which the lack of triadin expression induces elevation of myoplasmic [Ca2+]rest in this work, we independently assessed the contribution of extra- and intracellular Ca2+ sources.
TABLE 1.
Comparative effect of BTP-2 and overexpression of FKBP12.6 on resting free calcium concentrations of WT and triadin-null myotubes
Data represent Student's t test (Welch's correction) with respect to the corresponding WT value.
| Condition | Mean ± S.D. (n) |
p | |
|---|---|---|---|
| Wild type | Triadin-null | ||
| 2 mm Ca2+ | 116 ± 7 (27) | 181 ± 11 (65) | <0.001 |
| 0.5 mm Cd2+/ 0.1 mm La3+ | 98 ± 3 (10) | 153 ± 6 (17) | <0.001 |
| 2 μm BTP-2 | 97 ± 4 (5) | 142 ± 6 (7) | <0.001 |
| FKBP-12.6 | 112 ± 9 (22) | 134 ± 8 (31) | <0.001 |
| FKBP-12.6 + BTP-2 | 95 ± 6 (5) | 121 ± 4 (10) | <0.001 |
| FKBP-12.6 + Cd2+/La3+ | 92 ± 3 (5) | 96 ± 2 (10) | >0.05 |
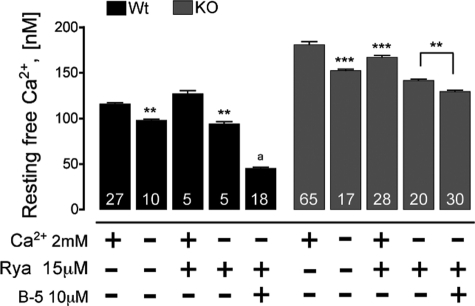
FIGURE 1.
Average resting free Ca2+ concentration measured from individual myotubes of WT and triadin-null myotubes in the presence (+) and in the absence (−) of the indicated concentrations of Ca2+, ryanodine (Rya), and bastadin-5 (B-5). Ca2+-free solutions were also supplemented with 0.5 mm Cd2+ and 0.1 mm La3+ to block Ca2+ entry. Calcium measurements were regularly acquired 5–10 min post-modification of the extracellular medium. Numbers on the bars indicate the number of cells analyzed per each condition. Data are presented as mean ± S.D. **, p < 0.01; ***, p < 0.001 with respect to untreated cells. a, data from Ref. 2.
As shown in Fig. 1, preventing the overall Ca2+ entry, by removing extracellular Ca2+ and supplementing the medium with 0.5 mm Cd2+ and 0.1 mm La3+, resulted in a significant reduction of [Ca2+]rest in both WT and triadin-null cells. However, despite this reduction, the [Ca2+]rest in triadin-null cells did not reach WT levels (98 ± 3 nm in WT cells versus 153 ± 6 nm in triadin-null cells), suggesting that the elevated [Ca2+]rest displayed by triadin-null cells is unlikely to be mediated solely by a dysregulation of Ca2+ entry pathway(s).
On the other hand, suppression of intracellular Ca2+ release from cytosolic stores by blocking RyR channels with ryanodine had a differential effect between triadin-null and WT myotubes. Whereas overnight incubation with 15 μm ryanodine, a condition that totally inhibited caffeine-induced Ca2+ release (data not shown), did not seem to affect [Ca2+]rest in WT cells (116 ± 7 nm versus 123 ± 8 nm, p > 0.05), the triadin-null myotubes showed a significant reduction in [Ca2+]rest from 181 ± 11 nm to 167 ± 10 nm (p < 0.05, Fig. 1), revealing an increased basal activity of the RyR1 channel population. However, ryanodine inhibition of RyR1 activity proved insufficient to return [Ca2+]rest to WT levels.
Interestingly, the combined effect of Cd2+/La3+ and ryanodine appears not to be additive as under these conditions; [Ca2+]rest in triadin-null cells still remained higher than those in WT cells (94 ± 5 nm in wild type versus 142 ± 6 nm in triadin-null, Fig. 1). To assess whether a ryanodine-resistant Ca2+ “leak” could be a source contributing to [Ca2+]rest, we tested whether addition of B5 in the presence of ryanodine could restore [Ca2+]rest to that measured in WT cells. B5 was previously shown to convert ryanodine-insensitive leak states of RyR1 into ryanodine-sensitive channels (2, 4, 21, 28). As shown in Fig. 1, 10 μm B5 reduced [Ca2+]rest in WT cells pretreated with ryanodine >50% (from 94 ± 5 to 45 ± 6 nm), confirming the existence of a Ca2+ leak mediated by RyR. Unlike WT cells, treatment of 10 μm B5 in combination with ryanodine had a much more modest reduction on [Ca2+]rest in triadin-null cells (from 142 ± 6 to 130 ± 8 nm; p > 0.05). These data show that the lack of triadin expression is associated with elevated [Ca2+]rest due to an enhanced Ca2+ leak through a ryanodine-resistant pathway that is less sensitive to B5 than WT cells.
Because of the well documented dependence of B5 effect on the presence of a functionally intact FKBP12-RyR1 complex (21, 29, 30), these data strongly suggest that the lack of triadin expression may impair FKBP12/RyR1 interactions that reduce responsiveness to B5 and contribute to elevated [Ca2+]rest. This possibility was tested by measuring expression of FKBP12 and several RyR regulatory proteins using Western blot analysis. Although triadin-null myotubes did not seem to differ from WT in expression of RyR1, DHPR α1S subunit, and junctin, a significant up-regulation of FKBP12 expression and a modest reduction in CSQ expression were noted (Fig. 2A). Immunoprecipitation studies with anti-RyR antibody 34C showed that, despite the up-regulation of FKBP12, RyRs from triadin-null SR vesicle seemingly co-precipitate less FKBP12 than those of WT muscles (Fig. 2B). This difference, however, was marginal and not statistically significant.
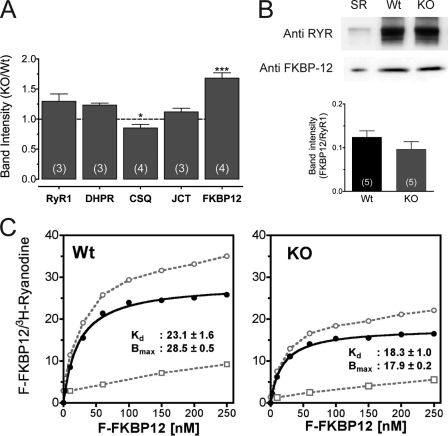
FIGURE 2.
Effect of triadin on FKBP12 expression and its binding to RyR1. A, equivalent amounts of crude membrane homogenates from WT and triadin-null myotubes were loaded and immunoblotted for expression of RyR1, DHPR α1S, CSQ-1, junctin, and FKBP12. Band intensity is expressed as fraction of the WT signal (dotted line). Numbers on the bars indicate the number of blots analyzed per protein. Data are presented as mean ± S.E. *, p < 0.05; ***, p < 0.01. B, Western blot analysis of the protein complex pulled down by 34C antibody from 300 μg of crude homogenates of WT and triadin-null (KO) muscles. SR, 2 μg of SR vesicle. Average band intensity of FKBP12 signal that co-precipitate with RyR1 in WT and triadin-null (KO) homogenates is expressed as fraction of the RyR1 signal (p > 0.05). C, binding of a fluorescent FKBP12 to crude membrane homogenates isolated from WT and triadin-null muscle (○, total binding; ●, specific binding; □, nonspecific binding). Data are expressed as a function of the relative amount of RyR1 measure by [3H]ryanodine binding. Values of Kd and Bmax (mean ± S.E.) are based on fits of averaged data (n = three experiments) to a model assuming a single, saturable binding site (SigmaPlot software, San Jose, CA).
To further assess whether the intrinsic capability of the RyR1 to bind exogenous FKBP12 may be altered in triadin-null muscle, we used a fluorescein-labeled F-FKBP12. Binding of F-FKBP12 to WT and triadin-null membrane vesicles was expressed as function of specific [3H]ryanodine bound in the same preparations (WT, 0.89 ± 0.06 pmol [3H]ryanodine/mg; triadin-null, 1.16 ± 0.09 pmol [3H]ryanodine/mg). Results in Fig. 2C show that F-FKBP12 bound with high affinity to both WT and triadin-null vesicles. However, the F-FKBP12 binding capacity of triadin-null vesicles was significantly reduced when normalized to the level of [3H]ryanodine binding (normalized Bmax, 28.5 ± 0.5 and 17.9 ± 0.2 for WT and triadin-null vesicles, respectively). These results indicate that the increased Ca2+ leak from triadin-null was associated with a lower capacity to bind FKBP12.
RyR1 Channel Activity of Triadin-null Cells
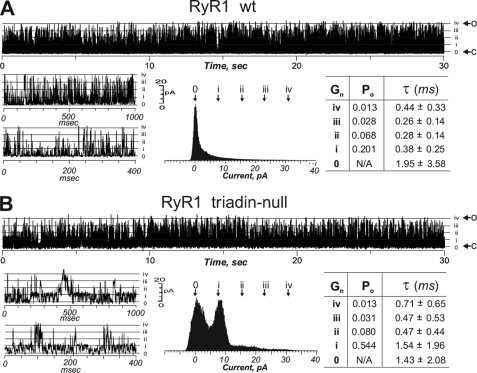
To more directly evaluate whether the lack of triadin influenced RyR1 channel activity, heavy SR membrane fractions were fused to BLM to reconstitute RyR1 channels from WT and triadin-null skeletal muscle. Fig. 3A shows representative traces of a WT Ca2+ channel in the presence of 1 μm Ca2+/2 mm ATP cis, and 100 μm Ca2+ trans. These channels displayed rapid gating behavior with frequent transitions from closed to the fully opened state (inset and histogram). Unlike their wild type counterpart, RyR1 channels reconstituted from triadin-null skeletal muscles displayed several atypical gating modes including stable transitions to multiple subconductance states (Gn of i, ii, iii, and iv) (Fig. 3B). Analysis of the current histogram of RyR1 channels from triadin-null muscle shown in Fig. 3B revealed the predominant distribution of the channel current at one-quarter of the full conductance level. Evaluating the open probability (Po) of WT and triadin-null channels, we found that at its full conductance level (Gn = iv), there was no difference in Po values between the genotypes, both Po = 0.013 (Fig. 3, inset). However, at Gn = i level (one-quarter of the full conductance state), the triadin-null RyR1 had 2.5-fold greater Po and a mean open dwell time ∼3-fold longer than corresponding values measured for WT-RyR1 channels, suggesting that absence of triadin enhances the stability of subconductance behavior. In support of our interpretation, analysis of n = 31 reconstituted triadin-null RyR1 channels under defined experimental cytoplasmic/luminal (cis/trans) conditions resulted in channels whose gating activity varied with Po values at current level Gn = i ranging from 28% to 260% of the corresponding level measured with WT channels under the same (cis/trans) experimental conditions. In addition to triadic-null channels exhibiting prominent stable one-quarter transitions (Gn = i; Fig. 3B), occasionally RyR1 channels reconstituted from triadin-null skeletal muscle exhibited exceptionally longer lasting subconductance reaching the one-half or three-quarter full conductance states during the recording (Gn = ii and Gn = iii, respectively; supplemental Fig. 1).
FIGURE 3.
RyR1 triadin-null channel exhibits more predominantly uncoordinated conduction. RyR1 single channels from either WT or triadin-null skeletal muscle membrane preps were induced to incorporate into BLM as described under “Experimental Procedures.” Free Ca2+ in cis and trans were 1 and 100 μm, respectively. Channel opening and closing fluctuations were indicated by an arrow with “o” and “c”, respectively. The tetrameric RyR1 channel subconductance levels (Gn = i, ii, iii, and iv) were indicated with dashed lines. Po, mean open and closed dwell times, and current amplitude histograms were obtained with the software pClamp 9.
These findings in addition to the limited influence of B5 on [Ca2+]rest (Fig. 1) suggest that the absence of triadin may either alter the interactions of FKBP12 with RyR1, impair the ability of FKBP12 to promote native gating behavior, or both. Therefore, as suggested by the F-FKBP12 binding study (Fig. 2C), absence of triadin may disrupt physical or/and functional FKBP12/RyR1 interactions, thereby destabilizing full conductance transitions and contribute to elevate [Ca2+]rest observed in intact cells.
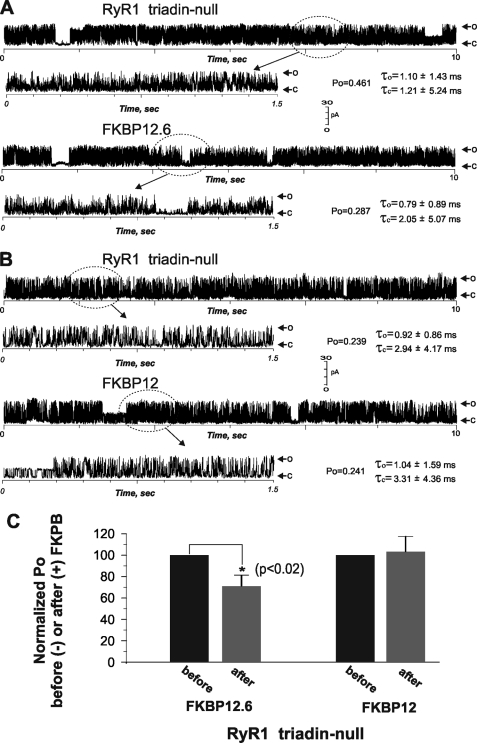
Because FKBP12.6 appears to have higher binding affinity for RyRs than FKBP12 (31, 32), we tested whether exogenously expressed recombinant FKBP12.6 and FKBP12 restored triadin-null RyR1 channel gating behavior to that observed in WT. Fig. 4, A and B show the gating behavior of a RyR1 channel reconstituted from triadin-null heavy SR fraction before and after addition of either recombinant FKBP12.6 or FKBP12 to the cis (cytoplasmic side) chamber. As above, before the addition of FKBP12.6, the triadin-null RyR1 channels have pronounced transitions to multiple subconductance levels (Fig. 4A). After 200 nm exogenous FKBP12.6 was introduced into the cis chamber (a concentration found to replace >95% of bound FKBP12, supplemental Fig. 3), the Po of the full conductance level significantly decreased from Po = 0.461 to Po = 0.287, a 38% reduction (Fig. 4A). However, the presence of FKBP12.6 did not eliminate the multiple subconductance gating behavior (n = 5).
FIGURE 4.
FKPB12.6 but not FKBP12 reduces Po of RyR1 triadin-null channels, although neither eliminates pronounced subconductance gating. RyR1 triadin-null channels were incorporated from fused heavy SR fraction vesicles in bilayers and were recorded for at least 2 min before being exposed to 200 nm FKBP12.6 or 400 nm FKBP12 cis. A, shown is a representative channel (from n = five independent single channel measurements) in the presence of 1/100 μm Ca2+ (cis/trans) and 2 mm Na2ATP (cis). B, shown is a representative single channel recorded (from n = four independent measurements) in the presence of 0.05/1 mm Ca2+ (cis/trans) and 2 mm Na2ATP (cis). C, the mean Po ± S.D. before and after addition of FKBP12.6 (n = 5) or FKBP12 (n = 4) is shown.
Fig. 4B shows a representative RyR1 triadin-null channel before and after the introduction of FKBP12 in the cis chamber. In four of four channels tested, FKBP12 neither altered Po nor prevented occurrence of pronounced subconductance gating behavior. Increasing cis FKBP12 stepwise to 600 nm failed to alter the gating properties of triadin-null channels (data not shown). The Po of triadin-null channels before and after addition 200 nm FKPB12.6 or FKBP12 are summarized in Fig. 4C.
In contrast, addition of 200 nm FKBP12 or 200 nm FKBP12.6 to control WT-RyR1 channels resulted in a significant reduction in Po (data not shown), a result consistent with previous reports (29, 33–35).
FKBP12.6 Expression Reduces SR Ca2+ Leak in Triadin-null Myotubes
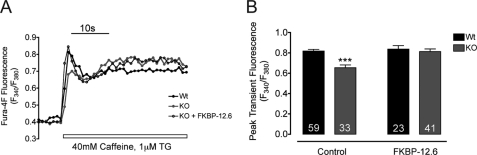
Previously, we reported that in comparison with the WT cells, triadin-null myotubes possessed reduced SR Ca2+ content (14). To further evaluate whether this reduced SR Ca2+ content is associated to the disruption of the FKBP12/RyR1 interaction, we used a lentivirus expression system to create primary WT and triadin-null myoblast lines that permanently overexpress FKBP12.6 and measured SR Ca2+ content after myotube differentiation (see “Experimental Procedures”). Overall SR Ca2+ content was estimated from the peak fluorescence produced by a 40 mm caffeine challenge in cells loaded with 5 μm Fura-4F. To eliminate the contribution of Ca2+ entry and SR Ca2+ uptake in estimating releasable SR Ca2+, all measurements were performed in the presence of Cd2+, La3+, and 1 μm thapsigargin. Fig. 5A shows representative fluorescence transients of WT and triadin-null cells with and without overexpression of FKBP12.6. As reported previously, triadin-null myotubes showed significantly smaller caffeine-induced Ca2+ transients than WT cells (14). Overexpression of FKBP12.6 in triadin-null myotubes significantly increased the peak amplitude of the SR Ca2+ release to levels similar to WT. Overexpression of FKBP12.6, in contrast, showed no detectable effect on average SR Ca2+ release in WT myotubes (Fig. 5B). These results indicate that overexpression of FKBP12.6 can restore SR Ca2+ content in triadin-null myotubes, likely by lowering the Po of RyR1 and abrogating SR Ca2+ leak.
FIGURE 5.
Triadin ablation reduces SR Ca2+ content in null myotubes. A, representative traces of caffeine-induced Ca2+ transients of Fura-4F loaded cells. B, effect of FKBP12.6 overexpression on the average SR Ca2+ content of WT and triadin-null myotubes, estimated from the peak Ca2+ transient amplitude. ***, p < 0.001. Data are presented as mean ± S.D. Numbers on the bars indicate the number of cells analyzed per condition.
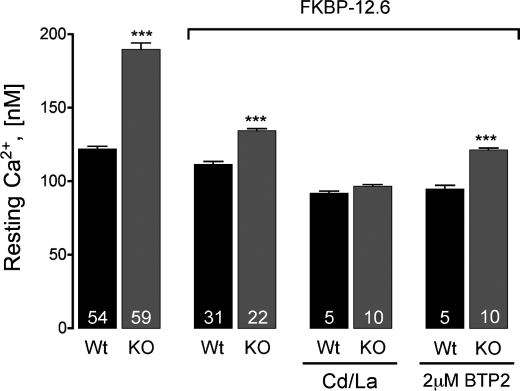
FKBP12.6 Expression Restores [Ca2+]rest in Triadin-null Myotubes
The effect of the stabilization of the RyR1 activity by FKBP12.6 on [Ca2+]rest was tested directly in WT and triadin-null myotubes that overexpress FKBP12.6 and are summarized in Table 1. As shown in Fig. 6, FKBP12.6 expression had virtually no effect on [Ca2+]rest of WT cells (116 ± 7 nm versus 112 ± 9 nm, with and without overexpression, respectively). However, overexpression of FKBP12.6 in triadin-null myotubes significantly lowered [Ca2+]rest from 181 ± 15 nm to 134 ± 8 nm. Consistent with incomplete recovery of WT gating behavior with channels reconstituted from triadin-null SR, FKBP12.6 overexpression in triadin-null myotubes did not lower [Ca2+]rest to WT levels. These results suggest that, even in the presence of FKBP12.6 overexpression, additional Ca2+ entry or release pathways remain active in triadin-null cells. Consistent with the former hypothesis, block of Ca2+ entry with 0.5 mm Cd2+ and 0.1 mm La3+ reduced [Ca2+]rest of triadin-null cells even further to levels similar to those of WT myotubes (92 ± 3 nm for wild type and 96 ± 2 nm for triadin-null cells, p > 0.05, Fig. 6).
FIGURE 6.
Effect of FKBP12.6 expression on resting Ca2+ levels in cultured myotubes. Overexpression of FKBP12 significantly lowered resting Ca2+ concentration in triadin-null myotubes but not in WT cells. Prevention of global Ca2+ entry with 0.5 mm Cd2+ and 0.1 mm La3+ lowered Ca2+ concentrations even further to levels similar to that of WT cells. Unlike Cd2+/La3+, the inhibition of SOCE with BTP-2 failed to restore normal resting Ca2+. ***, p < 0.001. Data are presented as mean ± S.D. Numbers on the bars indicate the number of cells analyzed per condition.
Triadin-null Myotubes Have Increased Ca2+ Entry at Rest
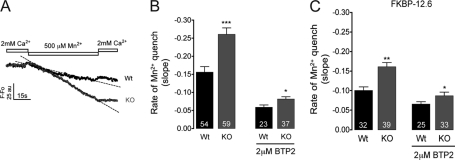
Partial depletion of SR Ca2+ stores in triadin-null myotubes is likely to enhance store-operated Ca2+ entry (SOCE) at rest. To further test this possibility, we measured rates of fluorescence quenching of Fura-2 produced by the entry of external Mn2+ into myotubes. Fig. 7, A and B, show that under resting conditions, triadin-null myotubes displayed greater rates of Mn2+ quench than WT myotubes, a result consistent with enhanced SOCE. Overexpression of FKBP12.6 itself caused a significant reduction in the rate of Mn2+ quench in both cell types (Fig. 7C), suggesting that immunophilin stabilized the RyR1 closed state and abrogated SOCE to a greater extent in resting triadin-null cells. However, this effect of FKBP12.6 on Ca2+ entry did not restore [Ca2+]rest to WT levels, suggesting that SOCE activity may be only partially responsible for the increased rates of Mn2+ quench observed in triadin-null cells. In support of this interpretation, the Transient Receptor Potential Channels (TRPC)/Orai-1 blocker BTP-2 (2 μm, supplemental Fig. 2) added to the external solution significantly reduced but failed to equalize Mn2+ quench rates in WT and triadin-null cells (Fig. 7B). BTP-2 in the presence of FKBP12.6 overexpression did not further reduce the corresponding Mn2+ quench rates compared with BTP-2 alone, nor did it equalize Mn2+ quench rates between the two cell types (Fig. 7C), suggesting that the enhanced Ca2+ entry at rest observed in triadin-null cells involve both SOCE-dependent and -independent mechanisms.
FIGURE 7.
Effect of BTP-2 and FKBP12.6 expression on Ca2+ entry in cultured myotubes. A, representative traces of Fura-2 fluorescence quench by 500 μm Mn2+ measured under resting condition. Triadin-null myotubes (KO) displayed faster rates of Mn2+ entry (slope indicated by dashed lines) than WT cells. B, comparison of the average rate of Mn2+ entry between WT and triadin-null myotubes before and after a 5-min incubation with 2 μm BTP-2. C, effect of overexpression of FKBP12.6 on average rate of Mn2+ entry in the absence and in the presence of BTP-2. *, p < 0.05; **, p < 0.01; ***, p < 0.001 with respect to untreated myotubes. Data are presented as mean ± S.D. Numbers on the bars indicate the number of cells analyzed per condition.
DISCUSSION
In this study, we show that triadin-null myotubes exhibit chronically elevated [Ca2+]rest that is primarily the result of destabilized RyR1 channel activity that promotes pronounced subconductance behavior. Furthermore, FKBP12.6 inhibits RyR1 channel activity and restores [Ca2+]rest in triadin-null myotubes. One consequence of destabilized RyR1 channels is reduced SR Ca2+ content and enhanced resting Ca2+ entry activity in triadin-null myotubes.
The novel effect of the triadin on RyR1 channel regulation is supported by both pharmacological and molecular manipulations. First, the significantly blunted ability of B5 to reduce [Ca2+]rest of triadin-null myotubes in the presence of ryanodine pretreatment, implicates a loss of functional regulation of RyR1 channels by FKBP12 compared with WT myotubes where B5 reduces [Ca2+]rest >50% in the presence of a blocking concentration of ryanodine (2, 28). Second, RyR1 channels reconstituted in BLM from triadin-null SR display pronounced subconductance transitions to Gn = ¼, ½, ¾, which can exhibit prolonged substate transitions. The dissociation of FKBP12 from RyR is known to promote subconductance behavior of RyR1 channels (33–35). Triadin-null RyR1 channels exhibit more frequent and much longer lived subconductances within typical recordings as contrasted by the brief subconductance transitions typically observed when FKBP12 is dissociated from RyR1 in the presence of triadin. This is consistent with our F-FKBP12 binding studies that found a significant disruption of FKBP12/RyR1 interaction in triadin-null muscle. Our results also show that addition of FKBP12.6, which has a higher affinity for RyR1 than FKBP12, partially stabilizes channel behavior by reducing Po. Lastly, cells overexpressing FKBP12.6 demonstrate a stabilizing effect on RyR1 in triadin-null myotubes that effectively attenuates both elevated [Ca2+]rest and enhanced Ca2+ entry.
Interestingly, pulldown assays and F-FKBP12 binding studies showed a reduction in FKBP12 occupancy in triadin-null muscle, revealing a important alteration of the structural and functional integrity of the FKBP12-RyR1 complex, suggesting that triadins regulate functional FKBP12/RyR1 interactions that are essential for setting [Ca2+]rest in WT myotubes. Although the mechanism by which triadin influences FKBP12/RyR1 interactions is unclear, the fact that triadin ablation did not significantly affect FKBP12 binding affinity seem to suggest that skeletal triadins may affect the association/dissociation (on/off) rates of the FKBP12-RyR1 complex. It is also clear from BLM studies that the absence of triadin also alters aspects of RyR1 function that cannot be completely overcome by addition of exogenous FKBP12.6 (such as subconductance behavior).
The current findings also provide insights into the mechanism by which triadin may modulate skeletal EC coupling. Indeed, several lines of evidence seem to support the idea that the disruption of the triadin/RyR1 interaction negatively regulates EC coupling, resulting in reduced depolarization-induced calcium release (9–11, 14). Coincidently, the disruption of FKBP12 binding to RyR1 in adult muscle (33, 36, 37) as well as cultured myotubes (37, 38) was shown to decrease the gain of EC coupling similar to that seen in triadin-null cells. Therefore, our data suggest that the effect of triadin on EC coupling may be indirectly mediated by the disruption of the normal interaction between FKBP12 and RyR1 and diminished SR Ca2+ load at rest.
Using Mn2+ quench imaging here we identify that at rest, triadin-null myotubes exhibited enhanced resting Ca2+ entry compared with their WT counterparts. Interestingly, the SOCE inhibitor BTP-2 was equally effective in reducing Ca2+ entry in both cell types, blocking Ca2+ influxes by ∼65%. Because BTP-2 blocks both TRPC and Orai-1 Ca2+ entry pathways, these data seem to suggest that both pathways may be more active in resting triadin-null cells. Ca2+ influx inhibition with BTP-2 also significantly reduced [Ca2+]rest, lowering free Ca2+ in WT and triadin-null cells by 15 and 22%, respectively, thereby reducing the intrinsic difference in [Ca2+]rest between WT and triadin-null cells from 65 to 45 nm. By comparison, overexpression of FKBP12.6 reduced this difference to 22 nm, showing a more robust effect than BTP-2 or Cd2+ and La3+ alone. These data strongly suggest that stabilization of the FKBP12/RyR1 interaction with FKBP12.6 seems to have a more profound effect on reducing [Ca2+]rest than the inhibition of Ca2+ entry. Therefore, it appears that the enhanced Ca2+ entry observed in triadin-null cells at rest may be a direct adaptation to the intrinsically higher RyR1 activity and the reduced SR Ca2+ content.
Interestingly, although the combined effects of BTP-2 and overexpression of FKBP12.6 reduced resting calcium levels even further, the effect was not additive, and the differences in [Ca2+]rest between both phenotypes persisted (∼26 nm). This difference was negated only after all sources of Ca2+ entry were blocked with addition of 0.5 mm Cd2+ and 0.1 mm La3+ to the external medium. This result is consistent with the observation that BTP-2, although effective in blocking SOCE, did not negate the differences in Ca2+ entry between WT and triadin-null cells and suggests that an additional BTP-2-insensitive Ca2+ entry pathway contributes to [Ca2+]rest in triadin-null cells. It is worth noting, however, that the attenuation of both SR Ca2+ leak and Ca2+ entry by the combined effect of FKBP12.6 overexpression and BTP-2, respectively, were effective in lowering [Ca2+]rest of triadin-null cells to levels observed in WT myotubes under normal resting conditions (121 ± 4 nm versus 116 ± 7 nm, respectively), supporting the idea that SR Ca2+ leak and enhanced resting Ca2+ entry are two of the primary pathways responsible for Ca2+ dysregulation in triadin-null cells.
Overall, our data support a molecular model in which the lack of triadin expression significantly destabilizes structural and functional FKBP12/RyR1 interactions, thereby causing an increased basal activity of RyR calcium channels. This in turn results in an increased SR Ca2+ leak that chronically elevates [Ca2+]rest. On the other hand, increased Ca2+ leak leads to a partial depletion of SR Ca2+ stores that activates Ca2+ entry through TRP/Orai-1 channels further sustaining elevated [Ca2+]rest. However, a role for intraluminal Ca2+ regulation of triadin-null RyR1s, although likely small, cannot be ruled out. On the other hand, an effect of triadin ablation on CSQ-mediated regulation could be expected to be minimal, given the fact that CSQ seems to play only a minor role, if any, in intraluminal regulation of RyR1 channels (39).
An intriguing extrapolation of this model is that [Ca2+]rest in skeletal muscle cells appears to be highly sensitive to the conformational states of RyR1 channels and its interactions with its protein binding partners within the Ca2+ release unit. Based on this premise, it is not surprising that ryanodine, at concentrations known to induce changes in activity and subconductance states of RyR1, had an effect on [Ca2+]rest of cultured myotubes comparable with that of triadin ablation (supplemental Fig. 4).
Because ultimately, [Ca2+]rest seems to be derived primarily from a complex steady state of Ca2+ fluxes across the plasma membrane (40), it appears that any unbalance of Ca2+ uptake and release within the SR (caused by changes in Ca2+ release unit function for instance) has the potential to reset new Ca2+ equilibria across the plasmalemma that eventually result in long term changes in [Ca2+]rest. Hence, the activity of regulatory components of the Ca2+ release unit, like triadin, although not directly involved in Ca2+ transport, can contribute a significant role fine-tuning Ca2+ homeostasis in skeletal muscles.
Supplementary Material
Acknowledgments
We thank Dr. Hongli Li for valuable help and technical expertise. We acknowledge the skillful contributions of Kim Truong with BLM measurements.
This work was supported, in whole or in part, by National Institute of Health Grants 5K01AR054818 (to C. F. P.), P01AR47605 (to P. D. A. and I. N. P.), and R01HL076433 (to B. R. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- SR
- sarcoplasmic reticulum
- BLM
- bilayer lipid membranes
- DHPR
- dihydropyridine receptor
- RyR
- ryanodine receptor
- F-FKBP12
- fluorescent FKBP12
- PIPES
- 1,4-piperazinediethanesulfonic acid
- SOCE
- store operated Ca2+ entry
- CSQ
- calsequestrin
- B5
- bastadin-5.
REFERENCES
- 1.Buck E. D., Nguyen H. T., Pessah I. N., Allen P. D. (1997) J. Biol. Chem. 272, 7360–7367 [DOI] [PubMed] [Google Scholar]
- 2.Eltit J. M., Yang T., Li H., Molinski T. F., Pessah I. N., Allen P. D., Lopez J. R. (2010) J. Biol. Chem. 285, 13781–13787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez J. R., Alamo L. A., Jones D. E., Papp L., Allen P. D., Gergely J., Sréter F. A. (1986) Muscle Nerve 9, 85–86 [PubMed] [Google Scholar]
- 4.Yang T., Esteve E., Pessah I. N., Molinski T. F., Allen P. D., López J. R. (2007) Am. J. Physiol. Cell. Physiol. 292, C1591–1598 [DOI] [PubMed] [Google Scholar]
- 5.Marty I., Fauré J., Fourest-Lieuvin A., Vassilopoulos S., Oddoux S., Brocard J. (2009) J. Physiol. 587, 3117–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt N. R., Caswell A. H., Wen S. R., Talvenheimo J. A. (1990) J. Membr. Biol. 113, 237–251 [DOI] [PubMed] [Google Scholar]
- 7.Kim K. C., Caswell A. H., Talvenheimo J. A., Brandt N. R. (1990) Biochemistry 29, 9281–9289 [DOI] [PubMed] [Google Scholar]
- 8.Motoike H. K., Caswell A. H., Smilowitz H. M., Brandt N. R. (1994) J. Muscle Res. Cell. Motil. 15, 493–504 [DOI] [PubMed] [Google Scholar]
- 9.Goonasekera S. A., Beard N. A., Groom L., Kimura T., Lyfenko A. D., Rosenfeld A., Marty I., Dulhunty A. F., Dirksen R. T. (2007) J. Gen. Physiol. 130, 365–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezgui S. S., Vassilopoulos S., Brocard J., Platel J. C., Bouron A., Arnoult C., Oddoux S., Garcia L., De Waard M., Marty I. (2005) J. Biol. Chem. 280, 39302–39308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Li X., Duan H., Fulton T. R., Eu J. P., Meissner G. (2009) Cell Calcium 45, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groh S., Marty I., Ottolia M., Prestipino G., Chapel A., Villaz M., Ronjat M. (1999) J. Biol. Chem. 274, 12278–12283 [DOI] [PubMed] [Google Scholar]
- 13.Ohkura M., Furukawa K., Fujimori H., Kuruma A., Kawano S., Hiraoka M., Kuniyasu A., Nakayama H., Ohizumi Y. (1998) Biochemistry 37, 12987–12993 [DOI] [PubMed] [Google Scholar]
- 14.Shen X., Franzini-Armstrong C., Lopez J. R., Jones L. R., Kobayashi Y. M., Wang Y., Kerrick W. G., Caswell A. H., Potter J. D., Miller T., Allen P. D., Perez C. F. (2007) J. Biol. Chem. 282, 37864–37874 [DOI] [PubMed] [Google Scholar]
- 15.Fodor J., Gönczi M., Sztretye M., Dienes B., Oláh T., Szabó L., Csoma E., Szentesi P., Szigeti G. P., Marty I., Csernoch L. (2008) J. Physiol. 586, 5803–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo W., Jorgensen A. O., Campbell K. P. (1996) Soc. Gen. Physiol. Ser. 51, 19–28 [PubMed] [Google Scholar]
- 17.Beard N. A., Sakowska M. M., Dulhunty A. F., Laver D. R. (2002) Biophys. J. 82, 310–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Györke I., Hester N., Jones L. R., Györke S. (2004) Biophys. J. 86, 2121–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eltit J. M., Lopez J. R., Wei F., Pessah I. N., Allen P. D., Perez C. F. (2010) Biophys. J. 98, 505a-510a20159146 [Google Scholar]
- 20.Cornea R. L., Nitu F. R., Samsó M., Thomas D. D., Fruen B. R. (2010) J. Biol. Chem. 285, 19219–19226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mack M. M., Molinski T. F., Buck E. D., Pessah I. N. (1994) J. Biol. Chem. 269, 23236–23249 [PubMed] [Google Scholar]
- 22.Laemmli U. K. (1970) Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 23.Perez C. F., López J. R., Allen P. D. (2005) Am. J. Physiol. Cell. Physiol. 288, C640–649 [DOI] [PubMed] [Google Scholar]
- 24.Lee E. H., Cherednichenko G., Pessah I. N., Allen P. D. (2006) J. Biol. Chem. 281, 10042–10048 [DOI] [PubMed] [Google Scholar]
- 25.Lee E. H., Rho S. H., Kwon S. J., Eom S. H., Allen P. D., Kim do H. (2004) J. Biol. Chem. 279, 26481–26488 [DOI] [PubMed] [Google Scholar]
- 26.Westerman K. A., Penvose A., Yang Z., Allen P. D., Vacanti C. A. (2010) Exp. Cell Res. 316, 1966–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks S. P., Storey K. B. (1992) Anal. Biochem. 201, 119–126 [DOI] [PubMed] [Google Scholar]
- 28.Pessah I. N., Molinski T. F., Meloy T. D., Wong P., Buck E. D., Allen P. D., Mohr F. C., Mack M. M. (1997) Am. J. Physiol. 272, C601–614 [DOI] [PubMed] [Google Scholar]
- 29.Chen L., Molinski T. F., Pessah I. N. (1999) J. Biol. Chem. 274, 32603–32612 [DOI] [PubMed] [Google Scholar]
- 30.Masuno M. N., Pessah I. N., Olmstead M. M., Molinski T. F. (2006) J. Med. Chem. 49, 4497–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeyakumar L. H., Ballester L., Cheng D. S., McIntyre J. O., Chang P., Olivey H. E., Rollins-Smith L., Barnett J. V., Murray K., Xin H. B., Fleischer S. (2001) Biochem. Biophys. Res. Commun. 281, 979–986 [DOI] [PubMed] [Google Scholar]
- 32.Xin H. B., Rogers K., Qi Y., Kanematsu T., Fleischer S. (1999) J. Biol. Chem. 274, 15315–15319 [DOI] [PubMed] [Google Scholar]
- 33.Brillantes A. B., Ondrias K., Scott A., Kobrinsky E., Ondriasová E., Moschella M. C., Jayaraman T., Landers M., Ehrlich B. E., Marks A. R. (1994) Cell 77, 513–523 [DOI] [PubMed] [Google Scholar]
- 34.Gaburjakova M., Gaburjakova J., Reiken S., Huang F., Marx S. O., Rosemblit N., Marks A. R. (2001) J. Biol. Chem. 276, 16931–16935 [DOI] [PubMed] [Google Scholar]
- 35.Mayrleitner M., Timerman A. P., Wiederrecht G., Fleischer S. (1994) Cell Calcium 15, 99–108 [DOI] [PubMed] [Google Scholar]
- 36.Lamb G. D., Stephenson D. G. (1996) J. Physiol. 494, 569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang W., Ingalls C. P., Durham W. J., Snider J., Reid M. B., Wu G., Matzuk M. M., Hamilton S. L. (2004) FASEB J. 18, 1597–1599 [DOI] [PubMed] [Google Scholar]
- 38.Avila G., Lee E. H., Perez C. F., Allen P. D., Dirksen R. T. (2003) J. Biol. Chem. 278, 22600–22608 [DOI] [PubMed] [Google Scholar]
- 39.Qin J., Valle G., Nani A., Chen H., Ramos-Franco J., Nori A., Volpe P., Fill M. (2009) Biophys. J. 97, 1961–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ríos E. (2010) J. Physiol. Sci. 60, 81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.