Abstract
A dynamic cycle of O-linked GlcNAc (O-GlcNAc) addition and removal is catalyzed by O-GlcNAc transferase and O-GlcNAcase, respectively, in a process that serves as the final step in a nutrient-driven “hexosamine-signaling pathway.” Evidence points to a role for O-GlcNAc cycling in diabetes and insulin resistance. We have used Drosophila melanogaster to determine whether O-GlcNAc metabolism plays a role in modulating Drosophila insulin-like peptide (dilp) production and insulin signaling. We employed transgenesis to either overexpress or knock down Drosophila Ogt(sxc) and Oga in insulin-producing cells (IPCs) or fat bodies using the GAL4-UAS system. Knockdown of Ogt decreased Dilp2, Dilp3, and Dilp5 production, with reduced body size and decreased phosphorylation of Akt in vivo. In contrast, knockdown of Oga increased Dilp2, Dilp3, and Dilp5 production, increased body size, and enhanced phosphorylation of Akt in vivo. However, knockdown of either Ogt(sxc) or Oga in the IPCs increased the hemolymph carbohydrate concentration. Furthermore, phosphorylation of Akt stimulated by extraneous insulin in an ex vivo cultured fat body of third instar larvae was diminished in strains subjected to IPC knockdown of Ogt or Oga. Knockdown of O-GlcNAc cycling enzymes in the fat body dramatically reduced neutral lipid stores. These results demonstrate that altered O-GlcNAc cycling in Drosophila IPCs modulates insulin production and influences the insulin responsiveness of peripheral tissues. The observed phenotypes in O-GlcNAc cycling mimic pancreatic β-cell dysfunction and glucose toxicity related to sustained hyperglycemia in mammals.
Keywords: Diabetes, Drosophila, Glucose, Glucose Metabolism, Glycogen, Insulin, Homeostasis, O-GlcNAc, Hexosamine, Trehalose
Introduction
Like protein phoshophorylation, the dynamic addition and removal of O-linked GlcNAc (O-GlcNAc)3 in over 500 intracellular proteins is a key regulator of nuclear and cytoplasmic protein activity (1, 2). The O-GlcNAc modification is partly driven by the levels of UDP-GlcNAc derived from the hexosamine biosynthetic pathway. This pathway is a nutrient-sensing pathway implicated in cellular signaling and insulin resistance. Two enzymes mediate cycling of O-GlcNAc: a glycosyltransferase (O-GlcNAc transferase; OGT) and a hexosaminidase (O-GlcNAcase; OGA). OGT-related sequences have been identified from Archaea to humans (3, 4). In plants, the OGT homolog Spindly and Secret Agent are involved in development (5, 6). These evolutionarily conserved proteins share a conserved structure. OGT contains tetratricopeptide repeats at the N terminus and a catalytic domain at the C terminus composed of Rossman-like folds. OGA, originally identified as meningioma-expressed autoantigen (termed MGEA5), is a member of the family 84 glycoside hydrolases (7). OGA is also highly conserved in eukaryotic evolution from insects to humans. In mammals, the MGEA5 gene encodes at least two alternatively spliced transcripts. The longer of these was originally designated OGA and has a C-terminal MYST family histone acetyltransferase-like domain (8–10). The N-terminal “hyaluronidase” domain has been proposed to be the catalytic site for OGA glycosidase activity and conforms to the Triosephosphate isomerase-barrel fold that typifies this class of glycosylhydrolases (1, 2).
Several independent lines of evidence point to a role for O-GlcNAc modification in diabetes and insulin resistance (11, 12). Overexpression of the OGT in muscle and fat leads to insulin resistance and hyperleptinemia (13). In addition, the O-GlcNAcase was recently shown to be a type 2 diabetes susceptibility gene associated with obesity in the Mexican-American population (14). Diabetes results from the failure of insulin production from the pancreatic β-cells and from insulin resistance in the peripheral tissues including liver, muscle, and white adipose tissue. Interestingly, transcripts encoding OGT are enriched in the pancreatic β-cells (15). Taken together, these studies suggest that nutrient-driven O-GlcNAcylation of proteins through hexosamine biosynthetic pathway in the β-cells could alter insulin production. However, a suitable whole animal model in which to examine this problem has been lacking.
Development of appropriate mouse knock-out models of the enzymes of hexosamine signaling has proven difficult because the pathway is essential in mammals. Knock-outs of OGT are embryonic and stem cell lethal (16), and conditional knock-outs of OGT in various tissues are complicated by induced tissue-specific lethality (17). Overexpression of OGA induces a mitotic exit phenotype (18), suggesting that it too may perform essential functions. We originally identified Caenorhabditis elegans OGT and OGA and have shown that knock-outs of ogt-1 and oga-1 in the nematode, although viable and fertile, have striking effects on insulin-like signaling and macronutrient storage (19, 20).
Our previous studies, and those of others, have demonstrated that many Drosophila proteins are modified by O-GlcNAc, suggesting that this modification may play a role in signaling in the fly model (21, 22). However, it is not clear how the cycling of O-GlcNAc on these target proteins is regulated by nutritional cues. Recently in Drosophila, ogt was identified as sxc (super sex combs), a gene regulating the homeotic genes that establish the adult body plan in the fly (23, 24). Flies harboring the loss of function alleles of ogt/sxc show arrested development. Null alleles of ogt/sxc arrest at third instar larvae or earlier and are thus not suitable for metabolic studies of the kind envisioned here.
Despite these limitations, the fly provides an excellent, unexplored genetic model for examining the influence of the hexosamine biosynthetic pathway on insulin signaling because the insulin-signaling pathway in Drosophila is highly conserved and similar to the mammalian insulin-signaling pathway (25, 26). The Drosophila genome contains several Drosophila insulin-like peptide genes (dilp) with significant homology to mouse and human insulin (25). Drosophila has insulin-producing cells (IPCs) in morphologically discrete regions of the brain (27). Ablation of these IPCs in the fly results in elevation of trehalose and glucose levels in the hemolymph similar to the hyperglycemia observed in mammalian diabetes mellitus (28, 29). Deletion of dilp1–5 causes delayed growth and increased carbohydrate levels in the hemolymph (30). Additionally, the Drosophila fat body is an organ that functions similarly to both mammalian liver and white adipose tissue (31).
In this study, we have explored the possibility that O-GlcNAc cycling in the insulin-producing cells is a key mediator of insulin production regulating the insulin-signaling pathway in the fly. We established several transgenic loss or gain of function models of sxc/ogt (CG10392) or oga (CG5871), using the GAL4-UAS system. Using these transgenic flies, we found that tissue-specific, O-GlcNAc modulation altered insulin secretion from the IPCs, carbohydrate content in the hemolymph, body size, and insulin sensitivity. These phenotypes are consistent with insulin resistance in response to altered O-GlcNAc cycling and with associated changes in glucose-insulin homeostasis.
EXPERIMENTAL PROCEDURES
Fly Stocks and Culturing for Experiments
The dILP2-GAL4 line was obtained from Dr. Eric Rulifson (University of California, San Francisco). The UAS-OGT line that contains Myc epitope tag in the N terminus (UAS-Myc-OGT) was made by P-element-mediated transformation. The Lsp2-GAL4 line (stock 6357) was obtained from Bloomington Stock center. UAS-RNAi lines of Ogt (stock 18611) and Oga (stock 41822) were obtained from Vienna Drosophila RNAi Center. w1118 crossed with dILP2-GAL4 or Lsp2-GAL4 was used as control as indicated. The stocks were maintained at 25 °C under constant humidity using standard sugar/yeast medium. In some experiments, adult flies were starved on 1.3% agar for 6 h.
Immunostaining
Brains of third instar larvae were dissected and fixed for 1 h with 4% paraformaldehyde in PBS at 25 °C, and then tissues were treated with 0.3% Triton X-100 and 5% normal goat serum in PBS for 4 h at 4 °C, as a block for nonspecific antibody binding. Affinity-purified rabbit anti-Drosophila insulin (DILP) antiserum (gift from Dr. Eric Rulifson) was used at 1:200 and incubated with samples at 4 °C for overnight and then incubated with Alexa Fluor 546 goat anti-rabbit as a secondary antibody. To evaluate cell toxicity in IPCs, brains were treated with TUNEL reaction solution from an in situ cell death detection kit (Roche Applied Science) for 1 h at 37 °C. For positive control, 1 unit of DNase I (Invitrogen) was incubated for 15 min at 37 °C prior to TUNEL reaction. For staining of nucleus, the brains were stained with DAPI for 15 min at 25 °C. Confocal imaging was carried out on an LSM 410 microscope (Zeiss).
Evaluation of Body Size
Individual adult flies were weighed three times for accuracy. To compare the body size of third instar larvae, larvae were collected according to the protocol described previously (28). The larvae were digitally photographed at 6.5× magnification. We used the iVision Scientific Image Processing software (Biovision Technology, Exton, PA) to measure the pixel area of whole bodies. We evaluated the relative size values compared with control larvae.
Quantitative Real Time PCR
Total RNA from whole bodies of wandering third instar larvae was extracted using the RNeasy mini kit (Qiagen) and reverse transcribed by the Superscript III system (Invitrogen). Quantitative PCR was performed using the 7900HT fast real time PCR system (Applied Biosystems, Foster City, CA). The primers for quantitative real time PCR were as follows: dilp2F, CCTGCAGTTTGTCCAGGAGTTC; dilp2R, TCGCAGCGGTTCCGATAT; dilp3F, CAAACTGCCCGAAACTCTCT; dilp3R, CTCTTGGTCATTGCGTTGAA; dilp5F, AATTCAATGTTCGCCAAACG; dilp5R, CGCCAAGTGGTCCTCATAAT; Actin42F, CCATGTACCCGGGAATCG; and Actin42R, GAGCCAACGCCGTGATTT.
Measurement of Hemolymph Carbohydrate
Hemolymph samples were collected as described (27). Carbohydrate levels were evaluated by Dionex high performance anion exchange equipped with a pulsed amperometric detector (model PAD 2) and pellicular anion exchange column (PA 10, 4 × 250 mm). The concentration of each carbohydrate levels was determined by the absolute value of the area of the peak at standard sample.
Western Blot Analysis
Whole bodies of 4-day-old adult flies were isolated in the protein extraction buffer (Pierce). Whole bodies or dissected fat bodies of wandering third instar larvae were isolated in the Drosophila homogenate buffer as described (29). Total proteins were separated by SDS-PAGE and then incubated with phospho-Drosophila Akt (Ser-505) antibody or Akt antibody (Cell Signaling, Danvers, MA) as primary antibody, and then membranes were incubated with IR800-labeled secondary antibodies (LI-COR). The blots were visualized using an imaging scanner (LI-COR).
Ex Vivo Culture of Fat Body
Fat bodies of wandering third instar larvae were dissected and cultured referring to the protocol (32). In brief, fat bodies were dissected in the Schneider's medium and incubated in the Schneider's medium for 4 h at 25 °C, and then 1 μg/ml insulin was administrated in the medium and cultured for another 15 min. Isolated proteins were separated by SDS-PAGE and immunoblotted with Drosophila-specific p-Akt antibody (Ser-505) or Akt antibody.
Statistical Analysis
The values are expressed as the means ± S.E., unless otherwise stated. The mean values were compared using Student's t test. An asterisk in the figures indicates a significant difference from control (p < 0.05).
RESULTS
Altered O-GlcNAc Cycling in IPCs Influences Growth
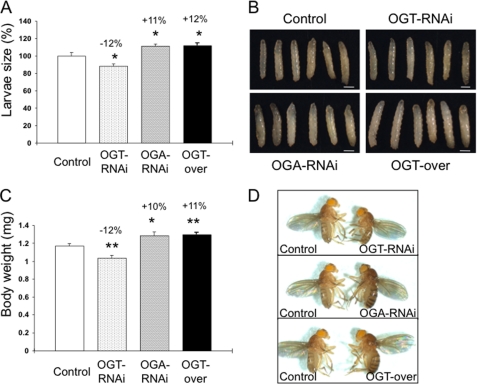
We analyzed the effects of IPC-specific knockdown of endogenous ogt or oga by using the same driver, dILP2-GAL4, as described above to express a UAS-RNAi hairpin directed against ogt or oga (dILP2GAL4/+;UAS-dOGTRNAi/+ and dILP2GAL4/+;UAS-dOGARNAi/+). This dILP2-GAL4 line has been extensively used to target the IPCs in the fly and was previously used to ablate the median neurosecretory cells (28). The RNAi construct targeted to ogt was tested for its effectiveness using a strong tub-Gal4, which phenocopied Sxc null alleles by producing no adult offspring. Previous studies have shown that ablation of IPCs induces a decrease in the body size, and overexpression of dilp2 rescues the body size (28). We analyzed the changes in body size in third instar larvae and adults, induced by interference with O-GlcNAc cycling. In third instar larvae, knockdown of Ogt significantly decreased body size (−12%) compared with control strains, whereas knockdown of Oga or overexpression of Ogt significantly increased the body size (+11 and +12%, respectively) (Fig. 1, A and B). The adult flies exhibited a similar modulation in body size (Fig. 1, C and D). These results demonstrate that altering O-GlcNAc cycling in the IPCs influences body size and suggests changes in DILP levels.
FIGURE 1.
Altered O-GlcNAc cycling influences Drosophila body size. Third instar larvae or 4-day-old adults of dILP2GAL4/+;UAS-dOGTRNAi/+ (OGT-RNAi), dILP2GAL4/+;UAS-dOGARNAi/+ (OGA-RNAi), dILP2GAL4/+;UAS-myc-dOGT/+ (OGT-Myc), or control strains were collected. A, measurement of larval size. Data are expressed as the means ± S.E. (n = 20). *, p < 0.05 versus control. B, representative images of third instar larvae are shown. C, body weight measurement of adult flies. Data are expressed as the means ± S.E. (n = 25). *, p < 0.05 versus control. D, representative images of adult flies are shown.
O-GlcNAc Cycling Modulates DILP Expression
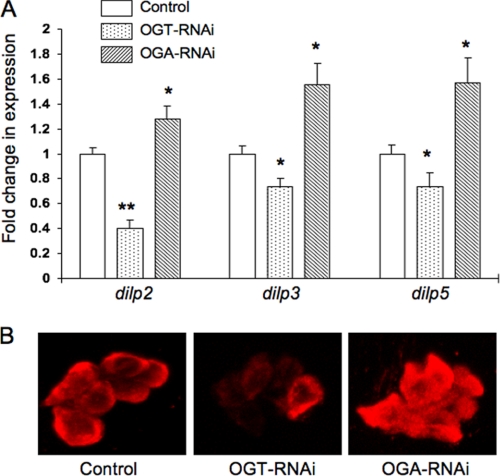
DILP2, DILP3, and DILP5 are expressed in the IPCs, and the levels of these peptides modulate growth and body size (33). Given the changes in body size that we detected in response to O-GlcNAc perturbation in the IPCs, we wanted to determine whether changes in O-GlcNAc cycling correlated with DILP levels. We measured dilp2, dilp3, and dilp5 transcripts by quantitative real time PCR using total RNA from wandering third instar larvae. Knockdown of Ogt decreased dilp2, dilp3, and dilp5 transcript levels compared with control (−60, −26, and −26%, respectively), whereas knockdown of Oga increased expression levels for all three dilps (+28, +55, and +57%, respectively) (Fig. 2A).
FIGURE 2.
Altered O-GlcNAc cycling modulates DILP expression. A, gene expression levels of dilp2, dilp3, and dilp5 in the third instar larvae were evaluated by quantitative real time PCR. Control experiments were carried out with Drosophila Actin42. Data are expressed as the means ± S.E. (n = 10). Dilp levels were decreased in dILP2GAL4/+;UAS-dOGTRNAi/+ (OGT-RNAi) but increased in dILP2GAL4/+;UAS-dOGARNAi/+ (OGA-RNAi) compared with control. *, p < 0.05; **, p < 0.001 versus the control. B, third instar larval brain was stained with rabbit anti-Drosophila insulin (DILP2) antibody. DILP2 staining in IPCs was decreased in OGT-RNAi but increased in OGA-RNAi.
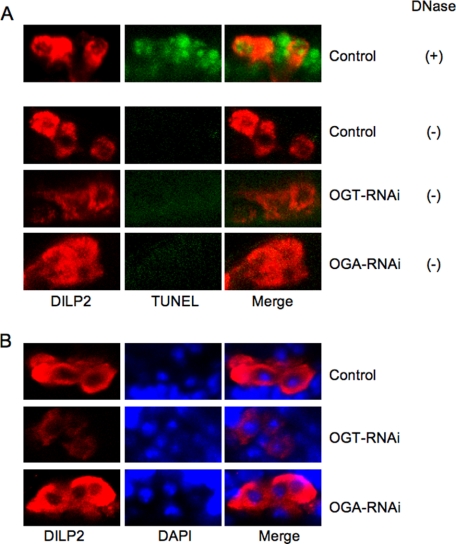
DILP2 protein expression in the brain of third instar larvae was evaluated using a DILP2-specific antibody. DILP2 staining decreased with the knockdown of Ogt (Fig. 2B, OGT-RNAi) and increased with the knockdown of Oga (Fig. 2B, OGA-RNAi) compared with control. The targeted knockdown of Ogt or Oga was not toxic to the IPCs. TUNEL assay and DAPI staining of the nuclei did not reveal changes in chromatin structure that would be consistent with apoptosis or necrosis (Fig. 3). Additionally, the morphology of IPCs did not change with RNAi of O-GlcNAc cycling enzymes. These results suggest that deregulation of O-GlcNAc cycling induced by knockdown of either enzyme significantly altered DILP expression in the absence of overt toxicity to the IPCs.
FIGURE 3.
The targeted knockdown of Ogt or Oga was not overtly toxic to the IPCs. Third instar larval brain from dILP2GAL4/+;UAS-dOGTRNAi/+ (OGT-RNAi), dILP2GAL4/+;UAS-dOGARNAi/+ (OGA-RNAi), or control strains was stained with rabbit anti-Drosophila insulin (DILP2) antibody. A, TUNEL reaction was performed using an in situ cell detection kit (Roche Applied Science) after DILP2 staining. For positive control, brain was incubated with DNaseI for 15 min before TUNEL reaction. No staining of DNA fragmentation was seen in the strains except for the positive control (DNase +) (middle column). Staining was merged (right column) with DILP2 staining (left column). B, DAPI staining was performed after DILP2 staining. DAPI staining of the nuclei did not reveal changes in chromatin structure (middle column). DAPI staining was merged (right column) with DILP2 staining (left column).
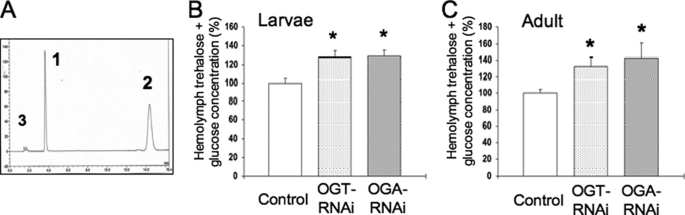
Both Knockdown Models of O-GlcNAc Cycling Increase Hemolymph Carbohydrate Levels
Insects contain two types of carbohydrate in circulating hemolymph: glucose and trehalose. The DILPs play an important role in regulating the levels of these circulating carbohydrates. To understand the role of altering DILP levels in this process, hemolymph carbohydrate levels for each strain were evaluated by high performance anion exchange-pulse amperometric detector. We have used this method to measure carbohydrate levels in C. elegans (19, 20). An example of the separation profile illustrating the clear resolution of trehalose and glucose is shown (Fig. 4A, peaks 1 and 2, respectively). In both third instar larvae and adult flies, knockdown of Ogt increased hemolymph carbohydrate levels compared with control strains (+27%, +32%, respectively) (Fig. 4, B and C). Interestingly, knockdown of Oga correlated with increased hemolymph carbohydrate levels (+28 and +43%, respectively) (Fig. 4, B and C), even with increased DILP levels (Fig. 2). Therefore, deregulation of O-GlcNAc cycling by targeted knockdown of either enzyme in the IPCs resulted in higher circulating carbohydrate levels. Although higher carbohydrate levels might be expected from the lower production of DILPs (Ogt knockdown), the elevated carbohydrate associated with higher DILPs expression in the Oga knockdown was surprising. One possibility is that chronically elevated DILPs might lead to changes in signaling in insulin-responsive tissues, which normally take up hemolymph carbohydrate, possibly representing a kind of insulin resistance. We have reported previously that carbohydrate uptake and storage are perturbed in transgenic models with altered O-GlcNAc cycling in both mice and C. elegans (13, 19, 20).
FIGURE 4.
Elevated hemolymph carbohydrate concentration in transgenic flies with altered O-GlcNAc cycling. A, hemolymph carbohydrate levels were determined by high performance anion exchange-pulse amperometric detector. The peaks represent trehalose (peak 1) and glucose (peak 2). Peak 3 indicates a nonspecific peak. B and C, hemolymph was collected from wandering third instar larvae (B) and 4-day-old adult flies starved for 6 h (C). Trehalose and glucose concentration in dILP2GAL4/+;UAS-dOGTRNAi/+ (OGT-RNAi), dILP2GAL4/+;UAS-dOGARNAi/+ (OGA-RNAi), or control were measured. Data are expressed as the means ± S.E. (third instar larvae; n = 8, adults; n = 10). Both OGT-RNAi and OGA-RNAi increased carbohydrate levels. *, p < 0.05 versus the control.
O-GlcNAc Cycling Influences the Insulin-signaling Pathway
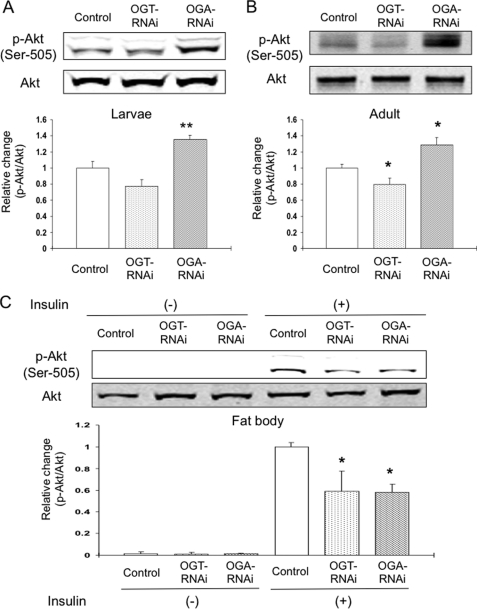
The insulin-signaling pathway is required for growth and the regulation of carbohydrate metabolism in Drosophila (34). To confirm the influence of altered O-GlcNAc cycling in the IPCs on the insulin-signaling pathway in the peripheral tissues, we used a Drosophila-specific phospho-Akt (p-Akt) antibody. Previous reports have shown that the phosphorylation site on Ser-505 in Drosophila Akt, corresponding to Ser-473 in mammalian Akt, is activated by extraneous insulin stimulation through the phosphatidylinositol (PI) 3-kinase pathway in Drosophila culture cells (35). In both third instar larvae and adult flies, targeted knockdown of Oga, but not knockdown of Ogt in the IPCs, increased the level of p-Akt compared with control (+35 and +29%, respectively) (Fig. 5, A and B). These results suggest that altering O-GlcNAc cycling in the IPCs affects the insulin-signaling pathway in peripheral tissues in a manner consistent with persistent elevated circulating DILP levels.
FIGURE 5.
Endogenous insulin signaling in the peripheral tissues and response of extraneous insulin stimulation in dissected fat bodies. A and B, bodies of wandering third instar larvae (A) and 4-day-old adult flies (B) in dILP2GAL4/+; UAS-dOGTRNAi/+ (OGT-RNAi), dILP2GAL4/+; UAS-dOGARNAi/+ (OGA-RNAi), or control were homogenized. C, fat bodies of OGT-RNAi, OGA-RNAi, or control were dissected and incubated for 4 h, and then 1 μg/ml of insulin was administrated in the medium and cultured for another 15 min. Western blot analysis was performed using Drosophila-specific phospho-Akt (p-Akt) antibody (Ser-505) and Akt antibody. Intensity of p-Akt was normalized by intensity of Akt. Data are expressed as the means ± S.E. (A, n = 8; B, n = 7; C, n = 7). The endogenous p-Akt level was higher in OGA-RNAi, but the level of p-Akt in the cultured insulin stimulation was less in both OGT-RNAi and OGA-RNAi. *, p < 0.05; **, p < 0.001 versus the control.
Extraneous Insulin Response Is Impaired in the Dissected Fat Body
In insects, the fat body is responsible for the uptake of hemolymph carbohydrate and for modulating energy storage (32). DILPs, in part, regulate carbohydrate uptake by the fat body. We examined the effect of extraneous insulin administration in ex vivo cultured fat bodies from third instar larvae using an established protocol (32). Fat bodies were dissected and cultured and then treated with 1 μg/ml of insulin for 15 min. We then evaluated the response in the insulin-signaling pathway using Western blot analysis with a p-Akt antibody. Under basal conditions (Fig. 5C, Insulin (−)), p-Akt was not detectable in any of the strains, although equivalent amounts of Akt were present as demonstrated using a pan-Akt antibody (Fig. 5C, Akt). In the control strain, insulin dramatically increased the level of p-Akt. However, the level of p-Akt was significantly less when either Ogt or Oga were knocked down in the IPCs (−41 and −42%, respectively) (Fig. 5C, Insulin (+)). Intriguingly, both elevation of DILPs by Oga knockdown and reduction in DILPs by Ogt knockdown lead to a reduction in insulin responsiveness in this target tissue. These results confirm that modulating O-GlcNAc cycling in the IPCs diminishes the acute response to insulin in the peripheral tissues.
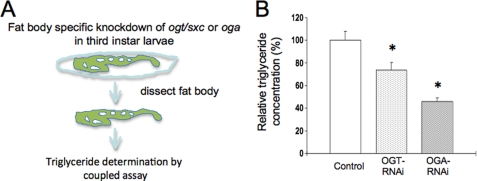
Previously, we have demonstrated that disrupted O-GlcNAc cycling alters insulin signaling in the worm and mouse (13, 19, 20). This phenomenon has also been observed in tissue culture models of insulin resistance. To more directly examine the role of O-GlcNAc cycling in peripheral tissues of the fly, we directed Oga and Ogt knockdown in the fat body using the Lsp2-GAL4 fat body-specific driver strain and examined neutral lipid storage (Fig. 6A). As shown in Fig. 6B, knockdown of either enzyme of O-GlcNAc cycling dramatically reduced the accumulation of neutral lipids in the fat body. Accompanying these macronutrient storage changes were alterations in the levels of several lipogenic and lipolytic enzymes including acetyl-CoA carboxylase, fatty acid synthase, lipase 4, and carnitine palmitoyltransferase I (supplemental Figs. S1 and S2). These transcriptional changes differed between starved (supplemental Fig. S1) and fed flies (supplemental Fig. S2), with Ogt knockdown having a greater effect in starvation, and the Oga knockdown producing more striking effects upon feeding. Taken together, these findings suggest that O-GlcNAc plays a role in mediating the response to nutrient flux in a peripheral tissue involved in fat storage; this function may complement its role in regulating insulin production in IPCs in response to nutrients. This suggests that deregulation of O-GlcNAc cycling could contribute to both altered insulin production and insulin signaling in peripheral tissues in a manner reminiscent of mammalian insulin resistance.
FIGURE 6.
Tissue-specific knockdown of ogt/sxc or oga alters lipid accumulation in the fat body. A, schematic representation of experimental design where third instar larval fat bodies were dissected from Lsp2GAL4/+;UAS-dOGTRNAi/+ (OGT-RNAi), Lsp2GAL4/+;UAS-dOGARNAi/+ (OGA-RNAi), or control strains and the triglyceride content measured by a coupled assay. B, triglyceride content is expressed as a relative percentage to the control strain.
DISCUSSION
Here we show that targeted disruption of O-GlcNAc cycling in the IPCs modulates the production of the DILPs. These perturbations are associated with profound changes in body size and growth, as well as insulin sensing in target tissue. These phenotypes suggest that IPCs are acutely sensitive to alterations in O-GlcNAc cycling.
To date, a variety of model systems have been examined with regard to the physiological role of O-GlcNAc cycling. In plants, the two OGT homologs Spindly and Secret Agent appear to play key roles in development (5, 6). The OGT knock-out in mice produces stem cell and embryonic lethality; the conditional knock-outs survive only until embryonic day 5.5 (16). We have previously shown that C. elegans null alleles of OGT and OGA were viable and fertile with changes in insulin-like signaling (19, 20). More recently we have used these mutants to evaluate the dynamic cycling of O-GlcNAc at promoters of genes involved in longevity, the stress response, and pathogen resistance. These genes become deregulated in the mutants leading to changes in longevity, dauer, and stress (37). The differences observed between the phenotypes in mammals and nematodes are likely due to the importance of O-GlcNAc cycling in early development. Thus, it has become important to examine the role of O-GlcNAc cycling more broadly, to define its role as a nutrient-sensing post-translational modification.
O-GlcNAc Cycling in Drosophila
In Drosophila, ogt/Sxc (CG10392) and oga (CG5871) are highly similar to the mammalian ncOGT and OGA genes, respectively. We have previously examined the results of Ogt/Sxc knockdown in Drosophila S2 cells (22). In addition, recent studies have linked Ogt/Sxc to the regulation of homeotic gene expression in Drosophila (23, 24). Therefore, Drosophila has emerged as a genetically amenable and convenient model system for evaluating the physiological role of O-GlcNAc.
In our current studies, perturbation of Drosophila O-GlcNAc cycling in IPCs by GAL4+UAS-RNAi results in the deregulation of Dilp production and increases in circulating carbohydrate levels in the hemolymph. These changes are not unlike the phenotypes of diabetes mellitus in mammals. We demonstrated that fly strains were still viable even with the targeted overexpression of Ogt or the knockdown of Ogt or Oga in DILP2-expressing cells. The generation of viable transgenic Drosophila strains overexpressing or depleting Ogt or Oga activity in discrete tissues allows a direct examination of the role that the O-GlcNAc modification may play in the metabolism and physiology of the fly.
O-GlcNAc Cycling Modulates Insulin Production in Drosophila Insulin-producing Cells
Previous studies suggested that in mammalian pancreatic β-cells, insulin expression and secretion might be linked to the hexosamine-signaling pathway. Transgenic mice overexpressing glutamine:fructose-6-phosphate amidotransferase, the rate-limiting enzyme for hexosamine-signaling pathway, in the pancreas exhibit increased insulin content in pancreatic islets and associated hyperinsulinemia (38). It has been shown that glucose-induced transient hyperglycemia in rats resulted in a corresponding transient increase in O-GlcNAc protein levels in pancreatic β-cells (39). Moreover, in the MIN6 β-cell line, depletion of OGT using siRNA markedly inhibited glucose-induced insulin release (40). These reports strongly suggest that glucose-stimulated insulin production may be modulated by the O-GlcNAc modification. The Drosophila genome contains seven dilp genes. Previous studies have shown that the expression of UAS-rpr by the IPCs-specific driver, dILP2-GAL4, decreased dilp2, dilp3, and dilp5 gene expression (28, 33). Additionally, deletion of dilps1–5 decreased body size and elevated carbohydrate levels (30). In our study, targeting only Ogt or Oga by RNAi modulated dilp2, dilp3, and dilp5 gene expression and altered body size, without overt changes in IPC viability. These results support the notion that O-GlcNAc modification is a key modulator for DILP production and release by Drosophila IPCs.
Deregulation of Carbohydrate Metabolism: The Fly as a Model of Glucose Toxicity?
The Drosophila fat body is responsible for the uptake of hemolymph carbohydrate and for modulating energy storage (32). Previous reports have shown that either ablation of IPCs or deletion of dilps in Drosophila induces elevated carbohydrate levels in the hemolymph (28–30). In mammals, insulin regulates the blood glucose level via translocation of GLUT4, the glucose transporter, to the plasma membrane in the insulin-responsive tissues. This mechanism is mediated through the PI3-kinase/Akt signaling pathway. The insulin-signaling pathway in Drosophila is highly conserved and similar to the mammalian insulin-signaling pathway (25, 31). PI3-kinase/Akt signaling in Drosophila peripheral tissues is required for growth. Overexpression of PTEN, the negative regulator of PI3-kinase, inhibited fly body size (41).
The role of O-GlcNAc cycling in insulin-glucose-homeostasis is more complex. In our studies, knockdown of Oga in the IPCs increased the endogenous activation of Akt in vivo; this was expected to occur given the elevation of insulin expression and increased body size. However, this strain also showed increased hemolymph carbohydrate levels similar to that seen with knockdown of Ogt in the IPCs. Both knockdown of Ogt and of Oga in the IPCs diminished the activation of Akt by extraneous acute insulin administration in the ex vivo cultured fat body. Although the mechanism of induction of this altered insulin response in the Drosophila peripheral tissues is not clear, these results suggest that both strains exhibit a reduction in the responsiveness of the insulin-signaling pathway. This pathway is known to be required for glucose uptake into peripheral tissues, such as the fat body.
Relationship between Fly and Mammalian Glucose Homeostasis
Diabetes in mammals involves altered insulin secretion in the pancreatic β-cell and peripheral insulin resistance. In mammals, insulin resistance causes impaired glucose tolerance and hyperglycemia because of reduction of glucose uptake via GLUT4 translocation mediated through impairment PI3-kinase/Akt signaling pathway (42, 43). Hyperglycemia is responsible for insulin resistance, mediated by several factors, such as oxidative stress, cytokine activation, and mitochondrial dysfunction. Some reports suggest that sustained hyperinsulinemia may also lead to insulin resistance (44, 45). Pirola et al. (46) reported that prolonged insulin treatment on L6 myoblasts desensitized PI3-kinase and insulin-induced glucose uptake. Egawa et al. (47) reported that persistent activation of PI3-kinase leads to desensitization of insulin action in 3T3-L1 adipocytes. Hyperglycemia then causes compensation of insulin production and deterioration of insulin resistance, ultimately leading to deregulation of insulin production. This vicious circle is known as glucose toxicity, which is essential in the progression of type 2 diabetes (36). Our results suggest that inhibition of O-GlcNAc levels by knockdown of Ogt in the IPCs induces hyperglycemia because of impairment of insulin production. Continuous increases in O-GlcNAcylation by knockdown of Oga in the IPCs mimic the compensation of insulin production for chronic hyperglycemic phenomena related to glucose toxicity.
Summary and Conclusions
In human genetic studies, a single nucleotide polymorphism in intron 10 of the MEGA5 gene encoding OGA is associated with diabetes mellitus and age of onset in the Mexican-American population. This polymorphism may interfere with OGA activity in the susceptible population (14). This report strongly suggests a possible relationship between polymorphisms in the human OGA gene and pancreatic β-cell function and insulin resistance in the peripheral tissues.
In our studies, deregulation of O-GlcNAc cycling in Drosophila insulin-producing cells produces a phenotype with striking parallels to human diabetes. The enzymes of O-GlcNAc cycling appear to act by “fine-tuning” the insulin production systems, providing additional levels of metabolic control in response to nutrient availability. This fine-tuning mechanism is likely to apply to other proposed functions of O-GlcNAc cycling in Drosophila, which include insulin signaling-regulated metabolic modulation in the fat body and other peripheral tissues. We found that specific knockdown of both Ogt and Oga in the fat body altered triglyceride levels (Fig. 6). This was associated with changes in gene expression of a number of lipolytic and lipogenic enzymes. These findings mirror observations we have previously made with null alleles of the enzymes of O-GlcNAc cycling in C. elegans (19, 20, 37). We conclude that the insect may be a very useful model for examining the production of insulin-like peptides in the insulin-producing cells induced by nutrient-sensitive perturbations in O-GlcNAc cycling. Alterations in this cycling may result in glucose toxicity and altered responsiveness of peripheral tissues to the normal metabolic regulation by insulin.
Supplementary Material
Acknowledgment
We thank Dr. Eric Rulifson for providing dILP2GAL4 line and DILP antiserum.
This work was supported, in whole or in part, by the National Institutes of Health NIDDK Intramural Research Program. This work was also supported by a scholarship from the Cell Science Research Foundation in Japan and the Manpei Suzuki Diabetes Foundation in Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1 and S2.
- O-GlcNAc
- O-linked GlcNAc
- OGT
- O-GlcNAc transferase
- OGA
- O-GlcNAcase
- IPC
- insulin-producing cell
- p-Akt
- phospho-Akt
- PI
- phosphatidylinositol.
REFERENCES
- 1.Hanover J. A. (2001) FASEB J. 15, 1865–1876 [DOI] [PubMed] [Google Scholar]
- 2.Love D. C., Hanover J. A. (2005) Sci. STKE re13. [DOI] [PubMed] [Google Scholar]
- 3.Lubas W. A., Frank D. W., Krause M., Hanover J. A. (1997) J. Biol. Chem. 272, 9316–9324 [DOI] [PubMed] [Google Scholar]
- 4.Kreppel L. K., Blomberg M. A., Hart G. W. (1997) J. Biol. Chem. 272, 9308–9315 [DOI] [PubMed] [Google Scholar]
- 5.Hartweck L. M., Scott C. L., Olszewski N. E. (2002) Genetics 161, 1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornton T. M., Swain S. M., Olszewski N. E. (1999) Trends Plant Sci. 4, 424–428 [DOI] [PubMed] [Google Scholar]
- 7.Heckel D., Comtesse N., Brass N., Blin N., Zang K. D., Meese E. (1998) Hum. Mol. Genet 7, 1859–1872 [DOI] [PubMed] [Google Scholar]
- 8.Toleman C. A., Paterson A. J., Kudlow J. E. (2006) J. Biol. Chem. 281, 3918–3925 [DOI] [PubMed] [Google Scholar]
- 9.Comtesse N., Maldener E., Meese E. (2001) Biochem. Biophys. Res. Commun. 283, 634–640 [DOI] [PubMed] [Google Scholar]
- 10.Toleman C., Paterson A. J., Whisenhunt T. R., Kudlow J. E. (2004) J. Biol. Chem. 279, 53665–53673 [DOI] [PubMed] [Google Scholar]
- 11.Wells L., Vosseller K., Hart G. W. (2001) Science 291, 2376–2378 [DOI] [PubMed] [Google Scholar]
- 12.Vosseller K., Wells L., Lane M. D., Hart G. W. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 5313–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClain D. A., Lubas W. A., Cooksey R. C., Hazel M., Parker G. J., Love D. C., Hanover J. A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 10695–10699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehman D. M., Fu D. J., Freeman A. B., Hunt K. J., Leach R. J., Johnson-Pais T., Hamlington J., Dyer T. D., Arya R., Abboud H., Göring H. H., Duggirala R., Blangero J., Konrad R. J., Stern M. P. (2005) Diabetes 54, 1214–1221 [DOI] [PubMed] [Google Scholar]
- 15.Hanover J. A., Lai Z., Lee G., Lubas W. A., Sato S. M. (1999) Arch Biochem. Biophys. 362, 38–45 [DOI] [PubMed] [Google Scholar]
- 16.Shafi R., Iyer S. P., Ellies L. G., O'Donnell N., Marek K. W., Chui D., Hart G. W., Marth J. D. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5735–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Donnell N., Zachara N. E., Hart G. W., Marth J. D. (2004) Mol. Cell. Biol. 24, 1680–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slawson C., Zachara N. E., Vosseller K., Cheung W. D., Lane M. D., Hart G. W. (2005) J. Biol. Chem. 280, 32944–32956 [DOI] [PubMed] [Google Scholar]
- 19.Hanover J. A., Forsythe M. E., Hennessey P. T., Brodigan T. M., Love D. C., Ashwell G., Krause M. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 11266–11271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsythe M. E., Love D. C., Lazarus B. D., Kim E. J., Prinz W. A., Ashwell G., Krause M. W., Hanover J. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11952–11957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly W. G., Hart G. W. (1989) Cell 57, 243–251 [DOI] [PubMed] [Google Scholar]
- 22.Jínek M., Rehwinkel J., Lazarus B. D., Izaurralde E., Hanover J. A., Conti E. (2004) Nat. Struct. Mol. Biol. 11, 1001–1007 [DOI] [PubMed] [Google Scholar]
- 23.Gambetta M. C., Oktaba K., Müller J. (2009) Science 325, 93–96 [DOI] [PubMed] [Google Scholar]
- 24.Sinclair D. A., Syrzycka M., Macauley M. S., Rastgardani T., Komljenovic I., Vocadlo D. J., Brock H. W., Honda B. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 13427–13432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E. (2001) Curr. Biol. 11, 213–221 [DOI] [PubMed] [Google Scholar]
- 26.Tatar M., Bartke A., Antebi A. (2003) Science 299, 1346–1351 [DOI] [PubMed] [Google Scholar]
- 27.Cao C., Brown M. R. (2001) Cell Tissue Res. 304, 317–321 [DOI] [PubMed] [Google Scholar]
- 28.Rulifson E. J., Kim S. K., Nusse R. (2002) Science 296, 1118–1120 [DOI] [PubMed] [Google Scholar]
- 29.Broughton S. J., Piper M. D., Ikeya T., Bass T. M., Jacobson J., Driege Y., Martinez P., Hafen E., Withers D. J., Leevers S. J., Partridge L. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3105–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H., Liu J., Li C. R., Momen B., Kohanski R. A., Pick L. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 19617–19622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giannakou M. E., Goss M., Jünger M. A., Hafen E., Leevers S. J., Partridge L. (2004) Science 305, 361. [DOI] [PubMed] [Google Scholar]
- 32.Géminard C., Rulifson E. J., Léopold P. (2009) Cell Metab. 10, 199–207 [DOI] [PubMed] [Google Scholar]
- 33.Ikeya T., Galic M., Belawat P., Nairz K., Hafen E. (2002) Curr. Biol. 12, 1293–1300 [DOI] [PubMed] [Google Scholar]
- 34.Teleman A. A. (2010) Biochem. J. 425, 13–26 [DOI] [PubMed] [Google Scholar]
- 35.Lizcano J. M., Alrubaie S., Kieloch A., Deak M., Leevers S. J., Alessi D. R. (2003) Biochem. J. 374, 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Rossetti L., Giaccari A., DeFronzo R. A. (1990) Diabetes Care 13, 610–630 [DOI] [PubMed] [Google Scholar]
- 37.Love D. C., Ghosh S., Mondoux M. A., Fukushige T., Wang P., Wilson M. A., Iser W. B., Wolkow C. A., Krause M. W., Hanover J. A. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 7413–7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang J., Neidigh J. L., Cooksey R. C., McClain D. A. (2000) Diabetes 49, 1492–1499 [DOI] [PubMed] [Google Scholar]
- 39.Konrad R. J., Janowski K. M., Kudlow J. E. (2000) Biochem. Biophys. Res. Commun. 267, 26–32 [DOI] [PubMed] [Google Scholar]
- 40.Andrali S. S., Qian Q., Ozcan S. (2007) J. Biol. Chem. 282, 15589–15596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goberdhan D. C., Paricio N., Goodman E. C., Mlodzik M., Wilson C. (1999) Genes Dev. 13, 3244–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zierath J. R., Krook A., Wallberg-Henriksson H. (2000) Diabetologia 43, 821–835 [DOI] [PubMed] [Google Scholar]
- 43.Garvey W. T., Maianu L., Zhu J. H., Brechtel-Hook G., Wallace P., Baron A. D. (1998) J. Clin. Invest. 101, 2377–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Del Prato S., Leonetti F., Simonson D. C., Sheehan P., Matsuda M., DeFronzo R. A. (1994) Diabetologia 37, 1025–1035 [DOI] [PubMed] [Google Scholar]
- 45.Miles P. D., Li S., Hart M., Romeo O., Cheng J., Cohen A., Raafat K., Moossa A. R., Olefsky J. M. (1998) J. Clin. Invest. 101, 202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pirola L., Bonnafous S., Johnston A. M., Chaussade C., Portis F., Van Obberghen E. L. (2003) J. Biol. Chem. 278, 15641–15651 [DOI] [PubMed] [Google Scholar]
- 47.Egawa K., Sharma P. M., Nakashima N., Huang Y., Huver E., Boss G. R., Olefsky J. M. (1999) J. Biol. Chem. 274, 14306–14314 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.