Abstract
Alarm calling is common in many species. A prevalent assumption is that calling puts the vocalizing individual at increased risk of predation. If calling is indeed costly, we need special explanations for its evolution and maintenance. In some, but not all species, callers vocalize away from safety and thus may be exposed to an increased risk of predation. However, for species that emit bouts with one or a few calls, it is often difficult to identify the caller and find the precise location where a call was produced. We analyzed the spatial dynamics of yellow-bellied marmot (Marmota flaviventris) alarm calling using an acoustic localization system to determine the location from which calls were emitted. Marmots almost always called from positions close to the safety of their burrows, and, if they produced more than one alarm call, tended to end their calling bouts closer to safety than they started them. These results suggest that for this species, potential increased predation risk from alarm calling is greatly mitigated and indeed calling may have limited predation costs.
Keywords: alarm communication, acoustic localization, yellow-bellied marmot, predation risk
Warning conspecifics with alarm calls is a common behavior in social animals. It is generally thought that alarm calls attract the attention of predators and thereby increase risks (e.g., Maynard-Smith 1965). These risks are offset by corresponding benefits, such as warning relatives, discouraging predation, or creating an opportunity to escape (Blumstein 2007). However, evidence that callers actually increase their exposure to predators is limited; great gerbils (Rhombomys optimus, Randall et al. 2000); black-tailed prairie dogs (Cynomys ludovicianus, Hoogland 1995), and yellow-bellied marmots (Marmota flaviventris, Blumstein et al. 1997) appear to call mostly from positions of safety. If callers incur only minimal costs, then it is doubtful that alarm calling requires reciprocal altruism or indirect kin selection to explain its evolution or maintenance. Alarm calls may function to discourage or distract predators (Sherman 1977), and calls emitted primarily by individuals who are unlikely to be successful targets of predation are consistent with these functions. Indeed, in sciurid rodents, calling apparently evolved to communicate to predators, not conspecifics, and later conspecific functions of calling are exapted from this initial function (Shelley & Blumstein 2005).
The location of calling individuals is one of several important factors in determining the risk associated with alarm calling behavior. If animals call from positions of safety, any risks of calling may be reduced. For instance, Belding's ground squirrels (Spermophilus beldingi) scurry to cover before emitting alarm calls in response to high-risk aerial predators (Sherman 1985). However, quantifying the location of callers with respect to safe locations is challenging in some species since callers vocalize infrequently, often emit only a single alarm call, and may move after calling. This makes it difficult to identify, with certainty, the calling individual when there are multiple potential callers in the area. Further, alarm calls often have acoustic features, such as narrow bandwidth and short duration, which makes them difficult to localize (Marler 1955). We addressed these problems by using an array of wireless acoustic sensors to localize the alarm calls of yellow-bellied marmots and to analyze the spatial dynamics of calling bouts with respect to the location of burrows.
Methods
Study site and array deployment
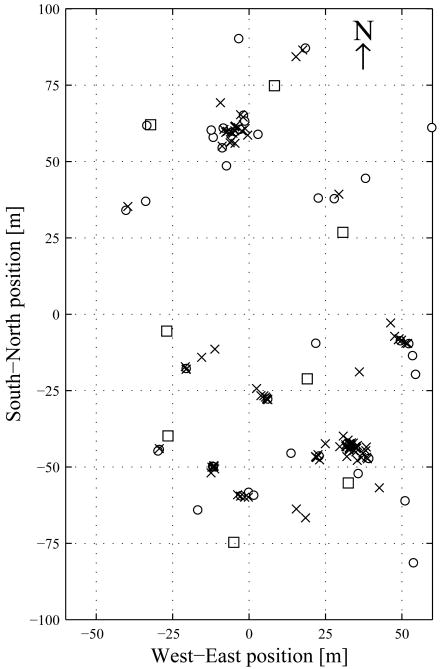
An array of eight VoxNet wireless sensor nodes (Ali et al. 2007; Allen et al. 2008) was established at ‘Marmot Meadow’ (figure 1; 38.9784 N, 106.9999 W), a long-term study site near the Rocky Mountain Biological Laboratory, Gothic, Colorado, USA. The yellow-bellied marmots at this site have been studied annually since 1962 (Armitage 1991). Yellow-bellied marmots are sciurid rodents with male dispersal who form colonies that contain one or a few matrilines, and typically a single territorial adult male (Arimtage 1991). During our study, all 28 marmots at the site were individually-marked (adults: 3♀ & 3♂, yearlings: 3♀ & 1♂, juveniles: 6♀ & 12♂); of these, we recorded calls from at least 15 different individuals (adults: 3♀ & 1♂, yearlings: 3♀ & 1♂, juveniles: 4♀ & 3♂).
Figure 1.
Overview of the acoustic array deployment. Sensor nodes are shown by □. Positions of safety are marked by ○. For each of the 98 bouts, the location of the first call is indicated with ×.
The monitored area (approximately 85 m × 150 m) contained two large burrow complexes consisting of numerous individual burrow openings. The area of the minimum convex hull enclosing the sensor nodes was 7483 m2, resulting in a deployment density (Collier et al. 2010) of 10.7 nodes/ha. Each sensor node consisted of four microphones, arranged in a 17 cm tetrahedron, that recorded sound as 16 bit, 48 kHz PCM files. Nodes were positioned on tripods approximately 1.5 m above the ground. A total of 21.8 h of recordings made from 1-11 August 2008 were analyzed. All recordings were band-pass filtered (2.5 to 5 kHz) to exclude sounds outside the range of the dominant frequencies of marmot calls.
The system used an acoustic self-survey system (Girod et al. 2006) to determine the location and orientation of each node. Each node emits a 0.11 second ‘ranging chirp’ sound that is detected by the other nodes. Range and bearing measurements between nodes from repeated cycles of ranging chirps are then used to solve for node positions and orientations using a multilateration algorithm. The locations of burrows and acoustic nodes were also surveyed using a Trimble GeoXT GPS, with 0.3 m horizontal accuracy.
We analyzed both naturally elicited bouts of calling and bouts elicited experimentally by revealing a taxidermy mounted badger (Taxidea taxus) 3 times, eliciting 3 bouts, and by running towards the marmots 15 times, eliciting 29 bouts. We only attempted to elicit calls when marmots were apparently relaxed with multiple individuals observed foraging far away from burrows to maximize the possibility of triggering calls away from positions of safety. Predators and other animals which naturally elicited observed calls included mule deer (Odocoileus hemionus), coyotes (Canis latrans), and red foxes (Vulpes vulpes), though in most cases neither the stimulus nor the caller were positively identified. Both acoustic array deployment and field observations were conducted by a single researcher.
Acoustic localization
Analysis had four stages: identifying alarm calls, localizing calls, assigning calls to bouts, and analysis of bouts. Identifying alarm calls was partially automated using a simple ‘power in band’ detector (Ali et al. 2007). The detector was run on a single channel from the 4 nodes identified in figure 2. Detected events were then individually scrutinized and erroneous or duplicate hits were removed. All calls noted by field observation as well as many which were missed were identified by the detector, including calls from another colony site ca. 500 m away across the valley.
Figure 2.
Pseudo-likelihood map produced by the first pass of the CES localization method for the marmot alarm call shown in figure 3. Sensor nodes are shown by □, with ■ denoting sensors used for initial call detection.
To determine the position each call was emitted from, we developed the correlation envelope sum (CES) acoustic localization method. This time-difference-of-arrival method calculates the most likely source location by summing the values of the Hilbert envelope (Bracewell & Kahn 1966; Feldman 1994) of the cross-correlation functions for each pairs of sensor at each candidate source point. CES is based upon the formally defined accumulated correlation method (Birchfield 2004), though differs in that the cross-correlation functions are temporally smoothed by taking their envelopes to allow a more efficient maximization search and achieve results which are more robust to noise and reverberations.
CES starts by computing the cross-correlation between the signal segment identified by detection and the recordings from all other microphones. The recording segment with the highest peak cross-correlation above a threshold value (0.75) is temporally masked by taking the time segment corresponding to the cross-correlation peak and the duration of the detected event. This masked segment is then cross-correlated with all recordings excluding the original key sensor. This process is repeated until no above threshold segment remains or the cross-correlations for all pairs of microphones are computed. This procedure is similar to the method employed by Mennill et al. (2006). Cross-correlations between microphones on the same nodes are discarded. For our eight nodes with four microphones each, a total of 448 cross-correlation functions are possible for each event; however the 0.75 threshold results in the number of cross-correlations used varying from event to event. The mean (±SE) number of cross-correlations used to localize the 2119 marmot alarm calls analyzed was 326.9±2.5 (median=364; mode=400).
The likelihood that the source (call) came from a given point in space is the sum of each cross-correlation envelop at the delay corresponding to the difference in distance between those sensors and the point. A two pass ‘brute force’ search method was used to locate the candidate source point with the maximum CES value. The first pass was over the 2D lattice covering the entire study area at a 0.5 m resolution (see figures 2 and 3). A 0.01 m resolution refinement pass was done over a 2×2 m lattice centered on the maximum value point from the first pass. The point with the maximum CES value in the refinement pass was taken as the final source localization result. All distances were computed in 3D, though the search was conducted over a 2D surface. The terrain was dominated by an upward slope from west to east. The elevation (Z) values used are 1.25 m below a surface fit to the self-survey locations of the nodes to approximate the typical calling height of a marmot.
Figure 3.
Time aligned spectrogram of the marmot alarm call from one channel of each node. The time of detection on the first channel of node 3 is set to 0. Call duration is 0.51 s. This is the same call localized in figure 2.
Bout assignment and analysis
A bout of alarm calling is used as the primary level of analysis for this study. We defined a bout as all the alarm calls produced by a single individual in response to a single stimulus. Calls that occurred within both two minutes and five meters of the preceding call were initially classified as belonging to the same bout. Initial bout assignments were verified by hand to detect erroneous groupings of calls from different individuals or failure to group calls from the same individual. Criteria included: detailed field notes taken during recording; a consideration of spectrogram cross-correlation values; and comparison of calls both visually and aurally.
Bouts are an appropriate level of analysis since each is a distinct instance of a marmot reacting to a perceived stimulus. However, potential pseudoreplication issues exist when inferring individual level behavior from bout level analysis. When possible, we address this by also analyzing a single bout per individual from the 19 bouts where the individual calling was positively identified. This set of 15 bouts from different individuals is referred to as the IDed bouts. Unfortunately, the power at this level of analysis is limited since attributing a bout to an individual was not possible in most cases using the data set and observations available. Ideally, a classification method such as discriminant function analysis could be used to identify individuals from the recordings themselves (Blumstein & Munos 2005), but sub-optimal recording conditions and an insufficient set of positively identified calls from all animals at the site made the development of such a system infeasible in this case.
Bouts were analyzed according to the number of calls, the positions the first and last calls were emitted from, and the maximum distance between successive calls. We assumed that marmots were safe when in close proximity to their burrows (Blumstein 2007). For each bout, the closest burrows to the first call and to the last call were identified, and the distances between those points and the locations of the calls were computed. Additionally, for each bout, the centroid of calls and distance of each call from the centroid were determined to gauge the precision of localization results.
All analyses employed Matlab (v. 6.0; Mathworks, Inc.) or Octave (v. 3.2.0; www.octave.org). R (v. 2.8.1; www.R-project.org) was used for two-tailed statistical tests with our alpha set to 0.05.
Results
Accuracy of acoustic localization
Even under greatly simplified assumptions, the accuracy of acoustic localization is a complex function that depends on the geometric relationship between the sensor positions and the source location (Chen et al. 2002). For this study, determining precise accuracy estimates is not critical. Excess errors would tend to bias results towards larger distance measures, and short distances between localized calls and surveyed locations support our conclusions. However, the acoustic localization system used here has previously demonstrated sub-meter accuracy in field conditions (e.g., neo-tropical rainforest) for sources (e.g., antbirds) located within the array (Ali et al. 2007; Collier et al. 2010). The accuracy of localization is degraded for sources outside the convex hull of the array, with more distant sources generally being worse. Some calls outside of the array were included in this study, but these were close and general accuracy was not compromised.
Detailed observations of longer calling bouts confirmed the accuracy of localizations. If the caller did not move during a multiple call bout, the distance of calls from the bout centroid is a measure of the localization precision. For the 36 multi-call bouts where animals were observed to be stationary, the mean (±SE) of this value was 0.477±0.013 m (n=1973 calls).
Number of calls per bout
We recorded 98 bouts comprising a total of 2119 alarm calls. The majority of bouts consisted of a single call (figure 4a; 50/98 bouts) and came from unidentified callers. Intentionally presented stimuli provoked fewer calls per bout (x̅=4.75±3.05; n=32) than did naturally occurring stimuli (x̅=29.80±8.79; n=66) (Mann-Whitney test: n=32,66, W=700.5, p=0.004, CI95=(-5, -5×10-4)). Possibly this was because disturbances to intentionally elicited calls were, by design, brief. The set of IDed bouts is skewed towards more calls due to the difficulty observing the caller in shorter bouts (x̅=91.60±33.28; n=15) (Mann-Whitney test: n=15,83, W=939, p=8.1×10-4, CI95=(4.0,86.0)). Only 4 out of these 15 bouts consist of a single call.
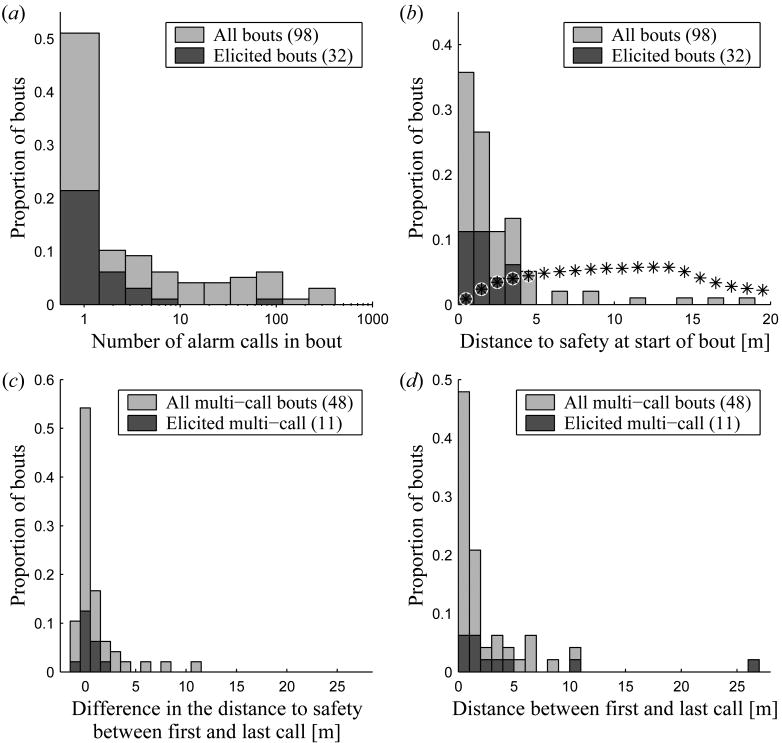
Figure 4.
Measures on bouts include: (a) the number of calls per bouts, (b) the distance to the closest point of safety from the first call of each bout, (c) the difference in distance to safety between the first and last calls, and (d) the distance between the first and last calls. The distribution of distances between a set of random points within the area marmots called from and the closest burrow to each point is shown in (b) by *.
‘Elicited’ bouts were in response to intentionally presented stimuli. Figures (c) and (d) are based on the subset of bouts with more than one call.
Distance to safety
The distance from the position of the first call of each bout to the nearest burrow is shown in figure 4b (x̅=2.48±0.25 m; median=1.46 m; n=98). For comparison, the distribution of distances between a set of random points from within the minimum convex hull containing all first call positions and the closest burrow is also shown in figure 4b (x̅=12.43±0.086 m; median=11.57 m; n=6.8×105). Marmots typically run between 3-5 m/s (Blumstein et al. 2004), and most bouts were started from safe positions from which a marmot would take less than 1 s to reach a burrow. Only 8/98 bouts had first call locations more than 5 m from a burrow (figure 4b).
Despite a conscious effort to elicit alarm calls when marmots were relatively far from their burrows, intentionally elicited and natural bouts were not initiated at significantly different distances from safety (figure 4b; Mann-Whitney test: n=32,66, W=967, p=0.502). Anecdotally, marmots were twice observed retreating to a burrow and then calling when experimentally alarmed. Bouts with multiple calls did not differ significantly from single call bouts with respect to burrow proximity (Mann-Whitney test: n=48,50, W=1104, p=0.497). IDed bouts seem to start closer to the nearest burrow (x̅=1.33±0.25 m; median=1.56 m; n=15) than the non-IDed bouts, but the difference is not statistically significant (Mann-Whitney test: n=15,83, W=470, p=0.134, CI95=(-1.29,0.15)).
Movement within bouts
We analyzed the overall pattern of movement for bouts of more than one call. The first call of a multi-call bout was more likely to be farther from a burrow than the final call (figure 4c; Wilcoxon paired signed rank test: n=48, V=801, p=0.029, CI95=(0.04, 0.74)). However, IDed multi-call bouts did not show a statistically significant difference between the distance of the first and last call to the nearest burrow (Wilcoxon paired signed rank test: n=11, V=29, p=0.76, CI95=(-0.28, 0.70)), but the power of this test was quite low due to sample size.
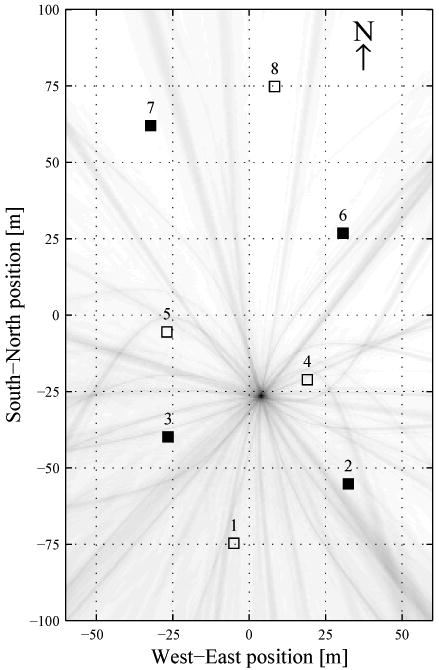
Most bouts were initiated near to a burrow and were relatively stationary, with 40 of the 48 multi-call bouts having <5 m between their first and last calls (figure 4d). For the 8 bouts with >5 m distance between the first and last calls (figure 5), an average of 94.2% (±1.4%) of that distance moved was directly towards the burrow closest to the final call. The identities of the marmots are unknown for these bouts, but they come from a minimum of 3 individuals. All 8 bouts consisted of 3 or more calls (median 6.5 calls). This allowed us to explore whether the marmots were calling while moving or calling, moving, and resuming calling once reaching their destination. Seven of the 8 bouts clearly showed the latter, with the maximum distance between two successive calls accounting for an average of 91.5% (±5.2%) of the overall distance moved. A marmot emitting one extremely rapid bout called while running; 8 calls were emitted in 3.4 s and were spaced over 11 m.
Figure 5.
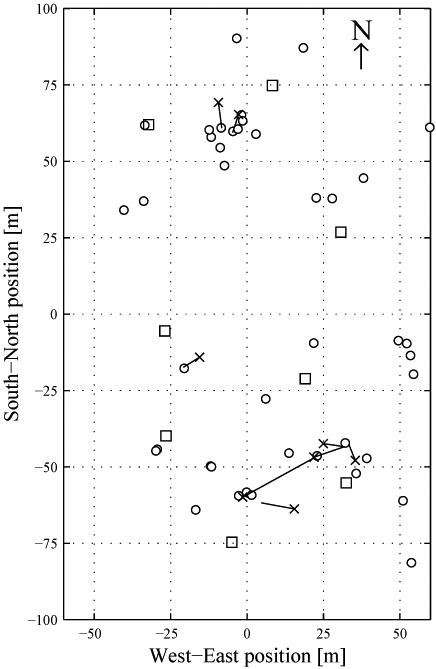
The 8 bouts with >5 m distance between the positions of the first and last call. Sensor nodes are shown by □. Positions of safety are marked by ○. The location of the first call of each bout is indicated with ×, and a line extends to the position of the last call.
Discussion
We found that yellow-bellied marmots primarily called from positions of safety (their burrows) and in those rare instances where they first called more than a second or so from a burrow, almost always moved towards their burrows after calling. The use of an acoustic array was both novel and essential to study this question accurately because most bouts consisted of only a single call, making them difficult to accurately locate using traditional methods since they are over before they are ever heard. Also, a single stimulus often elicits an alarm calling response from multiple marmots, resulting in many overlapping bouts that are difficult to accurately tease apart with normal field observations.
Acoustic localization by itself has significant limitations. Although traditional field observations were conducted while recording for this study, it was not possible to identify the calling marmot in most cases so individual level statistics were not possible. In the future, coupling localization with an automated classification system should be possible since marmot alarm calls contain individual, age, and sex specific information (Blumstein & Munos 2005). Additional acoustic factors such directionality (Yorzinski & Patricelli 2010) and call structure (Wilson & Hare 2006) may also be analyzed from acoustic array recordings.
This ALS, coupled with an automated classification system could also be used to better quantify the distribution of calling and identify factors that influence it with greater precision. For instance, it could be used to better understand the seasonal variation in alarm calling, how reproductive state influences calling, as well as how the presence of specific individuals (such as kin) influences calling. Thus, this technology could be used to generate a more comprehensive understanding of the benefits of calling.
Our results suggest that yellow-bellied marmots that emit alarm calls face little increased risk of predation while doing so because they call from positions of safety. Since calls are brief (20-45 ms; Blumstein & Munos 2005), and rarely emitted (Blumstein et al. 1997), they are likely to have inconsequential metabolic costs. Calling may have an opportunity cost, but most of this cost is likely born by the receiver, not the signaler because marmots that hear alarm calls typically interrupt their current activities to look around for a potential predator. Marmots have reduced this opportunity cost by assessing the reliability of individual callers and responding appropriately (Blumstein et al. 2004).
If alarm calling imposes little direct cost for the caller, the question of interest becomes why more animals do not call. Female marmots with pups above ground call more frequently (Blumstein et al. 1997), suggesting calls serve to protect vulnerable offspring. However, competition among even closely related marmots, including infanticide by females, has also been documented (Brody & Melcher 1985; Armitage 1986). Even if alarm calling is not dangerous, marmots may be reluctant to help conspecific competitors. While the localization of marmot alarm calls alone is insufficient to address this question, it is our hope that these results will be used to augment longer-term studies.
Acknowledgments
Research was conducted under permits from the Rocky Mountain Biological Laboratory, the UCLA Animal Research Committee, and the Colorado Division of Wildlife. We thank the NSF (award numbers 0410438 and 0754247), the National Geographic Society, and the UCLA Faculty Senate for support. We thank two anonymous reviewers for their very constructive comments.
Literature Cited
- Ali AM, Yao K, Collier TC, Taylor CE, Blumstein DT, Girod L. An empirical study of collaborative acoustic source localization. IPSN '07: Proceedings of the 6th international conference on information processing in sensor networks; New York, NY, USA. 2007. pp. 41–50. [Google Scholar]
- Allen M, Girod L, Newton R, Madden S, Blumstein DT, Estrin D. Voxnet: An interactive, rapidly-deployable acoustic monitoring platform. IPSN '08: Proceedings of the 7th international conference on information processing in sensor networks; Washington, DC, USA. 2008. pp. 371–382. [Google Scholar]
- Armitage KB. Marmot polygyny revisited: Determinants of male and female reproductive strategies. In: Rubenstein DS, Wrangham RW, editors. Ecological Aspects of Social Evolution. Princeton University Press; Princeton, NJ, USA: 1986. pp. 303–331. [Google Scholar]
- Armitage KB. Social and population dynamics of yellow-bellied marmots: results from long-term research. Annu Rev Ecol Syst. 1991;22:379–407. [Google Scholar]
- Birchfield ST. A unifying framework for acoustic localization. Proceedings of the 12th European Signal Processing Conference (EUSIPCO); Vienna, Austria. 2004. [Google Scholar]
- Blumstein DT. The evolution of alarm communication in rodents: Structure, function, and the puzzle of apparently altruistic calling in rodents. In: Wolff JO, Sherman PW, editors. Rodent societies. University of Chicago Press; Chicago, IL, USA: 2007. pp. 317–327. [Google Scholar]
- Blumstein DT, Steinmetz J, Armitage KB, Daniel JC. Alarm calling in yellow-bellied marmots: II. The importance of direct fitness. Anim Behav. 1997;53:173–184. [Google Scholar]
- Blumstein DT, Munos O. Individual, age and sex-specific information is contained in yellow-bellied marmot alarm calls. Anim Behav. 2005;69:353–361. [Google Scholar]
- Blumstein DT, Verneyre L, Daniel JC. Reliability and the adaptive utility of discrimination among alarm callers. Proc R Soc B. 2004;271:1851–1857. doi: 10.1098/rspb.2004.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracewell R, Kahn PB. The Fourier transform and its applications. Am J Phys. 1966;34(8):712–712. [Google Scholar]
- Brody AK, Melcher J. Infanticide in yellow-bellied marmots. Anim Behav. 1985;33:673–674. [Google Scholar]
- Chen JC, Hudson RE, Yao K. Maximum-likelihood source localization and unknown sensor location estimation for wideband signals in the near-field. IEEE T Signal Proces. 2002;50:1843–1854. [Google Scholar]
- Collier TC, Kirschel ANG, Taylor CE. Acoustic localization of antbirds in a Mexican rainforest using a wireless sensor network. J Acous Soc Am. 2010;128:182–189. doi: 10.1121/1.3425729. [DOI] [PubMed] [Google Scholar]
- Feldman M. Nonlinear system vibration analysis using the Hilbert transform- I. Free vibration analysis method ‘Freevib’. Mech Syst Signal Pr. 1994;8:119–127. [Google Scholar]
- Girod L, Lukac M, Trifa V, Estrin D. The design and implementation of a self-calibrating distributed acoustic sensing platform. SenSys '06: Proceedings of the 4th international conference on embedded networked sensor systems; New York, NY, USA. 2006. pp. 71–84. [Google Scholar]
- Hoogland JL. The black-tailed prairie dog: Social life of a burrowing mammal. University of Chicago Press; Chicago, IL, USA: 1995. [Google Scholar]
- Marler P. Characteristics of some animal calls. Nature. 1955;176:6–8. [Google Scholar]
- Maynard Smith J. The evolution of alarm calls. Am Nat. 1965;99:59–63. [Google Scholar]
- Mennill DJ, Burt JM, Fristrup KM, Vehrencamp SL. Accuracy of an acoustic location system for monitoring the position of duetting songbirds in tropical forest. J Acous Soc Am. 2006;119:2832–2839. doi: 10.1121/1.2184988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall JA, Rogovin KA, Shier DM. Antipredator behavior of a social desert rodent: footdrumming and alarm calling in the great gerbil. Rhombomys opiums Behav Ecol Sociobiol. 2000;48:110–118. [Google Scholar]
- Shelley EL, Blumstein DT. The evolution of vocal alarm communication in rodents. Behav Ecol. 2005;16:169–177. [Google Scholar]
- Sherman PW. Nepotism and the evolution of alarm calls. Science. 1977;197:1246–1253. doi: 10.1126/science.197.4310.1246. [DOI] [PubMed] [Google Scholar]
- Sherman PW. Alarm calls of Belding's ground squirrels to aerial predators: nepotism or self-preservation? Behav Ecol Sociobiol. 1985;17:313–323. [Google Scholar]
- Wilson DR, Hare JF. The adaptive utility of Richardson's ground squirrel (Spermophilus richardsonii) short-range ultrasonic alarm signals. Can J Zool. 2006;84:1322–1330. [Google Scholar]
- Yorzinski JL, Patricelli GL. Birds adjust acoustic directionality to beam their antipredator calls to predators and conspecifics. Proc R Soc Lond B. 2010;277:923–932. doi: 10.1098/rspb.2009.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]