Abstract
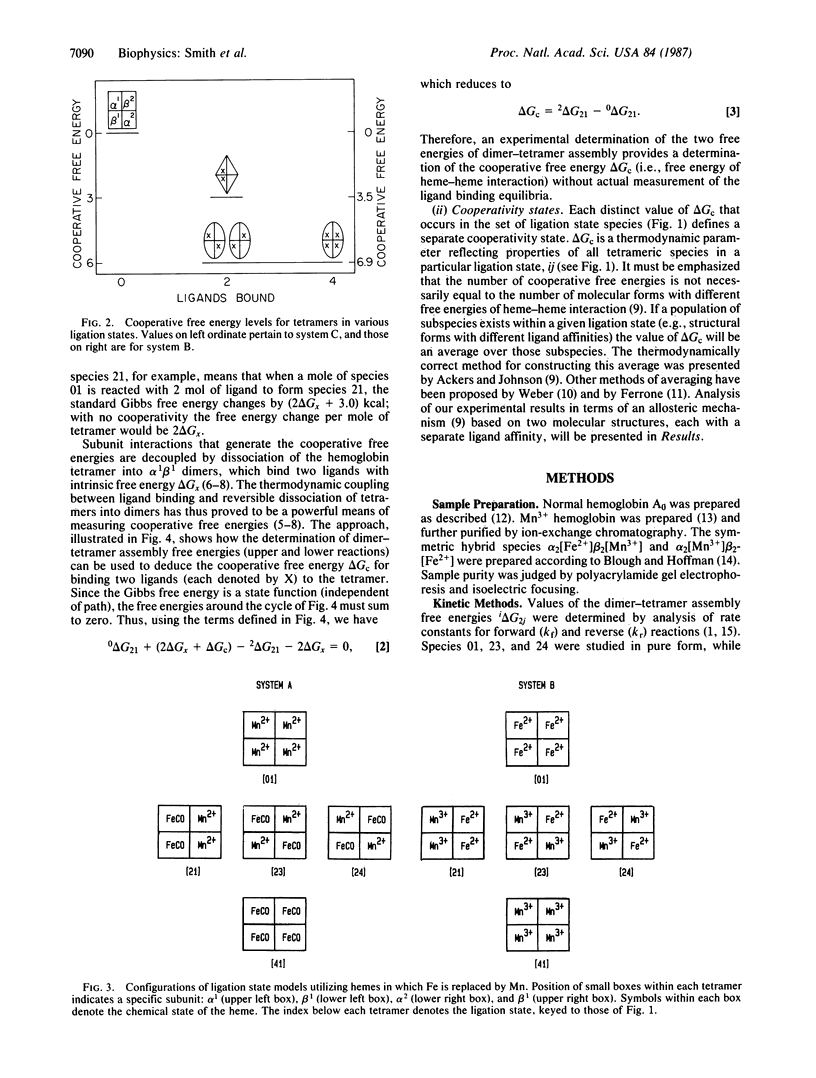
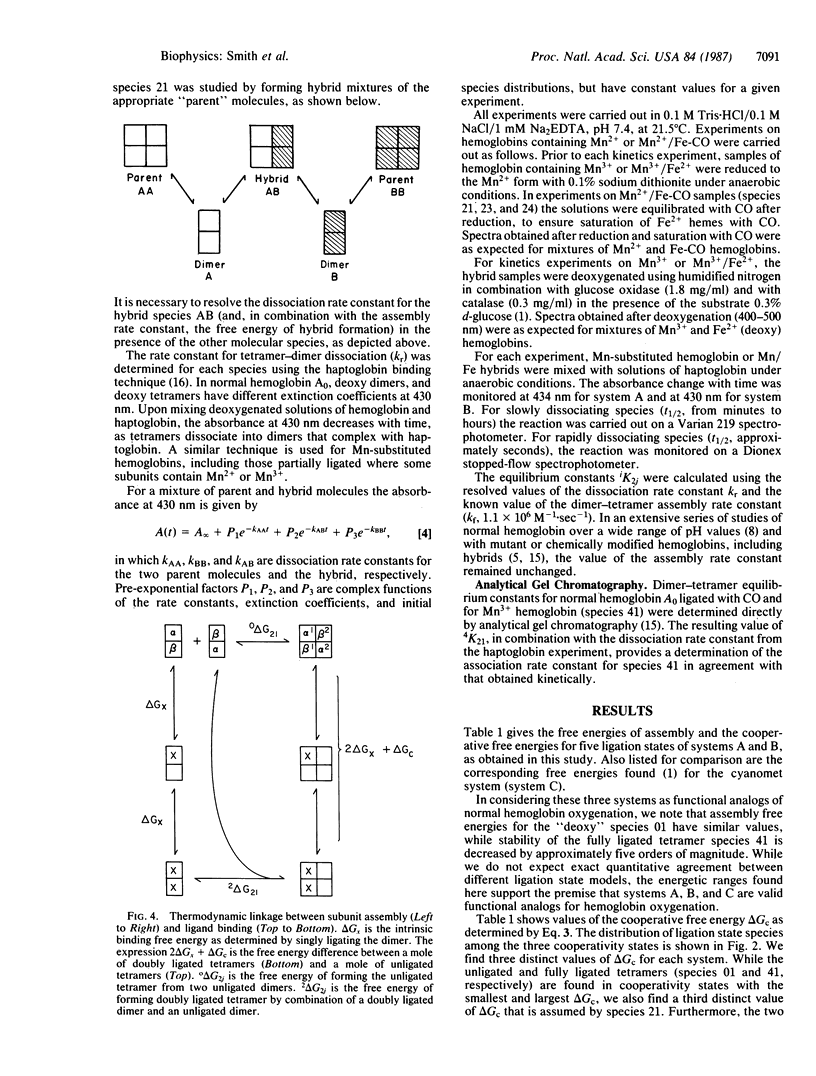
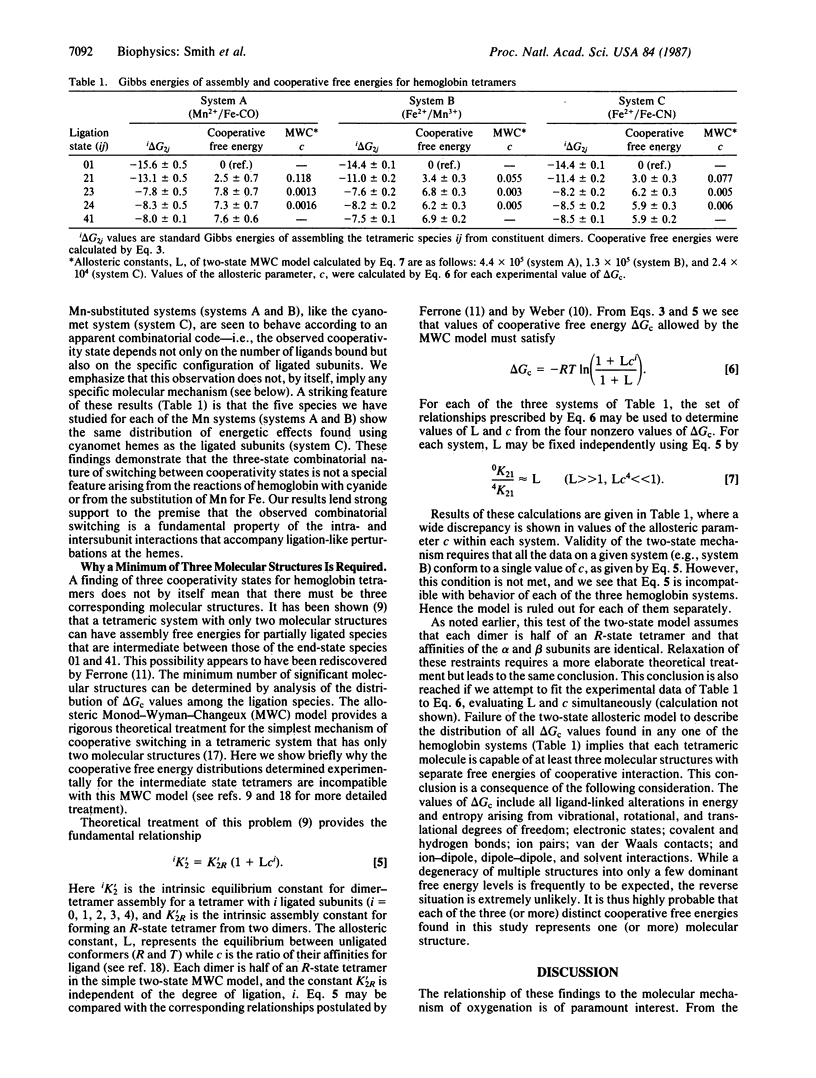
In a previous study on cyanomethemoglobin the 10 tetrameric species (each with a unique combination of ligated and unligated subunits) were found to exhibit three distinct free energies of cooperative interaction. The distribution of these free energies among the partially ligated species is incompatible with a two-state mechanism of molecular switching and requires a minimum of three molecular structures with distinctly different free energies of heme-heme interaction. Ligand-linked transitions between the three cooperativity states were found to be "combinatorial"--i.e., dependent upon changes in both the number and specific configuration of bound ligands. Here we present results from two other chemical systems that mimic intermediate oxygenation states. In these systems the heme iron is replaced by manganese in certain of the subunits. We find the same distribution of cooperative free energies as reported for the cyanomethemoglobin system. These results demonstrate that the three-state combinatorial nature of cooperative switching is neither a special feature of the cyanomet reactions nor of the substitution of manganese for iron, but reflects a fundamental property of hemoglobin. These findings are compared with crystallographic structural results on partially ligated hemoglobins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Halvorson H. R. The linkage between oxygenation and subunit dissociation in human hemoglobin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4312–4316. doi: 10.1073/pnas.71.11.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackers G. K., Johnson M. L. Linked functions in allosteric proteins. Extension of the concerted (MWC) model for ligand-linked subunit assembly and its application to human hemoglobins. J Mol Biol. 1981 Apr 25;147(4):559–582. doi: 10.1016/0022-2836(81)90400-9. [DOI] [PubMed] [Google Scholar]
- Ackers G. K., Smith F. R. The hemoglobin tetramer: a three-state molecular switch for control of ligand affinity. Annu Rev Biophys Biophys Chem. 1987;16:583–609. doi: 10.1146/annurev.bb.16.060187.003055. [DOI] [PubMed] [Google Scholar]
- Arnone A., Rogers P., Blough N. V., McGourty J. L., Hoffman B. M. X-ray diffraction studies of a partially liganded hemoglobin, [alpha(FeII-CO)beta(MnII)]2. J Mol Biol. 1986 Apr 20;188(4):693–706. doi: 10.1016/s0022-2836(86)80015-8. [DOI] [PubMed] [Google Scholar]
- Blough N. V., Hoffman B. M. Carbon monoxide binding to the ferrous chains of [Mn,Fe(II)] hybrid hemoglobins: pH dependence of the chain affinity constants associated with specific hemoglobin ligation pathways. Biochemistry. 1984 Jun 19;23(13):2875–2882. doi: 10.1021/bi00308a005. [DOI] [PubMed] [Google Scholar]
- Brzozowski A., Derewenda Z., Dodson E., Dodson G., Grabowski M., Liddington R., Skarzyński T., Vallely D. Bonding of molecular oxygen to T state human haemoglobin. Nature. 1984 Jan 5;307(5946):74–76. doi: 10.1038/307074a0. [DOI] [PubMed] [Google Scholar]
- Cassoly R. Use of nitric oxide as a probe for assessing the formation of asymmetrical hemoglobin hybrids. An attempted comparison between alphaNObetaNOalphadeoxybetadeoxy, alpha2NObeta2deoxy, and alpha2deoxybeta2NO hybrids. J Biol Chem. 1978 May 25;253(10):3602–3606. [PubMed] [Google Scholar]
- Ferrone F. A. Allosteric interpretation of the measurement of cooperative free energy in cyanomethemoglobin. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6412–6414. doi: 10.1073/pnas.83.17.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip S. H., Johnson M. L., Ackers G. K. Kinetics of deoxyhemoglobin subunit dissociation determined by haptoglobin binding: estimation of the equilibrium constant from forward and reverse rates. Biochemistry. 1976 Feb 10;15(3):654–660. doi: 10.1021/bi00648a032. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mills F. C., Johnson M. L., Ackers G. K. Oxygenation-linked subunit interactions in human hemoglobin: experimental studies on the concentration dependence of oxygenation curves. Biochemistry. 1976 Nov 30;15(24):5350–5362. doi: 10.1021/bi00669a023. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G. High resolution nuclear magnetic resonance spectra of hemoglobin. 3. The half-ligated state and allosteric interactions. J Mol Biol. 1972 Sep 28;70(2):315–336. doi: 10.1016/0022-2836(72)90542-6. [DOI] [PubMed] [Google Scholar]
- Pettigrew D. W., Romeo P. H., Tsapis A., Thillet J., Smith M. L., Turner B. W., Ackers G. K. Probing the energetics of proteins through structural perturbation: sites of regulatory energy in human hemoglobin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1849–1853. doi: 10.1073/pnas.79.6.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F. R., Ackers G. K. Experimental resolution of cooperative free energies for the ten ligation states of human hemoglobin. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5347–5351. doi: 10.1073/pnas.82.16.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. W., Pettigrew D. W., Ackers G. K. Measurement and analysis of ligand-linked subunit dissociation equilibria in human hemoglobins. Methods Enzymol. 1981;76:596–628. doi: 10.1016/0076-6879(81)76147-0. [DOI] [PubMed] [Google Scholar]
- Weber G. Order of free energy couplings between ligand binding and protein subunit association in hemoglobin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7098–7102. doi: 10.1073/pnas.81.22.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Tsay K. Y. A convenient chromatographic method for the preparation of human hemoglobin. Anal Biochem. 1973 Jul;54(1):137–145. doi: 10.1016/0003-2697(73)90256-x. [DOI] [PubMed] [Google Scholar]



