Abstract
Androgens contribute to the involution process of the aging thymus gland. However, molecular mechanisms behind this effect remain largely unknown. We have investigated the influence of testosterone on the ectopic synthesis of glucocorticoids (GCs) in thymocytes, an activity recently shown by us to be important for the homeostatic regulation of these cells. Castration, which leads to a strong increase in thymus tissue and function, was associated with a reduced GC release from thymocytes caused by down-regulated expression of several enzymes involved in GC synthesis, without affecting GC synthesis in the adrenals. Testosterone treatment of castrated male mice reversed these effects, also without affecting adrenal GC synthesis. The effects of testosterone in castrated mice on thymocyte homeostasis and GC release were strongly reduced in mice pretreated with the CYP11B1 enzyme inhibitor metyrapone, acting on the last step in the corticosterone synthesis. The androgen-induced thymic involution was dependent on GC action, because this was completely absent in mice lacking GC receptor (GR) expression specifically in thymocytes. We provide here an unrecognized mechanism how androgens contribute to thymic involution by stimulating local synthesis and release of GCs in the thymus.—Chen, Y., Qiao, S., Tuckermann, J., Okret, S., Jondal, M. Thymus-derived glucocorticoids mediate androgen effects on thymocyte homeostasis.
Keywords: testosterone, corticosterone, local glucocorticoid synthesis, glucocorticoid receptor-knockout mice, metyrapone
The thymus gland is composed of interdependent stromal and lymphoid compartments, organized into cortical and medullary parts, which promote the differentiation of bone-marrow-derived stem cells into fully immunocompetent naive T cells (1). The gradual loss of these naive “recent thymic emigrants” (RTEs) into the peripheral T-cell pool as a result of the age-related thymic involution process results in a restricted T-cell repertoire and defects in the cellular immune system in old age (2). This immunosenescence leads to an increased sensitivity to certain clinical conditions, in particular infections, autoimmunity, and possibly cancer in the elderly population (3).
An initial loss of thymocytes occurs early in life, at 6 wk of age in mice and at 1 yr of age in humans, and is followed by a more gradual reduction of cells throughout the rest of life (2, 4). Although the loss of thymic tissue is extensive, the gland retains some functional activity at old age. The cause of thymic involution, which occurs in most vertebrates, is unclear but is related to a reduced input of bone-marrow-derived stem cells into the gland, a loss of thymic epithelial cells (TECs), or a combination of both mechanisms (5). However, there is an acceleration of thymic decline after puberty, more pronounced in males compared to in females (6), suggesting that increasing levels of sex hormones contribute to the involution process. Among these hormones, androgens in particular exert considerable influence on the size and composition of the thymus. Exogenous administration of androgens in adult rodents results in an altered cell trafficking, reduced thymocyte proliferation, and an increase in thymocyte apoptosis (7). Conversely, removal of androgen by castration results in thymic enlargement and increased thymopoiesis (8). An enhanced thymic recovery and an increase in peripheral RTEs after bone-marrow transplantation can thus be achieved by physical or chemical castration, consistent with an overall augmented thymopoiesis after withdrawal of androgens (9). Despite the clinical importance, the exact mechanisms by which thymic homeostasis is regulated by androgens remain incompletely understood.
Glucocorticoids (GCs) are steroid hormones that affect a wide range of physiological and pathophysiological processes, including immune responses (10). GCs bind to, and activate, the intracellular GC receptor (GR). The activated receptor complex influences the expression of many genes important for a number of immune functions (10). Increased GC levels, triggered by stress and inflammatory cytokines through the hypothalamus-pituitary-adrenal axis, are anti-inflammatory and immunosuppressive. Increased GC levels also cause thymic involution, which involves a GR-dependent increase in thymocyte apoptosis (10, 11).
GCs are primarily synthesized in the adrenal glands, but an ectopic de novo synthesis has also been demonstrated in the brain, gastrointestinal tract, skin, and thymus (12). In the mouse the major GC is corticosterone (CS), derived from cholesterol through the sequential action of steroidogenic acute regulatory protein StAR and the steroidogenic enzymes CYP11A1, 3β-HSD, CYP21, and CYP11B1 (13). The tissue expression of 11β-HSD1 and 11β-HSD2 enzymes, which shuttle the hormone between an active (CS) and an inactive form (11-dehydrocorticosterone), is also important for setting the local GC concentration (13). The initial demonstration of GC synthesis in the thymus was found to occur in TECs and suggested to be important for the T-cell selection process that occurs during thymocyte differentiation (14). More recently, we have shown that thymocytes also synthesize GCs starting from around 4 wk after birth, in contrast to the GC synthesis in TECs, which decline with age (15). We have also found that GC synthesis in thymocytes is under the regulation of ACTH and important for the homeostatic regulation of these cells (16). Importantly, by using transgenic mice with an inducible increase in GC sensitivity selectively in thymocytes, we have clearly demonstrated that thymus-derived GCs are important in the regulation of thymocyte homeostasis (17).
We have proposed that thymocyte-synthesized GCs may contribute to the involution process that the thymus gland undergoes with age (15–18). We have now investigated if there is a link between the effects of testosterone on the thymus and GC synthesis in thymocytes as the latter is initiated around the time of puberty. We have found that a casual and mechanistic relationship exists between these two hormonal pathways as the effects of testosterone on the thymus are lost when GC synthesis in thymocytes is selectively down-regulated or when the GR is absent, specifically in thymocytes.
MATERIALS AND METHODS
Mice
We bred 11- to 12-wk-old male mice (C57BL/6) in the Animal House of the Department of Microbiology, Tumor, and Cell Biology at Karolinska Institutet (Stockholm, Sweden). Conditional T-cell-specific GR mutant (GRLckCre) and their wild-type littermates (GRflox) mice have been described previously (11). All experiments with mice were approved by the Animal Care and Use Committee at the Karolinska Institutet.
Castration procedure
Mice were anesthetized using ketamine/xylazine. The testicles were removed, and the spermatic cord was clamped and cauterized. Sham castration was performed using the same procedure but without removal of the testes.
Testosterone and metyrapone treatments
Testosterone (1 mg/mouse; Sigma-Aldrich, St. Louis, MO, USA) was dissolved in sesame oil (100 μl) and then injected intraperitonially (i.p.) once into mice 15 d after castration. Mice receiving injection with vehicle (100 μl sesame oil) only were used as controls. Mice were analyzed 2 d after testosterone injection.
Metyrapone (2-methyl-1,2-di-3-pyridyl-1-propanone; Sigma-Aldrich) was dissolved to a final concentration of 0.2 mg/ml in drinking water. After 10 d, castrated mice were injected i.p. with testosterone and sacrificed 2 d later. Thymi were isolated, and weight and thymocyte numbers were determined. Blood samples were collected at the time of sacrificed and serum CS levels were measured by ELISA (see below).
Flow cytometry
Single-cell suspensions of thymocytes and splenocytes were prepared and subjected to ammonium chloride lysis to remove red blood cells. Cells were counted and labeled using combinations of the following antibodies: CD4-FITC, CD8-PerCP, and CD62L-PE (eBioscience, San Diego, CA, USA). Three-color flow cytometry was performed on a FACScalibur using CellQuest software (BD Bioscience, San Jose, CA, USA), and all data were analyzed using WinMDI2.9 software (Verity Software House, Topsham, ME, USA).
BrdU incorporation and TUNEL staining assay
Mice were injected i.p. with BrdU (5-bromo-2′-deoxyuridine, 1 mg/mouse; Invitrogen, Carlsbad, CA, USA). Thymus glands were isolated after 24 h. Paraffin-embedded thymic blocks were cut to 3 μm and mounted on polylysine-charged glass slides. Cell proliferation was determinated by BrdU incorporation (BD Pharmingen, San Diego, CA, USA), and apoptosis was measured by TUNEL staining (Roche, Mannheim, Germany) according to the manufacturer's guidelines. Negative controls were processed in the same way by substituting the primary antibody with subclass- and concentration-matched rabbit or goat IgG1 isotype controls.
BrdU- or TUNEL-positive cells were determined by image analysis of histological sections. Stained thymus sections (10 sections/area for each mouse) were selected and photomicrographs were made from high-power fields (0.018 mm2) with a digital camera and analyzed using Image Pro-Plus 5.0 software (Media Cybernetics, Bethesda, MD, USA). The numbers of positive cells per high-power field were counted.
Measurement of T-cell receptor excision circles
For signal joint T-cell receptor rearrangement excision circle (sjTREC) assays, splenocytes, peripheral blood mononuclear cells, and thymocytes were collected, and genomic DNA was extracted using GenElute Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich) according to the manufacturer's instructions. The sjTREC level in each group was detected by real-time quantitative polymerase chain reaction (qPCR) using methods reported previously (19). The primers for sjTRECs were: sense, 5′-CATTGCCTTTGAA CCAAGCTG-3′; and antisense, 5′-TTATGCACAGGGTGCAGGTG-3′. All samples were run in duplicate.
Measurement of mRNA by quantitative real-time PCR
mRNA isolation, cDNA synthesis, and quantitative real-time PCR for detecting the expression of genes encoding steroidogenic enzymes was performed as described previously (15). The specificity of PCR product was examined by dissociation curves and results calculated by the 2−ΔΔCT method.
GR expression analysis
The GR mRNA transcription in thymocytes was detected by qPCR, and the primers were: sense, 5′-CAAGTGATTGCCGCAGTGAA-3′; and antisense, 5′-CATCCAGGTGTAAGTTTCTGAATCC-3′ (15). Moreover, the GR protein level in thymus glands was analyzed by immunohistochemistry using goat anti-mouse GR-specific antibodies (1:300, clone; M-20; Santa Cruz Biotechnology, Santa Cruz, CA, USA) according to the manufacturer's instructions.
GC reporter gene coculture assay
The GC-regulated luciferase (LUC) reporter gene assay was performed as described previously (15). Briefly, HEK-293 cells were stably transfected with a GR expression vector and a GC response element (GRE) -driven LUC reporter gene. The stably transfected HEK293 cells (1×105/well) were plated and allowed to attach before adding isolated thymocytes (3×106/well) for coculture. The supernatant was collected and cells were lysed after 20 h and applied to LUC activity measurements (Tecan Infinite M200, Männedorf, Switzerland). LUC activity is expressed as fold change relative to control.
CS determination by ELISA
Adrenal glands were isolated from experiment animals and directly put into DMEM medium with 1% dextran-coated charcoal-treated FCS. Culture supernatants were collected after 20 h. CS levels in serum and supernatants from tissue-cultured adrenal glands or thymocytes ex vivo were measured using the Correlate-EIA corticosterone enzyme immunoassay kit (Assay Designs, Ann Arbor, MI, USA) according to the manufacturer's instructions.
Statistical analysis
Data were analyzed using a 2-tailed Student's t test. The results were expressed as means ± sd. P < 0.05 was considered as significant.
RESULTS
Castration enhances thymopoiesis
We first confirmed the enhancing effects of castration on thymus tissue and function using male C57BL/6 mice. Thymus glands were isolated and analyzed 15 d after castration. There was a statistically significant increase in thymus weight, total thymocyte numbers, and all thymocyte subpopulations, including CD4/CD8 double-negative (DN), CD4/CD8 double-positive (DP), and CD4 as well as CD8 single-positive (SP) thymocytes compared to sham-castrated littermates (Supplemental Fig. 1).
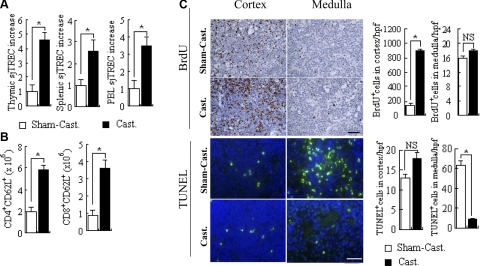
To assess whether the increase in thymus size and thymocyte numbers resulted in an increased thymic activity in terms of export of naive T cells to the periphery, we measured T-cell receptor (TCR) recombination activity by assessing the amount of sjTRECs present in thymocytes, splenocytes, and peripheral blood lymphocytes (PBLs). An augmented thymopoiesis was confirmed by a 4.7-fold increase of sjTREC in thymocytes following castration (Fig. 1A). This increase in thymic activity was accompanied by a 2.5- and 3.4-fold increase in the amount of sjTREC in splenocytes and PBLs, respectively (Fig. 1A), and of the proportion of naive T cells, measured as the expression of CD62L, in CD4- and CD8-positive splenocytes (Fig. 1B). These effects occurred as a result of a pronounced increase in the proliferation of cortical thymocytes, measured as increased BrdU incorporation and reduced rate of apoptosis in the medulla as analyzed by the TUNEL staining assay (Fig. 1C). These results confirmed that castration of male mice results in an enhanced thymopoiesis with a subsequent increase in the thymic output.
Figure 1.
Androgen withdrawal by castration leads to an enhanced thymic activity. A, B) TCR recombination activity in thymocytes, splenocytes, and PBL was measured by sjTREC expression (A), and numbers of naive CD62L+CD4+ and CD62L+CD8+ T cells in the spleen after castration (Cast.) or sham operation (Sham-Cast.) was determined by flow cytometry. C) Proliferation of thymocytes was analyzed by the determination of BrdU incorporation, and the rate of apoptosis in the cortex and the medulla of the thymus was analyzed by the TUNEL assay. *P < 0.05. NS, not significant. Scale bars = 20 μm. n = 8/group.
GC synthesis in thymocytes is regulated by testosterone
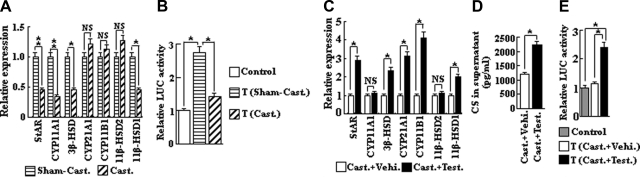
In addition to GC synthesis in the primary endocrine organ, the adrenals, we have earlier demonstrated a de novo GC synthesis in thymocytes, where GCs act locally (15, 16). We have now investigated whether androgen withdrawal by castration influenced this synthesis. A qPCR assay showed that the mRNA expression for genes involved in GC steroidogenesis, including StAR, CYP11A1, 3β-HSD, and 11β-HSD1, was significantly reduced in thymocytes from castrated mice as compared with thymocytes from sham-operated controls (Fig. 2A). As a consequence, GC synthesis in thymocytes was significantly reduced in castrated mice, as measured by the weakened ability of thymocytes to activate a GC-dependent reporter gene in a cellular coculture assay (Fig. 2B).
Figure 2.
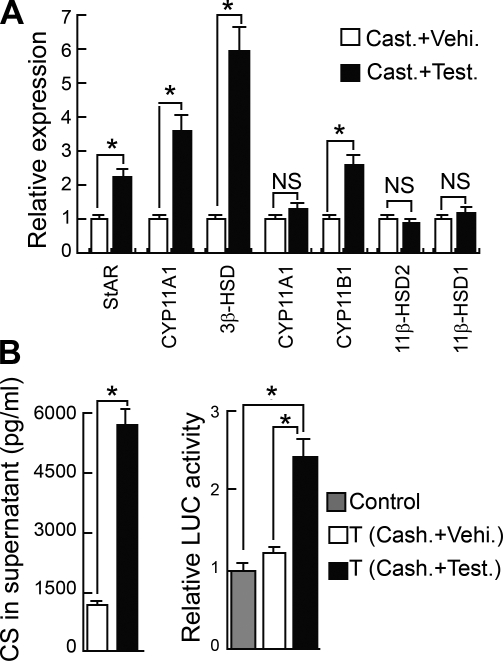
A) GC synthesis in thymocytes is regulated by androgens. Expression of genes encoding steroidogenic enzymes involved in GC synthesis was analyzed by qPCR in thymocytes from castrated mice (n=8) as compared to sham-operated controls (n=8). B) LUC activity stimulated by thymocytes derived from castrated mice vs. sham-operated control mice, as determined by the GC reporter gene coculture assay. C) Expression of genes encoding GC steroidogenic enzymes from castrated + testosterone treated mice (n=8), as compared to vehicle-treated castrated mice (n=8). D) CS secretion by cultured (20 h) thymocytes from castrated+testosterone-treated mice vs. castrated + vehicle-alone-treated mice, as determined by ELISA. E) LUC activity stimulated by thymocytes derived from castrated + testosterone-treated mice vs. castrated+vehicle-alone-treated mice. Control, reporter cells (transfected HEK293 cells) without thymocytes; T, reporter cells plus thymocytes. *P < 0.05; **P < 0.001. NS, not significant.
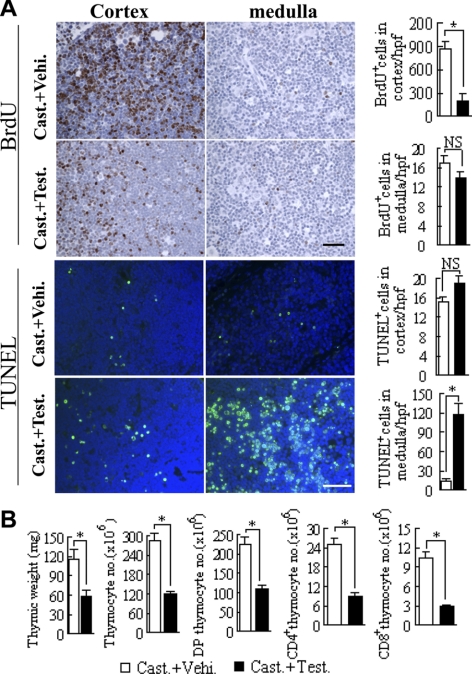
To further demonstrate that GC synthesis in thymocytes can be regulated by androgens in vivo, castrated mice were treated with testosterone and analyzed 48 h thereafter. Testosterone was found to reduce cortical thymocyte proliferation and to induce a strong apoptotic activity in the medulla without causing any significant alterations in medullary proliferation or cortical apoptosis (Fig. 3A). These effects most likely explain the decrease in thymus weight and in thymocyte numbers seen following testosterone administration (Fig. 3B). The mRNA expression of StAR, 3β-HSD, and 11β-HSD1, which was decreased in thymocytes in castrated mice, was significantly increased after testosterone treatment. In addition, in testosterone-treated mice, the amount of CYP21A and CYP11B1 mRNA in thymocytes was increased (Fig. 2C). The increase in the expression of these enzymes involved in GC steroidogenesis led to an increase in CS synthesis in thymocytes as measured both by the presence of CS in supernatants derived from tissue cultured thymocytes when measured by ELISA (Fig. 2D) and as the ability of thymocytes to activate a GC-dependent reporter gene in the coculture assay (Fig. 2E). These results clearly demonstrate that GC synthesis in thymocytes is under the regulation of testosterone.
Figure 3.
Testosterone decreases proliferation and increases apoptosis in the thymus of castrated mice. A) Proliferation of thymocytes was analyzed by the determination of BrdU incorporation after testosterone treatment of castrated mice and the rate of apoptosis in the cortex and the medulla from the thymus of the same mice was analyzed by the TUNEL assay. B) Thymic weight and total, DP, CD4, and CD8 SP thymocyte numbers were measured in castrated testosterone-treated mice vs. vehicle-treated control mice. *P < 0.05. NS, not significant. Scale bars = 20 μm. n = 8/group.
To exclude that androgen withdrawal affects adrenal CS synthesis and release, the major source of CS in mice, we analyzed GC synthesis and release from these glands following castration. Neither the expression of genes involved in GC synthesis nor CS levels derived from in vitro cultured adrenal organs or CS levels in serum was significantly changed following castration (Supplemental Fig. 2A–C). In addition, no influence on adrenal GC synthesis was found after testosterone treatment of castrated mice (Supplemental Fig. 2D–F). This demonstrates that the hormonal manipulations that we used in our system, to decrease or increase testosterone levels, selectively affected GC synthesis in the thymus.
Selective inhibition of GC synthesis in the thymus by metyrapone strongly reduces the effects of testosterone on thymocyte homeostasis
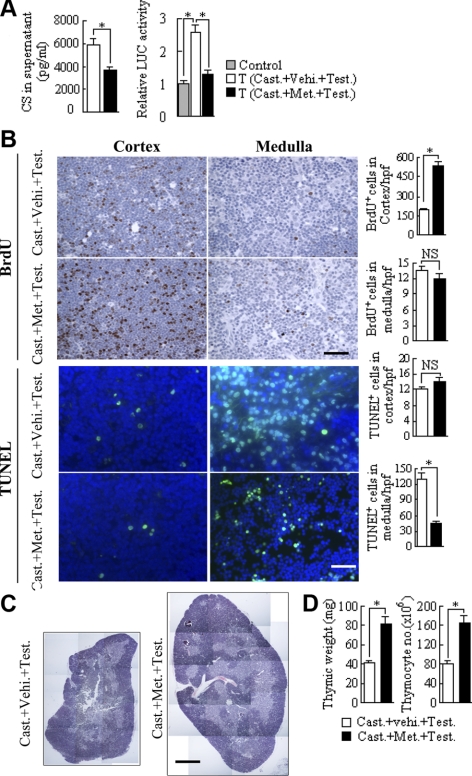
We have found that treatment of mice with a low dose of metyrapone, an inhibitor of CYP11B1, the last enzyme in steroidogenesis of CS, in the drinking water for 10 d results in a significant decrease in de novo GC synthesis in thymocytes, while leaving systemic CS levels unchanged (16). This approach was used to analyze whether testosterone-induced effects on the thymus were dependent on the induction of GC synthesis in the gland. Castrated mice were first treated with metyrapone in the drinking water for 10 d and thereafter with an intraperitoneal injection of testosterone. Metyrapone administration to mice was found to strongly reduce all effects of the testosterone treatment on the thymus in castrated mice, including induction of GC synthesis in thymocytes, loss of thymic tissue, inhibition of proliferation in the cortex, and induction of apoptosis in the medulla (Fig. 4). These results demonstrate that GC synthesis in the thymus is involved in mediating the effects of testosterone on the thymus.
Figure 4.
Effects of testosterone administration on the thymus in castrated mice are reduced by the CYP11B1 inhibitor metyrapone. A) Mice were treated with the CYP11B1 inhibitor metyrapone for 10 d prior to testosterone injection, and GC synthesis in thymocytes was determined by ELISA and GC-reporter gene coculture assay, respectively. B) Antiproliferative effect in the cortex and the apoptotic activity in the medulla triggered by testosterone in the presence or absence of metyrapone was determined by BrdU-labeling and TUNEL assay, respectively. C) Hematoxylin and eosin staining of thymic tissue sections from mice triggered by testosterone in the presence or absence of metyrapone. D) Thymic weight as well as total thymocyte number was measured from above treated mice. Control, reporter cells (transfected HEK293 cells) without thymocytes; T, reporter cells plus thymocytes. n = 8/group. *P < 0.05; **P < 0.001. NS, not significant. Scale bars = 20 μm (B); 400 μm (C).
GR expression in the thymus is not regulated by testosterone
GR is expressed in thymocytes and mediates the effects of GCs in these cells (13). A possible mechanism by which testosterone could increase the effects of a local GC synthesis in thymocytes could be by an increase in GR expression, and thereby augmented GC sensitivity in the cells. For that reason, we first investigated the expression of GR in thymocytes after castration and following castration plus testosterone treatment on the mRNA and protein levels by qPCR and by immunostaining, respectively. No alterations of GR expression under these different conditions was found, neither on the mRNA nor on the protein level (Supplemental Fig. 3). These results demonstrate that the expression of GR in the thymus is not regulated by androgens in our system.
Androgen-mediated effects on the thymus require expression of GR in the thymocytes
We next asked whether GR expression in thymocytes is involved in the androgen-induced thymocyte loss. To this end we used mice in which GR expression in the T-cell lineage, including thymocytes, is ablated (GRLckCre mice): that is, thymocytes that are insensitive to GCs (11). Lack of GR expression in the thymocytes of castrated GRLckCre mice completely abolished the effects of testosterone on the thymus seen in castrated wild-type control (GRflox) mice such as reduced thymic weight, loss of thymocytes, inhibition of the proliferation of cortical thymocytes, and the induction of apoptosis in medullary thymocytes (Fig. 5). However, lack of GR expression in thymocytes did not alter the testosterone-mediated increase in expression of genes involved in GC steroidogenesis as determined by qPCR (Fig. 6A) or GC synthesis in thymocytes as measured by ELISA of the culture supernatant (Fig. 6B) or by the GC reporter gene coculture assay (Fig. 6C). As in castrated wild-type mice (Supplemental Fig. 2D–F), adrenal GC synthesis was unaffected by testosterone treatment of GRLckCre mice (Supplemental Fig. 2G–I). Taken together, the testosterone-induced shrinkage of the thymus depends on a local production of GCs acting on the GR in thymocytes.
Figure 5.
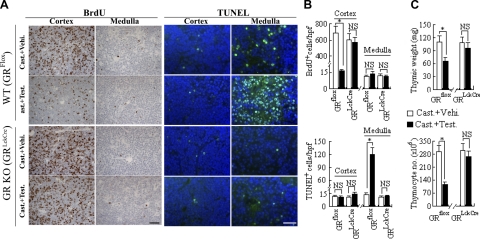
Effects of testosterone on the thymus in castrated mice require expression of GR. Castrated GRLckCre mice (n=7) lacking GR expression specifically in thymocytes and control GRflox mice (n=7) that do express GR in thymocytes were injected i.p. with testosterone or vehicle alone. A, B) At 2 d after treatment, proliferative and apoptotic activities in the thymic cortex and medulla were determined by BrdU staining and TUNEL assay, respectively (A), and quantified (B). C) Thymus weight or thymocyte numbers were determined. *P < 0.05. NS, not significant. Scale bars = 20 μm.
Figure 6.
Testosterone induces GC synthesis in GRLckCre thymocytes. A) Expression of genes encoding steroidogenic enzymes involved in GC synthesis was analyzed by qPCR in thymocytes from castrated + testosterone-treated GRLckCre mice (n=7) vs. castrated vehicle-treated GRLckCre mice (n=7). B) GC synthesis in thymocytes derived from castrated + testosterone-treated GRLckCre mice vs. castrated vehicle-treated GRLckCre mice as determined by ELISA using the thymocyte culture supernatants and the GC responsive reporter gene coculture assay, respectively. Control, reporter cells (transfected HEK293 cells) without thymocytes; T, reporter cells plus thymocytes. *P < 0.05. NS, not significant.
DISCUSSION
We have demonstrated that the withdrawal of testosterone following castration, which results in a pronounced increase of thymus tissue and thymopoesis, is correlated with a loss of GC synthesis in thymocytes. Reversely, testosterone treatment of castrated mice, which resulted in a loss of thymic tissue and thymopoiesis, was found to correlate with an induction of GC synthesis in thymocytes. Evidence that this local GC synthesis was responsible for the testosterone-induced effects on the thymus was obtained by pretreatment of castrated mice with an enzyme inhibitor that selectively affected thymic GC synthesis. This blockage of local GC synthesis clearly impaired the testosterone effects on the thymus. In addition, testosterone effects on the thymus were absent in mice that lacked expression of the GR specifically in thymocytes. The presence of GR is a prerequisite for GC-induced apoptosis of thymocytes (10). Our data therefore demonstrate an important role for the local GC synthesis on effects that androgens exert on the thymus. The results also imply that the accelerated thymic involution process that occurs during puberty may depend, at least in part, on an androgen-induced GC synthesis in the gland. In this respect, testosterone may have a more potent effect than estrogens because thymic activity declines earlier in males as compared to in females, measured as the output of RTEs (6). Testosterone-induced release of GCs from thymocytes may trigger an antiproliferative or an apoptotic reaction in these cells, through para- or autocrine mechanisms, when cells are exposed to (high) local concentrations of the hormone. Importantly, we have previously demonstrated using transgenic mice that the local thymic-derived GC production indeed participate in the regulation of thymic homeostasis in vivo (17).
Testosterone was found to regulate the expression of genes involved in GC steroidogenesis and thus the CS release from thymocytes. However, although the effects of androgen withdrawal by castration and testosterone treatment of castrated mice on GC synthesis in thymocytes were opposed to each other, the effects on mRNA expression for GC synthetic enzymes were only mirror-imaged for StAR, 3β-HSD, and 11β-HSD1 but not for CYP11A1, CYP21A, and CYP11B1, demonstrating that the regulatory effects of the whole testicular glands on the thymus are not directly comparable to the effects of the treatment with testosterone alone. In addition to testosterone, a number of other gonadal steroids are synthesized in small amounts in the testes such as dehydroepiandrosterone, androstenedione, and estrogens, which may also influence the expression pattern of these enzymes (20). Furthermore, why only some and not all enzymes involved in steroidogenesis are affected by androgen manipulations is unclear but may relate to differences in androgen receptor binding to the genes engaged in steroidogenesis or other genes that indirectly control expression of some but not all steroidogenic enzymes.
GC synthesis in the thymus has been demonstrated also in TECs, and a role for this synthesis as a mediator of testosterone effects on the thymus cannot be entirely excluded. However, it seems less likely as this synthesis declines with age, probably because of the early down-regulation of StAR and CYP11A1 enzymes (14), in contrast to GC synthesis in thymocytes, which is first detectable around 4 wk of age and increases at least until 22 wk of age (15). Thus, we believe that the effects of testosterone on the thymus that we see in our present work are mainly, if not exclusively, due to alterations of GC synthesis in the thymocyte population. Importantly, alteration in serum GC concentration or adrenal GC synthesis is not observed in our experiments following castration or testosterone administration, in line with previous observations (21).
In C57BL/6 mice the testosterone level, both in the testis and in serum, increases to a peak level between 3 and 8 wk of age, which correlates in time with a sharp drop in thymocyte numbers and TCR recombination activity (22). At the same time, there is a decline in the TEC population, reflected as a reduced stromal expression of genes coding for keratin 8 and the TEC transcriptional regulator FoxN1 (23). Androgens affects thymocytes in several ways, including an increase in apoptosis, a decrease in proliferation, and alterations in the differentiation/maturation of cells, all of these responses leading to a reduced output of RTEs into the peripheral T-cell system (22). An effect on differentiation by testosterone-induced GC may be partly explained by a higher sensitivity of double-positive, immature thymocytes to this hormone (24). In our present work, we detected the effect on proliferation mainly in the cortex and the effect on apoptosis mainly in the medulla. The mechanisms behind the antiproliferative effect of androgens are not yet defined but may include the synthesis transforming growth factor-β by TECs (25), which acts directly on thymocytes or indirectly through the release of cytokines such as IL-6 and leukemia inhibitory factor (26). In addition, earlier attempts to identify mechanisms behind the effect of testosterone on the thymus show that although both TECs and thymocytes express the androgen receptor (AR), only the AR expressed on TECs seem to mediate the effect of testosterone on the gland (27, 28). The removal of testosterone by castration has been found to lead to a proliferative response of both cortical and medullary TECs, which may contribute to the trophic effects on the thymus that we presently detect after orchidectomy (29). In support of this, in vitro experiments have shown that low concentrations of sex steroids cause a protein kinase C-dependent proliferative response in a TEC-derived cell line, whereas higher concentrations were found to be inhibitory (30). From this, it was concluded that high serum concentrations of sex steroids might function as a shutoff mechanism for TECs in vivo, a mechanism that most likely contributes to thymic involution as thymocytes and TECs are interdependent compartments.
Earlier data clearly demonstrate that the inflammatory cytokine TNF-α is involved in the thymus-involuting effect of androgens (31). One possibility is therefore that testosterone triggers the release of TNF-α from stromal cells that in turn act on thymocytes to increase GC synthesis in these cells. TNF-α can indeed induce a local GC synthesis as shown by Noti et al. (32), who demonstrated that LPS-induced TNF-α triggered an increased expression of enzymes involved in GC synthesis and thereby the release of GC from gastrointestinal epithelial cells. TNF-α and other members of the TNF-α family may in addition affect cells directly by binding to corresponding TNF receptors, which have been described to be express on thymocytes, thereby supplying a degree of redundancy in terms of effector mechanisms (33).
T-cell immunity is required for serological and cellular immune responses, two functions that are clearly reduced in the elderly. In mice, the age-associated decline in the T-cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza viruses (34). The importance of thymic involution for this process is clearly indicated by the premature T-cell immunosenescence seen in patients that have undergone thymectomy in early childhood (35). Various ways to regenerate thymus tissue and increase T-cell differentiation at old age, in addition to castration, have been defined in the mouse, including treatment with the cytokine IL-7 and with peptide hormones such as leptin, ghrelin, and growth hormone (GH) (36). Clinically, a trophic effect on T cells has been demonstrated by chemical castration in patients with prostate cancer and by GH treatment of adult patients suffering from GH deficiency (37). Our present results suggest an additional approach to reverse thymic involution, the selective inhibition of GC synthesis in the thymus. Preliminary experiments, in which we used metyrapone to pharmacologically achieve this, suggest that this may be possible. However, the quantitative and qualitative effects of such an approach on T cells, as well as on other leukocytes and on both peripheral immune and nonimmune tissues, remain to be established.
Supplementary Material
Acknowledgments
This work was supported by grants from the Swedish Cancer Society and the European Union FP7 project TOLERAGE (HEALTH-F4-2008-202156).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Rodewald H. R. (2008) Thymus organogenesis. Annu. Rev. Immunol. 26, 355–388 [DOI] [PubMed] [Google Scholar]
- 2.Dorshkind K., Montecino-Rodriguez E., Signer R. A. (2009) The ageing immune system: is it ever too old to become young again? Nat. Rev. Immunol. 9, 57–62 [DOI] [PubMed] [Google Scholar]
- 3.Linton P. J., Dorshkind K. (2004) Age-related changes in lymphocyte development and function. Nat. Immunol. 5, 133–139 [DOI] [PubMed] [Google Scholar]
- 4.Lynch H. E., Goldberg G. L., Chidgey A., Van den Brink M. R., Boyd R., Sempowski G. D. (2009) Thymic involution and immune reconstitution. Trends Immunol. 30, 366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudakov J. A., Khong D. M., Boyd R. L., Chidgey A. P. (2010) Feeding the fire: the role of defective bone marrow function in exacerbating thymic involution. Trends Immunol. 31, 191–198 [DOI] [PubMed] [Google Scholar]
- 6.Pido-Lopez J., Imami N., Aspinall R. (2001) Both age and gender affect thymic output: more recent thymic migrants in females than males as they age. Clin. Exp. Immunol. 125, 409–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen N. J., Viselli S. M., Fan J., Kovacs W. J. (1998) Androgens accelerate thymocyte apoptosis. Endocrinology 139, 748–752 [DOI] [PubMed] [Google Scholar]
- 8.Sutherland J. S., Goldberg G. L., Hammett M. V., Uldrich A. P., Berzins S. P., Heng T. S., Blazar B. R., Millar J. L., Malin M. A., Chidgey A. P., Boyd R. L. (2005) Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 175, 2741–2753 [DOI] [PubMed] [Google Scholar]
- 9.Van den Brink M. R., Alpdogan O., Boyd R. L. (2004) Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev. Immunol. 4, 856–867 [DOI] [PubMed] [Google Scholar]
- 10.Baschant U., Tuckermann J. (2010) The role of the glucocorticoid receptor in inflammation and immunity. J. Steroid Biochem. Mol. Biol. 120, 69–75 [DOI] [PubMed] [Google Scholar]
- 11.Tuckermann J. P., Kleiman A., Moriggl R., Spanbroek R., Neumann A., Illing A., Clausen B. E., Stride B., Förster I., Habenicht A. J., Reichardt H. M., Tronche F., Schmid W., Schütz. G. (2007) Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J. Clin. Invest. 117, 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt K. L., Pradhan D. S., Shah A. H., Charlier T. D., Chin E. H., Soma K. K. (2008) Neurosteroids, immunosteroids, and the balkanization of endocrinology. Gen. Comp. Endocrinol. 157, 266–274 [DOI] [PubMed] [Google Scholar]
- 13.Payne A. H., Hales B. D. (2004) Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 25, 947–970 [DOI] [PubMed] [Google Scholar]
- 14.Vacchio M. S., Papadopoulos V., Ashwell J. D. (1994) Steroid production in the thymus: implication for thymocyte selection. J. Exp. Med. 179, 1835–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiao S., Chen L., Okret S., Jondal M. (2008) Age-related synthesis of glucocorticoids in thymocytes. Exp. Cell. Res. 314, 3027–3035 [DOI] [PubMed] [Google Scholar]
- 16.Qiao S., Okret S., Jondal M. (2009) Thymocyte-synthesized glucocorticoids play a role in thymocyte homeostasis and are down-regulated by adrenocorticotropic hormone. Endocrinology 150, 4163–4169 [DOI] [PubMed] [Google Scholar]
- 17.Pazirandeh A., Jondal M., Okret S. (2005) Conditional expression of a glucocorticoid receptor transgene in thymocytes reveals a role for thymic-derived glucocorticoids in thymopoiesis in vivo. Endocrinology 146, 2501–2507 [DOI] [PubMed] [Google Scholar]
- 18.Jondal M., Pazirandeh A., Okret S. (2004) Different roles for glucocorticoids in thymocyte homeostasis? Trends Immunol. 25, 595–600 [DOI] [PubMed] [Google Scholar]
- 19.Sempowski G. D., Gooding M. E., Liao H. X., Le P. T., Haynes B. F. (2002) T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol. Immunol. 38, 841–848 [DOI] [PubMed] [Google Scholar]
- 20.Scott H. M., Mason J. I., Sharpe R. M. (2009) Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr. Rev. 30, 883–925 [DOI] [PubMed] [Google Scholar]
- 21.Da Silva J. A., Peers S. H., Perretti M., Willoughby D. A. (1993) Sex steroids affect glucocorticoid response to chronic inflammation and to interleukin-1. J. Endocrinol. 136, 389–397 [DOI] [PubMed] [Google Scholar]
- 22.Leposavić G., Perisić M. (2008) Age-associated remodeling of thymopoiesis: role for gonadal hormones and catecholamines. Neuroimmunomodulation 15, 290–322 [DOI] [PubMed] [Google Scholar]
- 23.Ortman C. L., Dittmar K. A., Witte P. L., Le P. T. (2002) Molecular characterization of the mouse involuted thymus: aberrations in expression of transcription regulators in thymocyte and epithelial compartments. Int. Immunol. 14, 813–822 [DOI] [PubMed] [Google Scholar]
- 24.Wiegers G. J., Knoflach M., Böck G., Niederegger H., Dietrich H., Falus A., Boyd R., Wick G. (2001) CD4(+)CD8(+)TCR(low) thymocytes express low levels of glucocorticoid receptors while being sensitive to glucocorticoid-induced apoptosis. Eur. J. Immunol. 31, 2293–2301 [DOI] [PubMed] [Google Scholar]
- 25.Olsen N. J., Zhou P., Ong H., Kovacs W. J. (1993) Testosterone induces expression of transforming growth factor-beta 1 in the murine thymus. J. Steroid Biochem. Mol. Biol. 45, 327–332 [DOI] [PubMed] [Google Scholar]
- 26.Sempowski G. D., Hale L. P., Sundy J. S., Massey J. M., Koup R. A., Douek D. C., Patel D. D., Haynes B. F. (2000) Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J. Immunol. 164, 2180–2187 [DOI] [PubMed] [Google Scholar]
- 27.Olsen N. J., Olson G., Viselli S. M., Gu X., Kovacs W. J. (2001) Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology 142, 1278–1283 [DOI] [PubMed] [Google Scholar]
- 28.Dulos G. J., Bagchus W. M. (2001) Androgens indirectly accelerate thymocyte apoptosis. Int. Immunopharmacol. 1, 321–328 [DOI] [PubMed] [Google Scholar]
- 29.Williams K. M., Hakim F. T., Gress R. E. (2007) T cell immune reconstitution following lymphodepletion. Semin. Immunol. 19, 318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izquierdo M., Redondo J. M., Balboa M. A., López-Rivas A., Cragoe E. J., Vázquez L., Fernández-Rañada J. M., López-Botet M. (1989) Deficient protein kinase C-dependent Na+/H+ exchanger activity in T cells from bone marrow transplantation recipients. J. Immunol. 143, 2185–2192 [PubMed] [Google Scholar]
- 31.Guevara Patiño J. A., Marino M. W., Ivanov V. N., Nikolich-Zugich J. (2000) Sex steroids induce apoptosis of CD8+CD4+ double-positive thymocytes via TNF-alpha. Eur. J. Immunol. 30, 2586–2592 [DOI] [PubMed] [Google Scholar]
- 32.Noti M., Corazza N., Tuffin G., Schoonjans K., Brunner T. (2010) Lipopolysaccharide induces intestinal glucocorticoid synthesis in a TNFalpha-dependent manner. FASEB J. 24, 1340–1346 [DOI] [PubMed] [Google Scholar]
- 33.Page D. M., Roberts E. M., Peschon J. J., Hedrick S. M. (1998) TNF receptor-deficient mice reveal striking differences between several models of thymocyte negative selection. J. Immunol. 160, 120–133 [PubMed] [Google Scholar]
- 34.Yager E. J., Ahmed M., Lanzer K., Randall T. D., Woodland D. L., Blackman M. A. (2008) Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J. Exp. Med. 205, 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauce D., Larsen M., Fastenackels S., Duperrier A., Keller M., Grubeck-Loebenstein B., Ferrand C., Debré P., Sidi D., Appay V. (2009) Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Invest. 119, 3070–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfister G., Savino W. (2008) Can the immune system still be efficient in the elderly? An immunological and immunoendocrine therapeutic perspective. Neuroimmunomodulation 15, 351–364 [DOI] [PubMed] [Google Scholar]
- 37.Morrhaye G., Kermani H., Legros J. J., Baron F., Beguin Y., Moutschen M., Cheynier R., Martens H. J., Geenen V. (2009) Impact of growth hormone (GH) deficiency and GH replacement upon thymus function in adult patients. PLoS ONE 22, e5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.