Abstract
Voltage-gated calcium channels conduct Ca2+ ions in response to membrane depolarization. The resulting transient increase in cytoplasmic free calcium concentration is a critical trigger for the initiation of such vital responses as muscle contraction and transcription. L-type Cav1.2 calcium channels are complexes of the pore-forming α1C subunit associated with cytosolic Cavβ subunits. All major Cavβs share a highly homologous membrane associated guanylate kinase-like (MAGUK) domain that binds to α1C at the α-interaction domain (AID), a short motif in the linker between transmembrane repeats I and II. In this study we show that Cavβ subunits form multimolecular homo- and heterooligomeric complexes in human vascular smooth muscle cells expressing native calcium channels and in Cos7 cells expressing recombinant Cav1.2 channel subunits. Cavβs oligomerize at the α1C subunits residing in the plasma membrane and bind to the AID. However, Cavβ oligomerization occurs independently on the association with α1C. Molecular structures responsible for Cavβ oligomerization reside in 3 regions of the guanylate kinase subdomain of MAGUK. An augmentation of Cavβ homooligomerization significantly increases the calcium current density, while heterooligomerization may also change the voltage-dependence and inactivation kinetics of the channel. Thus, oligomerization of Cavβ subunits represents a novel and essential aspect of calcium channel regulation.—Lao, Q. Z., Kobrinsky, E., Liu, Z., Soldatov, N. M. Oligomerization of Cavβ subunits is an essential correlate of Ca2+ channel activity.
Keywords: protein-protein interaction, high-order protein complexes, calcium channel regulation, calcium signaling
Functional calcium channels are clustered (1–4) complexes of the pore-forming α1 subunits with auxiliary α2δ and cytosolic Cavβ subunits (5). Each of the 4 known types of Cavβ, encoded by 4 different β1–4 genes (6), share a highly homologous central membrane associated guanylate kinase-like (MAGUK) domain (7–10) that binds to the α1 subunits at the conserved α-interaction domain (AID) situated in the linker between transmembrane repeats I and II (11). Association between α1 and Cavβ is essential for voltage gating, calcium-induced inactivation, and plasma membrane targeting of the channels (12). Earlier experiments showed that the α1/α2δ/Cavβ complexes are stable in nonionic detergents, and a stoichiometric ratio between α1, α2δ, and Cavβ in a purified functional channel is ∼1:1:1.3 (13). However, the exact ratio between the subunits in the plasma membrane-bound channels remains unclear. Overexpression of Cavβ was shown to affect the electrophysiological properties of the Cav1.2 (14–16) and Cav2 channels (17–19), and may have pathophysiological consequences (20–22). The nature and mechanisms of these effects remain largely unexplained. A stimulating hypothesis suggesting involvement of higher-order regulatory complexes (α1β)βn has been suggested by Tareilus et al. (18). This hypothesis becomes particularly attractive in light of a later finding that the purified Cavβs exhibit tendency to reversible aggregation (23). Our report provides evidence that Cavβ subunits in naturally occurring and recombinant Cav1.2 channels form oligomeric complexes and that the Cavβ oligomerization is a new molecular correlate of calcium channel regulation.
MATERIALS AND METHODS
Molecular biology
The plasmids coding for Flag-, V5-His-, and Venus-tagged proteins were constructed using p3×FLAG-Myc-CMVTM24 (Sigma, St. Louis, MO, USA), pCDNA3.1D/V5-His-TOPO (Invitrogen, Carlsbad, CA, USA), and monomeric mVenus- C1 vectors (24). In this study we used cDNAs coding for the following human Cavβ subunits: β1b (M92302), β2d (AF423191), and β3 (X76555). Flag-α1C, α1CAID, α1CAID/IQ, and α2δ-1 as well as red fluorescent protein (RFP)-, Cerulean-, and Venus-tagged Cavβs were prepared as described previously (1, 25). Flag-tagged Cavβ constructs were produced by PCR amplification of each cDNA with the respective linkers followed by incorporation into p3×FLAG-Myc-CMVTM24 at 5′-EcoRI/XbaI-3′ for β2d and β3, and at 5′-EcoRI/BamHI-3′ for β1b. Flag-β3ΔGK was produced by 2-step PCR as described previously (25). The inner primers were 5′-TATGACCGTGGTGCCCTCCCACCCAGCCCCTGGCCCCGGACTTCT-3′ (sense) and 5′-GGAGGGCACCACGTCATATGGGGG-3′ (antisense); the outer primers were 5′-atataaagcttATGTATGACGACTCCTACGTGCCC-3′ (sense) and 5′-atatatctagaGTAGCTATCCTTGGGCCAAGGCCG-3′ (antisense). The resulting PCR product was then incorporated into p3×FLAG-Myc-CMVTM24 at 5′-HindIII/XbaI-3′. The Flag tag is on the N termini of all the Flag-tagged proteins. V5-His-β3 and I-II linker were subcloned into the pCDNA3.1D/V5-His-TOPO vector according to standard PCR scheme, and the V5-His tag is on the C termini of the fusion proteins. The Flag-tagged GK fragments were constructed by PCR amplification of each fragment with linkers followed by subcloning into the vector at 5′-HindIII/EcoRI-3′ sites. The Venus-tagged GKC fragments were cloned by PCR amplification using 5′-BspEI/EcoRI-3′ sites of mVenus-C1 vector. QuickChange II Site-Directed Mutagenesis Kit and GeneMorph II EZClone Domain Mutagenesis Kit (Stratagene, La Jolla, CA, USA) were used for mutagenesis study. All recombinant plasmids were confirmed by DNA sequencing.
Cells and transfection
Primary human aortic smooth muscle cells were purchased from Lonza (Walkersville, MD, USA) and cultured for 5 d following the manufacturer's instructions. Cos7 cells were maintained in Dulbecco's modified Eagle medium (10% FBS, 4.5 g/L glucose) at 37°C in 5% CO2. Early passage cells (≈106/100-mm dish) were used for transfection by Effectene (Qiagen, Valencia, CA, USA). The cells were cultured for 72 h before use in experiments.
Coimmunoprecipitation (co-IP) and Western blot analysis
EZview Red FLAG M2 affinity gel was used for Flag IP with 3× Flag peptide (200 ng/μl) (Sigma) to elute the IP proteins. His pulldown was carried out with MagneHis (Promega, Madison, WI, USA) using 0.5–1 M imidazole in PBS for elution. A Cell Surface Protein Isolation Kit (Pierce Biotechnology, Rockford, IL, USA) was used for biotinylation of surface membrane proteins and isolation of the plasma membrane-bound calcium channel complexes. Standard heat denaturation at 95°C for 5 min in 1× Laemmli's sampling buffer was applied to the protein samples showing a “ladder” on Western blots. All other samples were treated by 50 mM DTT at 37°C for 30 min prior to heat denaturation. Tris-Acetate SDS-PAGE (3–8%) was used for the ladders, and 4–20% Tris-glycine SDS-PAGE was used for all other samples. Antibodies were obtained from Sigma (Flag, GFP), Invitrogen (V5; Invitrogen) and Millipore (α1C, β3; Millipore, Billerica, MA, USA). The dilution of primary antibodies for immunoblotting was as follows: 1:5000 for Flag (1:1000 for Flag-α1C), 1:10,000 for GFP, 1:3000 for V5, 1:1000 for β3, and 1:500 for α1C. For Far-Western blotting, Flag- and V5-His-tagged β3 were expressed separately in Cos7 cells and purified as described above. V5-His-β3 was resolved by SDS-PAGE and transferred to nitrocellulose membrane, followed by blocking with 3% BSA in PBS for 1 h and incubation of purified Flag-β3 (preheated at 65°C for 10 min) in 3% BSA at room temperature for 3 h. The membrane was then washed with TBS, blocked with 5% milk, and subjected to immunoblotting by an anti-Flag antibody. Blue native PAGE was performed as described previously (26) in 4–12% Bis-Tris gel, and Sigma MW-GF-1000 was used as molecular mass marker.
Colocalization of Venus-β3 and RFP-β3 by confocal microscopy
Cos7 cells were transfected with Venus-β3 and RFP-β3 (0.1 μg each vector/104 cells/slide). Colocalization of differentially labeled β3 was analyzed 72 h after transfection by the confocal laser scanning microscopy on a LSM510 confocal microscope (Carl Zeiss, Oberkochen, Germany) equipped with a ×63 1.4-numerical aperture (n.a.) oil DIC objective and multiple filter sets. The yellow fluorescent protein (YFP) cube (excitation 514 nm, emission 522–565 nm) was used to detect Venus-β3 with an argon laser; the RFP cube (excitation 561 nm, emission > 575 nm) was used to detect RFP-β3 with a diode-pumped solid-state (DPSS) 561-10 laser .
Colocalization of Venus-β3 and Flag-β3 by immunostaining and confocal microscopy
Cos7 cells were transfected with Venus-β3 and Flag-β3 (0.1 μg each vector/104 cells/slide). After 72 h in culture, transfected cells were fixed with 2% formaldehyde in PBS and permeabilized with 2.5% Triton-X in PBS. Subsequently, the cells were blocked with 5% BSA in PBS for 2 h at room temperature and immunostained with a mouse anti-Flag antibody (Sigma, 1:250 dilution) for 1 h, followed by standard immunostaining with rhodamine Red X-conjugated donkey anti-mouse antibody (Jackson ImmunoResearch, West Grove, PA) as the secondary antibody (at 1:2000 dilution). Colocalization of Venus-β3 and Flag-β3 was analyzed by confocal microscopy using YFP and RFP cubes, respectively, as described above. The fluorescent-positive cells were confirmed by phase-contrast microscopy. Before image recording, the noise was adjusted on nontransfected cells and the cells immunostained only with the secondary antibody.
Plasma membrane fluorescence resonance energy transfer (FRET) measurements
Cells were transfected with α1C, α2δ, Venus-β2d, and Cerulean-β2d at 1:1:1:1 mol/mol ratio as described previously (1). Images were recorded with a pixel size of 200 nm using a 14-bit Hamamatsu C9100–12 digital camera (Hamamatsu City, Japan) mounted on a Nikon TE2000 epifluorescent microscope (Nikon, Tokyo, Japan) equipped with a ×60 1.45-n.a. oil objective and multiple filter sets (Chroma Technology, Rockingham, VT, USA). Excitation light was delivered by a 175-W xenon lamp. Excitation filter sets were changed by a high-speed filter wheel system (Lambda 10-2; Sutter Instrument, Novato, CA, USA). The Dual-View system (Optical Insights, Santa Fe, NM, USA) was used for the simultaneous acquisition of 2 fluorescence images (donor and FRET). Images were collected and analyzed using C-Imaging (Compix, Cranberry Township, PA, USA) and MATLAB v.7.0.4 (The Mathworks, Natick, MA, USA). FRET was measured from the plasma membrane area with 3 filter sets: for the YFP cube, excitation filter 500/20 nm, dichroic beam splitter 515 nm, emission filter 535/30 nm; for the cyan fluorescent protein (CFP) cube, excitation filter 436/20 nm, dichroic beam splitter 505 nm, emission filter 480/40 nm. The average plasma membrane FRET efficiency was calculated as described elsewhere (1).
Cellular electrophysiology
Whole-cell recordings were performed exactly as described previously (12) with 20 mM CaC12 in bath medium. Calcium currents were evoked by stepwise depolarization applied from the holding potential of −90 mV.
RESULTS
Cavβs form homo- and heterooligomeric complexes
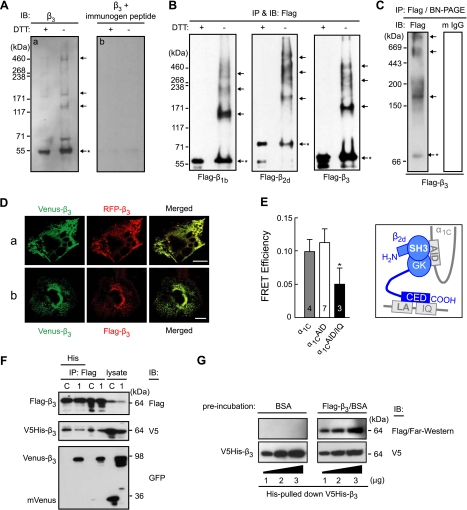
In our experiments we used primary human aortic smooth muscle cells expressing predominantly β3 calcium channel complexes (27). Cell lysate was immunoblotted with an anti-β3 antibody after standard heat denaturation (5 min, 95°C) with or without prior DTT treatment (Fig. 1A). DTT treatment revealed predominantly the monomeric β3 on the blot. However, in the absence of DTT treatment we observed ladder-like β3 immunoactive bands on the blot (Fig. 1Aa), suggesting the presence of β3 supramolecular complexes in naturally occurring human vascular calcium channels. The ladder-like bands are β3 specific, because preincubation of the β3 antibody with its immunogen peptide inhibited recognition of this pattern by the antibody (Fig. 1Ab).
Figure 1.
Oligomerization of Cavβ subunits. A) Immunoblotting (IB) of β3 in human aortic smooth muscle cells with an anti-β3 antibody after denaturation for 5 min at 95°C with or without prior treatment with DTT (a). To demonstrate the specificity of the antibody, in control (b), an anti-β3 antibody was preincubated with its immunogen peptide at room temperature for 1 h prior to the same immunoblotting procedure. B) Oligomerization of recombinant Cavβs expressed in Cos7 cells. Flag-labeled β1b, β2d, or β3 (0.5 μg each) was expressed in Cos7 cells and immunoprecipitated (IP) and immunoblotted with an anti-Flag antibody with or without prior DTT treatment. C) An aliquot of the Flag-β3 immunoprecipitate was analyzed without denaturation by blue native PAGE, followed by immunoblotting with an anti-Flag antibody (left panel) or with a rabbit anti-mouse IgG antibody (m IgG; right panel). Arrows mark oligomers; asterisk indicates monomer. D) Colocalization of Venus-β3 and RFP-β3 (a) or Venus-β3 and Flag-β3 (b) coexpressed in Cos7 cells. Images are confocal laser-scanning micrographs of representative cells obtained with YFP (Venus-β3) and RFP cubes (RFP-β3 and Flag-β3/Red-X). Merged images illustrate colocalization of differentially labeled β3 subunits. Scale bars = 20 μm. E) FRET between Venus- and Cerulean-tagged β2d subunits coexpressed in Cos1 cells with α2δ-1 and α1C (shaded bar) or its Ala-mutants α1CAID (α1C with disrupted AID region; open bar) or α1CAID/IQ (the same plus deleted LA/IQ region; solid bar). Inset: sketch of known interactions of α1C (gray) and β2d (blue) (24). FRET efficiency was measured as described previously (1) in live cells in the plasma membrane region of interest at −10 mV, 22°C. Number of tested cells is shown in the bars. *P < 0.05. F) Cavβ oligomers contain ≥3 β subunits. Flag-β3 and V5-His-β3 (0.5 μg each) were coexpressed in the absence (lane C, control) or presence (lane 1) of Venus-β3 (0.8 μg); the anti-Flag-IP fraction was subsequently pulled down on a His-binding matrix and immunoblotted with an anti-GFP antibody. G) Far-Western blotting. V5-His-β3 was pulled down by His; aliquots were separated by SDS-PAGE and probed by Far-Western blotting (see Materials and Methods) using 3% BSA (top right, control) or purified Flag-β3 in 3% BSA (top right). V5-His-β3 was verified by immunoblotting with an anti-V5 antibody after the Far-Western procedure (bottom panel). All plasmid DNA amounts in this study are reported per transfection per 106 cells/100-mm dish; 3 μg of Flag-β3 plasmid was used for purification of Flag-β3.
To exclude any possible effect of endogenous channel subunits, such as α1C (28), on Cavβ supramolecular complex formation, we then expressed recombinant Cavβs in calcium channels-free Cos7 cells (12). Epitope tags (Flag, His-V5 and GFP variants) were added to the recombinant Cavβs to ease purification and detection. In control experiments, we found that both anti-Flag and anti-β3 antibodies recognize identical ladder-like Flag-β3 bands (Supplemental Fig. S1A). All antibodies to tags used in this study are specific to their respective tagged β3 subunits, which are also well recognized by an anti-β3 antibody in the immunoblotting and immunoprecipitation assays (Supplemental Fig. S1B). Moreover, a ladder-like β3 pattern on the gel is not due to β3 overexpression, since it was also observed when the expression level of β3 was lowered to that of the mouse brain tissue level (Supplemental Fig. S2A, B). The efficiency and specificity of the Flag-IP/Western blot assay to study protein oligomerization sensitive to DDT treatment was demonstrated with the example of Flag-tagged bacterial alkaline phosphatase expressed in Cos7 cells (Supplemental Fig. S1C). In the absence of DTT treatment, a Flag-specific IP/Western blot analysis successfully detected a dimer and a trimer of the protein, a result consistent with previous reports (29, 30), while no reactivity was observed with the nontransfected cells (Supplemental Fig. S1C, panel NT).
To investigate whether the ladder-like pattern of β3 is common in all major Cavβs, Flag-tagged human β1b (31), β2d (32) (splice variants of β1 and β2, respectively; ref. 33), and β3 were expressed in Cos7 cells, immunoprecipitated as described in experimental procedures, and then immunoblotted with an anti-Flag antibody under the denaturing conditions described in Fig. 1A. A distinct ladder pattern was observed on the blots with all 3 types of Cavβ (Fig. 1B), suggesting their oligomerization.
The oligomerization of β3 was independently demonstrated by blue native PAGE technology (34) in the absence of denaturing (Fig. 1C). Flag-β3 was expressed in Cos7 cells, immunoprecipitated with an anti-Flag antibody, and subjected to blue native PAGE, followed by immunoblotting with an anti-Flag antibody. The result revealed that the Flag-β3 immunoprecipitate is composed of a mixture of the monomer and oligomers, the latter being predominant, as one can judge from staining intensity of the bands. However, these bands were not detectable when an aliquot of the same sample was immunoblotted with the secondary anti-mouse antibody (mIgG) alone, pointing to the lack of contamination by the immunoprecipitating antibody in the sample that may cause a high-molecular-weight shift.
Several independent methods confirmed the fact of Cavβ-Cavβ interaction. Consistent with Cavβ oligomerization, Venus-β3 colocalized with RFP-β3 (revealed by laser scanning microscopy, Fig. 1Da) or with Flag-β3 coexpressed in Cos7 cells (revealed by immunostaining, Fig. 1Db). An independent measurement of FRET between Venus-β2d and Cerulean-β2d associated with the plasma membrane-bound α1C also points to Cavβ oligomerization (Fig. 1E). FRET between Venus-β2d and Cerulean-β2d coexpressed with α1C (Fig. 1E, shaded bar) may be due to their oligomerization or the proximity of their binding sites in α1C. Indeed, it was established previously (25, 35) that α1C has 2 binding sites for Cavβs, the AID and IQ motifs (see Fig. 1D, inset). Using α1C mutants with disrupted AID region (α1CAID), we found that the IQ motif alone is sufficient to dock the oligomers of differentially labeled β2d (Fig. 1E, open bar). FRET between Venus- and Cerulean-labeled β2d docked at α1C is significantly decreased only when both AID and LA/IQ of α1C are disrupted (Fig. 1E, solid bar), preventing α1C/Cavβ interaction. The plasma membrane localization of α1C and its mutants is confirmed in Supplmental Fig. S3. Thus, 3 independent techniques, blue native PAGE, in vivo colocalization, and FRET, each confirmed our IP/Western blot data that Cavβs are subject to oligomerization, which is not affected by overexpression of proteins and the type of host cells.
We then determined that Cavβs form a complex of ≥3 individual molecules. Figure 1F illustrates the experiment where Flag-β3, Venus-β3, and V5-His-β3 were coexpressed in Cos7 cells and a 2-step pulldown procedure was applied to find out whether these 3 differentially labeled β3 molecules coprecipitate. The Flag-β3 complexes were immunoprecipitated by an anti-Flag antibody and eluted under native conditions, and then the His-V5-β3/Flag-β3 complexes were precipitated by His pulldown. Immunoblotting of these complexes with an anti-GFP antibody (Fig. 1F) revealed that Venus-β3 (lane 1), but not vector alone (lane C), survived every stage of this 2-stage pulldown procedure. Each of the steps of immunoprecipitation was β3-specific, as it was revealed by the Western blot assay of the aliquots with an antibody to β3 (Supplemental Fig. S1D). Taken together, this result provides evidence for the existence of β3 complexes composed of ≥3 individual β3 proteins. Thus, Cavβs show tendency to form multimolecular complexes.
To investigate whether the interactions between Cavβ subunits are direct rather than indirect, we performed a Far-Western blotting by detecting a purified V5-His-β3 with purified Flag-β3 as a probe (Fig. 1G). V5-His-β3 was purified by His pulldown from the lysates of the expressing cells and the aliquots of the purified protein were separated by SDS-PAGE and probed by Far-Western blotting using purified Flag-β3. Our results indicated that the interactions between different β3 molecules are direct and may occur in vitro. Taking into account the existence of β3 complexes containing ≥3 individual molecules (see Fig. 1F) and the apparent molecular masses of the oligomers (see Fig. 1C), these data suggest that the mixture of Cavβ oligomers contains trimers together with higher supramolecular complexes.
Homo- and heteromeric complexes of Cavβs
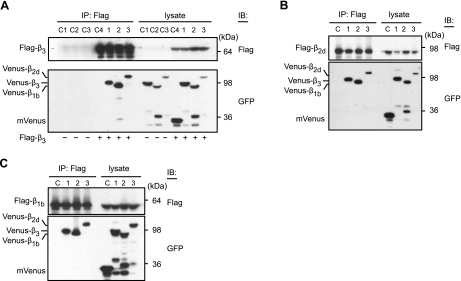
To examine whether Cavβs form both homo- and heteromeric complexes, we coexpressed Flag-β3 with mVenus-labeled β3, β1b, or β2d in Cos7 cells. Oligomeric complexes were immunoprecipitated with an anti-Flag antibody and analyzed on Western blots by anti-Flag and anti-GFP antibodies. In a typical experiment, shown in Fig. 2A, Flag-β3 coimmunoprecipitated with Venus-β3 (lane 1) as well as with mVenus-β1b (lane 2) and mVenus-β2d (lane 3). Because an anti-Flag antibody did not pull down the mVenus-labeled β subunits in the absence of Flag-β3 (Fig. 2A, lanes C1–C3), and Flag-β3 was not coprecipitated with mVenus (lane C4), this result clearly demonstrates that β3 forms complexes with β3, β1b, and β2d. Immunoblot analyses revealed similar results for Flag-β2d (Fig. 2B) and Flag-β1b (Fig. 2C), suggesting that all major Cavβ subunits have a tendency to form homo- and heteromeric complexes.
Figure 2.
Cavβs form both homo- and heterooligomers. A) Venus-labeled β3 (lanes C1, 1) β1b (lanes C2, 2), β2d (lanes C3, 3) (0.8 μg each), or mVenus (control, lane C4; 0.4 μg) were expressed in the absence (lanes C1–C3) or presence (lanes 1–3, C4) of Flag-β3 (0.5 μg) in Cos7 cells, immunoprecipitated by an anti-Flag antibody, and immunoblotted with anti-Flag and anti-GFP antibody. B, C) Venus-labeled Cavβs were also coimmunoprecipitated with Flag-β2d (B) or Flag-β1b (C). (Note that the presence of low-molecular-mass bands recognized by an anti-GFP antibody may represent degraded Cavβs).
Molecular structures responsible for Cavβ oligomerization
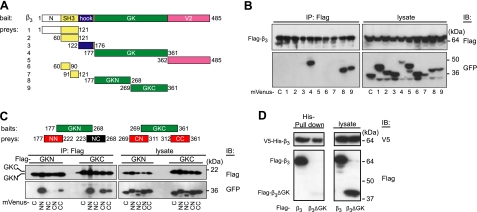
We next sought to determine whether β-subunit oligomerization originates from specific Cavβ structural domains (7, 36). Nine mVenus-labeled fragments of β3 (Fig. 3A) were coexpressed with Flag-β3 in Cos7 cells. Co-IP of β3 with an anti-Flag antibody and immunoblot analysis with an anti-GFP antibody (Fig. 3B) showed that Cavβ oligomerization does not involve the Src homology 3 (SH3) subdomain and is entirely due to the guanylate kinase (GK) subdomain of MAGUK. Because both the N- and C-terminal halves of GK (GKN and GKC, respectively) bind to β3 (Fig. 3B, lanes 8, 9), we further divided GK into 4 fragments (NN, NC, CN, and CC) for the analysis of binding activity (Fig. 3C). Results showed that NN and CC bind to GKN, while NN, CN, and CC bind to GKC. Thus, oligomerization of Cavβ subunits is mediated by multiple determinants residing in GK. Indeed, deletion of GK domain from the full length β3 completely abolished its binding to the full-size β3 (Fig. 3D). Mutagenesis analyses of the GK subdomain (see Supplemental Fig. S4) confirmed our conclusion that all 3 regions of GK, shown in Fig. 3C (red boxes), are responsible for the oligomerization. However, combined mutations of the residues that were found to be critical for the binding of GK fragments to β3 did not eliminate oligomerization of Cavβ3 (Supplemental Table S1). The β3 subunit contains 4 cysteine residues. Two of them are in the SH3 domain, which is not responsible for Cavβ oligomerization. We found that combined mutation of C328 and C346 in GK into alanines did not abolish oligomerization of β3 (Supplemental Table S1). Taken together, these data suggest that some yet unknown structural factors other than established residues and disulfide bonds contribute to the Cavβ oligomerization.
Figure 3.
Multiple determinants of Cavβ oligomerization. A) Domain structure of β3 (485 aa) with regard to the fragments tested for binding to β3. B) Co-IP of Flag-β3 with indicated mVenus-labeled fragments of β3 (lanes 1–9) and mVenus (lane C, control). C) Co-IP of Flag-labeled GKN and GKC with mVenus-labeled NN, NC, CN, and CC fragments or mVenus (lane C). D) Deletion of GK from the full-length β3 subunit abolished its binding with other β3 molecules. V5-His-β3 and Flag-β3 (left lanes) or Flag-β3ΔGK (right lanes) were coexpressed in Cos7 cells (see lysates, right panel). Unlike Flag-β3, Flag-β3ΔGK was not bound to V5-His-β3, as revealed by His pulldown (left panel). We used 0.5 μg of each plasmid for cotransfection.
Cavβ oligomers bind to the α1C subunit at its I-II linker
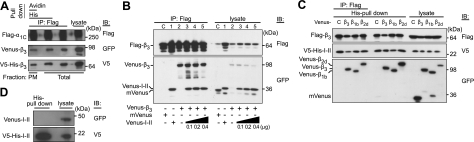
To find out whether binding to α1C affects oligomerization of Cavβ subunits, Flag-α1C, α2δ, V5-His-β3, and Venus-β3 were coexpressed in Cos7 cells, and the plasma-membrane proteins were labeled by extracellular biotinylation before cell lysis (Fig. 4A). Sequential His- and avidin-pulldown procedures identified differentially labeled β3 associated with Flag-α1C, thus confirming that β3 oligomers exist in the plasma-membrane-bound α1C/α2δ/β3 channel complexes. Because the AID region in α1C is the major binding site for Cavβ, we focused on the interaction of oligomeric β subunits with I-II linker (Fig. 4B). Flag-β3 and Venus-β3 were coexpressed in the absence (Fig. 4B, lane 2) or presence of the increasing amounts of Venus-I-II linker (25) (Fig. 4B, lanes 3–5). Immunoblot analysis with an anti-GFP antibody showed that the complexes pulled down by an anti-Flag antibody contained Venus-β3 independently on AID. The oligomerization of β3 was not affected by the presence of AID. In fact, I-II linker binds to homo- and heterooligomeric Cavβ complexes, as shown in Fig. 4C. Flag-β3 was coexpressed in Cos7 cells with Venus-β1b, Venus-β3 or Venus-β2d, or mVenus (Fig. 4C, lane C) in the presence of V5-His-I-II linker. Two-step purification of the oligomeric Cavβ complexes bound to I-II linker by His pulldown followed by immunoprecipitation with an anti-Flag antibody revealed the presence of homo- and heterooligomeric complexes composed of I-II linker and ≥2 individual β subunits. Since I-II linker does not oligomerize (Fig. 4D), these results suggest that I-II linker may dock complexes of Cavβ subunits. Taken together, our data show that Cavβs oligomerize at α1C subunits residing in the plasma membrane and bind to the AID.
Figure 4.
Cavβ oligomers bind to α1C at the α1C I-II linker. A) Oligomers of β3 exist in the plasma-membrane-bound α1C/α2δ/β3 channel complexes. Flag-α1C, α2δ, V5-His-β3, and Venus-β3 were coexpressed in Cos7 cells, and the plasma-membrane proteins were biotinylated. Anti-Flag IP of cell lysate isolated β3 bound to α1C. His pulldown confirmed the interaction of V5-His and Venus-β3 associated with α1C, while avidin pulldown confirmed it in the plasma-membrane fraction of α1C. B) Oligomerization of β3 is not affected by AID. Co-IP of homooligomers of Flag-β3 and Venus-β3 in the absence (lanes C, 2) or presence of increasing amounts of Venus-I-II linker containing AID (25) (0.1–0.4 μg/transfection, lanes 3–5). C) Evidence that I-II linker binds to Cavβ homo- and heterooligomers. Co-IP of Flag-β3 and V5-His-I-II linker with Venus-labeled β3, β1b, or β2d. Constructs were coexpressed in Cos7 cells. His pulldown was performed to isolate β subunits bound to AID, and the subsequent anti-Flag co-IP of the isolated β subunits was performed to check whether they were oligomers or monomers. D) Lack of oligomerization of I-II linker. His pulldown of V5-His-I-II linker and Venus-I-II linker coexpressed in Cos7 cells revealed the absence of Venus-I-II linker in the precipitate. Plasmids used for cotransfection were as following: Flag-α1C (1.2 μg), α2δ (1.0 μg), V5-His-β3 (0.5 μg), Venus-CaVβs (0.8 μg), V5-His-I-II linker (0.3 μg), and Venus-I-II linker (0.4 μg or as indicated).
Functional modulation of calcium channel associated with Cavβ oligomerization
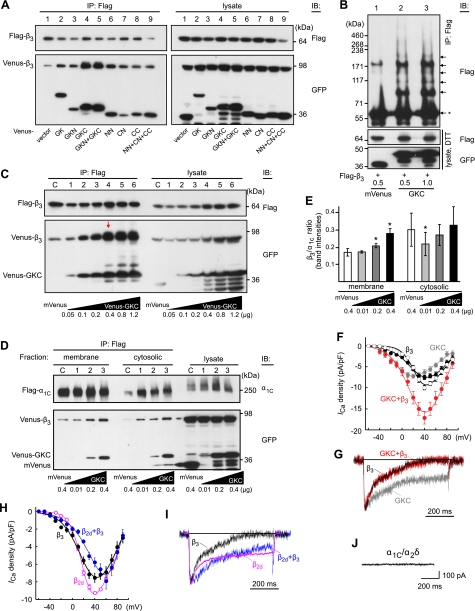
It is interesting that all active fragments of GK (see Fig. 3C) increased the binding between Venus- and Flag-labeled β3 as compared to control (Fig. 5A, lane 1), GKC showing the strongest enhancing activity. The non-GK fragments had no effect on the binding between different β3 molecules (Supplemental Fig. S5). Effect of GKC on β3 oligomerization was further confirmed by the enhancement of a ladder-like pattern on the blot (Fig. 5B, arrows), when Flag-β3 was coexpressed with GKC (Fig. 5B, lanes 2, 3). The maximum augmentation of β3 oligomerization was reached (Fig. 5C, red arrow) on cotransfection of β3 with GKC at 2:1 ratio (plasmid, w/w). The presence of GK fragments did not affect the interaction of β3 with I-II linker (Supplemental Fig. S6A), although some of these fragments exhibited direct binding activity to the I-II linker (Supplemental Fig. S6B). We then confirmed that coexpression of GKC stimulates β3–α1C association in the confines of the plasma membrane (Fig. 5D). Flag-α1C, α2δ, and Venus-β3 were coexpressed in Cos7 cells in the presence of increasing amounts of GKC, and the membrane bound complexes were labeled by cell surface biotinylation and isolated by avidin pulldown under conditions described in Fig. 4A. In the membrane fraction, increased expression of GKC leads to a significant increase in the amount of β3 coimmunoprecipitated with approximately the same amount of α1C (P<0.05, n=5) suggesting an increase in the β3/α1C ratio in the plasma membrane complexes. In the cytosolic fraction, there was no such increase in the β3/α1C ratio (Fig. 5D, E). Given the overexpression of β3 (as evident from the comparison of β3 and α1C ratio in the lysate with that in the coimmunoprecipitate), this result demonstrated that the coexpression of GKC with the calcium channel complex increases the efficiency of β3 association with the plasma membrane α1C molecules independently on the excess of β3 but in a GKC dose-dependent manner.
Figure 5.
Functional significance of Cavβ oligomerization. A-C) GKC increases oligomerization of β3. A) mVenus (lane 1) or indicated Venus-labeled fragments (lanes 2–9) were coexpressed with a mixture of Flag-β3 and Venus-β3. The β3 complexes were immunoprecipitated by an anti-Flag antibody and immunoblotted by an anti-GFP antibody. B) Flag-β3 (0.5 μg) was coexpressed with mVenus (lane 1) or increasing amounts of Venus-GKC (lanes 2, 3). Top panel: anti-Flag IP and immunoblotting without DTT treatment. Arrows mark oligomers; asterisk indicates monomer. C) Mixture of Flag-β3 (0.3 μg) and Venus-β3 (0.5 μg) plasmids was supplemented with mVenus (lane C, control) or increasing amounts of Venus-GKC vector (0.05–1.2 μg/transfection, lanes 1–6). Flag-β3 was immunoprecipitated from Cos7 cells by an anti-Flag antibody and immunoblotted with Flag or GFP antibodies as indicated. Red arrow indicates an apparent maximum augmentation of β3 oligomerization. D, E) Coexpression of GKC stimulates β3-α1C association in the plasma membrane. D) Flag-α1C, α2δ, and Venus-β3 (1.2, 1.0, and 0.8 μg, respectively) were coexpressed in Cos7 cells in the presence of mVenus (control) or increasing amounts of Venus-GKC (0.01–0.4 μg/transfection). Plasma membrane fraction of the Flag coimmunoprecipitates was isolated by avidin pulldown, as described in Fig. 4A, and the flow-through fractions were analyzed for cytosolic complexes. Resulting immunoblot is representative of 5 independent experiments, which exhibited similar levels of band intensity. E) Band intensities of β3 were normalized to the respective band intensities of α1C to estimate the β3/α1C ratio in the channel complex. Histogram is average of 5 measurements with Image J to quantify the band intensities (mean±sd); Students' t test was used for statistical analysis in comparison to control. *P < 0.05; paired 2-tailed distribution. F, G) Augmentation of β3 homooligomerization enhances the calcium current density. Cos7 cells were transfected with α1C (1.2 μg), α2δ (1 μg), and either 0.8 (black circles) or 1.6 μg (open circles) of β3, GKC (0.4 μg, gray), or a mixture of 0.8 μg β3 and 0.4 μg GKC (red). Shown are respective current-voltage relationships (F) and representative traces of the Ca2+ currents recorded in response to a stepwise shift of membrane potential from the holding potential of −90 to +20 mV and scaled to the peak current (G). H, I) Effect of heterooligomerization on current-voltage relationship (H) and kinetics of inactivation of the Ca2+ current (I). Cos7 cells were transfected as above except using 0.4 μg each β3 and β2d (blue). For comparison, respective β2d channel data are shown in magenta (25). J) Lack of calcium channel activity (at +20 mV) in Cos7 cell expressing α1C (1.2 μg) and α2δ (1 μg).
Coexpression of the calcium channel with β3 and GKC (2:1, plasmid w/w) in Cos7 cells resulted in a 2.3-fold increase of the calcium current density (red; n=10) as compared to β3 (n=5, P<0.01; Fig. 5F, solid circles), whereas doubling the cDNA amount of β3 in the α1C/α2δ/β3 cotransfection in the absence of GKC lead to only a slight increase in the current density (n=5; Fig. 5F, open circles). In the control experiment, coexpression of α1C and α2δ in the absence of Cavβ produced silent channels (Fig. 5J) (12). Coexpression of α1C and α2δ with GKC in the absence of β3 generated a low-amplitude calcium current (Fig. 5G, gray trace) characterized by the voltage dependence shifted by 20 mV to the hyperpolarizing direction and a notably slower inactivation kinetics of the calcium current as compared to β3 or β3 + GKC (Fig. 5F, G). No significant changes in reversal potential (104.5±3.4 mV), voltage at 50% of current activation (V0.5=25.9±0.9 mV), slope factor kI-V (−16.8±0.3), and kinetics of the current decay (Fig. 5G, black and red traces) were observed. In contrast, heterooligomerization of β3+β2d (1:1; Fig. 5H, blue) caused a 20-mV depolarization shift of the peak I-V curve (V0.5=58.1±6.9 mV, kI-V=−20.0±1.4, n=9) with regard to those of β3 (Fig. 5H, black solid circles) and β2d (Fig. 5H, magenta open circles; ref. 25). The rate of inactivation (Fig. 5I, blue trace) was not intermediate to that of β3 (Fig. 5I, black trace) and β2d (Fig. 5I, magenta trace) but notably reduced. Thus, Cavβ homo- and heterooligomerization significantly affect different functional properties of calcium channels.
DISCUSSION
The major conclusions of our study are that all major Cavβ subunits form oligomers in both naturally occurring and recombinant Cav1.2 calcium channels and that Cavβ homooligomerization affects the calcium current density, while heterooligomerization may also affect voltage-dependence and inactivation kinetics of the current. These findings support the hypothesis of Tareilus et al. (18) and add new dimensions to our understanding of the roles of Cavβs. First of all, Cavβs modulate the calcium channel functions by direct association with α1 at multiple sites. In the Cav1.2 channel, those sites include AID (11) and IQ motifs (35), while β2 subunits, in addition, bind with their unique C-terminal domain to the pre-IQ LA region (25). As we have shown previously (25), each of these 3 determinants is sufficient to support the calcium current in the presence of Cavβ (e.g., β2d). Because Cavβ oligomerization occurs independently on association with α1C (Fig. 4B), each of these sites probably interacts with higher order Cavβ complexes rather than monomers. Thus, multifaceted Cavβ modulation extends into a realm where homo- or heteromeric Cavβ complexes of variable size mediate the regulatory functions, but how many complexes are involved is less clear.
Another critical element of α1C-Cavβ interaction seems to be their ratio. A stoichiometric 1:1:1.3 ratio of α1B, α2δ, and β3 is sufficient for the purified and reconstituted N-type Cav2.2 calcium channel to retain such essential functional properties of the native channel as distinct voltage dependence and sensitivity to ω-conotoxin blockade (13). The finding of Cavβ oligomerization puts a new twist on the role of Cavβ while maintaining the basic structural principles of α1C-Cavβ interaction. In a double-transgenic mouse model (22), an enhanced expression of β2 over constitutive Cav1.2 caused increase of the single channel activity, suggesting a mechanistic link to the development of heart failure. In a different study (20), an adenovirus-assisted overexpression of β2a in ventricular myocytes caused a 3-fold increase in the calcium current density. These results may well be explained by the homo- or heterooligomerization of Cavβ subunits. Indeed, an augmentation of β3 homooligomerization increased the β3/α1C ratio on the plasma membrane as well as the calcium current density without significant changes in voltage-dependence and kinetics of the current (Fig. 5F, G). Thus, we have a case where Cavβ oligomerization may provide a mechanistic explanation for the relationship between the properties of the channel and levels of Cavβ expression. This basic mechanism may have a role in both the developmental-dependent and pathophysiological changes of calcium channel functioning such as increased frequency of Cav1.2 coupled gating events in hypertensive smooth muscle cells and Timothy syndrome (LQT8) (37). Moreover, Cavβ heterooligomerization may contribute to the remodeling of calcium channel properties in response to the increased expression of β2 subunits in heart failure (22) and be a pathogenic factor in hypertension (38) and other pathophysiological conditions.
Binding studies (Fig. 1D) showed that ≥3 individual β3 proteins coassemble to from oligomeric complex. However, the mechanism of Cavβ oligomerization remains unresolved, although 3 determinants appear to be identified in our study. The oligomerization of “classic” MAGUK (e.g., PSD-95) is mediated by swapping of complementary SH3 subdomains (39). Surprisingly, domain mapping experiments did not show an involvement of the SH3 subdomain of β3 in intermolecular interaction, all 3 identified determinants of Cavβ oligomerization being localized to GK (Fig. 3). These results are consistent with the previous report that Cavβ GK fragments expressed in bacteria dimerize and may modulate Cav2.3 channel gating (40). Although alanine mutations of each of the identified determinants inhibited binding of the respective fragments to β3 (Supplemental Fig. S4), the combined mutation of all 3 regions was not sufficient to eliminate oligomerization of β3. Which unidentified yet structural factors contribute to assembly of Cavβ subunits, why some isolated GK fragments (e.g., GKC; Fig. 5C) promote Cavβ oligomerization, and how these properties contribute to Cavβ regulation of calcium signaling are questions remaining to be answered.
We have shown previously that the structural organization of Cav1.2 channels in the plasma membrane depends on the type of Cavβ subunits present (1). Although all major types of Cavβ tend to form oligomers, the extent of oligomerization may vary. Modulation of Ca2+ channel through oligomerization of Cavβs, described here for Cav1.2, may provide a common mechanism in muscle, secretory, and neuronal Cav1 and Cav2 channels, which also require Cavβs for function. The recent finding of interaction between β1b subunits of Cav2 calcium channels revealed recently by the yeast 2 hybrid technique (see Supplemental Table 1 in ref. 41) supports this hypothesis.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Aging Intramural Research Program (Z01 AG000294–08 to N.M.S.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Kobrinsky E., Abrahimi P., Duong S. Q., Thomas S., Harry J. B., Patel C., Lao Q. Z., Soldatov N. M. (2009) Effect of Cavβ subunits on structural organization of Cav1.2 calcium channels. PLoS ONE 4, e5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. Y., Tsien R. W. (1988) Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc. Natl. Acad. Sci. U. S. A. 85, 2398–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navedo M. F., Amberg G. C., Votaw V. S., Santana L. F. (2005) Constitutively active L-type Ca2+ channels. Proc. Natl. Acad. Sci. U. S. A. 102, 11112–11117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westenbroek R. E., Ahlijanian M. K., Catterall W. A. (1990) Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature 347, 281–284 [DOI] [PubMed] [Google Scholar]
- 5.Catterall W. A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 [DOI] [PubMed] [Google Scholar]
- 6.Dolphin A. C. (2003) β Subunits of voltage-gated calcium channels. J. Bioenergetics Biomembranes 35, 599–620 [DOI] [PubMed] [Google Scholar]
- 7.McGee A. W., Nunziato D. A., Maltez J. M., Prehoda K. E., Pitt G. S., Bredt D. S. (2004) Calcium channel function regulated by the SH3-GK module in β subunits. Neuron 42, 89–99 [DOI] [PubMed] [Google Scholar]
- 8.Opatowsky Y., Chen C.-C., Campbell K. P., Hirsch J. A. (2004) Structural analysis of the voltage-dependent calcium channel β subunit functional core and its complex with the α1 interaction domain. Neuron 42, 387–399 [DOI] [PubMed] [Google Scholar]
- 9.Van Petegem F., Clark K. A., Chatelain F. C., Minor D. L., Jr. (2004) Structure of a complex between a voltage-gated calcium channel β-subunit and an α-subunit domain. Nature 429, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y.-H., Li M.-H., Zhang Y., He L.-L., Yamada Y., Fitzmaurice A., Shen Y., Zhang H., Tong L., Yang J. (2004) Structural basis of the α1-β subunit interaction of voltage-gated Ca2+ channels. Nature 429, 675–680 [DOI] [PubMed] [Google Scholar]
- 11.Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T. P., Campbell K. P. (1994) Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature 368, 67–70 [DOI] [PubMed] [Google Scholar]
- 12.Ravindran A., Lao Q. Z., Harry J. B., Abrahimi P., Kobrinsky E., Soldatov N. M. (2008) Calmodulin-dependent gating of Cav1.2 calcium channels in the absence of Cavβ subunits. Proc. Natl. Acad. Sci. U. S. A. 105, 8154–8159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witcher D. R., De Waard M., Sakamoto J., Franzini-Armstrong C., Pragnell M., Kahl S. D., Campbell K. P. (1993) Subunit identification and reconstitution of the N-type Ca2+ channel complex purified from brain. Science 261, 486–489 [DOI] [PubMed] [Google Scholar]
- 14.Colecraft H. M., Alseikhan B., Takahashi S. X., Chaudhuri D., Mittman S., Yegnasubramanian V., Alvania R. S., Johns D. C., Marban E., Yue D. T. (2002) Novel functional properties of Ca2+ channel β subunits revealed by their expression in adult rat heart cells. J. Physiol. 541, 435–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miriyala J., Nguyen T., Yue D. T., Colecraft H. M. (2008) Role of Cavβ subunits, and lack of functional reserve, in protein kinase A modulation of cardiac Cav1.2 channels. Circ. Res. 102, e54–64 [DOI] [PubMed] [Google Scholar]
- 16.Wei S.-K., Colecraft H. M., DeMaria C. D., Peterson B. Z., Zhang R., Kohout T. A., Rogers T. B., Yue D. T. (2000) Ca2+ channel modulation by recombinant auxiliary β subunits expressed in young adult heart cells. Circ. Res. 86, 175–184 [DOI] [PubMed] [Google Scholar]
- 17.Canti C., Davies A., Berrow N. S., Butcher A. J., Page K. M., Dolphin A. C. (2001) Evidence for two concentration-dependent processes for β-subunit effects on α1B calcium channels. Biophys. J. 81, 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tareilus E., Roux M., Qin N., Olcese R., Zhou J., Stefani E., Birnbaumer L. (1997) A Xenopus oocyte β subunit: evidence for a role in the assembly/expression of voltage-gated calcium channels that is separate from its role as a regulatory subunit. Proc. Natl. Acad. Sci. U. S. A. 94, 1703–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasuda T., Lewis R. J., Adams D. J. (2004) Overexpressed Cavβ3 inhibits N-type (Cav2.2) calcium channel currents through a hyperpolarizing shift of “ultra-slow” and “closed-state” inactivation. J. Gen. Physiol. 123, 401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X., Zhang X., Kubo H., Harris D. M., Mills G. D., Moyer J., Berretta R., Potts S. T., Marsh J. D., Houser S. R. (2005) Ca2+ Influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ. Res. 97, 1009–1017 [DOI] [PubMed] [Google Scholar]
- 21.Hullin R., Asmus F., Ludwig A., Hersel J., Boekstegers P. (1999) Subunit expression of the cardiac L-type calcium channel is differentially regulated in diastolic heart failure of the cardiac allograft. Circulation 100, 155–163 [DOI] [PubMed] [Google Scholar]
- 22.Hullin R., Matthes J., von Vietinghoff S., Bodi I., Rubio M., D'Souza K., Khan I. F., Rottländer D., Hoppe U. C., Mohacsi P., Schmitteckert E., Gilsbach R., Bünemann M., Hein L., Schwartz A., Herzig S. (2007) Increased expression of the auxiliary β2-subunit of ventricular L-type Ca2+ channels leads to single-channel activity characteristic of heart failure. PLoS ONE 2, e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neely A., Garcia-Olivares J., Voswinkel S., Horstkott H., Hidalgo P. (2004) Folding of active calcium channel β1b-subunit by size-exclusion chromatography and its role on channel function. J. Biol. Chem. 279, 21689–21694 [DOI] [PubMed] [Google Scholar]
- 24.Koushik S. V., Chen H., Thaler C., Puhl H. L., III, Vogel S. S. (2006) Cerulean, Venus, and VenusY67C FRET reference standards. Biophys. J. 91, L99–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lao Q. Z., Kobrinsky E., Harry J. B., Ravindran A., Soldatov N. M. (2008) New determinant for the Cavβ2 subunit modulation of the Cav1.2 calcium channel. J. Biol. Chem. 283, 15577–15588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wittig I., Braun H.-P., Schägger H. (2006) Blue native PAGE. Nat. Protocols 1, 418–428 [DOI] [PubMed] [Google Scholar]
- 27.Hullin R., Singer-Lahat D., Freichel M., Biel M., Dascal N., Hofmann F., Flockerzi V. (1992) Calcium channel β subunit heterogeneity: functional expression of cloned cDNA from heart, aorta and brain. EMBO J. 11, 885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh C. P., Davies A., Butcher A. J., Dolphin A. C., Kitmitto A. (2009) Three-dimensional structure of Cav3.1: comparison with the cardiac L-type voltage-gated calcium channel monomer architecture. J. Biol. Chem. 284, 22310–22321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akiyama Y., Ito K. (1993) Folding and assembly of bacterial alkaline phosphatase in vitro and in vivo. J. Biol. Chem. 268, 8146–8150 [PubMed] [Google Scholar]
- 30.Yeh M. F., Trela J. M. (1976) Purification and characterization of a repressible alkaline phosphatase from Thermus aquaticus. J. Biol. Chem. 251, 3134–3139 [PubMed] [Google Scholar]
- 31.Powers P. A., Liu S., Hogan K., Gregg R. G. (1992) Skeletal muscle and brain isoforms of a β-subunit of human voltage-dependent calcium channels are encoded by a single gene. J. Biol. Chem. 267, 22967–22972 [PubMed] [Google Scholar]
- 32.Herzig S., Khan I. F. Y., Grundemann D., Matthes J., Ludwig A., Michels G., Hoppe U. C., Chaudhuri D., Schwartz A., Yue D. T., Hullin R. (2007) Mechanism of Cav1.2 channel modulation by the amino terminus of cardiac β2-subunits. FASEB J. 21, 1527–1538 [DOI] [PubMed] [Google Scholar]
- 33.Foell J. D., Balijepalli R. C., Delisle B. P., Yunker A. M. R., Robia S. L., Walker J. W., McEnery M. W., January C. T., Kamp T. J. (2004) Molecular heterogeneity of calcium channel β-subunits in canine and human heart: evidence for differential subcellular localization. Physiol. Genomics 17, 183–200 [DOI] [PubMed] [Google Scholar]
- 34.Swamy M., Siegers G. M., Minguet S., Wollscheid B., Schamel W. W. A. (2006) Blue native polyacrylamide gel electrophoresis (BN-PAGE) for the identification and analysis of multiprotein complexes. Sci. STKE 2006, pl4. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R., Dzhura I., Grueter C. E., Thiel W., Colbran R. J., Anderson M. E. (2005) A dynamic α-β inter-subunit agonist signaling complex is a novel feedback mechanism for regulating L-type Ca2+ channel opening. FASEB J. 19, 1573–1575 [DOI] [PubMed] [Google Scholar]
- 36.Hanlon M. R., Berrow N. S., Dolphin A. C., Wallace B. A. (1999) Modelling of a voltage-dependent Ca2+ channel β subunit as a basis for understanding its functional properties. FEBS Lett. 445, 366–370 [DOI] [PubMed] [Google Scholar]
- 37.Navedo M. F., Cheng E. P., Yuan C., Votaw S., Molkentin J. D., Scott J. D., Santana L. F. (2010) Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ. Res. 106, 748–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy D., Ehret G. B., Rice K., Verwoert G. C., Launer L. J., Dehghan A., Glazer N. L., Morrison A. C., Johnson A. D., Aspelund T., Aulchenko Y., Lumley T., Kottgen A., Vasan R. S., Rivadeneira F., Eiriksdottir G., Guo X., Arking D. E., Mitchell G. F., Mattace-Raso F. U. S., Smith A. V., Taylor K., Scharpf R. B., Hwang S.-J., Sijbrands E. J. G., Bis J., Harris T. B., Ganesh S. K., O'Donnell C. J., Hofman A., Rotter J. I., Coresh J., Benjamin E. J., Uitterlinden A. G., Heiss G., Fox C. S., Witteman J. C. M., Boerwinkle E., Wang T. J., Gudnason V., Larson M. G., Chakravarti A., Psaty B. M., van Duijn C. M. (2009) Genome-wide association study of blood pressure and hypertension. Nat. Genet. 41, 677– 687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGee A. W., Dakoji S. R., Olsen O., Bredt D. S., Lim W. A., Prehoda K. E. (2001) Structure of the SH3-guanylate kinase module from PSD-95 suggests a mechanism for regulated assembly of MAGUK scaffolding proteins. Mol. Cell 8, 1291–1301 [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Gutierrez G., Miranda-Laferte E., Nothmann D., Schmidt S., Neely A., Hidalgo P. (2008) The guanylate kinase domain of the β-subunit of voltage-gated calcium channels suffices to modulate gating. Proc. Natl. Acad. Sci. U. S. A. 105, 14198–14203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Page K. M., Heblich F., Margas W., Pratt W. S., Nieto-Rostro M., Chaggar K., Sandhu K., Davies A., Dolphin A. C. (2010) N Terminus is key to the dominant negative suppression of Cav2 calcium channels. Implications for episodic ataxia type 2. J. Biol. Chem. 285, 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.