Abstract
Fluid balance is critical to life and hence is tightly controlled in the body. Angiotensin II (ANGII), one of the most important components of this regulatory system, is recognized as a dipsogenic hormone that stimulates vasopressin (VP) expression and release. However, detailed mechanisms regarding how ANGII brings about these changes are not fully understood. In the present study, we show initially that the osmoregulatory functions of secretin (SCT) in the brain are similar to those of ANGII in mice and, more important, we discovered the role of SCT as the link between ANGII and its downstream effects. This was substantiated by the use of two knockout mice, SCTR−/− and SCT−/−, in which we show the absence of an intact SCT/secretin receptor (SCTR) axis resulted in an abolishment or much reduced ANGII osmoregulatory functions. By immunohistochemical staining and in situ hybridization, the proteins and transcripts of SCT and its receptor are found in the paraventricular nucleus (PVN) and lamina terminalis. We propose that SCT produced in the circumventricular organs is transported and released in the PVN to stimulate vasopressin expression and release. In summary, our findings identify SCT and SCTR as novel elements of the ANGII osmoregulatory pathway in maintaining fluid balance in the body.—Lee, V. H. Y., Lee, L. T. O., Chu, J. Y. S., Lam, I. P. Y., Siu, F. K. Y, Vaudry, H., Chow, B. K. C. An indispensable role of secretin in mediating the osmoregulatory functions of angiotensin II.
Keywords: fluid homeostasis, secretin receptor-deficient mice, circumventricular organs, vasopressin
Regulation of body fluid solute concentrations is critical to recovering from dehydration. Dehydration causes a rise in plasma osmolality and a volume reduction of extracellular fluid. In either situation, both physiological and behavioral responses, including release of the antidiuretic hormone vasopressin (VP) to prevent further water loss in the kidney (1) and the dipsogenic angiotensin II (ANGII) to increase fluid intake (2), are vital for maintaining a constant body fluid content.
ANGII functions to augment thirst, VP release, and blood pressure via binding on its receptors (AT1 and AT2). The AT1 receptor appears to be responsible for most key ANGII responses, including water and blood pressure homeostasis (3). In the brain, both plasma hyperosmolality and ANGII stimulate the lamina terminalis, consisting of the subfornical organ (SFO), median preoptic nucleus (MnPO), and organum vasculosum of the lamina terminalis (OVLT), which has been recognized as a critical site for regulating water intake and VP secretion (1, 4). The SFO and OVLT are referred to as circumventricular organs (CVOs) located outside the blood-brain barrier and equipped with osmoreceptors (4), AT1 receptors, and angiotensinergic nerve endings (5). Thus, they are capable of sensing osmolality and hormonal fluctuations. Different components of the lamina terminalis are reciprocally connected (6). The CVOs send direct efferent neural signals to VP-synthesizing magnocellular neurons in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) (7), and also via MnPO neurons, which also integrates neural signals from CVOs with other inputs such as those from vascular baroreceptors (7). Taken together, the key question in understanding water homeostasis in the body is the mechanism of how ANGII regulates water intake and VP release in the central nervous system (CNS).
Secretin (SCT) is a classical gastrointestinal hormone that has recently been suggested to act as a neuropeptide (8–10) and an antidiuretic hormone in the neurohypophysial-renal axis (11). The roles of SCT in renal water reabsorption have remained controversial (12) until recent data from the SCT receptor-knockout (SCTR−/−) mice showing that SCT, acting via a VP-independent mechanism, is an alternative pathway to stimulate renal water reabsorption (11). Regarding the source of SCT, evidence was recently revealed (13) showing that SCT could be released from the posterior pituitary into circulation on dehydration or direct electrical stimulation of the PVN. In rat hypothalamus, increased expressions of SCT, SCTR, and VP genes were observed during dehydration. In addition, intracerebroventricular (i.c.v.) SCT injection has been shown to stimulate VP expression in the hypothalamus and VP release into circulation in rats.
In this study, we provide evidence that SCT is the missing link that connects ANGII with its effects in the CNS. On top of its antidiuretic role in the kidney, SCT is a neuroactive peptide that directly stimulates VP expression and release, as well as water intake, in mice. To achieve this, two recently developed mouse models, SCTR−/− and SCT−/−, were employed. These models provide unique physiological environments to investigate SCT-specific functions by showing the absence of effects in the SCTR−/− and recovery of effects in the SCT−/− mice by introduction of synthetic SCT.
MATERIALS AND METHODS
Animals
Procedures of animal care and handling were in accordance with the protocols approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong. All experiments were carried out using adult mice (20–25 g) of at least N5 generation, which were kept in a temperature-controlled room with a 12-h light-dark cycle. Unless stated otherwise, mice were fed ad libitum with standard rodent chow (no. 5010; Test Diet, Richmond, IN, USA) and water.
Construction of the SCT−/− and SCTR−/− mice
The development of the SCTR−/− mice has previously been described (11). To achieve specific knockout of the SCT gene, the SCT-targeting vector containing 2 loxP sites flanking the SCT gene and a frt-neofrt cassette at nt −439 and +1186, relative to the transcriptional start site of the SCT gene, was constructed. The targeting vector was linearized and transfected into embryonic stem (ES) cells by electroporation. Positive clones were selected and microinjected into blastocysts from C57BL/6 female mice, which were subsequently implanted into the uterus of ICR foster mother mice. Male chimeric offspring were backcrossed with C57BL/6 female mice. For the generation of SCT-null mice (Supplemental Fig. 1A), male agouti mice derived from genetically modified ES cells (n=1) were mated with female β-actin Cre mice. SCT+/− mice were backcrossed with female C57BL/6 mice to purify the mixed genetic background; each backcross was indicated by an increase in N number. SCT−/− mice were obtained by mating with the corresponding heterozygous mice. Genotypes of the mice were characterized by southern blotting (Supplemental Fig. 1B) with specific probes and PCR (Supplemental Fig. 1C).
Water deprivation and saline drinking
For water deprivation, the water bottle was removed for 24 h. To induce serum hypertonicity, drinking water was replaced by 2% saline. After 1 or 5 d, the control and saline-drinking mice were sacrificed, and their brains and blood samples were collected. Brains of control, dehydrated, and saline-drinking mice were used for laser-capture microdissection (LCM); blood serum osmolality was analyzed using a vapor pressure osmometer (Vapro 5520; Wescor, Inc., Logan, UT, USA), and plasma SCT and VP protein concentrations were measured by enzyme immunoassays (Phoenix Pharmaceuticals Inc., Burlingame, CA, USA) as described previously (13).
I.c.v. incannulation and drug administration
Mice were anesthetized and surgery was conducted in aseptic conditions. The coordinates of cannula implantation were determined according to mouse brain atlas. The cannula (11 mm long, 21-gauge stainless steel tubing) was stereotaxically placed (Bregma: 0.5 mm, lateral: 1.0 mm, depth: 2.0 mm) and secured using Loctite instant adhesive (Henkel, Folsom, CA, USA) and dental acrylic. Artificial cerebrospinal fluid (ACSF; 2 or 5 μl), SCT (500 ng/5 μl; 60677; AnaSpec, Fremont, CA, USA), and ANGII (100 ng/2 μl; 002–12; Phoenix Pharmaceuticals) were injected. For chronic injection, a brain infusion cannula (Brain Infusion Kit 3; Alzet, Cupertino, CA, USA) connected to a 7-d osmotic minipump (1007D; Alzet) was used. SCT and ANGII concentrations were 20.8 and 2.1 ng/h, respectively. For water intake measurements, mice were single-housed in metabolic cages after injection. In time course measurements, water was supplied via a plastic burette (0.1 ml graduation) attached to a spout (14), and water intake was read directly from the burette. For chronic injection study, the amount of consumed water was measured by weighing the bottle before and after a 24 h period.
LCM and quantitative real-time PCR
Cells of lamina terminalis, PVN, and cortex were isolated by LCM as described elsewhere (13). RNA of captured cells was prepared using the PicoPure RNA Isolation Kit (Molecular Devices, Silicon Valley, CA, USA). First-strand cDNAs were preamplified with TaqMan PreAmp Master Mix kit (Applied Biosystems, Foster City, CA, USA), followed by TaqMan real-time PCR with specific probes (GAPDH:4352339E; SCT:Mm01279165_g1; SCTR:Mm01290790_m1; and VP:Mm00437761_g1). Fluorescence signals were measured during the extension step by the 7300 Real-Time PCR System (Applied Biosystems). The threshold cycle (Ct) was defined as the fractional cycle number at which the fluorescence signal reached 10-fold sd of the baseline (from cycles 2 to 10). The ratio change in the target gene relative to the GAPDH control gene was determined by the 2−ΔΔCt method (15).
Immunohistochemical (IHC) staining
IHC staining was performed essentially as described elsewhere (11, 13). Brains isolated from mice were fixed in 3.7% formalin, embedded in paraffin, and sectioned (7 μm). Paraffin sections were dewaxed and rehydrated in graded ethanol. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide. Blocking of nonimmunological binding was performed with 5% normal goat serum for 2 h. Sections were then incubated with rabbit anti-SCT IgG (1:300 dilution; Millipore, Billerica, MA, USA), rabbit anti-SCTR IgG (1:500 dilution; Invitrogen, Carlsbad, CA, USA), or rabbit anti-cFos IgG (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. Knockout mouse brain sections were used as negative controls (SCT−/− for SCT and SCTR−/− for SCTR staining). The immunoreactive signals were detected by using the Vectastain ABC Elite kit (PK-6101, Vector Laboratories, Burlingame, CA, USA). In brief, after several washes with PBS, sections were treated with anti-rabbit biotinylated secondary antibody (1:200 dilution), followed by application of the avidin-biotin-horseradish peroxidase complex reagent. Finally, signals were visualized in light brown color by 1× DAB substrate (Roche Diagnostics, Shanghai, China) and counterstained with hematoxylin (Zymed Laboratories, San Francisco, CA, USA). For fluorescence staining, antibodies used were rabbit anti-AT1 IgG (1:100 dilution; Millipore), Alexa Fluor594 chicken anti-rabbit IgG (1:500 dilution; Invitrogen), rabbit anti-SCT IgG (1:300 dilution), and Alexa Fluor488 chicken anti-goat IgG (1:500 dilution; Invitrogen). Images were captured with the Zeiss LSM510 Meta computerized image analysis system (Carl Zeiss, Oberkochen, Germany).
In situ hybridization
For in situ hybridization, the wild-type (WT) mouse brains were used, while brains from the SCTR−/− and SCT−/− were used as controls. Paraffin sections were dewaxed, rehydrated, and treated with 0.5 M HCl, 5 μg/ml proteinase K, and 0.08 M triethanolamine/0.25% acetic anhydride. After 1 h of prehybridization (10 mM Tris, pH 7.5; 600 mM NaCl; 1 mM EDTA; 0.25% SDS; 10% dextran sulfate; 1× Denhardt's solution; 200 μg/ml yeast tRNA; and 50% formamide) without probes in a humidified chamber at 50°C, sections were incubated with hybridization buffer containing the labeled antisense SCT or SCTR probes (0.2 ng/μl) overnight at 50°C. For negative controls, an antisense probe without DIG-labeling (30×) was used to compete with the labeled probes. The fragments of mouse SCT (456 bp; nt −7 to +449 relative to ATG) and SCTR (501 bp; nt −33 to +468 relative to ATG) were used for generating the labeled antisense and unlabeled sense probes by the DIG RNA labeling kit (Roche) according to the manufacturer's instructions. On the next day, slides were washed in 2× SSC for 10 min (3 times), 1× SSC for 10 min, and 0.1× SCC for 10 min (3 times). Sections were blocked with 3% FBS for 1 h and incubated with anti-DIG antibody (dilution 1:500; Roche) overnight at 4°C. On the third day, the chromogenic substrate (Roche) NBT/BCIP was used for generating signals.
Statistical analysis
All data are shown as means ± se. The deviations between groups were analyzed using Prism 3.0 software (GraphPad Software Inc., San Diego, CA, USA). Unpaired t test was performed when 2 groups were under consideration, whereas data from >2 groups were analyzed by 1-way ANOVA, followed by Dunnett's test.
RESULTS
Functional SCT and SCTR are important for mediating the effects of the dipsogenic peptide ANGII
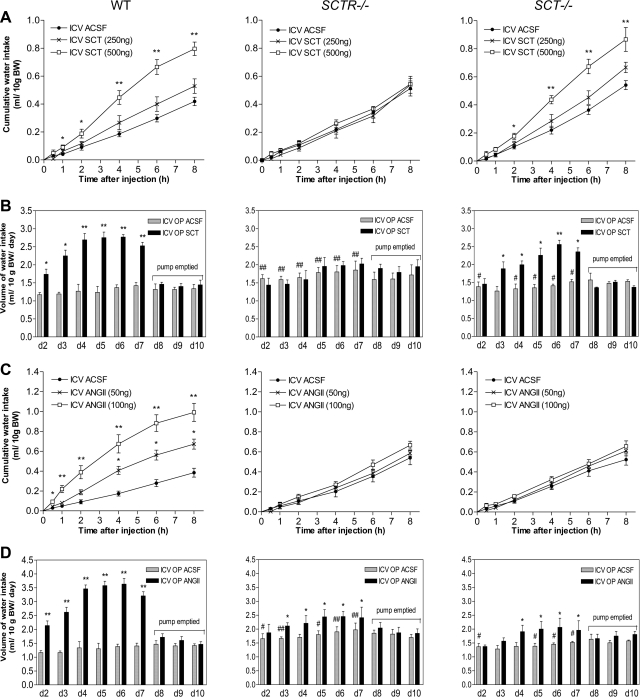
We have recently shown that, in rats, SCT is released from the posterior pituitary (13), and that the released SCT is able to regulate aquaporin-2 expression and translocation in kidney tubules (11). To investigate potential SCT-specific functions in the CNS to control water intake, a SCT−/− mouse line was generated for this study (Supplemental Fig. 1) and was used in conjunction with the SCTR−/− mice (11). Our data show that i.c.v. SCT stimulated drinking in WT mice (Fig. 1A) in a dose-dependent manner. Water intake was significantly increased 1 h postinjection (500 ng SCT; an increase of 0.05 ml/10 g body weight), and this difference became larger until the eighth hour. I.c.v. injection of half the amount of SCT (250 ng) also increased drinking, but the volume increments were insignificant. These effects of SCT were specific since the stimulated water intake was not observed in SCTR−/− mice, but was recovered when SCT was centrally injected into SCT−/− mice as in WT (Fig. 1A). To investigate a more long-term effect of i.c.v. SCT in regulating water intake, the peptide was continuously infused, using osmotic minipumps, for 7 d. Consistently, WT mice with continuous central SCT infusion exhibited significantly elevated daily water intake from d 2 to 7 (Fig. 1B). From d 8 to 10, as the SCT peptide in the pumps had been used up, water intake in the experimental group was dropped to control levels. In SCTR−/− mice, continuous i.c.v. SCT had no significant effect on water-drinking behavior, while it stimulated water intake in SCT−/− mice as in WT (Fig. 1B).
Figure 1.
Dipsogenic effects of central SCT or ANGII injection in mice. A, C) Time-course study on the effects of single-dose i.c.v. SCT (250 and 500 ng; A), ANGII (50 and 100 ng; C) or control ACSF injection into lateral ventricle on cumulative water intake at various times (0.5, 1, 2, 4, 6, and 8 h) in WT, SCTR−/−, and SCT−/− mice (n=10/group). B, D) Effects of continuous i.c.v. SCT (B), ANGII (D), or ACSF infusion on daily water intake in WT, SCTR−/−, and SCT−/− mice (n=6/group). Data are expressed as means ± se. *P < 0.05, **P < 0.001 vs. respective control; #P < 0.05, ##P < 0.001 vs. WT control.
I.c.v. ANGII is renowned as a dipsogenic factor to induce drinking responses, thus it would be of great interest to examine this central effect of ANGII in SCTR−/− and SCT−/− mice. Consistent with the increase in the daily water intake (16), a single i.c.v. ANGII injection significantly induced drinking in WT mice within 30 min using 100 ng peptide and from the fourth hour using 50 ng peptide (Fig. 1C). Dipsogenic action of ANGII was surprisingly completely abolished in SCTR−/− and SCT−/− mice (Fig. 1C). Similarly, while continuous i.c.v. ANGII infusion in WT mice caused a robust increase in drinking (Fig. 1D), it induced a much lower response in SCTR−/− and SCT−/− mice. This observation strongly indicates that the ANGII-mediated dipsogenic effects are dependent on an intact SCT/SCTR system in mice. These data also provide a possible explanation for the observed regulated fluid intake in mice lacking ANG (17), as SCT/SCTR may provide an alternative pathway.
SCT and SCTR are needed to mediate the ANGII actions in activating VP expression in the hypothalamus and release from the pituitary
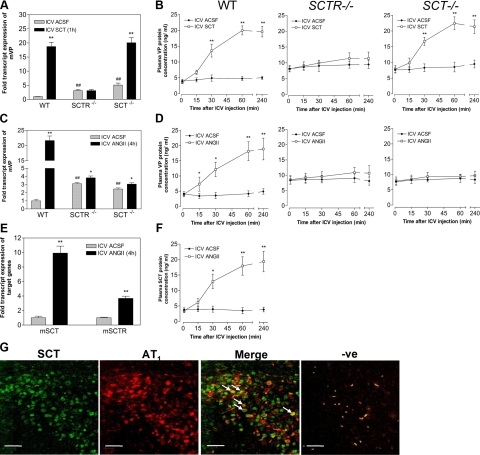
ANGII has long been shown to stimulate hypothalamic magnocellular neurons to release VP in hypertonic conditions; we have therefore investigated connections between ANGII, SCT, and VP using SCTR−/− and SCT−/− mice. Central SCT injection significantly activated VP expression in PVN (18.70-fold; Fig. 2A) and plasma VP release (2.75-fold at 30 min; Fig. 2B) compared to control. These effects were found to be dependent on the presence of a functional SCTR, as they were not observed in SCTR−/− (Fig. 2A, B), but could be reproduced in SCT−/− mice. These data are consistent with our previous findings in rats (13), confirming SCT's role via its receptor as a regulator of VP expression and VP release in rodents.
Figure 2.
Effects of central SCT or ANGII injection on VP and SCT expression and release. A–F) Effects of i.c.v. SCT (500 ng; A, B), ANGII (100 ng; C–F), or ACSF on mouse VP (A, C) and SCT expression levels (E) in the PVN, and plasma VP (B, D) and SCT levels (F) postinjection (15 and 30 min, 1 and 4 h) in WT, SCTR−/−, and SCT−/− mice. mRNA levels of target gene were normalized with GAPDH levels. Data are expressed as means ± se (n=6–8/group). *P < 0.05, **P < 0.001 vs. respective control; #P < 0.05, ##P < 0.001 vs. WT control. G) Double immunofluorescence staining shows colocalization of SCT and AT1 receptors in the PVN of mouse brain. White arrows indicate cell where SCT and AT1 receptor are expressed. Scale bars = 40 μm.
In WT mice, i.c.v. ANGII also drastically elevated VP expression in the PVN (21.63-fold; Fig. 2C), and these effects were almost abolished in SCTR−/− (1.23-fold) and SCT−/− (1.25-fold). Similar to i.c.v. SCT, central ANGII injection also triggered VP release (Fig. 2D) 15 min postinjection (2.14-fold). Stimulation of VP release also depended on SCT and SCTR, as much lowered plasma VP levels were found in SCTR−/− and SCT−/− mice (Fig. 2D), even after i.c.v. ANGII. These findings are important, showing that the SCT system is largely responsible for mediating functions of ANGII in PVN.
To confirm the role of SCT as a mediator of ANGII's actions, we next investigated whether centrally injected ANGII could alter SCT expression and SCT release. ANGII injection caused significant augmentations in both SCT and SCTR expression in the PVN of WT mice (9.92- and 3.67-fold; Fig. 2E). Apart from that, i.c.v. ANGII increased plasma SCT levels in WT 30 min after injection (3.27-fold; Fig. 2F), and the rise continued until the fourth hour. Using double immunofluorescence staining (Fig. 2G), AT1 receptor and SCT were found to colocalize in magnocellular neurons within the PVN, thus providing anatomical evidence for a possible regulation of SCT expression by ANGII. Collectively, these findings show the involvement and importance of central SCT in mediating ANGII's actions to activate VP expression and VP release.
SCT is responsible for stimulating VP expression in the PVN and VP release on hyperosmolality
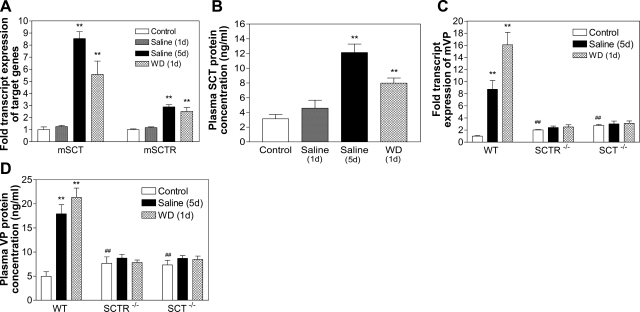
With the data showing that central SCT functions to mediate activities of ANGII, we next sought to investigate the effects of salt loading, which could produce a physiological rise in serum osmolality (18), on expressions of SCT, SCTR, and VP in the PVN. It should be noted that 1 d saline drinking does not affect serum osmolality, as shown previously (18); thus, data from both expression and release studies were similar to controls. Salt loading for 5 d, however, significantly increased serum osmolality in WT, SCTR−/−, and SCT−/− mice (Supplemental Fig. 2), and as a result, SCT and SCTR expression in the PVN (Fig. 3A) as well as plasma SCT concentrations (Fig. 3B) of WT mice were elevated when compared to controls given plain water. On the other hand, overnight dehydration already significantly up-regulated SCT and SCTR transcript levels in the PVN (Fig. 3A) and plasma SCT-IR (Fig. 3B). These data show that hyperosmolality induced by long-term salt loading and overnight water deprivation could induce SCT and SCTR expression in the PVN, and also SCT release into the circulation.
Figure 3.
Effects of hyperosmolality on SCT and SCTR expression and SCT release. Effects of salt loading (2%; 1 or 5 d) and water deprivation (1 d) on SCT and SCTR (A) and VP mRNA levels (C) in the PVN; and plasma SCT (B) and VP levels (D) of WT mice. mRNA levels of target genes are normalized with GAPDH levels. Data are expressed as means ± se (n=10/group). *P < 0.05, **P < 0.001 vs. respective control.
To study the role of SCT in mediating the effects of salt loading on VP expression and VP release, WT, SCTR−/−, and SCT−/− mice were subjected to 5 d saline drinking or overnight water deprivation. As expected in WT (19), drastically elevated VP expression in the PVN (8.74- and 16.10-fold, respectively; Fig. 3C) and VP release from the neurohypophysis (3.76- and 4.30-fold, respectively; Fig. 3D) were observed. In contrast, in SCTR−/− and SCT−/− mice, despite significant increases in serum osmolality (Supplemental Fig. 2), salt loading had little effect on VP expression and VP release (Fig. 3C and 3D). These data strongly indicate that, in response to hyperosmolality, SCT and VP are not utilizing parallel pathways; instead, an intact SCT/SCTR system in the PVN is a prerequisite for regulating VP expression and VP release.
Involvement of SCT in the lamina terminalis in hyperosmolality or direct ANGII stimulation
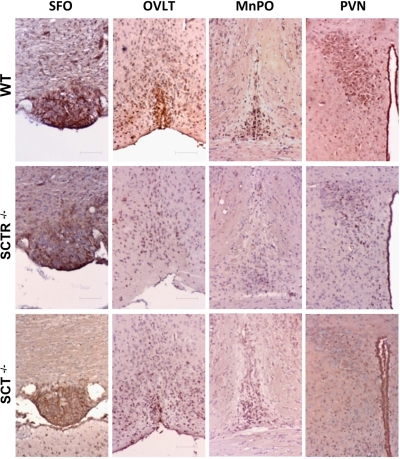
As shown in previous studies, there are abundant angiotensinergic neurons and AT1 receptors in the lamina terminalis and PVN (5). In addition, both i.c.v. ANGII and intravenous NaCl infusion increase Fos-IR in these regions (20, 21). We have also tested cFos stimulation in the lamina terminalis in response to increased serum osmolality via salt loading, and we found more cells with Fos-IR in the lamina terminalis and PVN (Supplemental Fig. 3A). Consistent with previous data in rats (13), i.c.v. SCT could activate Fos expression in both lamina terminalis and PVN (Supplemental Fig. 3B), indicating a potential link between dehydration, ANGII, and SCT with Fos expression. I.c.v. ANGII also stimulated Fos expression in these brain regions, as expected (Fig. 4); however, the activated Fos expression was reduced in the brains of knockout mice. This indicates that lamina terminalis and PVN are important sites where interaction of ANGII and SCT occurs. In addition, immunohistochemistry and in situ hybridization revealed the presence of SCT and SCTR proteins (Supplemental Fig. 4) and transcripts (Supplemental Fig. 5) in the lamina terminalis and PVN, providing evidences for the potential functions of SCT in upstream osmoregulatory sites.
Figure 4.
Changes in Fos immunoreactivity in the lamina terminalis and PVN on i.c.v. ANGII. Immunohistochemical staining shows Fos immunoreactivity in the SFO, MnPO, OVLT, and PVN on i.c.v. ANGII injection in WT, SCTR−/−, and SCT−/− mice. Scale bars = 6 μm.
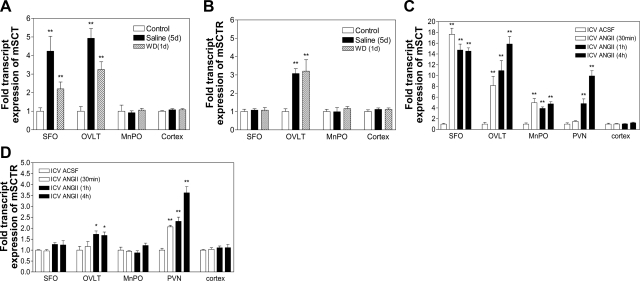
To further investigate the role of the SCT system in osmoregulation, WT mice were subjected to either saline drinking, dehydration or i.c.v. ANGII, and changes in SCT and SCTR expression in the lamina terminalis were measured. In WT, saline drinking and dehydration activated SCT expression in the SFO (4.24- and 2.20-fold; Fig. 5A) and OVLT (4.93- and 3.25-fold), while no significant changes were detected in the MnPO and the control cortex. Salt loading and dehydration also caused induction of SCTR expression in OVLT (3.08- and 3.21-fold, respectively; Fig. 5B), whereas there were no effects in other brain areas tested.
Figure 5.
SCT and SCTR expression in lamina terminalis on hyperosmolality and i.c.v. ANGII. Effects of (a–b) salt loading (2%; 5 d), water deprivation (1 d), and (c–d) i.c.v. ANGII or ACSF injection (30 min, 1 and 4 h) on mSCT and mSCTR expressions in the SFO, OVLT, MnPO, and cortex of WT brains. Data are expressed as means ± se (n = 6/group). *P < 0.05, **P < 0.001 vs. respective control.
I.c.v. ANGII caused a dramatic upsurge of SCT expression in the SFO 30 min postinjection (17.65-fold), and the level remained significantly high until the fourth hour (Fig. 5C). In OVLT, there was also induction of SCT at 30 min, with a continuous increase in the next hour. In MnPO, SCT expression was increased, but to a lesser extent when compared to other sites. In PVN, i.c.v. ANGII caused a delayed stimulation; significant changes in SCT expression were seen 1 h postinjection (4.81-fold), suggesting that the observed effects were secondary, possibly due to changes in the upstream sites, such as the SFO and OVLT. As a control, SCT expression in the cortex was examined and was not influenced by ANGII. On the other hand, i.c.v. ANGII led to an increase in SCTR expression only in the OVLT and PVN, but not in the SFO, MnPO, or cortex (Fig. 5D).
DISCUSSION
SCT is the mediator of the angiotensinergic osmoregulatory functions in mice
ANGII is well established as a dipsogenic hormone that initiates thirst and stimulates VP synthesis and release; however, mechanisms that mediate these actions of ANGII are not completely elucidated. In the present study, we discovered that central responses triggered by ANGII and SCT are similar. More important, deficiency of either component in the SCT/SCTR system resulted in an abolishment of all or most of the ANGII-induced effects. These findings provide clear evidence that ANGII actions are largely dependent on a functional SCT/SCTR axis.
Genetically modified mice lacking either angiotensinogen (AGT), which is the sole source of ANGII peptide, or AT1 receptor, which is the dominant receptor for ANGII, have been developed in the past decade (22). Consistent with the role of SCT in linking ANGII with its downstream effects, SCTR−/−, SCT−/−, AGT−/−, and AT1−/− mice shared some phenotypes that are related to osmoregulation. For example, SCTR−/− (11), AGT−/−, and AT1−/− mice (23) were found to produce significantly larger volumes of more dilute urine. Also, SCTR−/− mice exhibit abnormalities in renal cortex and medulla, typically characterized by the increased mesangial area and urinary space, frequent tubular dilation, and hypertrophy in the collecting tubules of medulla (11). All these suggest that SCTR−/− mice are defective in glomerular filtration and renal water reabsorption, similar to AGT−/− and AT1−/− mice (23).
SCTR−/− and SCT−/− mice drink more water than WT mice, presumably due to water loss from the kidney. The reduced water reabsorption capability of the kidneys causes a considerable water loss; consequently, the mice need to drink more water to compensate. One may also argue that polydipsia is the cause, rather than the consequence, of polyuria and reduced urine osmolality. This idea, however, is rejected by the pathological renal structures that indicate dysfunction of SCTR−/− kidneys. Also, Li et al.(24) suggested that hypotension in the AT1−/− mice could stimulate thirst and thus was another reason for polydipsia. Although we have not measured blood pressure changes in SCTR−/− and SCT−/− mice, SCT has previously been shown to regulate arterial blood pressure (25). The relationships between SCT, SCTR, hypotension, and polydipsia remain to be investigated. Taken together, the observed phenotypes in SCTR−/− and SCT−/− mice have unveiled the critical roles of SCT in osmoregulation in the brain and kidney.
Knowing that SCT/SCTR is essential to central actions of ANGII, notably, chronic central ANGII injection in SCTR−/− and SCT−/− mice could still result in significant, although much lowered, water intake increments (Fig. 1D). These observations suggest that ANGII could utilize SCT-independent pathways that work in parallel with SCT to carry out its central effects, although the contributions from these SCT-independent pathways were far less important than the SCT-mediated pathway. Interestingly, existence of SCT-independent pathways may also provide an explanation for the observation that, under normal or control conditions, the knockout mice had increased water intake, VP expression in the PVN, and VP levels in plasma, and this is consistent with our previous study that shows polydipsia phenotype in SCTR−/− mice (11). The absence of a functional SCT/SCTR axis in the knockout mice may provoke the SCT-independent pathways, potentially achieved by up-regulating other components of RAS. For instance, brain AT1a receptor transcript levels in the knockout mice were found to be higher than those in WT mice (Supplemental Fig. 6). This adaptive response in the knockout mice is essential to maintaining a proper body fluid environment. ANGII is known to reduce plasma osmolality (26), and since SCT can also induce drinking and VP release as ANGII does, it is therefore reasonable to believe that SCT could also decrease plasma osmolality. If this is true, SCTR−/− and SCT−/− mice should have higher plasma osmolality than WT mice, but we found that these knockouts could maintain serum osmolality at normal levels (Supplemental Fig. 2). This could also be explained again by the provoked SCT-independent pathways in the knockouts to compensate for the lack of SCT/SCTR.
Potential role of SCT in the neural pathway along the lamina terminalis and PVN
In the present study, specific mRNA expressions of SCT and its receptor in lamina terminalis and PVN were revealed by both in situ hybridization and LCM coupled with real-time PCR. Interestingly, we observed a time-dependent activation of SCT in different brain areas after i.c.v. ANGII. In CVOs, SCT expression was greatly increased 30 min postinjection, while a much delayed and more gradual increase was observed in PVN, suggesting that the effects observed in PVN are secondary and likely due to changes in the upstream CVO sites. In addition, the observed increase in SCT expression in MnPO was small compared to CVOs. Taken together, we propose here that ANGII's stimulatory effect on SCT expression is predominantly originated from SFO, and via OVLT to PVN.
We have previously shown the synthesis and endogenous release of SCT at somatodendritic terminals of Purkinje neurons (9, 10), and the released SCT functions as a retrograde messenger to stimulate presynaptic SCTR to trigger GABA release from basket cells. In addition, SCT has also been shown to be released from the hypothalamus, and this process involves high-voltage-activated calcium channels (13). Taken together, these data indicate the function of SCT as a neurotransmitter in the CNS. Similar to ANGII (27), in this study, SCT-IR was also detected in lamina terminalis and PVN. Together with the temporal activation of SCT gene expression in lamina terminalis and PVN as discussed earlier, it is therefore possible that SCTergic and ANGergic pathways overlap and that SCT is utilized presumably as a neurotransmitter like ANGII for transducing signals throughout the osmoregulatory pathway. Single-cell patch-clamp studies can be carried out in the future to identify cellular signaling mechanisms utilized by cells in the lamina terminalis that mediate SCT's actions in response to ANGII.
In summary, SCT is shown to play an indispensable role in mediating the central effects of ANGII involving osmoregulation (Supplemental Fig. 7). In SFO and OVLT, ANGII via AT1 receptor stimulates SCT expression. SCT produced in the CVOs then stimulates VP expression and VP release. The centrally injected ANGII can interact with the SCT/SCTR axis at multiple sites to trigger SCT and SCTR expression. Our findings identify novel elements of the ANGII pathway that provide new insights in understanding fluid homeostasis.
Supplementary Material
Acknowledgments
The authors thank Prof. Cheah K. S. (University of Hong Kong; supported by HKU 02/02C) for the development of the SCT−/− mice, Prof. Chung S. K. (University of Hong Kong) for the development of SCTR−/− mice, and Dr. Neal Copeland (National Cancer Institute, Frederick, MD, USA) for providing the bacterial recombination cells and vectors.
This study was supported by the Hong Kong Research Grants Council (GRF7686/08 to L.T.O.L. and GRF738/09 to B.K.C.C.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.MacGregor D. P., Murone C., Song K., Allen A. M., Paxinos G., Mendelsohn F. A. (1995) Angiotensin II receptor subtypes in the human central nervous system. Brain Res. 675, 231–240 [DOI] [PubMed] [Google Scholar]
- 2.Wilson W. L., Roques B. P., Llorens-Cortes C., Speth R. C., Harding J. W., Wright J. W. (2005) Roles of brain angiotensins II and III in thirst and sodium appetite. Brain Res. 1060, 108–117 [DOI] [PubMed] [Google Scholar]
- 3.Saavedra J. M. (1992) Brain and pituitary angiotensin. Endocr. Rev. 13, 329–380 [DOI] [PubMed] [Google Scholar]
- 4.McKinley M. J., Mathai M. L., McAllen R. M., McClear R. C., Miselis R. R., Pennington G. L., Vivas L., Wade J. D., Oldfield B. J. (2004) Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J. Neuroendocrinol. 16, 340–347 [DOI] [PubMed] [Google Scholar]
- 5.Ferguson A. V., Washburn D. L., Latchford K. J. (2001) Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp. Biol. Med. (Maywood) 226, 85–96 [DOI] [PubMed] [Google Scholar]
- 6.Johnson A. K., Gross P. M. (1993) Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 7, 678–686 [DOI] [PubMed] [Google Scholar]
- 7.Lind R. W., Thunhorst R. L., Johnson A. K. (1984) The subfornical organ and the integration of multiple factors in thirst. Physiol. Behav. 32, 69–74 [DOI] [PubMed] [Google Scholar]
- 8.Bolbecker A. R., Hetrick W. P., Johannesen J. K., O'Donnell B. F., Steinmetz J. E., Shekhar A. S. (2009) Secretin effects on cerebellar-dependent motor learning in schizophrenia. Am. J. Psychiatry 166, 460–466 [DOI] [PubMed] [Google Scholar]
- 9.Lee S. M., Chen L., Chow B. K., Yung W. H. (2005) Endogenous release and multiple actions of secretin in the rat cerebellum. Neuroscience 134, 377–386 [DOI] [PubMed] [Google Scholar]
- 10.Yung W. H., Leung P. S., Ng S. S., Zhang J., Chan S. C., Chow B. K. (2001) Secretin facilitates GABA transmission in the cerebellum. J. Neurosci. 21, 7063–7068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu J. Y., Chung S. C., Lam A. K., Tam S., Chung S. K., Chow B. K. (2007) Phenotypes developed in secretin receptor-null mice indicated a role for secretin in regulating renal water reabsorption. Mol. Cell. Biol. 27, 2499–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng C. Y., Chu J. Y., Chow B. K. (2009) Vasopressin-independent mechanisms in controlling water homeostasis. J. Mol. Endocrinol. 43, 81–92 [DOI] [PubMed] [Google Scholar]
- 13.Chu J. Y., Lee L. T., Lai C. H., Vaudry H., Chan Y. S., Yung W. H., Chow B. K. (2009) Secretin as a neurohypophysial factor regulating body water homeostasis. Proc. Natl. Acad. Sci. U. S. A. 106, 15961–15966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews E. C., Rowland N. E. (2005) Role of angiotensin in body fluid homeostasis of mice: effect of losartan on water and NaCl intakes. Am. J. Physiol. 288, R638–644 [DOI] [PubMed] [Google Scholar]
- 15.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 16.Denton D. A., Blair-West J. R., McBurnie M., Osborne P. G., Tarjan E., Williams R. M., Weisinger R. S. (1990) Angiotensin and salt appetite of BALB/c mice. Am. J. Physiol. 259, R729–735 [DOI] [PubMed] [Google Scholar]
- 17.McKinley M. J., Walker L. L., Alexiou T., Allen A. M., Campbell D. J., Di Nicolantonio R., Oldfield B. J., Denton D. A. (2008) Osmoregulatory fluid intake but not hypovolemic thirst is intact in mice lacking angiotensin. Am. J. Physiol. 294, R1533–1543 [DOI] [PubMed] [Google Scholar]
- 18.Chen Y., da Rocha M. J., Morris M. (2003) Osmotic regulation of angiotensin AT1 receptor subtypes in mouse brain. Brain Res. 965, 35–44 [DOI] [PubMed] [Google Scholar]
- 19.Lauand F., Ruginsk S. G., Rodrigues H. L., Reis W. L., de Castro M., Elias L. L., Antunes-Rodrigues J. (2007) Glucocorticoid modulation of atrial natriuretic peptide, oxytocin, vasopressin and Fos expression in response to osmotic, angiotensinergic and cholinergic stimulation. Neuroscience 147, 247–257 [DOI] [PubMed] [Google Scholar]
- 20.Mahon J. M., Allen M., Herbert J., Fitzsimons J. T. (1995) The association of thirst, sodium appetite and vasopressin release with c-fos expression in the forebrain of the rat after intracerebroventricular injection of angiotensin II, angiotensin-(1–7) or carbachol. Neuroscience 69, 199–208 [DOI] [PubMed] [Google Scholar]
- 21.Han L., Rowland N. E. (1996) Dissociation of Fos-like immunoreactivity in lamina terminalis and magnocellular hypothalamic nuclei induced by hypernatremia. Brain Res. 708, 45–49 [DOI] [PubMed] [Google Scholar]
- 22.Tanimoto K., Sugiyama F., Goto Y., Ishida J., Takimoto E., Yagami K., Fukamizu A., Murakami K. (1994) Angiotensinogen-deficient mice with hypotension. J. Biol. Chem. 269, 31334–31337 [PubMed] [Google Scholar]
- 23.Lochard N., Silversides D. W., van Kats J. P., Mercure C., Reudelhuber T. L. (2003) Brain-specific restoration of angiotensin II corrects renal defects seen in angiotensinogen-deficient mice. J. Biol. Chem. 278, 2184–2189 [DOI] [PubMed] [Google Scholar]
- 24.Li X. C., Shao Y., Zhuo J. L. (2009) AT1a receptor knockout in mice impairs urine concentration by reducing basal vasopressin levels and its receptor signaling proteins in the inner medulla. Kidney Int. 76, 169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sitniewska E. M., Wisniewska R. J., Wisniewski K. (2002) Diabetes-induced changes of nitric oxide influence on the cardiovascular action of secretin. Regul. Pept. 105, 163–172 [DOI] [PubMed] [Google Scholar]
- 26.Johnson A. K., Cunningham J. T., Thunhorst R. L. (1996) Integrative role of the lamina terminalis in the regulation of cardiovascular and body fluid homeostasis. Clin. Exp. Pharmacol. Physiol. 23, 183–191 [DOI] [PubMed] [Google Scholar]
- 27.Healy D. P., Printz M. P. (1984) Distribution of immunoreactive angiotensin II, angiotensin I, angiotensinogen and renin in the central nervous system of intact and nephrectomized rats. Hypertension 6, I130–136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.