Abstract
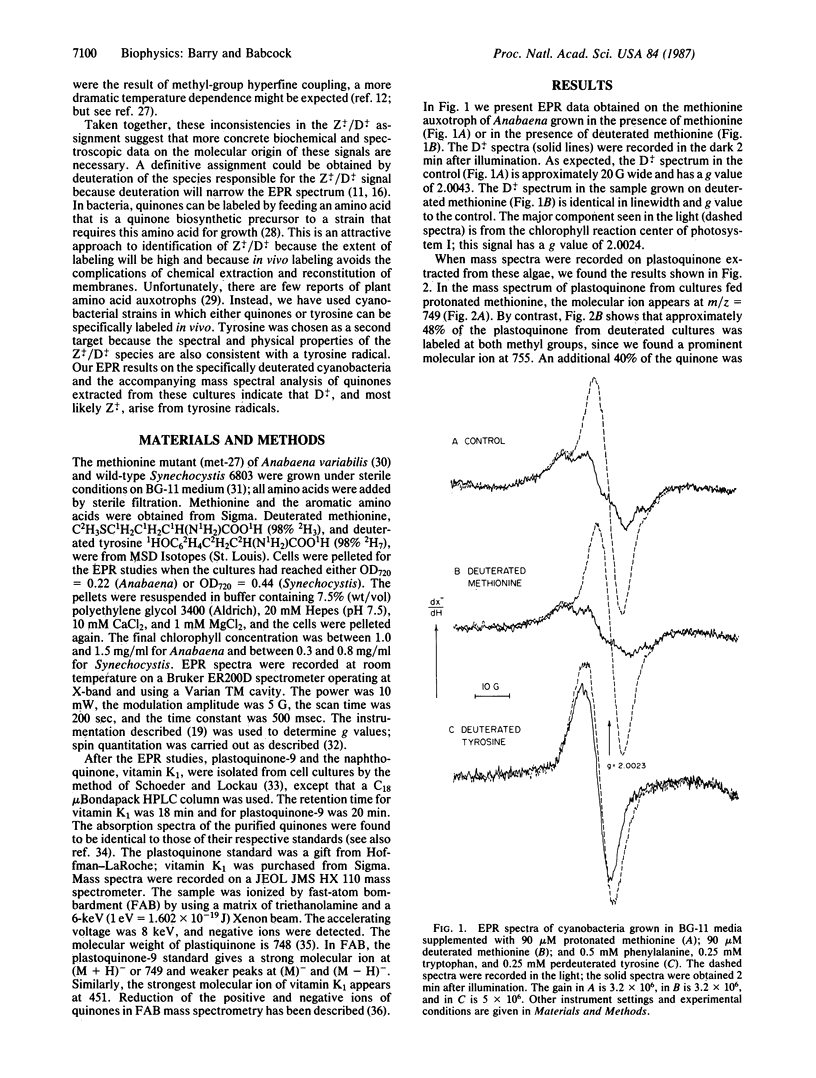
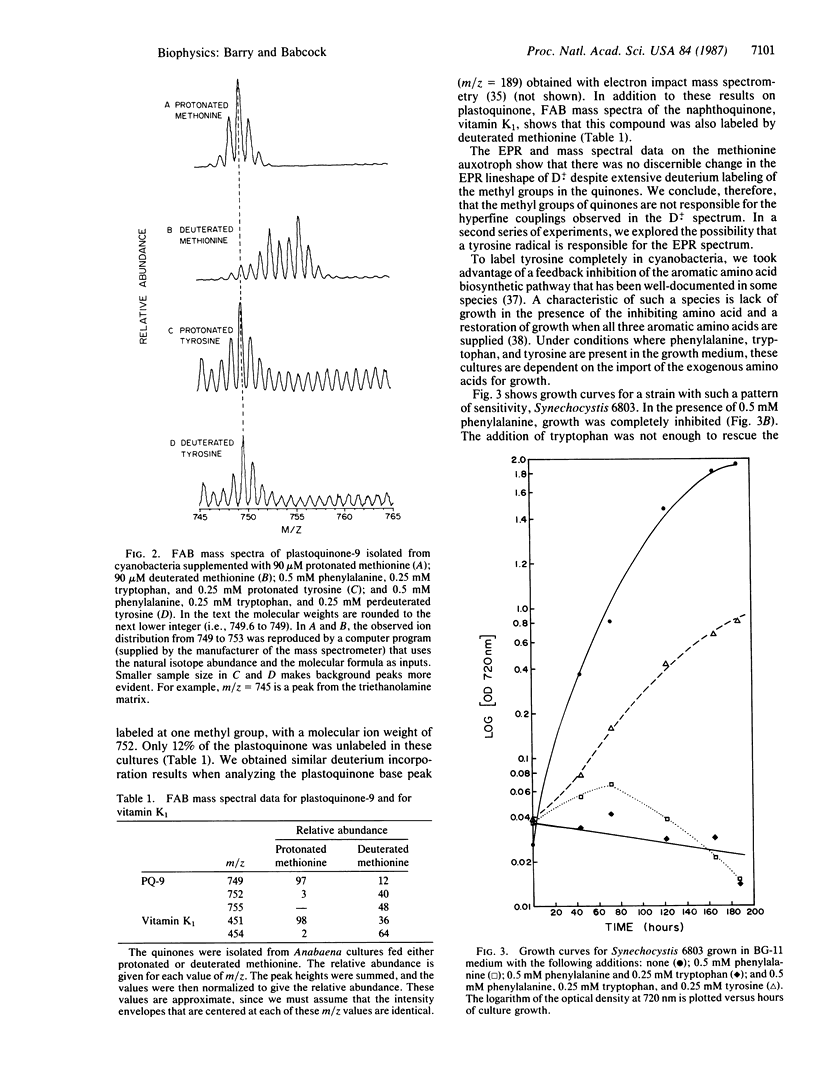
In addition to the reaction-center chlorophyll, at least two other organic cofactors are involved in the photosynthetic oxygen-evolution process. One of these cofactors, called "Z," transfers electrons from the site of water oxidation to the reaction center of photosystem II. The other species, "D," has an uncertain function but gives rise to the stable EPR signal known as signal II. Z+. and D+. have identical EPR spectra and are generally assumed to arise from species with the same chemical structure. Results from a variety of experiments have suggested that Z and D are plastoquinones or plastoquinone derivatives. In general, however, the evidence to support this assignment is indirect. To address this situation, we have developed more direct methods to assign the structure of the Z+./D+. radicals. By selective in vivo deuteration of the methyl groups of plastoquinone in cyanobacteria, we show that hyperfine couplings from the methyl protons cannot be responsible for the partially resolved structure seen in the D+. EPR spectrum. That is, we verify by extraction and mass spectrometry that quinones are labeled in algae fed deuterated methionine, but no change is observed in the line shape of signal II. Considering the spectral properties of the D+. radical, a tyrosine origin is a reasonable alternative. In a second series of experiments, we have found that deuteration of tyrosine does indeed narrow the D+. signal. Extraction and mass spectral analysis of the quinones in these cultures show that they are not labeled by tyrosine. These results eliminate a plastoquinone origin for D+.; we conclude instead that D+., and most likely Z+., are tyrosine radicals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Sauer K. A rapid, light-induced transient in electron paramagnetic resonance signal II activated upon inhibition of photosynthetic oxygen evolution. Biochim Biophys Acta. 1975 Feb 17;376(2):315–328. doi: 10.1016/0005-2728(75)90024-9. [DOI] [PubMed] [Google Scholar]
- Blankenship R. E., Babcock G. T., Warden J. T., Sauer K. Observation of a new EPR transient in chloroplasts that may reflect the electron donor to photosystem II at room temperature. FEBS Lett. 1975 Mar 1;51(1):287–293. doi: 10.1016/0014-5793(75)80909-4. [DOI] [PubMed] [Google Scholar]
- Botham K. M., Pennock J. F. The biosynthesis of tocopherols and related compounds in the blue-green alga Anabaena variabilis. Biochem J. 1971 Mar;122(1):127–128. doi: 10.1042/bj1220127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Kinetic models for the electron donors of photosystem II of photosynthesis. Biochim Biophys Acta. 1980 Dec;594(2-3):85–103. doi: 10.1016/0304-4173(80)90006-3. [DOI] [PubMed] [Google Scholar]
- Commoner B., Heise J. J., Townsend J. LIGHT-INDUCED PARAMAGNETISM IN CHLOROPLASTS. Proc Natl Acad Sci U S A. 1956 Oct;42(10):710–718. doi: 10.1073/pnas.42.10.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R., Unger S. Structure of the quinone antibiotic EM5519 and the behavior of quinones in fast atom bombardment mass spectrometry. J Antibiot (Tokyo) 1985 Jan;38(1):24–30. doi: 10.7164/antibiotics.38.24. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Haury J. F., Wolk C. P. Isolation and preliminary characterization of auxotrophs of a filamentous Cyanobacterium. J Bacteriol. 1977 Mar;129(3):1556–1562. doi: 10.1128/jb.129.3.1556-1562.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B. C., Lounasmaa M., Tendille C., Lederer E. Mass spectrometry of plastoquinones. The structure of the plastoquinones B,C and D. Biochem Biophys Res Commun. 1965 Nov 22;21(4):318–322. doi: 10.1016/0006-291x(65)90195-6. [DOI] [PubMed] [Google Scholar]
- Hall G. C., Flick M. B., Gherna R. L., Jensen R. A. Biochemical diversity for biosynthesis of aromatic amino acids among the cyanobacteria. J Bacteriol. 1982 Jan;149(1):65–78. doi: 10.1128/jb.149.1.65-78.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. C., Jensen R. A. Enzymological basis for growth inhibition by L-phenylalanine in the cyanobacterium Synechocystis sp. 29108. J Bacteriol. 1980 Dec;144(3):1034–1042. doi: 10.1128/jb.144.3.1034-1042.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman L. M., O'Brien I. G., Cox G. B., Gibson F. Methionine as the source of methyl groups for ubiquinone and vitamin K: a study using nuclear magnetic resonance and mass spectrometry. Biochim Biophys Acta. 1967 Jun 13;141(1):1–7. doi: 10.1016/0304-4165(67)90239-5. [DOI] [PubMed] [Google Scholar]
- Kohl D. H., Townsend J., Commoner B., Crespi H. L., Dougherty R. C., Katz J. J. Effects of isotopic substitution on electron spin resonance signals in photosynthetic organisms. Nature. 1965 Jun 12;206(989):1105–1110. doi: 10.1038/2061105a0. [DOI] [PubMed] [Google Scholar]
- Kohl D. H., Wood P. M. On the Molecular Identity of ESR Signal II Observed in Photosynthetic Systems: The Effect of Heptane Extraction and Reconstitution With Plastoquinone and Deuterated Plastoquinone. Plant Physiol. 1969 Oct;44(10):1439–1445. doi: 10.1104/pp.44.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi N., Hoff A. J., van der Waals J. H. Electron spin echo studies on chloroplasts. Spectral characteristics of electron transport components and light-induced transients. Biochim Biophys Acta. 1980 Mar 7;590(1):74–88. doi: 10.1016/0005-2728(80)90147-4. [DOI] [PubMed] [Google Scholar]
- Reichard P., Ehrenberg A. Ribonucleotide reductase--a radical enzyme. Science. 1983 Aug 5;221(4610):514–519. doi: 10.1126/science.6306767. [DOI] [PubMed] [Google Scholar]
- Sayre R. T., Andersson B., Bogorad L. The topology of a membrane protein: the orientation of the 32 kd Qb-binding chloroplast thylakoid membrane protein. Cell. 1986 Nov 21;47(4):601–608. doi: 10.1016/0092-8674(86)90624-0. [DOI] [PubMed] [Google Scholar]
- Sjöberg B. M., Reichard P., Gräslund A., Ehrenberg A. The tyrosine free radical in ribonucleotide reductase from Escherichia coli. J Biol Chem. 1978 Oct 10;253(19):6863–6865. [PubMed] [Google Scholar]
- Threlfall D. R., Whistance G. R., Goodwin T. W. Biosynthesis of phytoquinones. Incorporation of L-[Me-14C,3H]methionine into terpenoid quinones and chromanols in maize shoots. Biochem J. 1968 Jan;106(1):107–112. doi: 10.1042/bj1060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEAVER E. C. Possible interpretation of the slow-decaying EPR signal in algal suspensions. Arch Biochem Biophys. 1962 Oct;99:193–196. doi: 10.1016/0003-9861(62)90262-x. [DOI] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R. Biosynthesis of phytoquinones. Biosynthetic origins of the nuclei and satellite methyl groups of plastoquinone, tocopherols and tocopherolquinones in maize shoots, bean shoots and ivy leaves. Biochem J. 1968 Oct;109(4):577–595. doi: 10.1042/bj1090577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R. Biosynthesis of phytoquinones. Homogentisic acid: a precursor of plastoquinones, tocopherols and alpha-tocopherolquinone in higher plants, green algae and blue-green algae. Biochem J. 1970 Apr;117(3):593–600. doi: 10.1042/bj1170593. [DOI] [PMC free article] [PubMed] [Google Scholar]



