Abstract
Kidney damage due to injury rarely resolves completely, and there are currently no therapies capable of promoting repair. In addition to understanding mechanisms by which tissues are damaged, illuminating mechanisms of repair and regeneration is also of great importance. Here we show that the melanoma-associated, transmembrane glycoprotein, Gpnmb, is up-regulated 15-fold following ischemic damage in kidney tissue and by more than 10-fold in macrophages and 3-fold in surviving epithelial cells. Gpnmb-expressing macrophages and epithelial cells were found to contain apoptotic bodies at 3 times the rate of nonexpressing cells. Either mutation of Gpnmb or ablation of inflammatory macrophages prevents normal repair of the kidney. Significantly, the kidneys from postischemic Gpnmb mutant mice exhibited a 5-fold increase in apoptotic cellular debris compared to wild-type mice. These mice also experienced an 85% increase in mortality following bilateral ischemic kidney. Finally, we demonstrate that Gpnmb is a phagocytic protein that is necessary for recruitment of the autophagy protein LC3 to the phagosome where these proteins are colocalized and for lysosomal fusion with the phagosome and hence bulk degradation of their content. Therefore, Gpnmb is a novel prorepair gene that is necessary for crosstalk between the macroautophagic degradation pathway and phagocytosis.—Li, B., Castano, A. P., Hudson, T. E., Nowlin, B. T., Lin, S.-L., Bonventre, J. V., Swanson, K. D., Duffield, J. S. The melanoma-associated transmembrane glycoprotein Gpnmb controls trafficking of cellular debris for degradation and is essential for tissue repair.
Keywords: autophagy, macrophages, phagocytosis
The mechanisms by which organs undergo repair following injury is of increasing therapeutic interest because targeting either dysregulated or failed repair mechanisms is an attractive alternative to targeting injury mechanisms. Proof of principle that targeting such repair mechanisms can augment normal repair has been established (1). However, there is a paucity of knowledge about normal repair and dysregulation of normal repair in chronic inflammatory diseases. Working toward this aim, we have recently described a key role for epithelial cell phagocytosis in repair and remodeling of the functional units of the kidney, the nephrons, following severe ischemic injury, a model for acute tubular necrosis in humans (2). In addition to this role for epithelial cells in repair and regeneration, there is now an established literature indicating that in certain circumstances macrophages (Mφs) perform crucial roles in repair, not only by engaging in scavenger and debris-clearing functions, but also through paracrine signaling to parenchymal cells that support tissue regeneration (3–7).
To identify genes that may play roles in repair rather than in injury we performed representational difference analysis of normal rat kidney and kidney subjected to ischemia reperfusion injury (IRI) during the phase of organ repair (8). This analysis revealed that the gene encoding the transmembrane protein Kim1 (also known as Tim1; see ref. 9) was highly up-regulated during repair. Subsequent analysis indicated that Kim1 functions in repair, at least in part, by functioning as a scavenger receptor on injured epithelial cells (2). In addition to the Kim1 gene, this analysis identified that the gene encoding glycoprotein nonmetastatic melanoma B (Gpnmb) was also markedly up-regulated following injury.
Gpnmb (reported elsewhere as DC-HIL or osteoactivin) is a heavily N-glycosylated type I transmembrane domain protein with a short cytoplasmic domain containing an endosomal sorting motif that was originally described in melanoma and retinal pigment epithelial cells. It has subsequently been described in dendritic cells (DCs) and most recently in thioglycholate-elicited peritoneal Mφs (TEPM) (10–15). The extracellular domain of Gpnmb possesses a polycystin domain (PKD) whose function remains unknown despite being highly conserved. It has been suggested that Gpnmb may function as either a heparin receptor or a regulator of cytokine release in DCs and Mφs (10, 15), although these studies have not been reproduced. A spontaneous mutation within the Gpnmb locus that creates a premature stop codon in exon 4 resulting in a truncated form of Gpnmb has been identified in mice. Mice homozygous for this allele exhibit autoimmune pigmentary glaucoma and compromising ocular immunosuppression, which is manifested by deficient anterior chamber-associated immune deviation (14). These reports suggest that Gpnmb may play a role in normal handling of self-antigen and avoidance of aberrant adaptive immune responses (14, 16). Furthermore, retinal pigment epithelial (RPE) cells normally express Gpnmb, and the accumulation of pigment in Mφs recruited to the eye in these mice points to a possible role for Gpnmb in the normal clearance of photoreceptor outer segment debris and iris pigment debris by RPE cells. Gpnmb expression has also been reported to be increased in neoplasms including glioblastoma, but its function here is obscure (17).
In the present study, we offer compelling evidence that Gpnmb plays a critical role in kidney repair following injury, and we show that it functions by regulating degradation of phagosomes through recruitment of macroautophagic bulk degradation proteins and mechanisms. These studies link a phylogenetically conserved pathway of self-eating, autophagy, with the regulation of “eating from the outside” (i.e., phagocoytosis and endocytosis) and highlight novel crosstalk between 2 pathways that have until now been considered independent.
MATERIALS AND METHODS
Materials
Reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Fisher Scientific (Pittsburgh, PA, USA) unless otherwise stated. All cell culture media were from Cellgro (Mediatech, Inc., Manassas, VA, USA), and cell culture plastics were from Corning (Corning, NY, USA).
Animal models
Rat and mouse kidney ischemia reperfusion injury was performed in 190- to 275-g male SD rats (bilateral model ischemic time 30 min), 25- to 30-g C57BL/6 male mice (Charles River Laboratories, Wilmington, MA, USA; bilateral model ischemic time 25 min, unilateral model 27 min), DBA/2 male mice (bilateral model ischemic time 20 min, unilateral model 23 min), or FVB/N mice (bilateral model ischemic time 27 min), as described previously (18). Liver fibrosis was generated in FVB/N mice by iterative administration of CCl4 over 12 wk as described previously (5). Aortic arch atheroma was from ApoE−/− mice fed a high-fat diet for 165 d (gift of Dr. Peter Libby, Harvard Medical School). Cd11b-DTR mice (FVB/N) were generated as described previously (19). Gpnmb−/− (DBA/2) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA), and Gpnmb+/+ (DBA/2) strain-matched mice from Charles River Laboratories. Gpnmb−/− mice were confirmed to have mutant Gpnmb by generating PCR fragments of Exon 5 from genomic DNA using primers 5′-CTCGACTGTTTGTTCTTGGTT-3′ and 5′-AAAACCAAACCAAGTGTGTG-3′ and an annealing temperature of 48°C. The PCR fragment (207 bp) was digested using PvuII. Mutated exon 4 has a novel PvuII site yielding fragments of 102 and 105 bp (not shown). All procedures were carried out according to approved protocols by the Harvard Animal Research and Comparative Medicine Committee.
DNA constructs and antisera
Mouse Gpnmb cDNA from Mφs and rat Gpnmb cDNA from rat ischemic kidney was cloned into pcDNA3.1. To generate a GFP-Gpnmb fusion protein, the ORF of rat Gpnmb was subcloned into pEGFP-N1, replacing the stop codon with alanine. RFP-Gpnmb fusion protein was generated by cloning the ORF of rat Gpnmb into pmRFP1.3-N1 (gift of Dr. Jagesh S. Shah, Harvard Medical School) (20). Gpnmb-Fc fusion protein was generated by subcloning the extracellular domain of rat Gpnmb into CA117 pIg vector containing the Fc domain of human IgG1 (21). Amphotropic retrovirus expressing Gpnmb was generated by subcloning mouse or rat Gpnmb ORF into both pMSCV-IRES-GFP and pMXs-puro (gift of Dr. Toshio Kitamura, University of Tokyo, Tokyo, Japan). To express GFP-tagged rat LC3, GFP-LC3 in pEGFPC1 vector (gift of Dr. Noburu Mizushima, Tokyo Metropolitan Institutes of Science, Tokyo, Japan) was subcloned into pMXs-puro (2). The scavenger receptor, SRA-II ORF, was cloned from cDNA prepared from mouse peritoneal Mφs (BALB/c) into pCDNA3.
Polyclonal antibodies against rat Gpnmb were generated in rabbit using peptide no. 14 SRGDREKDPLLQDKPWC from the C terminus (cytoplasmic domain), cross-linked to Keyhole Limpet Hemocyanin (KLH; Pierce, Rockford, IL, USA), and peptide no. 15 KRFRDVLGHEQYPDHMRC from the N terminus (Ecto domain). Fifty micrograms of KLH-peptide conjugate was used to immunize a rabbit as described previously (22). Antibodies were affinity purified using the original peptides conjugated to sepharose beads (Pierce). The specificity of these antibodies was compared against in situ hybridization of rat kidney sections (not shown). In tissue sections, both antibodies recognize rat Gpnmb, but only the anti-Ecto domain antibody recognizes mouse Gpnmb. By Western blot analysis, both antibodies recognize rat and mouse Gpnmb.
Cell culture, viral transduction, and cell-based assays
The porcine kidney proximal tubule cell line (LLC-PK1), 293T human embryonic kidney cells, NIH-3T3 mouse fibroblast cells, RAW 247.1 mouse splenic Mφ cell line, and Cos-1 cells were from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in DMEM with 10% FBS. Primary epithelial cells were cultured as described previously (2). Transfection of cell lines was performed by generating plasmid DNA/polyethylenimine complexes using methods modified from previous studies (23). Stable cell lines were generated and characterized as described previously (2) or by transducing cells with amphotropic retrovirus, followed by selection in puromycin (5 μg/ml) for 3 d. For cells expressing fluorescently tagged proteins, stable cell lines were generated by flow cytometric sorting of highly fluorescent cells 14 d after transfection or 4 d after viral transduction. To induce autophagy, cells were amino acid starved by washing 3 times with PBS, then cultured in either HBSS with 1 mM HEPES or with rapamycin (400 nM) in serum-free medium for 6 h. Mouse bone marrow-derived macrophages (BMMφs) were cultured from whole bone marrow (BM) as described previously and used between d 7 and d 10 (1). Mφs were purified from kidney, using methods previously described (3, 19). The single-cell suspension of kidney cells was incubated 1 h in serum-fee medium on glass cover slips. Nonadherent cells were then removed by vigorous washing 5 times prior to fixation and immunolabeling.
For phagosome scoring assays, cultured cells were imaged at ×400 in a masked fashion. Images were assessed in a masked fashion for number of labeled endosomes per cell. Phagocytosis assays were carried out in 12-well plates using CMFDA-labeled apoptotic thymocytes. After washing away noningested apoptotic cells, live cells were assessed by flow cytometry as described previously (2). Apoptotic thymocytes were generated by dexamethasone treatment as described previously (1). For imaging studies, cells were cultured on glass cover slips with or without apoptotic thymocytes in a 10:1 ratio for 1 h or zymosan particles (100 μg in 200 μl) for 1 h or polystyrene beads (0.5 μm) (Polysciences, Warrington, PA, USA). Cells were washed 5 times with ice-cold PBS cells and fixed as described above. To quantify degradation of apoptotic thymocytes in BMMφs, subsequent to removal of noningested apoptotic cells by washing with PBS, BMMφs were returned to 37°C incubator for 1, 2, 4, 8, or 24 h and then lysed. To quantify autophagy, LC3-II was identified by immunofluorescence (see below) or by the pattern of expression of GFP-LC3. Cells were quantified as autophagic if they had LC3-II vesicles. To assess lysosomal fusion with phagosomes, LysoTracker Red (Molecular Probes, Eugene, OR, USA) was applied to cells (30 min, 37°C, 200 nM).
Amphotropic retroviruses were generated as follows: 293T17 cells in 100-mm dishes were cotransfected with pCLampho (Imgenex, San Diego, CA, USA) and pMSCV-IRES-GFP, pMSCV-Gpnmb-IRES-GFP, pMXs-puro, or pMXs-GFPLC3-puro in equimolar concentrations. After 16 h, virus was collected in 6 ml of Mφ medium or other cell culture medium for 6 or 24 h. The virus was harvested and filtered through a 0.45-μm filter. For Mφ retroviral transduction, bone marrow cells were cultured for 3 d in Mφ medium, and then the precursor cells remaining in suspension were collected and cultured in retrovirus-conditioned Mφ medium containing polybrene (14 μg/ml) for 16 h. Transduced cells were then incubated in regular Mφ medium, and cells were cultured until d 7. For transduction of LLC-PK1 cells, cells were cultured with retrovirus containing medium with polybrene 8 μg/ml, for 24 h. One day later, cells were selected with puromycin 3 μg/ml for 72 h.
To assess autophagy, cells were incubated with bafilomycin A1 with rapamycin (200 nM, 4 h) or bafilomycin A1 with chloroquine (50 μM, 4 h). Alternatively cells were amino acid starved in HEPES-buffered HBSS for 1, 2, or 4 h and then fixed with 2% PFA for 5 min.
Immunofluorescence detection and microscopy
Mouse and rat tissues were prepared either by perfusion fixation using 4% paraformaldehyde, l-lysine, or periodate (PLP) or by perfusion of organs with ice-cold PBS, followed by dissection of organs and fixing in PLP for 2 h, followed by 18% sucrose for 16 h prior to cryosectioning at 5 μm as described previously (18). For immunofluorescence, rat anti-mouse CD68 (1:200), mouse anti-rat CD68 antibody (ED1; 1:200; ABD-Serotec, Kidlington, UK), rat anti-F4/80 (1;200; eBioscience, San Diego, CA, USA), mouse anti-vimentin antibodies (1:200; Sigma), anti-human LAMP-1 hybridoma supernatant (1:200; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA), anti-human EEA-1 antibodies (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-ATG12 (1:100), rabbit anti-LC3 (1:200; (Abgent, San Diego, CA, USA), anti-Gpnmb cyto (1:500), or anti-Gpnmb ecto (1:500) were applied to cryosections in blocking medium for 2 h at room temperature, followed by washing. Cy2- or Cy3-conjugated affinity-purified secondary antibodies were applied in a blocking medium (Jackson ImmunoResearch, West Grove, PA, USA) using methods described previously (18). After washing, slides were mounted with Vectashield containing DAPI (200 nM). LysoTracker Red (Molecular Probes) was applied to live epithelial cells or Mφs in culture for 0.5–2 h at 37°C at a concentration of 50–200 nM. Filipin (Sigma) 50 ng/ml was applied to fixed cells for 2 h and 25°C using methods adapted from established methods (24), and images were visualized using fluorescence microscopy. Confocal images were generated by taking 30 or 40 3-channel fluorescent image stacks (Nikon TE2000 microscope; Nikon, Tokyo, Japan) 0.2 μm apart, then generating confocal images using deconvolution software (Autodeblur; Media Cybernetics, Bethesda, MD, USA) as described previously (18). For Gpnmb detection in kidney sections, confocal images were generated using a Nikon C1 D-Eclipse confocal microscope. Projection images were generated from 10 Z-stack images that were acquired at 0.1-μm steps. To allow comparison between sections, all confocal settings were kept constant between sections.
For transmission electron microscopy, nonadherent cells were collected in supernatants, and adherent cells collected after cell scraping. After washing, they were fixed in glutaraldehyde/paraformaldehyde, then prepared using standard methods (25).
Quantitative PCR
Total RNA was purified from whole kidney and other organs using Trizol (5) and from cells using the RNA Easy kit (Qiagen, Valencia, CA, USA). cDNAs were generated using random hexamers and poly dT primers and iScript (Bio-Rad, Hercules, CA, USA) according to the manufacturers' instructions. Q-PCR was carried out as described previously using Bio-Rad iQ5 system and SYBR green-based quantification (Bio-Rad) (3). Primer pairs for mGpnmb were the following: 5′-TCATGGAAGTGACTGTCTTT-3′, 5′-CAGACAAGTTCCTGTCATTC-3′; and 5′-GATGTCCTCATTCATGATCC-3′, 5′-TTCAAAGTGTGATTGTTGGA-3′ were tested. Both pairs gave solitary specific bands and comparable results using an annealing temperature of 51°C. Primer pairs for Gapdh were 5′-CTGAGAAAACCTGCCAAGTA-3′, 5′-AAGAGTGGGAGTTGCTGTTG-3′.
Flow cytometry
Single-cell suspensions were prepared from blood and kidney as described previously (3, 19). In brief, whole blood was citrated, then PBMCs were separated using Ficoll density gradient (300 g, 20 min), followed by PBS wash 2 times. PBMCs were added to tissue culture wells in DMEM/F12 and washed to remove nonadherent cells after 1 h, or alternatively, were sorted using FACSaria (BD Biosciencesm San Jose, CA, USA), selecting the high-FSC, low-SSC population of monocytes. Whole kidney was minced and then digested using Liberase CI and DNase (Roche, Basel, Switzerland) in HBSS for 30 min at 37°C, then filtered (30 μm). Resuspended cells were added to tissue culture wells in complete DMEM/F12, and nonadherent cells washed after 1 h. Alternatively filtered single cells were resuspended in FACS buffer (PBS, 0.1% BSA) (3), labeled with lotus lectin-FITC 1:200 (Dako, Copenhagen, Denmark) and anti-CD45-PE, 1:200 (eBioscience), or anti-Kim-1-biotin-antibodies, 1:200 (RMT1–4, eBioscience) followed by streptavidin-APC (Jackson ImmunoResearch), or labeled with F4/80-APC antibodies (eBioscience). Cells were sorted by FACSAria for Kim-1+ CD45-cells, LTL+ CD45-cells, or F4/80+ cells. Sorted cells were immediately lysed and RNA purified using the RNA Easy (Qiagen) system. To determine the presence of cell surface Gpnmb expression, anti-Gpnmb Ecto antibodies or control rabbit IgG, 1:400, were applied to LLCPK1 cells expressing Gpnmb (30 min, 4°C) in FACS buffer, then after washing, anti-rabbit-biotin antibodies were applied (30 min, 4°C), followed by washing and incubation of streptavidin-APC, 1:2000 (20 min, 4°C), followed by washing and fixation. Fluorescence was compared using FACSCalibur flow cytometry. To detect acid load of Mφ lysosomes, cultured BMMφs were treated with LTR as described above, then assessed by flow cytometry in the FL3 channel for fluorescence compared with unlabeled controls.
Immunoblotting
Bone marrow Mφs (107/condition; d 7–10) were stimulated with 100 ng/ml LPS, 100 U/ml IL4, 500 U/ml IFNγ, vehicle, or 5 nM dexamethasone for 24 h, then membrane proteins were purified as described above for immunoprecipitation. Then 25–100 μg protein/condition was denatured with Laemmli buffer, separated by SDS-PAGE, and transferred to PVDF membrane. The membranes were probed with anti-Gpnmb antibodies as described above. The membrane was stripped and reprobed using anti-ERK antibodies (Cell Signaling, Danvers, MA, USA) to assess loading, followed by anti-rabbit secondary antibodies. To detect thymocyte CD3ζ chain in BMMφs, protein lysates were prepared, resolved by SDS-PAGE as above, and transferred to PVDF membranes that were probed with anti-CD3-ζ chain antibodies (1:1000; 70619; Santa Cruz Biotechnology), followed by goat anti-mouse secondary antibodies. Blots were stripped and reprobed using anti-ERK antibodies as above.
Statistical analysis
All values are given as means ± se. The Mantel-Cox log-rank test was used to analyze survival. The nonparametric Mann-Whitney U test was used for group comparisons. Analyses were performed using Prism software (GraphPad, San Diego, CA, USA).
RESULTS
Gpnmb expression is increased in inflammatory Mφs and injured epithelial cells during repair following injury of the kidney
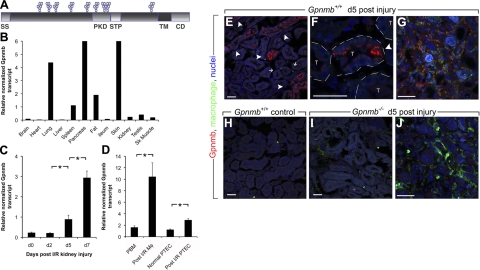
To identify genes that are up-regulated during tissue repair after injury, we used representational difference analysis, a PCR-based method of subtraction, to analyze populations of cDNAs generated from normal and postischemic rat kidneys (8) and identified Gpnmb as being highly expressed following ischemic injury (26). Gpnmb is a type I transmembrane glycoprotein with an endosomal sorting signal in the cytoplasmic domain and a PKD of unknown function (Fig. 1A). In healthy mice, Gpnmb transcript is normally expressed in skin, spleen, lung, pancreas, and fat and is present at lower levels in kidney, testis, and skeletal muscle (Fig. 1B). However, consistent with our cDNA screen, Gpnmb is markedly up-regulated during the phase of repair (24 h to 7 d) following IRI in mouse kidney (Fig. 1C). Purified single-cell suspensions of proximal tubule epithelial cells (PTECs) and Mφs from d 5 post-IRI kidney had up-regulated Gpnmb transcripts compared with healthy PTECs and monocytes from the blood of post-IRI mice, respectively (Fig. 1D). Using polyclonal antibodies generated against the N terminus of Gpnmb, immunofluorescence analysis of mouse kidneys undergoing repair following IRI-identified Gpnmb expression in intratubular epithelial phagocytes, repairing epithelial cells and a subpopulation of interstitial Mφs 5 d post-IRI (Fig. 1E–G). Expression was detected at greater levels in regenerating tubules 7 d post-IRI as well as interstitial Mφs (Supplemental Fig. S1A–D) (27). By contrast, no Gpnmb staining was detectable in unchallenged kidney (Fig. 1H). In post-IRI kidneys, Gpnmb was observed in intracellular compartments of epithelial cells and appeared to localize to both large and small cytoplasmic vesicles (Fig. 1F and Supplemental Fig. S1A–D). Unlike blood monocytes and resident kidney Mφs, many post-IRI kidney interstitial Mφs also expressed Gpnmb in intracellular vesicles (Fig. 1G and Supplemental Fig. S1D). In the outer medulla, which represents the region of most intense initial injury, more of the F4/80+ inflammatory Mφs expressed Gpnmb, whereas in the outer cortex, where initial injury was mild, the proportion that coexpressed Gpnmb and F4/80+ inflammatory Mφs was lower (Table 1). The specificity of the N-terminal antibodies was confirmed by the lack of reactivity on post-IRI kidneys from Gpnmb−/− mice (Fig. 1I, J).
Figure 1.
Kidney epithelial cells and inflammatory Mφs express Gpnmb in response to ischemic injury. A) Domain model of Gpnmb. B) Representative quantitative PCR (qPCR) of Gpnmb transcripts in normal mouse tissues. C) qPCR for Gpnmb transcript from sham surgery mouse kidney (d 0) and from time points following IRI kidney. D) qPCR for Gpnmb transcript from d 5 post-IRI kidney Mφs compared with peripheral blood monocytes (PBM), and injured proximal tubule epithelial cells (PTEC) compared with normal PTEC. E–J) Confocal images of Gpnmb detected by immunofluorescence in Mφs in normal kidney (H) and d 5 post-IRI mouse kidney outer medulla (E, F) and inner medulla (G). Injured, regenerating epithelial cells express Gpnmb (arrowheads) as well as intratubule epithelial phagocytes. Although inner medulla macrophages coexpress Gpnmb (F), cortical macrophages (E, small arrows) do not. Note Gpnmb+ intracellular vesicles in regenerating epithelial cells (F). T, tubule. In Gpnmb−/− post-IRI kidneys (I, J), outer medulla epithelium (I) and inner medulla Mφs (J) do not express Gpnmb. Scale bars =5 0 μm. *P < 0.05 (n=4/group).
Table 1.
Percentage of F4/80-positive cells in the outer medulla or outer cortex of the d 7 post-IRI kidney that also express Gpnmb
| Parameter | Medulla | Cortex |
|---|---|---|
| NMB+ cells (%) | 74.0 ± 4.0 | 38 ± 5.0 |
To determine the normal pattern of Gpnmb expression in the mouse, we next performed immunohistochemical staining of healthy tissues. Gpnmb staining was detected in discrete subsets of both resident and inflammatory Mφs in disparate tissues. Notably, alveolar Mφs expressed Gpnmb, but resident Mφs in other organs such as liver, heart, or kidney did not (Supplemental Fig. S1E). In lymphoid organs, the Gpnmb-expressing subpopulations of Mφs and dendritic cells in spleen (Supplemental Fig. S1F) and thymus co-expressed the hemoglobin scavenger receptor CD163 expression. However, not all of the Gpnmb+ cells expressed the Mφ marker CD68, strongly suggesting that a proportion of the Gpnmb+ myeloid cells were DCs. Tingible body Mφs in the splenic white pulp, identified because of numerous phagosomes containing degraded lymphocytes in the cytoplasm, also expressed Gpnmb (Supplemental Fig. S1F). Although we did not identify Gpnmb in normal liver, it was highly up-regulated in a subpopulation of Mφs that we have previously shown to mediate regression of fibrosis in the liver by degradation and phagocytosis of extracellular matrix (Supplemental Fig. S1H) (5, 6). Furthermore, Gpnmb was highly expressed in foam cells (Mφs) of atherosclerotic plaques, which are involved in clearance of oxidized lipids (Supplemental Fig. S1G). Gpnmb expressed by cultured BMMφs was as 2 discrete bands in whole-cell lysates by both anti-C-terminal or N-terminal antibodies (Supplemental Fig. S1I, J). In BMMφs cultured from Gpnmb−/− mice, neither of these proteins were detectable (Supplemental Fig. S1I). Gpnmb was expressed basally in BMMφs but was down-regulated in M1 Mφs activated with the Toll-like receptor 4 ligand LPS or IFNγ (Supplemental Fig. S1J). IL-4 stimulation did not increase Gpnmb expression in these primary cells, but cultured BMMφs expressed Gpnmb at much higher levels than circulating monocytes (Supplemental Fig. S1K), suggesting the BMMφs may already have some degree of M2 polarization. Together, these observations implicate Gpnmb in repair processes where scavenging functions are proposed, as well as other functions that require the uptake large amounts of extracellular material, such as apoptotic lymphocytes in the spleen and photoreceptor outer segments in the eye by RPEs (11, 28). Further, these findings suggest Gpnmb marks a distinct population of wound healing or reparative Mφs and that it may play a role in the clearance of debris within wounded tissue (29, 30).
Gpnmb is critically important in repair following injury
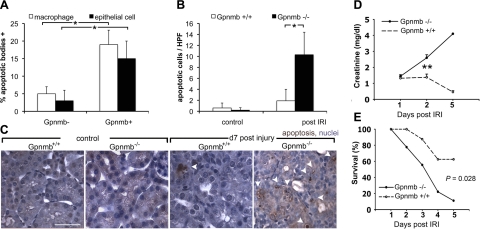
The pattern of expression in the kidney during repair post-IRI implicates Gpnmb as an effector protein of wound-healing Mφs. In addition to Mφs, there has recently been increased emphasis on the role injured epithelia in clearance of debris and dying cells by phagocytosis during repair and regeneration (2, 31). To test the possible relationship between Gpnmb and these processes, Gpnmb+ Mφs and epithelial cells in the kidney were examined microscopically for the presence of apoptotic bodies (Fig. 2A) (2). Both Gpnmb+ Mφs and epithelial cells contained more apoptotic bodies than their counterparts lacking Gpnmb. To test the significance of this observation to tissue repair, IRI was induced in kidneys of Gpnmb−/− mice and strain-matched controls. Histological assessment of kidneys from mice with unilateral IRI, d7 following injury, showed a significant increase in apoptotic cells in the kidney tubules and interstitium in homozygous Gpnmb mutant animals relative to wild-type (WT) controls (Fig. 2B, C), indicating that Gpnmb was functionally important for clearance of injury-induced debris. Even more strikingly, when bilateral kidney IRI was induced in Gpnmb−/− mice or WT controls, even though the initial injury phase in kidneys from both mice was similar (Fig. 2D), the Gpnmb−/− mice failed to repair normally, as quantified by the marker of kidney function, plasma creatinine level (Fig. 2D). Plasma creatinine levels in mutant mice remained elevated and increased during the normal repair phase while it showed the expected initial rise followed by a return to base line in the WT controls. None of the Gpnmb−/− mice that received bilateral IRI were able to recover function (Fig. 2D) and consequently exhibited increased mortality compared to control mice (Fig. 2E). Collectively, these findings demonstrate that Gpnmb plays a vital role in either the regulation of apoptotic cell death or in the removal of dead cells by Mφs and epithelial cells following IRI.
Figure 2.
Gpnmb is necessary for postischemia reperfusion kidney repair and is required for normal apoptotic cell clearance. A) Proportion of post-IRI d 7 kidney Mφs or epithelial cells in the outer medulla containing apoptotic bodies segregated by expression of Gpnmb. *P < 0.05. B, C) Apoptotic cells seen in the normal and d 7 postunilateral IRI kidneys of Gpnmb−/− mice and controls. Note an abundance of apoptotic bodies (arrowheads) in the kidney outer medulla. D) Plasma creatinine levels in Gpnmb−/− and Gpnmb+/+ mice following bilateral IRI (n=9/group). **P < 0.001. E) Kaplan-Meier survival curves for Gpnmb−/− and Gpnmb+/+ mice following bilateral IRI. P = 0.028
Inflammatory Mφs mediate repair of the kidney post-IRI
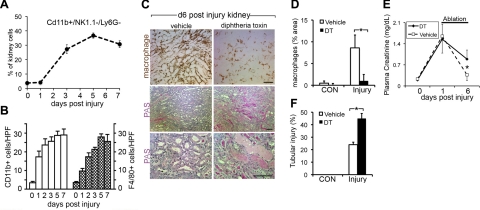
In recent studies we have highlighted a key role for injured epithelial cells in repair and regeneration of the kidney through phagocytosis and endocytosis of debris in the tubule lumen (2). Given the observed up-regulation of Gpnmb in Mφ in the post-IRI kidneys and motivated by recent studies of the role of inflammatory Mφs during the repair of liver, heart, and skeletal muscle (4, 6, 7), we analyzed Mφ content in IRI kidneys by flow cytometry. Whereas the number of monocyte/Mφs (CD11b+, NK1.1−, Ly6G−) in the kidneys increased little over 24 h following injury; by 72 h following injury, we observed a marked increase in Mφs within the tissue (Fig. 3A). This increase persisted through 7 and 10 d post-IRI. Thus, the kinetics of Mφ recruitment coincided with repair, not injury, and these data were further supported by immunostaining and scoring tissue sections from parallel samples for CD11b+ cells (detecting neutrophils and monocytes/Mφs) or F4/80+ (Mφs only) cells (Fig. 3B). In contrast to Mφ populations, neutrophil recruitment occurred earlier following injury and did not persist and was thus more consistent with an injury response (not shown). Therefore, to study the function of Mφs during repair of the kidney, conditional Mφ ablation in vivo was performed using the Cd11b-DTR transgenic mouse model we developed previously (5). In this in vivo model, administration of minute amounts of diphtheria toxin (DT) results in rapid specific ablation of monocytes and kidney Mφs (3–5, 19, 32). Cohorts of mice with identical IRI were randomized at 1 d post-IRI to receive DT from 2–6 d or vehicle (Fig. 3C–F). Ablation was successful when F480+ kidney Mφs were quantified (Fig. 3C, D). In those mice that retained the normal complement of Mφs there was recovery of injury as assessed by plasma creatinine level and tubular injury score. However, in mice that had Mφ ablation, plasma creatinine levels did not recover. Coincident with this, tubules showed severe persistent injury, and the tubule injury score remained also markedly elevated compared with mice that had the normal Mφ complement (Fig. 3C, F). Tubules remained severely injured with flattened morphology, and there was a persistence of debris within the tubules. These observations provide strong evidence that Mφs are functioning in a reparative capacity in this model.
Figure 3.
Mφ ablation in Cd11b-DTR mice prevents normal kidney repair following IRI. A) Percentage of kidney cells that are CD11b+, NK1.1−, Ly6G− Mφs, as assessed by flow cytometry. B) CD11b+ and F4/80+ cells in the kidney following IRI, quantified by immunostaining of kidney sections. C) Photomicrographs of F4/80 staining and PAS staining of kidneys at d 6 post-IRI following no Mφ ablation (vehicle) or Mφ ablation with DT from d 3 to 6. D) F4/80 area in control kidneys or d 6 post-IRI kidneys following ablation. E) Effect of Mφ ablation from d 3 to 6 post-IRI on plasma creatinine. Note that following Mφ ablation there is a higher creatinine level on d 6 than in mice with normal Mφ compliment. F) tubule injury score in d 6 post-IRI kidneys following ablation. Scale bars = 100 μm. *P < 0.05.
Gpnmb localizes to LC3-containing vesicle compartments
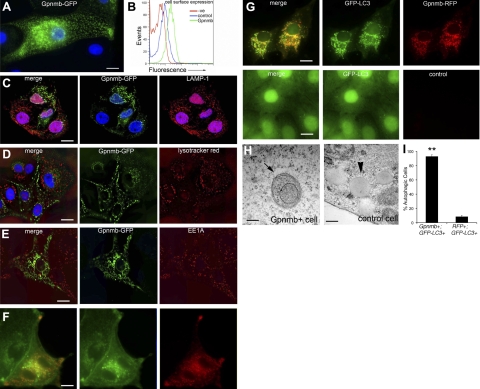
To further dissect the cellular mechanism by which Gpnmb promotes repair, we determined its subcellular localization. Untagged Gpnmb or Gpnmb fused to either GFP or RFP (Gpnmb-GFP or Gpnmb-RFP) was expressed in the porcine kidney epithelial cell line LLC-PK1. Both the untagged (detected by antibody; not shown) and fluorescently tagged Gpnmb proteins localized to intracellular vesicles (Fig. 4A). The protein was primarily detected associated with the membranes of these vesicles. To test whether Gpnmb was also expressed at low levels at the cell surface, cells were stained with anti-Gpnmb antibodies directed against its ectodomain, and detection was amplified by secondary antibodies and biotin-avidin complexes. Compared with nonimmune controls, anti-Gpnmb antibodies specifically identified Gpnmb on the cell surface (Fig. 4B).
Figure 4.
Gpnmb localizes to autophagosomes in epithelial cells and is expressed at low levels at the plasma membrane. A) Gpnmb-GFP is localized to membranes of a network of intracellular vesicles when expressed in kidney epithelial cells in vitro. B) Cell surface expression of Gpnmb, assessed by flow cytometry. Cells were stained either with rabbit IgG (control) or anti-Gpnmb ectodomain-specific antibodies. C) Confocal images of Gpnmb-GFP (green) in cells expressing the transgene and costained with antibodies against LAMP-1 (red). Note that despite extensive networks of both LAMP-1 and Gpnmb compartments, there is no colocalization. D) Confocal images of Gpnmb-GFP-expressing cells stained with LysoTracker Red. Note again there is no colocalization. E) Confocal images of Gpnmb-GFP cells stained with antibodies against the early endosomal marker EEA1 (red). Note there is no colocalization. F) Fluorescence images of Gpnmb-RFP cells stained with the cholesterol marker filipin (false green). Note the extensive colocalization. G) Fluorescence images of Gpnmb-RFP+ GFP-LC3 cells (top panels), and control GFP-LC3 cells (bottom panels). Note extensive colocalization of these 2 proteins and the reorganization of LC3 in Gpnmb-expressing cells. H) Electron micrographs showing the double membrane autophagosome in the cytoplasm of a Gpnmb expressing cell (arrow), and absence of autophgosomes, but the presence of lipid droplets (arrowhead) in control cell. I) Percentage of autophagic cells stably coexpressing Gpnmb-RFP and GFP-LC3 compared with cells expressing GFP-LC3 and control vector RFP. Scale bars = 10 μm (A–G); 100 nm (H).
To identify the intracellular Gpnmb-containing compartment in resting cells, we stained Gpnmb-GFP-expressing LCC-PK1 epithelial cells with the lysosomal markers LAMP-1 and LysoTracker Red. However, we failed to detect overlap between the extensive Gpnmb staining and either of these lysosomal markers (Fig. 4C, D). Similarly, antibodies to the early endosomal marker EEA1 (Fig. 4E) and the peroxisomal marker catalase (not shown) also failed to colocalize with the tagged Gpnmb. Thus, Gpnmb did not localize to lysosomal, early-endosomal, and/or peroxisomal compartments. However, Gpnmb colocalized with both filipin (Fig. 4F) and oil red O (not shown), demonstrating that Gpnmb localized to cholesterol-rich vesicular membranes. Since the identity of Gpnmb+ vesicles was not apparent and since autophagosomes can form during cellular stress in the kidney epithelium (Supplemental Fig. S2A), we explored whether Gpnmb was detected in autophagosomes. A GFP-tagged form of the autophagy protein Atg8 (LC3) was stably expressed in epithelial cells. In cells not expressing recombinant Gpnmb, GFP-LC3 was localized diffusely in the cytoplasm and nucleus (Fig. 4G), but in Gpnmb+ cells or Gpnmb-RFP+ cells, GFP-LC3 was relocalized to intracellular compartments (Fig. 4G) and colocalized with Gpnmb-RFP. This pattern of GFP-LC3 was similar to its reorganization induced by amino acid starvation (not shown). Gpnmb+ epithelial cells were quantified for autophagy by scoring the presence of LC3+ vesicles (Fig. 4I), revealing a clear difference compared with the control cells. Thus, Gpnmb colocalized with LC3 to a vesicular compartment, suggesting that it may localize to autophagosomes. Gpnmb-expressing cells but not control cells exhibited many double membrane vesicles by electron microscopy (EM) consistent with autophagosomes (Fig. 4H). Analysis of random EM fields (×20,000) of Gpnmb+ LLCPK1 cells revealed 1.0 ± 0.3 autophagosomes/field, whereas no autophagosomse were found in control cells. In rapamycin-treated cells, we observed 0.5 ± 0.2 autophagosomes/field. When Gpnmb+ cells were incubated with chloroquine to inhibit autophagsome degradation, Gpnmb+ vesicles rapidly expanded in size (Supplemental Fig. S2C). Moreover, the Gpnmb-RFP fusion protein accumulated rapidly in response to chloroquine treatment (Supplemental Fig. S2D). The combination of these findings, namely, the presence of Gpnmb in the membranes of intracellular vesicles, its colocalization with LC3 in these vesicular membranes, and the accumulation of vesicles and membrane content on chloroquine treatment, strongly suggests that Gpnmb colocalizes to autophagosomes.
These observations in cultured epithelial cells are consistent with the observation that post-IRI kidney epithelial tubules have autophagosomes detected by reorganization of native LC3 to vesicles in sections stained with anti-LC3 antibodies (Supplemental Fig. S2A) as well as detection of increased transcripts for the autophagy proteins Atg5 and Atg7 (Supplemental Fig. S2B).
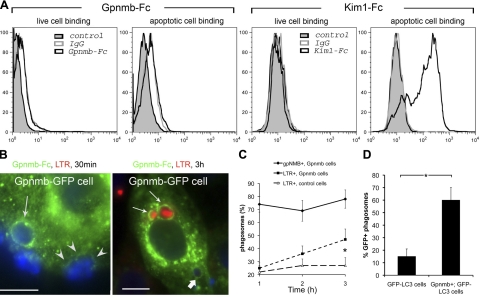
Gpnmb is recruited to the phagocytic cup and promotes phagosomal acidification in epithelial cells
Macroautophagy is a bulk degradation pathway involved in the disposal of used macromolecular structures within the cell via lysosomal degradation (33). Since Gpnmb colocalizes with autophagy proteins and Gpnmb−/− mice have impaired kidney repair characterized by an excess of dead cells, we hypothesized that Gpnmb regulates clearance and/or degradation of apoptotic bodies in the kidney by targeting them for lysosomal degradation. Therefore, we tested whether Gpnmb was a phagocytic receptor that could deliver cellular debris to the lysosomal machinery to facilitate its degradation. However, unlike in the case of the extracellular domain of the apoptotic cell receptor Kim-1/Tim-1 (2), Gpnmb-Fc fusion proteins did not bind to apoptotic cells (Fig. 5A). In addition, Gpnmb-Fc did not bind fluorescently tagged oxidized LDL and Gpnmb expression in both epithelial cells, and fibroblasts did not confer enhanced capacity for phagocytosis (data not shown), further underscoring the lack of apoptotic cell receptor function. Despite this lack of avidity for apoptotic cells, in cell culture, Gpnmb-GFP was rapidly recruited to the phagocytic cup during apoptotic cell phagocytosis (Fig. 5B) and remained an integral part of the phagosome membrane throughout phagosome maturation (Fig. 5B, C). The rate of acidification of phagosomes as a consequence of lysosomal fusion was significantly enhanced by expression of Gpnmb (Fig. 5C). Since autophagic proteins are also required for recruitment of lysosomes to autophagosomes during bulk degradation (34) and Gpnmb colocalized with the autophagy protein LC3 (Fig. 4), we tested whether Gpnmb expression also resulted in the recruitment of LC3 to phagosomes (Fig. 5D). In epithelial cells coexpressing Gpnmb and LC3-GFP there was early recruitment of LC3-GFP to the phagosome, whereas in the absence of Gpnmb, LC3 recruitment was reduced. Together these findings suggest that in epithelial cells, Gpnmb plays a role in the coordinated recruitment of LC3 to the phagosome. LC3 recruitment in turn is required for lysosomal fusion with the phagosome (35).
Figure 5.
Gpnmb is recruited to the phagocytic cup and directs phagocytic trafficking to a lysosomal degradation pathway A) Binding of Gpnmb-Fc or IgG to live thymocytes or apoptotic thymocytes (left panels) and binding of Kim1-Fc or IgG (right panels). Kim1-Fc specifically binds to apoptotic cells, whereas Gpnmb-Fc does not. B) Gpnmb-GFP-expressing epithelial cells incubated with apoptotic thymocytes for 30 min or 1 h, followed by 2 h incubation. Gpnmb-GFP is recruited to an intact phagosome (arrow) and is also seen at the base of the phagocytic cup (arrowheads) before internalization. Image shows apoptotic bodies within Gpnmb phagosomes at 3 h (arrows). Early phagosomes are not stained with LysoTracker Red (thick arrow) whereas late phagosomes are (thin arrows). C) Time course of Gpnmb association with phagosomes compared with LysoTracker Red association with phagosomes in Gpnmb-GFP cells or control cells. Note that cells not expressing Gpnmb-GFP fail to acidify phagosomes. D) Graph of GFP-LC3 recruitment to phagosomes after 1 h of incubation in GFP-LC3 cells compared with Gpnmb+ GFP-LC3 cells. *P < 0.05 (n=5/group).
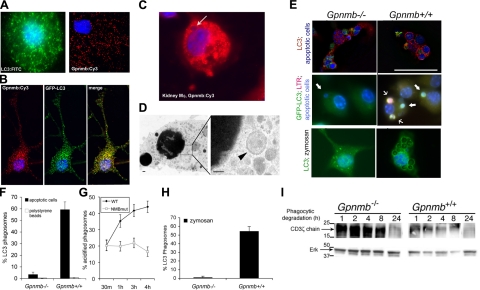
Endogenous Gpnmb promotes LC3 endosomal fusion and lysosomal acidification of phagosomes in Mφs
To test whether endogenous Gpnmb functioned similarly to expressed-recombinant Gpnmb, and Gpnmb functioned similarly in myeloid cells compared with epithelial cells, cultured, unstimulated BMMφs expressing endogenous Gpnmb (Fig. 6A and Supplemental Fig. S1) were immunolabeled for subcellular localization of Gpnmb, which was detected in a vesicular compartment in a similar pattern to endogenous LC3 protein (Fig. 6A). Furthermore, endogenous Gpnmb colocalized with retrovirally expressed LC3-GFP-labeled vesicles (Fig. 6B). Gpnmb expression in Mφs purified from the repairing kidney also had a vesicular pattern, and endogenous Gpnmb was identified in phagosome membranes (Fig. 6C). Similar phagosomal localization was observed in BMMφs on ingestion of apoptotic cells in vitro (not shown). To test whether these LC3+/Gpnmb+ compartments were autophagosomes, the cytoplasm of BMMφs that had phagocytosed apoptotic cells was sequentially analyzed by electron microscopy for the presence of double-membrane endosomes (Fig. 6D). Although autophagosomes were detected in small numbers surrounding apoptotic bodies (1–3/total cell cross section; Fig. 6D), they were present in insufficient numbers to account for all the LC3+/GFP+ vesicles seen in BMMφs, indicating that many of the LC3+/Gpnmb+ vesicles in BMMφs are not autophagosomes despite containing the autophagy protein LC3. BMMφs from Gpnmb−/− mice or strain-matched Gpnmb+/+ (WT) mice were then coincubated with apoptotic cells (thymocytes), and the resulting phagocytosis was quantified in a flow cytometric-based assay (2). Consistent with observations in epithelial cells, the absence of Gpnmb had no effect on phagocytic rate in vitro (Table 2). However, phagocytosis of both fluorescently labeled apoptotic thymocytes (Fig. 6E, F) and unlabeled zymosan particles (Fig. 6E, H) by Gpnmb−/− Mφs was accompanied by poor recruitment of LC3 to the phagosomes, but robust LC3 recruitment in Gpnmb+/+ Mφs. Inert polystyrene beads (Fig. 6F) did not recruit LC3 regardless of the expression of Gpnmb, indicating that other recognition factors must play a role in the coordinated recruitment of Gpnmb and autophagy proteins to phagosomes. To assess the kinetics of LC3 recruitment and lysosomal fusion with apoptotic cell phagosomes, after 1 h of phagocytosis in vitro, LC3+ and LTR+ phagosomes were quantified. We found that 68 ± 13% of the phagosomes had recruited LC3 in Gpnmb+/+ Mφs, whereas only 36 ± 8% of LC3+ phagosomes also contained the LTR, indicating that in BMMφ, LC3, and Gpnmb were components of the early phagosome prior to lysosomal fusion with the phagosome. This is similar to our findings in epithelial cells. To determine the functional consequence of failed LC3 recruitment to the apoptotic cell in the absence of Gpnmb, the rate of lysosomal fusion with phagosomes in Gpnmb−/− and WT Mφs was compared. Lysosomal fusion was substantially delayed in Gpnmb−/− Mφs (Fig. 6G), confirming the importance of Gpnmb in recruiting lysosomes to the phagosome. To explore this observation further we analyzed the ability of BMMφs from Gpnmb−/− and Gpnmb+/+ mice to degrade the CD3ζ-chain protein of apoptotic thymocytes following uptake. In these experiments, apoptotic thymocytes were incubated with BMMφs for 1 h to allow for their phagocytosis, followed by washing to remove the noninternalized cells. There was no observable difference in phagocytic uptake rate between mutant and WT BMMφs (Table 2). At increasing times after phagocytosis, BMMφ cell lysates were examined for content of the thymocyte ζ-chain by Western blot analysis. Whereas ζ-chain was rapidly degraded in WT Mφs, it was degraded much more slowly in Gpnmb mutant Mφs (Fig. 6I). Nevertheless, after 24 h, the ζ-chain was degraded in both mutant and WT Mφs.
Figure 6.
Endogenous Mφ Gpnmb colocalizes with autophagic proteins and promotes phagosome acidification and degradation. A) Representative confocal images of BMMφs showing endogenously expressed LC3 and Gpnmb localization to intracellular vesicles. B) Representative confocal image of BMMφs transduced with GFP-LC3 and labeled with anti-Gpnmb antibodies, revealing a high degree of colocalization. C) Representative image of kidney Mφs from d 5 post-IRI kidney labeled with antibodies to Gpnmb (red) and colabeled with DAPI. Note the apoptotic body in a Gpnmb phagosome (arrow). D) Electron micrograph of healthy BMMφs showing double-membraned autophagosome adjacent to a single-membrane, apoptotic body phagosome. E) Confocal images of native LC3 immunostaining (red; top panels) or retrovirally transduced GFP-LC3 (green; middle and bottom panels) after 1 h of phagocytosis of apoptotic cells (blue) or zymosan by Gpnmb−/− or Gpnmb+/+ BMMφs. Note that the phagosome frequently contains membrane-associated LC3 in WT Mφs but rarely in Gpnmb−/− Mφs. Cells were coincubated with LysoTracker Red. Note some LC3+ phagosomes in Gpnmb+/+ Mφs show red color, indicative of early lysosome interaction. F) Percentage apoptotic body or polystyrene bead phagosomes in Gpnmb−/− or Gpnmb+/+ Mφs that show ring enhancement due to LC3 in the membrane after 1 h incubation. G) Percentage of zymosan phagosomes in Gpnmb−/− or Gpnmb+/+ Mφs that show ring enhancement due to LC3 in the membrane. H) Percentage apoptotic body phagosomes that label with LysoTracker Red with time in Gpnmb−/− or Gpnmb+/+ Mφs. I) Immunoblot measuring Mφ content of the thymocyte-specific protein CD3 ζ-chain at time points following 1 h of phagocytosis of apoptotic thymocytes. Scale bars = 100 nm (D); 50 μm (E).
Table 2.
Percentage apoptotic cell phagocytosis by either epithelial cell lines or Mφs in vitro
| Parameter | LLCPK1 cells |
Mφs |
||
|---|---|---|---|---|
| Control | Gpnmb-expressing | Gpnmb+/+ | Gpnmb−/− | |
| Phagocytosis (%) | 4.2 ± 0.3 | 4.6 ± 0.4 | 32.6 ± 5.9 | 28.4 ± 8.6 |
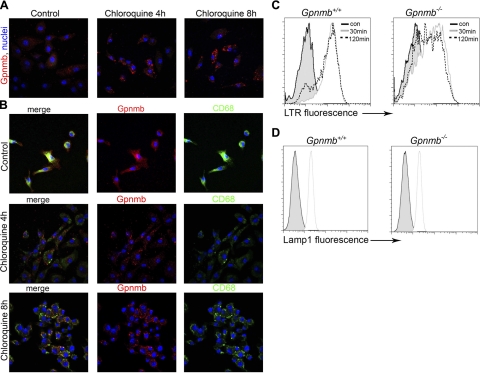
Our studies in BMMφs indicated that Gpnmb played a similar role in phagosomal acidification as in Gpnmb-expressing epithelial cells. However in unstimulated Mφs, the vast majority of Gpnmb+, LC3+ vesicles are not autophagosomes (Fig. 6). To determine the nature of these vesicles further, BMMφs were treated with chloroquine to permit accumulation of autophagosomes. Similarly to observations in LCC-PK1 cells (Supplemental Fig. S2), Gpnmb expression increased markedly in response to chloroquine treatment in Mφs (Fig. 7A) and accumulated in the membranes of large vesicles, consistent with the expected behavior of autophagosomes. The lysosome marker CD68 was detected in separate compartments from Gpnmb in normal BMMφs and predominantly in separate compartments in those cells treated with chloroquine for 4 h. However, after 8 h of treatment, CD68 lysosomal protein was readily detectable in Gpnmb compartments (Fig. 7B). Although LTR was detected in a separate compartment from Gpnmb in healthy BMMφs, after 4 h of chloroquine treatment there was significant acidification in Gpnmb compartments consistent with recruitment of lyosomal or late endosomal content to Gpnmb compartments (Supplemental Fig. S4A). It was not possible to colocalize Atg12 with Gpnmb because of lack of directly conjugated antibodies. Although Atg12 was very weakly detected only in healthy BMMφs, it was more clearly detected in LTR+ acid compartments in chloroquine-treated BMMφs, consistent with Atg12 localizing to Gpnmb compartments (Supplemental Fig. S4B). Although the total LAMP-1 expression, detected by flow cytometry, is similar in WT and Gpnmb−/− healthy BMMφs, reflecting similar lysosomal content, Gpnmb−/− BMMφs had an intracellular compartment with normal acid load and a compartment with low acid load (Fig. 7C, D).
Figure 7.
Characterization of intracellular compartments in Mφs. A) Confocal images of d 7 BMMφs, untreated or treated with chloroquine, then immunolabeled for Gpnmb. B) Split-panel confocal images of CD68 and Gpnmb in BMMφs untreated or treated with chloroquine. C, D) Flow cytometric histogram plots of LysoTracker Red fluorescence (C) and LAMP-1 immunofluorescnce (D) in WT and Gpnmb mutant BMMφs after 30 or 120 min loading. Note a population of LTR dim cells in Gpnmb mutants.
Gpnmb-deficient mice exhibit delayed phagosomal acidifcation in vivo during apoptotic cell clearance by peritoneal Mφs
Our studies in vitro point to a functional role for Gpnmb recruitment and LC3 recruitment to the phagosome. These findings explain the in vivo observations in the kidney (Figs. 1 and 2). However, we were unable to test the kinetics of apoptotic cell clearance in the kidney following injury. Gpnmb is expressed in elicited TEPMs, and the peritoneum is accessible for study of clearance kinetics (36). Therefore, to test whether Gpnmb promotes acidification of phagosomes in vivo, apoptotic cells were applied to the peritoneum where they were phagocytosed by TEPMs. TEPMs were harvested at time points following injection of apoptotic cells, and acidification of phagosomes was assessed. Although no detectable difference of phagocytic rate in TEPMs from WT and mutant mice was observed, the absence of Gpnmb resulted in delayed phagosome acidification (Supplemental Fig. S3). This observation points to a generalized role for Gpnmb recruitment to the apoptotic cell phagosome in regulating phagosome maturation and cargo degradation consistent with our observation of differences in ζ-chain degradation above.
DISCUSSION
We show that Gpnmb performs a previously unappreciated and vital function in tissue repair following injury of the kidney by linking the clearance of phagocytosed debris to its recruitment into vesicles containing autophagy proteins in both injured epithelial cells and inflammatory Mφs recruited to sites of inflammation. These findings not only highlight the important role of inflammatory Mφs in tissue repair following injury, but also underscore the role of surviving epithelial cells in promoting tissue repair through activation of a phagosomal pathway to which internalized contents are directed for degradation. The fact that Gpnmb is expressed by Mφs in other inflammatory settings including populations of Mφs and DC in atherosclerotic plaques, thymus, and spleen implicate Gpnmb in clearance and degradation of inflammatory oxidized lipids, and foreign or self peptides under homeostatic conditions. It has been reported that Gpnmb is expressed in RPE cells, which are highly phagocytic, a process necessary for clearing the constantly shed outer photoreceptor segments (11, 14). Our studies therefore also provide a possible mechanism whereby Gpnmb is important for maintaining retinal homeostasis by facilitating the uptake and disposal of this debris and sheds light on the mechanism underlying the retinal pathology observed in Gpnmb−/− mice (14).
Mechanisms by which tissues repair and regenerate following injury is of great interest because such mechanisms are likely to provide targets for novel therapeutic strategies as opposed to simply preventing further injury or deleterious inflammation in the damaged tissue (1). The findings presented here have uncovered a novel pathway that promotes or enhances repair: regulation of degradation of internalized debris and dead cells. Further, although it has long been supposed that phagocytosis is a key step in preventing inflammation and autoimmunity, an assertion recently supported by in vivo studies (37), relatively little attention has been given to the intracellular process of degradation of phagocytosed contents on inflammation or aberrant antigen presentation. The present studies provide a compelling link between macroautophagy proteins and tissue repair. Macroautophagy is a phylogenetically preserved, well-described bulk degradation pathway leading to clearance of intracellular organelles, by which cells generate autophagosomes that subsequently recruit lysosomes (33).
The current study reveals that epithelial cells up-regulate Gpnmb, and this likely coordinates lysosomal fusion with phagosomes in these cells. In previous studies we have shown that injured epithelial cells up-regulate expression of phagocytic receptors and become semiprofessional phagocytes (2). This injury-induced phagocytic state promotes recanalization of the epithelial lumen and is therefore a key process in epithelial repair. These studies therefore add to the understanding of epithelial function following injury because here we identify a mechanism by which injured epithelial cells can process phagocytosed cellular debris. The normal function of epithelial cells includes substantial endosomal transportation of small molecules across the polarized cell, but following injury a major function is the clearance and degradation of intraluminal debris. Therefore, the coordinated up-regulation of phagocytic receptors and the machinery to target and degrade internalized contents rather than transport them across the cell is paramount to that function. It therefore makes sense that injured epithelial cells utilize the macroautophagy pathway to achieve this end. The importance of Gpnmb to such repair processes is amply demonstrated by the failure to undergo repair and injury resolution in the Gpnmb mutant mice following kidney IRI challenge, demonstrating that Gpnmb function is essential to proper repair.
Although autophagosomes were identified in unstimulated Mφs, many vesicles containing both the autophagic protein LC3 and Gpnmb were not double-membrane structures (i.e., autophagosomes) and were also not lysosomes (38). In myeloid cells, therefore, Atg proteins may serve more complex functions than formation of classical autophagosomes, but may form an integral part of early phagosome maturation in Mφs. These studies therefore promote an important new concept: Mφs have coopted the phylogenetically ancient system of autophagic degradation for the specialized processes of endocytosis and phagocytosis. In resting Mφs in vitro and in subpopulations of phagocytosing Mφs in vivo, Gpnmb colocalizes in vesicles with autophagosome proteins including Atg8 (and probably Atg12). Under the conditions we studied, delivery of autophagic proteins to phagosomes is dependent on Gpnmb. In an analogous processes in lower order animals and plants, autophagosomes may fuse with one another to form single-membrane autophagic bodies or autophagic vacuoles or similarly may fuse with endosomes to form amphisomes (39–42). In mammals, failure to rapidly clear phagocytosed apoptotic cells, and other debris, may lead to premature death of Mφs, failure to clear inflamed sites, aberrant cytokine release, or aberrant presentation of antigens, as has been implicated by reports of aberrant dendritic cell function in the Gpnmb−/− mouse (14). Further studies will be required to understand the consequences to the Mφ of failure to recruit autophagic proteins. Recently the RAW Mφ cell line has been reported to show recruitment of autophagosome proteins to the phagosome in a toll-like receptor-dependent manner, lending weight to the studies reported here in Mφs (38). Further, in early embryogenesis, the absence of the autophagic protein Atg5 or Beclin1 leads to failure of phagocytosis of apoptotic bodies in mammals in the embryoid body (43), also in keeping with our studies and lending support to the notion that Atg proteins are important factors in phagocytic clearance. However, our studies identify an additional component of the complex clearance pathways in the mononuclear phagocyte system. The importance of TLR ligation in regulating phagosome maturation has recently been exemplified (38, 44, 45), yet recruitment of Gpnmb to apoptotic cell phagosomes and hence Atg proteins is not a TLR-dependent step but nevertheless regulates the rate of degradation of cargo in the phagosome. It is tempting to speculate that Gpnmb deficiency may predispose to aberrant presentation of self peptides as has been suggested by others (14). The consequence of this for autoimmunity remains to be explored.
Although other studies have suggested Gpnmb colocalizes with lysosomes in nonstressed cells, our studies do not confirm those findings, but in situations of cellular stress or phagocytosis, contents of membrane proteins from lysosomes may be delivered to Gpnmb compartments (46, 47). It is striking that in chloroquine-treated BMMφs in which autophagosomes both enlarge and accumulate, the autophagosomes have Gpnmb in the membranes. It is therefore likely that in healthy BMMφs the Gpnmb+, LC3+ vesicles fuse with forming autophagosomes as well as with maturing phagosomes. Further studies are required to define better the Gpnmb+, LC3+ vesicles in Mφs and determine the role of LC3 in acidification since LC3 is not known to be required for autophagosome fusion per se.
In conclusion, the present study reveals that Gpnmb is highly up-regulated in the kidney during repair and promotes normal repair, by regulating degradation of phagocytosed material in inflammatory reparative, wound-healing Mφs and reparative epithelial cells through coordinated engagement of bulk degradative pathways.
Supplementary Material
Acknowledgments
The authors thank Dr. Ira Tabas (Columbia University, New York, NY, USA) for advice, Dr. Eileen O'Leary [Harvard Medical School (HMS)] for assistance with constructs, Dr. Peter Libby (HMS) and Galina Sukhova (HMS) for mouse aorta tissue sections, Dr. Noburu Mizushima (Tokyo Metropolitan Institutes of Science, Tokyo, Japan) for the LC3-EGFP-C1 construct, Dr. Jagesh S. Shah (HMS) for the RFP construct, Dr. Ben Humphreys (HMS) for KIM-1-GFP vector, Dr. Toshio Kitamura (University of Tokyo, Tokyo, Japan) for pMXs-puro vector, Dr. Martin Pollak (HMS) and Dr. Johannes Schlondorff (HMS) for advice, Dr. Joel Henderson and Dr. Helmut Rennke (HMS) for assistance with electron microscopy, and Dr. Lewis Cantley for his support of K.D.S.
This work was supported by U.S. National Institutes of Health (NIH) grants DK073299 and DK84077, a Gottschalk award from the American Society of Nephrology, Genzyme Renal Initiatives Program, and a Kidney Research UK Senior Fellowship. K.D.S. was supported by an NIH grant (GM041890) to Lewis C. Cantley.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Duffield J. S., Hong S., Vaidya V. S., Lu Y., Fredman G., Serhan C. N., Bonventre J. V. (2006) Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 177, 5902–5911 [DOI] [PubMed] [Google Scholar]
- 2.Ichimura T., Asseldonk E. J., Humphreys B. D., Gunaratnam L., Duffield J. S., Bonventre J. V. (2008) Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest. 118, 1657–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castano A. P., Lin S. L., Surowy T., Nowlin B. T., Turlapati S. A., Patel T., Singh A., Li S., Lupher M. L., Jr., Duffield J. S. (2009) Serum amyloid P inhibits fibrosis through Fc gamma R-dependent monocyte-macrophage regulation in vivo. Sci. Transl. Med. 1, 5ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R. K., Chazaud B. (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duffield J. S., Forbes S. J., Constandinou C. M., Clay S., Partolina M., Vuthoori S., Wu S., Lang R., Iredale J. P. (2005) Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallowfield J. A., Mizuno M., Kendall T. J., Constandinou C. M., Benyon R. C., Duffield J. S., Iredale J. P. (2007) Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 178, 5288–5295 [DOI] [PubMed] [Google Scholar]
- 7.Nahrendorf M., Swirski F. K., Aikawa E., Stangenberg L., Wurdinger T., Figueiredo J. L., Libby P., Weissleder R., Pittet M. J. (2007) The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichimura T., Bonventre J. V., Bailly V., Wei H., Hession C. A., Cate R. L., Sanicola M. (1998) Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 273, 4135–4142 [DOI] [PubMed] [Google Scholar]
- 9.McIntire J. J., Umetsu S. E., Akbari O., Potter M., Kuchroo V. K., Barsh G. S., Freeman G. J., Umetsu D. T., DeKruyff R. H. (2001) Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2, 1109–1116 [DOI] [PubMed] [Google Scholar]
- 10.Ripoll V. M., Meadows N. A., Raggatt L. J., Chang M. K., Pettit A. R., Cassady A. I., Hume D. A. (2008) Microphthalmia transcription factor regulates the expression of the novel osteoclast factor GPNMB. Gene 413, 32–41 [DOI] [PubMed] [Google Scholar]
- 11.Bachner D., Schroder D., Gross G. (2002) mRNA expression of the murine glycoprotein (transmembrane) nmb (Gpnmb) gene is linked to the developing retinal pigment epithelium and iris. Brain Res. Gene Expr. Patterns 1, 159–165 [DOI] [PubMed] [Google Scholar]
- 12.Weterman M. A., Ajubi N., van Dinter I. M., Degen W. G., van Muijen G. N., Ruitter D. J., Bloemers H. P. (1995) nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int. J. Cancer 60, 73–81 [DOI] [PubMed] [Google Scholar]
- 13.Ahn J. H., Lee Y., Jeon C., Lee S. J., Lee B. H., Choi K. D., Bae Y. S. (2002) Identification of the genes differentially expressed in human dendritic cell subsets by cDNA subtraction and microarray analysis. Blood 100, 1742–1754 [PubMed] [Google Scholar]
- 14.Mo J. S., Anderson M. G., Gregory M., Smith R. S., Savinova O. V., Serreze D. V., Ksander B. R., Streilein J. W., John S. W. (2003) By altering ocular immune privilege, bone marrow-derived cells pathogenically contribute to DBA/2J pigmentary glaucoma. J. Exp. Med. 197, 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shikano S., Bonkobara M., Zukas P. K., Ariizumi K. (2001) Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J. Biol. Chem. 276, 8125–8134 [DOI] [PubMed] [Google Scholar]
- 16.Anderson M. G., Smith R. S., Hawes N. L., Zabaleta A., Chang B., Wiggs J. L., John S. W. (2002) Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat. Genet. 30, 81–85 [DOI] [PubMed] [Google Scholar]
- 17.Kuan C. T., Wakiya K., Dowell J. M., Herndon J. E., 2nd, Reardon D. A., Graner M. W., Riggins G. J., Wikstrand C. J., Bigner D. D. (2006) Glycoprotein nonmetastatic melanoma protein B, a potential molecular therapeutic target in patients with glioblastoma multiforme. Clin. Cancer Res. 12, 1970–1982 [DOI] [PubMed] [Google Scholar]
- 18.Duffield J. S., Park K. M., Hsiao L. L., Kelley V. R., Scadden D. T., Ichimura T., Bonventre J. V. (2005) Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J. Clin. Invest. 115, 1743–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin S. L., Castano A. P., Nowlin B. T., Lupher M. L., Jr., Duffield J. S. (2009) Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J. Immunol. 183, 6733–6743 [DOI] [PubMed] [Google Scholar]
- 20.Shaner N. C., Campbell R. E., Steinbach P. A., Giepmans B. N., Palmer A. E., Tsien R. Y. (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 [DOI] [PubMed] [Google Scholar]
- 21.Hung C. C., Ichimura T., Stevens J. L., Bonventre J. V. (2003) Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J. Biol. Chem. 278, 29317–29326 [DOI] [PubMed] [Google Scholar]
- 22.Sigel M. B., Sinha Y. N., VanderLaan W. P. (1983) Production of antibodies by inoculation into lymph nodes. Methods Enzymol. 93, 3–12 [DOI] [PubMed] [Google Scholar]
- 23.Boussif O., Lezoualc'h F., Zanta M. A., Mergny M. D., Scherman D., Demeneix B., Behr J. P. (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U. S. A. 92, 7297–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee S., Zha X., Tabas I., Maxfield F. R. (1998) Cholesterol distribution in living cells: fluorescence imaging using dehydroergosterol as a fluorescent cholesterol analog. Biophys. J. 75, 1915–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills K. R., Reginato M., Debnath J., Queenan B., Brugge J. S. (2004) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc. Natl. Acad. Sci. U. S. A. 101, 3438–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ichimura T., Duffield J. S., Hung C.-C., Bonventre J. V. (2004) NMB, a PKD domain membrane glycoprotein, is highly expressed in macrophages during development and in the kidney after renal injury, and is localized to the cell surface and intracellular vesicles. J. Am. Soc. Nephrol. 15, 716A [Google Scholar]
- 27.Oda T., Hotta O., Taguma Y., Kitamura H., Sugai H., Onodera S., Horigome I., Suzuki K., Shouji Y., Furuta T., Chiba S., Yoshizawa N., Nagura H. (1998) Clinicopathological significance of intratubular giant macrophages in progressive glomerulonephritis. Kidney Int. 53, 1190–1200 [DOI] [PubMed] [Google Scholar]
- 28.Kevany B. M., Palczewski K.Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 25, 8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A., Sica A., Locati M. (2005) Macrophage polarization comes of age. Immunity 23, 344–346 [DOI] [PubMed] [Google Scholar]
- 30.Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rees A. J., Kain R. (2008) Kim-1/Tim-1: from biomarker to therapeutic target? Nephrol. Dial. Transplant. 23, 3394–3396 [DOI] [PubMed] [Google Scholar]
- 32.Duffield J. S., Tipping P. G., Kipari T., Cailhier J. F., Clay S., Lang R., Bonventre J. V., Hughes J. (2005) Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am. J. Pathol. 167, 1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Codogno P., Meijer A. J. (2005) Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 12(Suppl. 2), 1509–1518 [DOI] [PubMed] [Google Scholar]
- 34.Kuma A., Hatano M., Matsui M., Yamamoto A., Nakaya H., Yoshimori T., Ohsumi Y., Tokuhisa T., Mizushima N. (2004) The role of autophagy during the early neonatal starvation period. Nature 432, 1032–1036 [DOI] [PubMed] [Google Scholar]
- 35.Jager S., Bucci C., Tanida I., Ueno T., Kominami E., Saftig P., Eskelinen E. L. (2004) Role for Rab7 in maturation of late autophagic vacuoles. J. Cell Sci. 117, 4837–4848 [DOI] [PubMed] [Google Scholar]
- 36.Taylor P. R., Carugati A., Fadok V. A., Cook H. T., Andrews M., Carroll M. C., Savill J. S., Henson P. M., Botto M., Walport M. J. (2000) A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J. Exp. Med. 192, 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanayama R., Miyasaka K., Nakaya M., Nagata S. (2006) MFG-E8-dependent clearance of apoptotic cells, and autoimmunity caused by its failure. Curr. Dir. Autoimmun. 9, 162–172 [DOI] [PubMed] [Google Scholar]
- 38.Sanjuan M. A., Dillon C. P., Tait S. W., Moshiach S., Dorsey F., Connell S., Komatsu M., Tanaka K., Cleveland J. L., Withoff S., Green D. R. (2007) Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450, 1253–1257 [DOI] [PubMed] [Google Scholar]
- 39.Arnoult D., Tatischeff I., Estaquier J., Girard M., Sureau F., Tissier J. P., Grodet A., Dellinger M., Traincard F., Kahn A., Ameisen J. C., Petit P. X. (2001) On the evolutionary conservation of the cell death pathway: mitochondrial release of an apoptosis-inducing factor during Dictyostelium discoideum cell death. Mol. Biol. Cell. 12, 3016–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otto G. P., Wu M. Y., Kazgan N., Anderson O. R., Kessin R. H. (2003) Macroautophagy is required for multicellular development of the social amoeba Dictyostelium discoideum. J. Biol. Chem. 278, 17636–17645 [DOI] [PubMed] [Google Scholar]
- 41.Klionsky D. J., Abeliovich H., Agostinis P., Agrawal D. K., Aliev G., Askew D. S., Baba M., et al. (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zatloukal K., French S. W., Stumptner C., Strnad P., Harada M., Toivola D. M., Cadrin M., Omary M. B. (2007) From Mallory to Mallory-Denk bodies: what, how and why? Exp. Cell. Res. 313, 2033–2049 [DOI] [PubMed] [Google Scholar]
- 43.Qu X., Zou Z., Sun Q., Luby-Phelps K., Cheng P., Hogan R. N., Gilpin C., Levine B. (2007) Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell 128, 931–946 [DOI] [PubMed] [Google Scholar]
- 44.Blander J. M., Medzhitov R. (2006) On regulation of phagosome maturation and antigen presentation. Nat. Immunol. 7, 1029–1035 [DOI] [PubMed] [Google Scholar]
- 45.Blander J. M., Medzhitov R. (2004) Regulation of phagosome maturation by signals from toll-like receptors. Science 304, 1014–1018 [DOI] [PubMed] [Google Scholar]
- 46.Furochi H., Tamura S., Mameoka M., Yamada C., Ogawa T., Hirasaka K., Okumura Y., Imagawa T., Oguri S., Ishidoh K., Kishi K., Higashiyama S., Nikawa T. (2007) Osteoactivin fragments produced by ectodomain shedding induce MMP-3 expression via ERK pathway in mouse NIH-3T3 fibroblasts. FEBS Lett. 581, 5743–5750 [DOI] [PubMed] [Google Scholar]
- 47.Hoashi T., Sato S., Yamaguchi Y., Passeron T., Tamaki K., Hearing V. J.Glycoprotein nonmetastatic melanoma protein b, a melanocytic cell marker, is a melanosome-specific and proteolytically released protein. FASEB J. 24, 1616–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.