Abstract
Bone marrow-derived progenitor cells can fuse with cells of several different tissues, including lung, especially following injury. Despite many reports of cell fusion, few studies have examined the function of the resulting hybrid cells. We cocultured human multipotent stromal cells (hMSCs) and normal human bronchial epithelial cells (NHBEs) and observed the formation of hMSC/NHBE heterokaryons. The heterokaryons expressed several proteins characteristic of epithelial cells, such as keratin and occludin. Hybrid cells also expressed the mRNAs and proteins for 2 important ion channels that maintain bronchial and alveolar fluid balance: the cystic fibrosis transmembrane conductance regulator (CFTR) and the amiloride-sensitive epithelial Na+ channel (ENaC). By immunocytochemistry, CFTR was expressed in many hybrid cells but was absent or low in others. Whole-cell patch-clamp recordings demonstrated a glibenclamide-sensitive current in the presence of barium chloride, consistent with functional CFTR channels, in control NHBEs and hMSC/NHBE heterokaryons. Total cell capacitance measurements showed that the membrane surface area of heterokaryons was similar to that of NHBEs. Heterokaryons expressed the α- and γ-ENaC subunits but did not express the β-ENaC subunit, indicating the inability to form a complete ENaC channel. In addition, hybrid cells formed by the fusion of hMSCs with immortalized bronchial cells that expressed CFTR ΔF508 did not lead to reprogramming of the hMSC nucleus and expression of wild-type CFTR mRNA. Our data show that reprogramming can be incomplete following fusion of adult progenitor cells and somatic cells and may lead to altered cell function.—Curril, I. M., Koide, M., Yang, C. H., Segal, A., Wellman, G. C., Spees, J. L. Incomplete reprogramming after fusion of human multipotent stromal cells and bronchial epithelial cells.
Keywords: cell fusion, progenitor cell, CFTR, ENaC
Cell fusion has become an important issue in adult stem/progenitor cell biology, in particular for bone marrow-derived (BMD) cells. Some of the reported plasticity of BMD cells has been demonstrated to arise from fusion of BMD cells with injured or existing tissue-resident cells rather than through the process of homing, engraftment, and differentiation (1). Fusion between BMD cells, including BMD stem/progenitor cells, and differentiated cells occurs in a variety of tissues, including the heart (2, 3), brain (4, 5, 6), skeletal muscle (7, 8), intestine (9), liver (2, 10), and lung (3, 11). Cell fusion events in uninjured tissues are typically rare (2) and difficult to detect (12). In contrast, fusion events may increase markedly in the presence of tissue injury (5, 6, 13, 14). For some cell types, such as lung alveolar type II cells, the majority of bone marrow derivatives that contribute to the pool of type II cells may arise from cell fusion events. For example, Herzog et al. (11) found that >50% of the BMD surfactant protein C-expressing cells (type II cells) in the lung resulted from cell fusion following bone marrow transplantation of wild-type marrow into SPC-null recipients.
Whether cell fusion has a beneficial or detrimental impact on steady-state tissue dynamics or disease pathology is still not clear. In some cases, cell fusion has been shown to preserve cerebellar Purkinje neurons that have a limited adult population size (4, 5, 6, 13) or even to reverse a heritable disease phenotype in a mouse model of tyrosinemia type I (10, 15). In other cases, cell fusion has been shown to play a role in disease pathology (9). Although cell fusion has been documented in many tissues, few studies assess functional aspects of the resulting hybrid cells.
Previously, cell fusion has been reported to occur in cocultures of human mesenchymal stem cells (hMSCs; multipotent stromal cells) and small airway epithelial cells (SAECs) during the repair of injured epithelial layers (16). Time-lapse photomicroscopy demonstrated that over the course of 4 d following the initial fusion events, the hybrid cells took on the morphology of lung epithelial cells. Phenotyping studies further demonstrated the expression of mRNAs and proteins characteristic of differentiated epithelial cells in the resulting hMSC/SAEC hybrids (16).
Here we performed cocultures with genetically tagged hMSCs and normal human bronchial epithelial cells (NHBEs). To examine cell function, we first examined the expression of mRNAs and proteins for 2 important ion channels that maintain bronchial and alveolar fluid balance: the cystic fibrosis transmembrane conductance regulator (CFTR) and the amiloride-sensitive epithelial Na+ channel (ENaC). We then performed whole-cell patch-clamp measurements to compare CFTR function in hMSC/NHBE heterokaryons with that of control NHBEs. Attempts to rescue a common genetic defect of cystic fibrosis patients (CFTR ΔF508) by reprogramming of hMSC nuclei in cell fusion products were unsuccessful. Our results reveal incomplete reprogramming following the fusion of adult BMD progenitor cells with somatic cells.
MATERIALS AND METHODS
Coculture of NHBE cells and hMSCs
hMSCs were obtained from healthy donors by bone marrow aspiration under an Institutional Review Board–approved protocol (Tulane University Health Sciences Center, New Orleans, LA, USA). Primary cultures of hMSCs were lentivirally transduced to express green fluorescent protein (GFP) and mitochondria-targeted DsRed2 as in Spees et al. (17): donor 1 (GFP-labeled), donor 2 (GFP-labeled), and donor 3 (DsRed2-Mito- and GFP-labeled). The GFP and the DsRed2-mito lentivectors expressed fluorescent proteins from the EF1 α-short promoter for ubiquitous expression in mammalian cell types. Both vectors were constructed using the original Trono lab pWPT-GFP lentivector backbone (http://www.addgene.org/didier_trono).
Vials containing 1 × 106 cells each were frozen at −80°C for later use. For coculture experiments, frozen vials were thawed and plated in 20 ml of complete culture medium (CCM) in 150-cm2 culture dishes (Nunc; Thermo Fisher Scientific, Rochester, NY, USA). CCM consisted of α-MEM (Invitrogen, Carlsbad CA, USA) supplemented with 20% fetal calf serum (Life Technologies, Inc.; Invitrogen), 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 mM l-glutamine (Invitrogen). After 1 d on recovery plates, the hMSCs were split 1:5, and the culture medium was changed every 3 d until the cells reached 70–80% confluence. NHBEs (Clonetics, San Diego, CA, USA) were plated in 75-cm2 filter-top flasks (Nunc). They were grown in serum-free basal medium with supplements [BEGM with Singlequots (NHBE growth medium); Clonetics], until they achieved 70–80% confluence (∼1×106 to 1.5×106 cells). All cells were incubated at 37°C with 5% humidified CO2. To initiate cocultures, passage 4–5 hMSCs (2.5×105 cells) were added to flasks containing adherent passage 2–3 NHBEs (the ratio of cells was ∼1:4 to 1:6; hMSCs to NHBEs).
For live cell counts of GFP-positive epithelial heterokaryons, grids of 1-cm2 squares were drawn onto the bottoms of T75 flasks before cocultures. Three coculture flasks were counted for each hMSC donor. Individual squares (5) were chosen at random for counting from each flask. Within each of the 5 squares, 10 random fields were chosen, and the total numbers of GFP-positive cells and GFP-positive heterokaryons were counted. The cell percentages calculated from 10 fields, 5 squares, and 3 flasks were averaged for each hMSC donor separately. For immunocytochemistry studies, cells were cocultured in flasks for 4 d, lifted with trypsin, and cultured in Labtek chamber slides (Nunc) for 2–3 d before fixation.
Coculture of mutant CFT1 cells (CFTR ΔF508) with hMSCs
Immortalized human bronchial epithelial (HBE1) cells and immortalized tracheal epithelial (CFT1) cells harboring the CFTR ΔF508 mutation were cultured in DMEM/F12 medium (Life Technologies; Invitrogen) supplemented with 7.5% bovine serum albumin (Invitrogen), 20 μg/ml cholera toxin (Sigma, St. Louis, MO, USA), 10 μg/ml EGF (Sigma), 2 mg/ml insulin (Sigma), 2.5 mg/ml transferrin (Sigma), 0.05 mM dexamethasone (Sigma), 10 mg/ml brain pituitary extract (Invitrogen), 50 U/ml penicillin (Invitrogen), and 50 μg/ml streptomycin (Invitrogen). CFTR mutant cells were transduced with the DsRed2-mito lentivector, purified by FACS, and expanded. The DsRed2-mito CFTR mutant cells were then cocultured with GFP-positive hMSCs and treated with polyethylene glycol 1500 (PEG 1500; Roche Applied Science, Mannheim, Germany) to induce cell fusion by the Blau Laboratory protocol for production of heterokaryons (http://www.stanford.edu/group/blau/protocols/heterokaryon.html). After 4–5 d, fused cells (red and green) were isolated from the cultures by FACS. CFTR mRNA was analyzed by RT-PCR and DNA sequencing from control HBE1 cells and hMSC/CFTR ΔF508 hybrids.
Immunocytochemistry and photomicrographs
Cells cultured in Labtek chamber slides were washed with PBS and fixed with 4% paraformaldehyde in PBS for 10 min. After blocking with 5% normal goat serum in PBS containing 0.4% Triton X 100, slides were incubated overnight with primary antibodies in blocking buffer. The primary antibodies were as follows: rabbit polyclonal keratin 19 antibody (1:100; Spring Biosciences, Pleasanton, CA, USA), mouse monoclonal occludin antibody (1:400; Zymed, San Francisco, CA, USA), rabbit polyclonal α-ENaC antibody (1:400; CalBiochem, San Diego, CA, USA), mouse monoclonal CFTR antibody (1:50; C-terminal antigen; Millipore, Billerica, MA, USA), or rabbit polyclonal CFTR antibody (1:1000, N-terminal antigen; Affinity Bioreagents; Thermo Fisher Scientific). After 3 washes with PBS, secondary antibody was applied in blocking buffer for 1 h (1:800, goat-anti-rabbit or goat-anti-mouse Alexa 594; Invitrogen). After 3 additional washes with PBS, the slides were coverslipped and mounted using Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA). Phase photomicrographs were taken with a Zeiss Axiovert 200 microscope (Carl Zeiss MicroImaging, Thornwood, NY, USA) equipped with an Axiocam camera with Axiovision software (Carl Zeiss MicroImaging). Immunocytochemical stains were photographed with a Leica DM6000B microscope equipped with a DFC350 FX camera and Leica FW4000 software (Leica Microsystems, Bannockburn, IL, USA).
Cell sorting
To analyze GFP-positive cells and GFP-positive hMSC/NHBE hybrid cells, total GFP-positive cells were isolated from 7 d cocultures by fluorescence-activated cell sorting (FACS, BD FACSAria; Becton-Dickinson, Franklin Lakes, NJ, USA). To increase cell purity, the GFP-positive cells were sorted a second time (efficiency 97%). Postsorting, the cells were transferred to PBS and lysed for total RNA extraction.
RNA isolation and RT-PCR
Total RNA was isolated from pure cultures of control cells and from GFP-positive cells isolated following coculture. In brief, adherent cells were washed with 1× PBS and recovered by incubation with 0.25% (w/v) trypsin and 1 mM EDTA (Fisher Biosciences, Pittsburgh, PA, USA) for 5 min at 37°C. After centrifugation (1000 g) for 8 min, the cells were harvested and placed on ice to prevent sample degradation. Total RNA was isolated from cell pellets with a commercial kit (RNAqueous; Ambion, Austin, TX, USA). The total RNA was treated with DNase before reverse transcription (Turbo DNase; Ambion). Reverse transcription was performed with Superscript III (Invitrogen) in the presence of RNase inhibitor (RNaseOUT, Invitrogen). PCR was carried out with an Eppendorf Master Cycler EP thermal cycler (Eppendorf North America, Westbury, NY). Control RT-PCR reactions included template samples in which the reverse transcriptase was omitted from the single strand synthesis reaction. Target sequences were denatured at 94°C (2 min) followed by 40 amplification cycles of 94°C (30 s), anneal temp (30 s), and 72°C (1 min). The last PCR step extended the products at 72°C for 7 min. RT-PCR products were analyzed on 1% agarose gels. For CFTR, nested primers were used to further amplify 1 μl of an initial reaction of a larger amplicon (both reactions were 40 cycles). The primer sequences, annealing temperatures, and expected product sizes were as follows: α-ENaC, forward 5′-GTTGAGAACCTTTACCCTTCA-3′ and reverse 5′-TAGCTGGTCACGCTGCATG-3′, 56°C, 245 bp; β-ENaC, forward 5′-GACTATGTAGCCTCAACAGGA-3′ and reverse 5′-TGATATTGGTGCTTTGGTC-3′, 54°C, 876 bp; γ-ENaC, forward 5′-GTGCCAATCAGGAACATCTA-3′ and reverse 5′-CAGAAGCATCTCAATACTG-3′, 53°C, 450 bp; CFTR (large), forward 5′-CAGAACTGAAACTGACTCG-3′ and reverse 5′-TAGTTTTGTTAGCCATCAGT-3′, 49.7°C, 818 bp; CFTR (nested), forward 5′-AAGACTTCACTTCTAATGGTG-3′ and reverse 5′-GCTTTGATGACGCTTCTGTA-3′, 49°C, 179 bp.
Sequencing of the α-ENaC subunit expressed by hMSCs
RT-PCR for α-ENaC was performed with cDNA isolated from 3 hMSC donors. Bands of the correct amplicon size were gel extracted, purified, and sequenced (Vermont Cancer Center DNA Analysis Facility, University of Vermont, Burlington, VT, USA).
Preparation of cells for electrophysiological measurements
Sterile coverslips (0.5–1.0 cm2) were coated with a mixture of fibronectin (5 μ/ml; Sigma) and gelatin (0.01%, Sigma) at 37°C for 6–24 h. After 4–5 d of coculture, hMSCs/NHBEs were trypsinized and plated on small fragments of the glass coverslips in 100-cm2 dishes containing NHBE growth medium. The coverslip fragments with adherent cells were used for electrophysiological measurements. Membrane currents were measured in cells 2–4 d after plating. For long-term storage, aliquots of some 4–5 d cocultures were frozen in 1 ml of freezing medium (10% DMSO and 30% FBS in α-MEM) in cyrovials (Nunc) at −80°C overnight and then stored over liquid nitrogen. We found that fused cells from frozen cocultures could be reliably assayed by whole-cell patch-clamp recording after 2 d in culture in NHBE growth medium.
Electrophysiological measurements of CFTR channel currents
Coverslips with attached cells were placed in a recording chamber mounted to an inverted microscope for whole-cell patch-clamp measurements (Zeiss Axiovert 40CFL or Zeiss Axiovert 25 microscope; Carl Zeiss MicroImaging). Patch pipettes were adjusted with a micromanipulator (MP-285; Sutter Instrument Company, Novato, CA, USA). The patch-clamp setup consisted of an Axopatch 200A (Axon CNS; Molecular Devices, Sunnyvale, CA, USA) with a Digidata 1322A digitizer and pClamp9.2 software (Axon CNS; Molecular Devices). When indicated, the bath solution consisted of NaCl bath containing 140 mM NaCl, 4 mM KCl, 1.8 mM CaCl2·2H2O, 1 mM MgCl2·6H2O, and 10 mM HEPES (pH 7.4) or Na-gluconate bath containing 140 mM Na gluconate, 10 mM HEPES, 5 mM NaCl, 4 mM KCl, 1.8 mM CaCl2·2H2O, and 1 mM MgCl2·6H2O (pH 7.4). Patch pipettes (4–6 MΩ) were filled with an internal solution that consisted of 140 mM KCl, 10 mM HEPES, 5 mM BaCl2, 1 mM MgCl2·6H2O, and 2–4 mM Mg-ATP. To increase CFTR activity, cells were dialyzed with 50 U/ml bovine protein kinase A (catalytic subunit of PKA, csPKA; Calbiochem) by inclusion of this compound in the patch pipette solution. The sulfonylurea glibenclamide (100 uM) was used to inhibit CFTR activity (glybenclamide; Sigma). As glibenclamide is also known to inhibit ATP-sensitive K+ (KATP) channels (18), BaCl2 (5 mM) was added to the bath, to inhibit KATP channels, before the addition of glibenclamide. All electrophysiological experiments were performed at room temperature (20–22°C).
Statistics
Data are presented as means ± se. Statistical significance between groups was determined by 2-tailed Student's t test. Values of P < 0.05 were considered significant.
RESULTS
Morphological and molecular characteristics of hMSC/NHBE heterokaryons
To study the function of hybrid pulmonary cells resulting from the fusion of bone marrow progenitor cells and lung epithelial cells, we established a coculture system that consisted of lentivirally transduced hMSCs and NHBEs. Cell fusion events were observed as early as 1 d following the initiation of the cocultures. Hybrid cells were identified by the presence of fluorescent tags (GFP and/or DsRed2-mito) derived from the hMSC donors, the presence of 2 or more nuclei, and by their distinct epithelial morphology, e.g., radial mitochondrial arrangement (Fig. 1A–C). Unfused hMSCs had an elongated fibroblastic morphology, a longitudinal mitochondrial arrangement, and single nuclei (Fig. 1A–C). After 7 d, we determined the number of GFP-positive epithelial heterokaryons in living cocultures with inverted epifluorescence microscopy. For cocultures derived from hMSC donor 1, there were 4.6 ± 1.1% fused cells (n=3); for cocultures derived from hMSC donor number 2, there were 3.2 ± 0.23% fused cells (n=3).
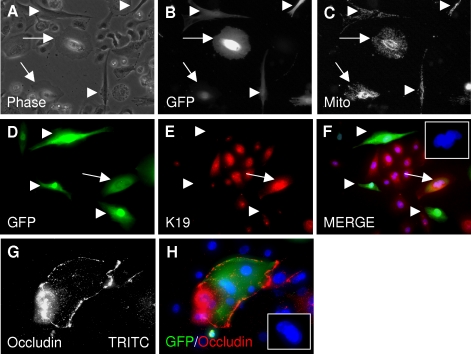
Figure 1.
Fusion of hMSCs with NHBE cells results in heterokaryons with an epithelial morphology and an epithelial mitochondrial arrangement. A–C) Coculture experiments were performed using hMSCs that were double-transduced with lentiviral vectors for GFP and for DsRed2-mito, a mitochondrial targeting vector, and photographed after 1 wk. A) Phase-contrast microscopy of live cells: fused cells have an epithelial morphology. Arrows: hMSC/NHBE heterokaryons. Arrowheads: normal GFP-hMSCs. B) Epifluorescent microscopy of A for FITC channel: heterokaryons express GFP. C) Epifluorescent microscopy of A for TRITC channel: heterokaryons express Dsred2-mito. Note radial arrangement of mitochondria in fused cells (arrows) as compared with the longitudinal arrangement of mitochondria in unfused hMSCs (arrowheads). D–F) Immunocytochemistry of fixed cells from 7 d coculture. Arrow: GFP-positive heterokaryon expressing Keratin 19 (red). Arrowheads: hMSCs did not express Keratin 19. G, H) GFP-positive heterokaryon expressing the tight junction protein occludin. TRITC channel is shown in white in G for clarity. Insets (F, H): DAPI staining demonstrates multiple nuclei in fused cells.
Immunocytochemistry of fixed 7-d cocultures demonstrated that hybrid cells expressed the intermediate filament protein keratin 19, characteristic of bronchial epithelial cells (Fig. 1D–F). The tight junction protein occludin was also expressed in some hybrid cells, indicating their capability to form adherens junctions with NHBEs (Fig. 1G, H). Hybrid cells also expressed CFTR and the α-subunit of ENaC channels (CFTR, Fig. 2A–I and Supplemental Fig. 1; α-ENaC, Fig. 3A–C). The α-ENaC protein was also detected in unfused hMSCs, whereas CFTR was not (Figs. 2 and 3).
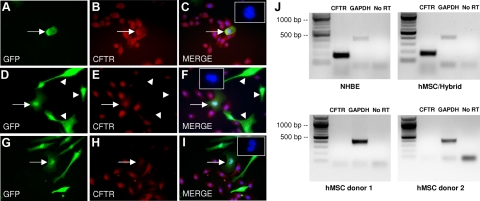
Figure 2.
CFTR expression in NHBEs, hMSCs, and hMSC/NHBE hybrid cells. A–F) Examples of GFP-positive heterokaryons that express CFTR (red). Arrowheads (D-F): hMSCs do not express CFTR. G–I) Some GFP-positive heterokaryons express low or undetectable levels of CFTR (arrow). Insets (C, F, I): DAPI staining demonstrates multiple nuclei in fused cells. J) NHBE control cells and hMSC/NHBE hybrid cells express CFTR mRNA transcripts, but hMSCs cultured alone do not. GAPDH amplification and a GAPDH PCR reaction in which the reverse transcriptase was omitted (No RT) are shown as positive and negative PCR controls.
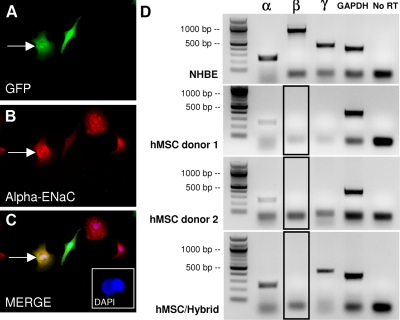
Figure 3.
ENaC expression in NHBEs, hMSCs, and hMSC/NHBE hybrid cells. A–C) GFP-positive heterokaryon expressing α-ENaC (red). Note that unfused hMSC (fibroblastic) also expresses a low level of α-ENaC. Inset: DAPI staining showing 2 nuclei in fused cell. D) Top panel: control NHBE cells express robust levels of α-, β-, and γ-ENaC. Middle panels: hMSCs express α-ENAC before coculture (2 donors shown) but not β-ENaC or γ-ENaC. Note that α-ENaC mRNA expressed by hMSCs was confirmed by cDNA sequencing. Bottom panel: hybrid cells express α- and γ- but not β-ENaC. Boxes with black outline indicate the lane where a β-ENaC band is not present (compare with NHBE β-ENaC band above). GAPDH and No RT are positive and negative controls.
Notably, while 100% of cultured NHBEs expressed similar levels of CFTR by immunocytochemistry, its expression in hMSC/NHBE heterokaryons was variable. Qualitatively, some heterokaryons expressed CFTR at levels comparable to neighboring NHBEs (Fig. 2A–C), some expressed detectable CFTR at levels less than adjacent NHBE cells (Fig. 2D–F), and some expressed very low or undetectable levels of CFTR (Fig. 2G–I). Under epifluorescence microscopy, we counted the number of heterokaryons that expressed CFTR in 7-d hMSC/NHBE cocultures derived from 2 different hMSC donors (n=3 for each). Because immunocytochemistry is not a sensitive method to detect low levels of membrane protein, we scored GFP-positive heterokaryons as “CFTR positive” or “CFTR low/negative.” Based on these criteria, cocultures derived from hMSC donor 1 contained 64.7 ± 4.9% CFTR-positive heterokaryons, while cocultures derived from hMSC donor 2 had 48.7 ± 5.9% CFTR-positive heterokaryons. Therefore, ∼35 to 50% of heterokaryons expressed low to undetectable levels of CFTR.
To confirm the expression of CFTR and ENaC ion channels in the hybrid cells, RT-PCR assays were performed with RNA isolated from control NHBEs, control hMSCs, and hybrid cells that were enriched by a double FACS isolation for GFP. The cells isolated by FACS consisted of a mixture of GFP-positive hMSCs and GFP-positive hybrid cells. By nested RT-PCR, control NHBEs and the mixed GFP-positive cells from cocultures expressed CFTR mRNA, although control (noncocultured) GFP-positive hMSCs did not (Fig. 2J). We then examined the mRNA expression for all 3 ENaC subunits (α, β, and γ) by RT-PCR (Fig. 3D). The mRNAs for all of the ENaC subunits were present at relatively high levels in NHBE control cells (Fig. 3D). Before coculture, hMSCs were found to express α-ENaC mRNA that was identical to that expressed in NHBEs (by sequencing analysis and Blast homologous alignment, Supplemental Fig. 2A) but not mRNA for the β- or γ-subunits (Fig. 3D). In contrast to the NHBE controls, the hybrid cells expressed mRNA for the α-ENaC subunit and the γ-ENaC subunit mRNA but did not express mRNA for the β-ENaC subunit (Fig. 3D). We assayed GFP-positive cells from cocultures generated with 3 different hMSC donors and none of the cell isolates expressed detectable β-ENaC mRNA. Accordingly, heterokaryon cells from our cocultures would not be expected to form a complete ENaC channel. Attempts to detect ENaC by either whole-cell patch-clamp or inside-out patch recordings were unsuccessful (data not shown). To control for possible effects of GFP, cell handling, or FACS on β-ENaC mRNA levels, we transduced pure cultures of NHBEs with GFP lentivirus, sorted GFP-positive and GFP-negative NHBEs, and ran RT-PCR assays for all of the ENaC subunits. The results did not differ from those obtained with control (unsorted) NHBEs (data not shown).
CFTR function in control NHBEs and hMSC/NHBE heterokaryons
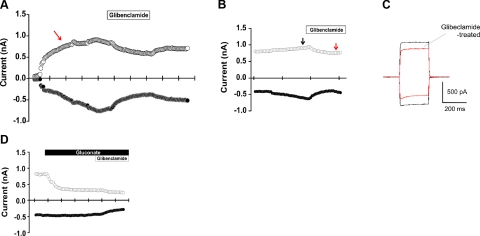
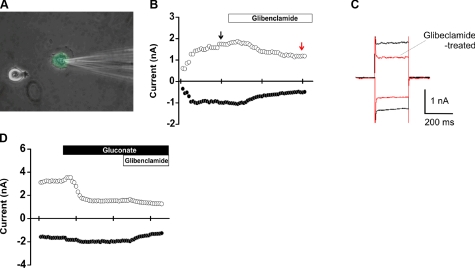
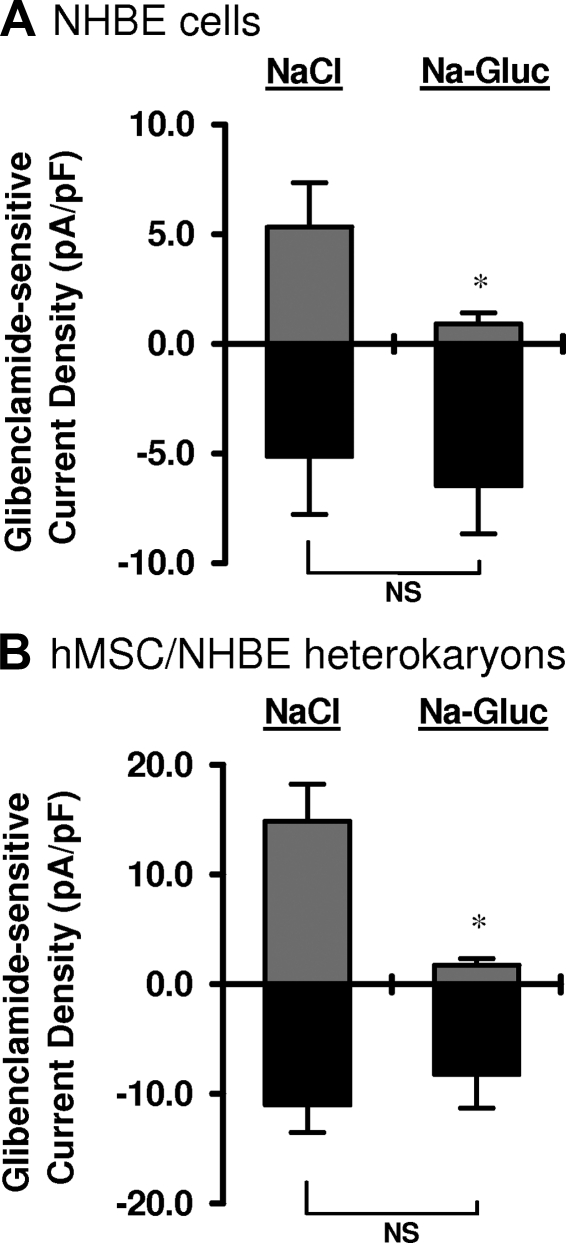
To assess CFTR function in hMSC/NHBE heterokaryons, CFTR channel activity was measured using the conventional whole-cell configuration of the patch-clamp technique. Since CFTR activity is enhanced by PKA, 1000 U of csPKA was loaded in the pipette solution. csPKA increased both outward currents (at +60 mV) and inward currents (at −60 mV) for NHBEs (Fig. 4A, red arrow) and heterokaryons (Fig. 5B, black arrow). Glibenclamide, an inhibitor of CFTR (19), decreased outward and inward currents stimulated by csPKA (Figs. 4A–C and 5B, C). As glibenclamide can also inhibit KATP channels (18), cells were incubated with BaCl2 before the addition of glibenclamide to exclude KATP currents. After washout of the first glibenclamide treatment (e.g., Fig. 4A), extracellular Cl− was decreased by iso-osmotic replacement of NaCl with Na gluconate. A second glibenclamide treatment was then performed in the Na gluconate bath. Under these conditions (low extracellular Cl−), glibenclamide-sensitive outward currents were greatly reduced in both NHBEs as well as hMSC/NHBE heterokaryons (Figs. 4D and 5D). These results demonstrate the presence of chloride currents due to functional CFTR channels in hMSC/NHBE heterokaryons as well as in control NHBEs. However, some heterokaryons and NHBEs did not respond to the glibenclamide treatment, indicating an absence of CFTR membrane currents (data not shown). The percentage of NHBE cells that responded to glibenclamide was 46.6% (n=10/21), and the percentage of heterokaryons that responded was 28.6% (n=10/35). For cells exhibiting glibenclamide-sensitive currents, current density was calculated by dividing cell membrane current by the cell capacitance for each cell. Decreasing extracellular Cl− (Na-gluconate treatment) led to a significant reduction in outward current density in NHBE cells [current density at +60 mV: 5.31±1.4 pA/pF (n=7) and 0.90±0.52 pA/pF (n=9) in NaCl and Na-gluconate, respectively; P<0.05; Fig. 6A]. Interestingly, a greater reduction in the outward current density was observed for heterokaryons when the bath solution was changed from NaCl to Na-gluconate in the presence of gliblenclamide [from current density at +60 mV: 14.88±3.36 pA/pF (n=10) in NaCl bath to 1.73± 0.59 pA/pF (n=5) in Na-gluconate; P<0.05; Fig. 6B]. The inward current responses of NHBE cells and heterokaryons to glibenclamide in the NaCl bath or in the Na-gluconate bath (at −60 mV) were not significantly different (P>0.05 in both cases; Fig. 6A, B).
Figure 4.
Electrophysiological measurements of CFTR activity in NHBE cells. Currents were measured using the conventional whole-cell configuration of the patch-clamp technique. Command potential is −60 mV or +60 mV as indicated. A) csPKA in the pipette stimulates activity of the CFTR channel. Note an increase in current before glibenclamide treatment (red arrow). B, C) Glibenclamide (100 uM) treatment inhibits CFTR activity (red arrow). Outward and inward currents were both decreased (C) after the addition of glibenclamide in the presence of BaCl2 (5 mM). D) Decrease in inward Cl− movement through CFTR after change of bath solution from NaCl to Na-gluconate. Note that all glibenclamide treatments were made in the presence of BaCl2.
Figure 5.
Electrophysiological measurements of CFTR activity in hMSC/NHBE heterokaryons. A) GFP-positive heterokaryon with electrode (patched). Note the presence of 2 nuclei and round epithelial morphology. B, C) Glibenclamide (100 uM) inhibits CFTR activity in a heterokaryon (decrease; red arrow). Outward and inward currents were both decreased (C) after the addition of glibenclamide in the presence of BaCl2 (5 mM). D) Decrease in inward Cl− movement through CFTR after change of bath solution from NaCl to Na-gluconate. Note that all glibenclamide treatments were made in the presence of BaCl2.
Figure 6.
Summary of glibenclamide-sensitive current densities for NHBE cells and hMSC/NHBE heterokaryons. A) Switching from NaCl bath to Na-gluconate bath (Na-Gluc) depletes available Cl− and results in a significant reduction in outward current density in NHBE cells (controls). B) A similar and significant reduction in outward current density was observed for heterokaryons after the switch from NaCl bath to Na-gluconate bath. All measurements were made in the presence of BaCl2 (n=17 NHBE cells; n=15 heterokaryons). *P < 0.05.
Membrane capacitance of NHBEs and hMSC/NHBE heterokaryons
Cell membrane capacitance is proportional to the total membrane surface area and is useful to normalize ionic conductance to account for cell surface variation (20). The capacitance of NHBEs (61.3±5.18 pF; n=17 cells) was not significantly different from the capacitance of heterokaryons (63.7±6.91; n=15 cells). These data indicate that at the time of patch-clamp measurements the membrane area of heterokaryons was similar to that of NHBEs.
CFTR mRNA expressed by hMSC/CFT1 (ΔF508) hybrid cells is mutant and not wild type
To determine whether cell fusion would result in reprogramming of the hMSC nucleus to express wild-type CFTR, we cocultured GFP-positive hMSCs with immortalized tracheal epithelial cell line CFT1 from a cystic fibrosis patient (ΔF508) that was labeled by DsRed2-mito. After PEG 1500 treatment and 4–5 d of coculture, green/red cells were isolated by FACS and analyzed by RT-PCR and DNA sequencing. The results demonstrated that hMSC/CFT1 hybrids expressed mutant CFTR transcripts and not wild-type CFTR transcripts (Supplemental Fig. 2B).
DISCUSSION
ENaC and CFTR channels regulate lung fluid volume at the pulmonary epithelial surface by controlling ion and water secretion and absorption. The equilibrium established for positive and negative ions (Na+ and Cl−) maintains a thin layer of fluid at the epithelial surface, determining the viscosity of the mucus layer and allowing cilia to beat properly for clearance. We detected CFTR protein and mRNA in hybrid hMSC/NHBE cells by both immunocytochemistry and RT-PCR assays. The function of the CFTR channel in heterokaryons was determined by whole-cell patch clamping and compared with that of control NHBEs. CFTR requires PKA activation to alter Cl− conductance (21). Therefore, csPKA was added to the pipette solution to stimulate CFTR activity. In addition to showing increasing outward and inward currents in the presence of csPKA in the pipette, our data demonstrated that many NHBEs and hMSC/NHBE heterokaryons possessed glibenclamide-sensitive ion channels in the presence of BaCl2 in the bath. Significant decreases in the outward current density observed in response to glibenclamide as well as to Na-gluconate incubation indicated the presence of functional CFTR in both NHBEs and hMSC/NHBE heterokaryons. Aside from the epithelial morphology of hMSC/NHBE heterokaryons, cell capacitance measurements confirmed that the fused cells in our studies had adapted their membrane surface areas to match those of NHBEs.
With these observations, one might conclude that cultured hMSC/NHBE heterokaryons are “normal” and would function similarly to typical bronchial epithelial cells. However, by staining we found that 35–50% of heterokaryons expressed low or undetectable levels of CFTR, whereas 100% of NHBE cells expressed CFTR. Although these assays do not specifically measure membrane levels of CFTR to correlate with channel activity, they do indicate that CFTR may be silenced at the transcriptional or translational level in hMSC/NHBE heterokaryons. We also observed that CFTR currents were significantly larger (2- to 3-fold) in heterokaryons compared with NHBEs. This difference may be attributed to increased activity of CFTR in heterokaryons to counteract a reduced or absent β-ENaC subunit. By RT-PCR assays, we found that hybrid cells expressed the mRNAs for α-ENaC and γ-ENaC subunits but did not express detectable mRNA for the β-subunit.
ENaC is a heterotetrameric protein (2 α-subunits, 1 β-subunit, and 1 γ-subunit) that forms a channel at the apical cell surface and regulates Na+ absorption. Interestingly, similar to NHBE cells, before coculture hMSCs expressed both α-ENaC mRNA and protein. Cell surface expression of the α-ENaC subunit alone can be sufficient to create a small amiloride-sensitive sodium current, but for maximal channel activity both β-ENaC and γ-ENaC are also required (22). The importance of having all 3 subunits was recently demonstrated by Caci et al. (23) in experiments that silenced each of the ENaC subunits by siRNA. Knockdown with siRNAs complimentary to either α-, β-, or γ-ENaC subunits resulted in similar effects, all reducing the ENaC activity of human bronchial epithelial cells (23). In addition, homozygous mutant mice in which β-ENaC expression was decreased by 90% in lung epithelial cells were shown to possess dysfunctional pulmonary fluid clearance (24).
The lack of β-ENaC mRNA expression in hMSC/NHBE heterokaryons may be due to epigenetic silencing. Both methylation-induced silencing of gene expression and demethylation to activate previously silent genes have been reported for somatic cell heterokaryons generated by cell fusion (25). Recent cell fusion studies (26) with primary myoblasts and keratinocytes demonstrated that nuclear reprogramming in heterokaryons can be bidirectional. Based on species-specific transcriptome amplification assays, Palermo et al. (26) reported that the ratio of fusion partners can influence the direction of reprogramming and differentiation in heterokaryons. In their experiments, the cocultured cell type in the highest proportion (keratinocyte>myoblast) dictated the degree of reprogramming toward a particular cell fate (keratinocyte). The end result was shown to be reversible by biasing the starting cell ratio in the opposite direction (26).
The results of Palermo et al. (26)suggested that somatic cell reprogramming in heterokaryons was an all-or-none phenomenon in which 1 cellular phenotype was dominant over the other. In contrast, our data show that incomplete reprogramming can occur in hMSC/NHBE heterokaryons and is likely to alter cellular function. The incomplete reprogramming in our system with hMSCs/NHBEs could have occurred for many reasons. Reprogramming kinetics may differ in a heterokaryon formed from the fusion of 2 differentiated cell types compared with a heterokaryon derived from the fusion of a progenitor cell with a differentiated cell. Also, complete expression of α-, β-, and γ-ENaC mRNAs could occur at a time point later than we assayed. Although the majority of genes may be expressed for the dominant phenotype in somatic cell heterokaryons, the demethylation/methylation states of some genes or histone acetylation in some chromatin regions may be context dependent, and some genes may require cellular engraftment in vivo to be properly expressed.
Based on their ENaC gene expression phenotype, it would be of interest to determine the degree of ENaC function in hMSC/NHBE heterokaryons. However, the formation of mature ENaC channels is highly context dependent. While CFTR function is readily assayed in cultured and passaged bronchial epithelial cells, ENaC function is typically measured from freshly isolated primary cells that are cultured on porous permeable cell culture inserts (Transwells) to promote apical/basal membrane polarity and a fully differentiated epithelial phenotype (23). Despite numerous attempts, we were unable to detect amiloride-sensitive currents in hMSC/NHBE heterokaryons cultured on glass coverslips or on Transwell inserts. ENaC function can also be measured with an Ussing chamber, a device that determines conductance across a confluent epithelium (with tight junctions and transepithelial resistance), rather than by whole-cell patch-clamp assays. However, due to the rarity of fused cells in hMSC/epithelial cocultures (16), FACS purification of sufficient numbers of heterokaryons to generate the pure and confluent cell layer required for Ussing chamber measurements remains a technical challenge. Notably, although they did not appear to be reprogrammed completely in culture, we do not know whether or not the hMSC/NHBE heterokaryons would acquire β-ENaC expression and function normally in vivo. Bae et al. (27) reported that heterokaryons derived from murine MSCs and Purkinje neurons had functional synapses and electrical activity in vivo.
Our data emphasize the importance of measuring multiple parameters to evaluate cell identity and function, in particular for heterokaryons derived from cell fusion. Cell morphology and single or perhaps even multiple mRNA or proteins markers can not imply cell function. In some cell types, multiple inputs and outputs, such as ion channels, may work in concert to determine overall cellular function, and several components, such as protein subunits, may be required for optimal activity. Thus, while cell fusion may be able to rescue some systems with single gene mutations or deletions (e.g., tyrosinemia type I; refs. 10, 15), other disease phenotypes where multiple genes must be reprogrammed efficiently for interacting proteins may be difficult to rescue by cell fusion. Based on our results, it may be important to consider the function of heterokaryons and hybrid cells formed by cell fusion in vivo after administration of adult stem/progenitor cells for cell therapy.
Supplementary Material
Acknowledgments
The authors thank Colette Charland (University of Vermont) for technical assistance with FACS. The authors thank Dr. Darwin Prockop (Texas A&M University Health Science Center, Temple, TX, USA) for providing hMSCs. The CFT1 cell line was created by Dr. James Yankaskas (University of North Carolina, Chapel Hill, NC, USA). The authors thank Dr. Albert Van der Vliet (University of Vermont) for providing and assistance with the CFTR δF508 cell line and Dr. Alexander Aronshtam (University of Vermont) for help with DNA sequence analysis.
This work was supported in part by U.S. National Institutes of Health/National Heart, Lung, and Blood Institute grant R01-HL-085210 (to J.L.S.) and National Center for Research Resources Centers of Biomedical Research Excellence grant P20 RR-155557 (Vermont Lung Center, Charles Irvin, principal investigator). I.C. was supported by the Vermont Lung Center T32 Pulmonary Training Program.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Herzog E. L., Chai L., Krause D. S. (2003) Plasticity of marrow-derived stem cells. Blood 102, 3483–3493 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Dolado M., Pardal R., Garcia-Verdugo J. M., Fike J. R., Lee H. O., Pfeffer K., Lois C., Morrison S. J., Alvarez-Buylla A. (2003) Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968–973 [DOI] [PubMed] [Google Scholar]
- 3.Spees J. L., Whitney M. J., Sullivan D. E., Lasky J. A., Laboy M., Ylostalo J., Prockop D. J. (2008) Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 22, 1226–1236 [DOI] [PubMed] [Google Scholar]
- 4.Weimann J. M., Johansson C. B., Trejo A., Blau H. M. (2003) Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat. Cell Biol. 5, 959–966 [DOI] [PubMed] [Google Scholar]
- 5.Bae J. S., Furuya S., Shinoda Y., Endo S., Schuchman E. H., Hirabayashi Y., Jin H. K. (2005) Neurodegeneration augments the ability of bone marrow-derived mesenchymal stem cells to fuse with Purkinje neurons in Niemann-Pick type C mice. Hum. Gene Ther. 16, 1006–1011 [DOI] [PubMed] [Google Scholar]
- 6.Johansson C. B., Youssef S., Koleckar K., Holbrook C., Doyonnas R., Corbel S. Y., Steinman L., Rossi F. M., Blau H. M. (2008) Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat. Cell Biol. 10, 575–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camargo F. D., Green R., Capetanaki Y., Jackson K. A., Goodell M. A. (2003) Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat. Med. 9, 1520–1527 [DOI] [PubMed] [Google Scholar]
- 8.Doyonnas R., LaBarge M. A., Sacco A., Charlton C., Blau H. M. (2004) Hematopoietic contribution to skeletal muscle regeneration by myelomonocytic precursors. Proc. Natl. Acad. Sci. U. S. A. 101, 13507–13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizvi A. Z., Swain J. R., Davies P. S., Bailey A. S., Decker A. D., Willenbring H., Grompe M., Fleming W. H., Wong M. H. (2006) Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc. Natl. Acad. Sci. U. S. A. 103, 6321–6325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X., Willenbring H., Akkari Y., Torimaru Y., Foster M., Al-Dhalimy M., Lagasse E., Finegold M., Olson S., Grompe M. (2003) Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422, 897–901 [DOI] [PubMed] [Google Scholar]
- 11.Herzog E. L., Van Arnam J., Hu B., Zhang J., Chen Q., Haberman A. M., Krause D. S. (2007) Lung-specific nuclear reprogramming is accompanied by heterokaryon formation and Y chromosome loss following bone marrow transplantation and secondary inflammation. FASEB J. 21, 2592–2601 [DOI] [PubMed] [Google Scholar]
- 12.Wagers A. J., Sherwood R. I., Christensen J. L., Weissman I. L. (2002) Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297, 2256–2259 [DOI] [PubMed] [Google Scholar]
- 13.Wiersema A., Dijk F., Dontje B., van der Want J. J., de Haan G. (2007) Cerebellar heterokaryon formation increases with age and after irradiation. Stem Cell Res. 1, 150–154 [DOI] [PubMed] [Google Scholar]
- 14.Nygren J. M., Liuba K., Breitbach M., Stott S., Thorén L., Roell W., Geisen C., Sasse P., Kirik D., Björklund A., Nerlov C., Fleischmann B. K., Jovinge S., Jacobsen S. E. (2008) Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat. Cell Biol. 10, 584–592 [DOI] [PubMed] [Google Scholar]
- 15.Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., Wang X., Finegold M., Weissman I. L., Grompe M. (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 6, 1229–1234 [DOI] [PubMed] [Google Scholar]
- 16.Spees J. L., Olson S. D., Ylostalo J., Lynch P. J., Smith J., Perry A., Peister A., Wang M. Y., Prockop D. J. (2003) Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc. Natl. Acad. Sci. U. S. A. 100, 2397–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spees J. L., Olson S. D., Whitney M. J., Prockop D. J. (2006) Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. U. S. A. 103, 1283–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang C. S., Chen C. C., Lin C. C., Chang N. C., Lee T. M. (2009) Effect of ATP-sensitive potassium channel agonists on sympathetic hyperinnervation in postinfarcted rat hearts. Am. J. Physiol. Heart Circ. Physiol. 296, H1949–H1959 [DOI] [PubMed] [Google Scholar]
- 19.Melin P., Hosy E., Vivaudou M., Becq F. (2007) CFTR inhibition by glibenclamide requires a positive charge in cytoplasmic loop three. Biochim. Biophys. Acta 1768, 2438–2446 [DOI] [PubMed] [Google Scholar]
- 20.Moody W. J., Lansman J. B. (1983) Developmental regulation of Ca2+ and K+ currents during hormone-induced maturation of starfish oocytes. Proc. Natl. Acad. Sci. U. S. A. 80, 3096–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. (1991) Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253, 202–205 [DOI] [PubMed] [Google Scholar]
- 22.Firsov D., Gautschi I., Merillat A. M., Rossier B. C., Schild L. (1998) The heterotetrameric architecture of the epithelial sodium channel (ENaC). EMBO J. 17, 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caci E., Melani R., Pedemonte N., Yueksekdag G., Ravazzolo R., Rosenecker J., Galietta L. J., Zegarra-Moran O. (2009) Epithelial sodium channel inhibition in primary human bronchial epithelia by transfected siRNA. Am. J. Respir. Cell Mol. Biol. 40, 211–216 [DOI] [PubMed] [Google Scholar]
- 24.Randrianarison N., Clerici C., Ferreira C., Fontayne A., Pradervand S., Fowler-Jaeger N., Hummler E., Rossier B. C., Planès C. (2008) Low expression of the beta-ENaC subunit impairs lung fluid clearance in the mouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L409–L416 [DOI] [PubMed] [Google Scholar]
- 25.Zhang F., Pomerantz J. H., Sen G. (2007) Active tissue-specific DNA demethylation conferred by somatic cell nuclei in stable heterokaryons. Proc. Natl. Acad. Sci. U. S. A. 104, 4395–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palermo A., Doyonnas R., Bhutani N., Pomerantz J., Alkan O., Blau H. M. (2009) Nuclear reprogramming in heterokaryons is rapid, extensive, and bidirectional. FASEB J. 23, 1431–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae J. S., Han H. S., Youn D. H., Carter J. E., Modo M., Schuchman E. H., Jin H. K. (2007) Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells 25, 1307–1316 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.