Abstract
Introduction
It has been reported that individuals with Autism Spectrum Disorder (ASD) have abnormal reactions to the sensory environment and visuo-perceptual abnormalities. Electrophysiological research has provided evidence that gamma band activity (30-80 Hz) is a physiological indicator of the co-activation of cortical cells engaged in processing visual stimuli and integrating different features of a stimulus. A number of studies have found augmented and indiscriminative gamma band power at early stages of visual processing in ASD; this may be related to decreased inhibitory processing and an increase in the ratio of cortical excitation to inhibition. Low frequency or ‘slow’ (≤1HZ) repetitive transcranial magnetic stimulation (rTMS) has been shown to increase inhibition of stimulated cortex by the activation of inhibitory circuits.
Methods
We wanted to test the hypothesis of gamma band abnormalities at early stages of visual processing in ASD by investigating relative evoked (i.e. ~ 100 ms) gamma power in 25 subjects with ASD and 20 age-matched controls using Kanizsa illusory figures. Additionally, we wanted to assess the effects of 12 sessions of bilateral ‘slow’ rTMS to the dorsolateral prefrontal cortex (DLPFC) on evoked gamma activity using a randomized controlled design.
Results
In individuals with ASD evoked gamma activity was not discriminative of stimulus type, whereas in controls early gamma power differences between target and non-target stimuli were highly significant. Following rTMS individuals with ASD showed significant improvement in discriminatory gamma activity between relevant and irrelevant visual stimuli. We also found significant improvement in the responses on behavioral questionnaires (i.e., irritability, repetitive behavior) as a result of rTMS.
Conclusion
We proposed that ‘slow’ rTMS may have increased cortical inhibitory tone which improved discriminatory gamma activity at early stages of visual processing. rTMS has the potential to become an important therapeutic tool in ASD treatment and has shown significant benefits in treating core symptoms of ASD with few, if any side effects.
Keywords: Autism, EEG, gamma oscillations, visual processing, evoked potentials
Introduction
Autism Spectrum Disorder (ASD) includes three conditions sharing a similar core symptomatology: Autism, Asperger syndrome, and Pervasive Developmental Disorders (PDD). It has been reported that individuals with ASD have abnormal reactions to the sensory environment (Charman, 2008) and visuo-perceptual abnormalities (Happe, 1999). In fact, sensory-perceptual abnormalities have been found to be present in approximately 90% of individuals with Autism (Gomes, Pedroso, & Wagner, 2008). Aversive reactions to visual, auditory, and tactile stimuli commonly recorded in autistic individuals may be due to higher-than-normal cortical noise, as an increase in the ratio of cortical excitation to cortical inhibition has been reported in ASD (Casanova, Buxhoeveden, & Gomez, 2003) as well as a higher incidence of epilepsy (Gillberg & Billstedt, 2000) and abnormal epileptiform activity during sleep (Lewine et al., 1999).
One possible explanation for higher-than-normal cortical noise in ASD is the recent finding of minicolumnar abnormalities. Minicolumns are considered the basic anatomical and physiological unit of the cerebral cortex (Mountcastle, 2003); they contain pyramidal cells that extend throughout laminae IIVI and are surrounded by a neuropil space consisting of, among other elements, several species of GABAergic, inhibitory interneurons (i.e., double-bouquet, basket, and chandelier cells) (Casanova, 2007). Double-bouquet cells provide a “vertical stream of negative inhibition” (Mountcastle, 1997, 2003) surrounding the minicolumnar core and maintain a constant geometric orientation perpendicular to the pial surface (Douglas & Martin, 2004). Our preliminary studies indicate that minicolumns in the brains of autistic patients are narrower and contain less peripheral, neuropil space (Casanova, 2006a). A lack of appropriate neuropil space and associated lateral inhibition may adversely affect the functional distinctiveness of minicolumnar activation and could result in enhanced localized activation in the context of a lack of associated inhibition (Rippon, Brock, Brown, & Boucher, 2007). In addition to signal amplification the effect of loss of surround inhibition may result in the loss of information as information for the brain is based on contrast: Going from an analog (Mexican hat) signal to a digital (stovepipe hat) one results in lost information (Casanova, 2006a). The orchestration of an appropriate signal-to-noise ratio is imperative for the output of any network to be sufficiently robust and distinct enough to successfully achieve necessary processing (Rippon et al., 2007; Shadlen & Movshon, 1999; Treisman, 1999). Behaviorally speaking signal/sensory amplification may impair functioning, raise physiological stress, and adversely affect social interaction in patients with ASD (Ratey, 1998).
It is well known that networks of inhibitory interneurons acting as GABA-gated pacemakers are critically involved in gamma EEG (30-80Hz) oscillations (Grothe & Klump, 2000; Whittington, Traub, Kopell, Ermentrout, & Buhl, 2000). Electrophysiological research has provided evidence that gamma activity is a physiological indicator of the co-activation of cortical cells engaged in processing visual stimuli (Keil, Gruber, & Müller, 2001; Singer & Gray, 1995; Tallon-Baundry & Bertrand, 1999) and integrating different features of a stimulus (Müller, Gruber, & Keil, 2000). The onset of a visual stimulus gives rise to a burst of gamma activity over occipital sites and when more complex tasks are undertaken, discrete bursts of gamma activity have been identified overlying cortical regions thought to be engaged in those tasks (Brown, Gruber, Boucher, Rippon, & Brock, 2005). For example, tasks involving attention modulation or the top-down integration of features give rise to simultaneous bursts of gamma over frontal and occipito-parietal regions (Müller, 2000; Müller & Gruber, 2001; Rodriguez et al., 1999). Kanizsa illusory figures (Kanizsa, 1976) have been shown to readily produce gamma oscillations during visual cognitive tasks (Hermann, Mecklinger, & Pfeifer, 1999; Tallon-Baundry, Bertrand, Delpuech, & Pernier, 1996): Kanizsa stimuli consist of inducer disks of a shape feature and either constitute an illusory figure (square, triangle) or not (colinearity feature); in non-impaired individuals, gamma activity has been shown to increase during ‘target-present’ compared to ‘target-absent’ trials (Brown et al., 2005; Müller et al., 1996; Tallon-Baundry et al., 1996).
Gamma band activity can be divided into either evoked or induced: Evoked gamma band activity has been identified at a latency of around 100 msec after stimulus onset (Bertrand & Tallon-Baundry, 2000; Herrmann & Mecklinger, 2000) and is highly phase locked to the onset of the stimulus; induced gamma band activity occurs later with a variable onset although it has been reported to start at around 250 msec (Brown et al., 2005). It has been proposed that evoked gamma band activity reflects the effect of attention on early visual processing and the binding of perceptual information within the same cortical area (i.e., intra-areal), whereas induced gamma band activity reflects the binding of feed-forward and feed-back processing in a whole network of cortical areas (corticocortical) (Brown et al., 2005; Müller et al., 2000; Shibata et al., 1999). Variations of such activity have been termed event-related synchronization and desynchronization (ERS/ERD) (Pfurtscheller & Aranibar, 1977) or Event Related Spectral Perturbations (ERSP) (Makeig, Debener, Onton, & Delorme, 2004) and have been associated with the activation of task-relevant neuronal assemblies (Pfurtscheller & Lopes da Silva, 1999; Rippon et al., 2007).
A number of studies have found abnormal gamma band activity in individuals with ASD. Brown et al. (2005) showed that autistic participants had higher parietal gamma power than controls in an experiment using Kanizsa, visual illusions; additionally, in this study, individuals with ASD showed a very early burst of gamma activity between 80 and 120 ms, and later gamma (around 300 ms) was found to occur earlier and be more powerful in the autistic patients. Grice et al. (2001) compared gamma band activity over frontal regions during a face discrimination task in adults with Autism and controls. The control subjects showed clear discriminative increases in frontal gamma activity when the faces were presented upright compared to inverted; while in the autistic group the extent of gamma activity did not differ significantly between the upright and inverted faces. These findings suggest that in ASD gamma activity is augmented and indiscriminative. According to Brown et al. (2005) this may reflect decreased ‘signal to noise’ due to decreased inhibitory processing: Uninhibited gamma activity suggests that none of the circuits in the brain can emerge to dominate and constrain perceptual processing because too many of them are active simultaneously.
Recently there has been considerable interest on the effects of repetitive transcranial magnetic stimulation (rTMS) on cortical excitability. TMS operates based on Faraday's law of electromagnetic induction (Faraday, 1831) which describes the process by which a changing magnetic field induces the flow of electric current in a nearby conductor preferentially standing at 90 degrees to the magnetic field. Studies have indicated that low-frequency or ‘slow’ rTMS (≤1Hz) increases inhibition of stimulated cortex (e.g., Boroojerdi, Prager, & Muellbacher, 2000), whereas high-frequency rTMS (>1Hz) increases excitability of stimulated cortex (e.g., Pascual-Leone et al., 1994). It has been proposed that the effect of ‘slow’ rTMS arises from increases in the activation of inhibitory circuits (Pascual-Leone, Walsh, & Rothwell, 2000). Hoffman and Cavus (2002) in their review of ‘slow’ rTMS studies propose that long-term depression and long-term depotentiation may be models for understanding the mechanism of ‘slow’ rTMS. We theorize that contrary to other inhibitory cells (i.e., basket and chandelier), whose projections keep no constant relation to the surface of the cortex, the geometrically exact orientation of double-bouquet cells and their location at the periphery of the minicolumn (inhibitory surround) makes them an appropriate candidate for induction by a magnetic field applied parallel to cortex. Over a course of treatment ‘slow’ rTMS may selectively depotentiate enhanced synaptic weights associated with pathological conditions, and in the case of ASD, may lower the ratio of cortical excitation to cortical inhibition. We therefore hypothesize that individuals with ASD will show amplified and indiscriminative evoked gamma power in response to illusory figures reflecting ‘noisy’ and uninhibited cortical activity at early stages of visual processing. Additionally we hypothesize that 12 sessions of bilateral, ‘slow’ rTMS stimulation applied to the dorsolateral prefrontal cortices (DLPFC) will attenuate amplified, early gamma activity and improve discriminatory gamma activity between relevant and irrelevant visual stimuli (i.e., target vs. non-target stimuli).
Materials and Methods
Participants
Participants with Autism Spectrum Disorder (ASD) (age range 9 to 26 years) were recruited through the University of Louisville Weisskopf Child Evaluation Center (WCEC). Diagnosis was made according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (APA, 2000) and further ascertained with the Autism Diagnostic Interview – Revised (ADI-R) (LeCouteur, Lord, & Rutter, 2003). They also had a medical evaluation by a developmental pediatrician. All subjects had normal hearing based on past hearing screens. Participants either had normal vision or wore corrective lenses. Participants with a history of seizure disorder, significant hearing or visual impairment, a brain abnormality conclusive from imaging studies or an identified genetic disorder were excluded. All participants were assessed for IQ using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV, Wechsler 2003) or the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler 2004).
Controls were recruited through advertisements in the local media. All control participants were free of neurological or significant medical disorders, had normal hearing and vision, and were free of psychiatric, learning, or developmental disorders based on self- and parent reports. Subjects were screened for history of psychiatric or neurological diagnosis using the Structured Clinical Interview for DSM-IV Non-Patient Edition (SCID-NP, First, Spitzer, Gibbon, & Williams, 2001). Participants within the control and ASD groups were attempted to be matched by age, full scale IQ, and socioeconomic status of their family. Socioeconomic status of ASD and control groups was compared based on parent education and annual household income. Participants in both groups had similar parent education levels.
Participating subjects and their parents (or legal guardians) were provided with full information about the study including the purpose, requirements, responsibilities, reimbursement, risks, benefits, alternatives, and role of the local Institutional Review Board (IRB). The consent and assent forms approved by the IRB were reviewed and explained to all subjects who expressed interest to participate. All questions were answered before consent signature was requested. If the individual agreed to participate, she/he signed and dated the consent form and received a copy countersigned by the investigator who obtained consent.
EEG Data Acquisition and Signal Processing
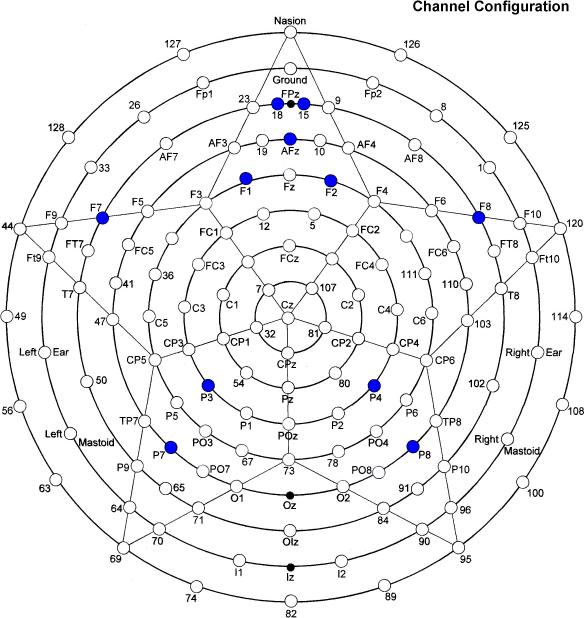
Dependent measures in EEG gamma band were recorded continuously with an EGI (Electrical Geodesics, Inc.) 128-electrode net, referenced to vertex (impedances < 50 kohm; sampling rate 500 Hz; 0.1–200 Hz online bandpass). EEG was segmented to obtain epochs starting 0–180 ms following stimulus onset. Extraction of evoked gamma band power (30-45 Hz) in 30 trials for each stimulus type was performed with Morlet wavelet analysis (Goupillaud, Grossman, & Morlet, 1984) using MATLAB. We selected the following channels: FPz (EGI channels to left (18) and right (15) of FPz) and AFz (16) from the midline prefrontal area, F1 (20), F2 (4), F7 (34), F8 (122) from the frontal area, and P3 (53), P4 (87), P7 (59), P8 (92) from the parietal area (Figure 1); this channel configuration allowed us to analyze gamma band activity over both hemispheres. All recorded signals were first automatically and then manually inspected for artifacts and rejected if eye movement artifacts, gross movements or EEG sensor drifts were detected. For automatic detection, we computed the standard in a moving time window and the normalized cross correlation coefficient between the current recoded signal and previous succeeded trials; the current recorded signal was rejected if thresholds exceeded two standard deviations or exceeded normalized cross correlation. The standard deviation threshold was in the 35–50 μV range, and normalized cross correlation was approximately 0.5. To accurately find the features that discriminate autistic subjects from controls and autistic subjects before and after rTMS using recorded EEG signals, we calculated relative power of gamma (i.e., 30-45 Hz) within the entire spectrum.
Figure 1.
Sensor layout of the 128-channel Geodesic net (EGI, Eugene, Oregon) with selected channels labeled.
Kanizsa Illusory Figure Test
In this task subjects have to respond with a button-press to rare (25% probability) Kanizsa squares (targets) among Kanizsa triangles (rare non-target distracters, 25% probability) and non-Kanizsa figures (standards, 50% probability). The stimuli are presented for 250 ms with inter-trial intervals (ITI) varying in the range of 1,100–1,300 ms. A fixation point (cross) was presented during ITI. Black figures are displayed on a white background on a flat 19” color LCD. Subjects are instructed to press the first button on a 5-button keypad with their right index finger when a target appears, and ignore when the non-target Kanizsa or standard stimuli appear. All stimulus presentation and behavioral response (reaction time [RT], accuracy) collection was controlled by a PC computer running E-prime software (Psychology Software Tools, PA). Subjects were instructed to remain as still as possible with their eyes on the fixation mark and to refrain from blinking. Autistic patients had at least one session for EEG net conditioning and getting familiar with the experimental room.
The stimulus types used in the experiment are Kanizsa square (target), Kanizsa triangle (non-target), non-Kanizsa square, and non-Kanizsa triangle (standards). The non-target Kanizsa triangle is introduced to differentiate the processing Kanizsa figures and targets. The stimuli consist of either three or four inducer disks which are considered the shape feature, and they either constitute an illusory figure (square, triangle) or not (collinearity feature) (Figure 2). One block of 240 trials was presented. Subjects with Autism were administered the Kanizsa, illusory figure test before (pre-TMS) and after (post-TMS) treatment. There was also a randomly assigned waiting-list group where individuals with ASD were administered the same Kanizsa illusory figure test twice (with an 8 week interval) to control for the TMS treatment. Control subjects were administered the Kanizsa illusory figure test once; our previous study (Sokhadze et al., 2009b) indicated that in control subjects repeated exposure to the same test did not significantly affect gamma band power in response to Kanizsa illusory figures.
Figure 2.
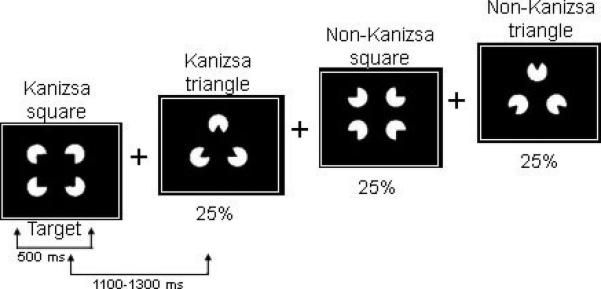
We used Kanizsa and non-Kanizsa figures as stimulus material in this experiment. In particular, the stimulus types are Kanizsa square (target), Kanizsa triangle, non-Kanizsa square, and non-Kanizsa triangle. The non-target Kanizsa triangle is introduced to differentiate processing of Kanizsa figures and targets. The stimuli consist of either three or four inducer disks which are considered the shape feature, and they either constitute an illusory figure (square, triangle) or not (collinearity feature).
TMS Procedure
A trained electrophysiologist delivered rTMS using a Magstim Rapid (Model 220) instrument (Magstim Corporation, Sheffield, England) with a 70-mm wing span figure-eight coil. Motor threshold (MT) was determined for each hemisphere in all individuals by gradually increasing the output of the machine by 5% until a 50 μV deflection or a visible twitch in the First Dorsal Interosseous (FDI) muscle was identified in 2 out of 3 trials of stimulation over the cortical area controlling the contralateral FDI. Electromyographic responses were monitored on a continuous base with a C-2 J&J Engineering physiological monitor (Poulsbo, WA). Motor evoked potentials were recorded from the hand contralateral to stimulation using the C2 J&J system with USE-2 Physiodata software applications. We also recorded heart rate, heart rate variability, skin conductance, and skin temperature. EMG and other physiological recordings were stored for later analysis. Autistic patients were encouraged to visit the laboratory at least once beforehand, in order to get familiar with the TMS procedure.
The TMS treatment course was administered once per week for 12 weeks (a total of twelve 1 Hz rTMS treatments); the first six treatments were over the left DLPFC while the remaining six were over the right DLPFC. The site for stimulation was found by placing the coil 5 cm anterior, and in a parasagital plane, to the site of maximal FDI stimulation. The figure-eight coil, with a 70-mm wing diameter was kept flat over the scalp. Subjects were wearing a swimming cap on their head. Stimulation was done at 1Hz and 90% MT, with a total of 150 pulses/day (fifteen 10 s trains with a 20–30 s interval between the trains). We selected 1 Hz as the stimulation frequency as studies have shown that low-frequency rTMS (≤1Hz) increases inhibition of stimulated cortex (e.g., Boroojerdi et al., 2000); there is also a lower risk for seizures the lower the rTMS frequency. Selection of 90% of the MT was based on the experience of numerous publications where rTMS was used for the stimulation of DLPFC in different psychiatric and neurological conditions (for reviews see Daskalakis, Christensen, Fitzgerald, & Chen, 2002; Gershon, Dannon, & Grunhaus, 2003; Greenberg, 2007; Holtzheimer, Russo, & Avery, 2001; Loo & Mitchell, 2005; Rosenberg et al., 2002; Wassermann & Lisanby, 2001). We also wanted to keep the stimulation power below MT as an extra safety precaution due to the increased risk of seizure within this study population. The minimal number of TMS pulses during a TMS session has varied from 30 to 2,000 pulses/per session on a once-per-week over 8 weeks to twice-a-day basis over 10 days (Daskalakis et al., 2002). It has been concluded that less than 100 pulses/per session is not very promising in terms of therapeutic efficacy (see Helmich, Siebner, Bakker, Munchau, & Bloem, 2006 for review).
Pre- and Post-TMS Behavioral Measures
Social and behavioral functioning for participants was evaluated utilizing caregiver report and clinician ratings of improvement. Participants were evaluated prior to receiving TMS and 2 weeks following treatment. Measures included: Aberrant Behavior Checklist (ABC). The ABC (Aman & Singh, 1994) is a clinician administered rating scale assessing five problem areas: Irritability, Lethargy/Social Withdrawal, Stereotypy, Hyperactivity, and Inappropriate Speech based on caregiver report. Each area contains multiple items receiving a rating from 0 to 3. Items are summed and high scores for each area reflect severity of the problem area. The ABC has been shown to be effective in assessing behavior changes in autism (Aman, 2004). Specifically, for this study we used the Irritability and Hyperactivity subscales of the ABC as outcome measures. Social Responsiveness Scale (SRS). The SRS (Constantino & Gruber, 2005) is a caregiver completed rating scale assessing social interest and interaction. The scale provides a dimensional measure of social interaction allowing the rating of social skills in Autism as well as non-autistic individuals. For this study we used the Social Awareness subscale of the SRS as an outcome measure. Repetitive Behavior Scale—Revised (RBS). The RBS (Bodfish, Symons, & Lewis, 1999) is a caregiver completed rating scale assessing repetitive and restricted behavior patterns. The RBS is a measure of different behaviors: stereotyped, self-injurious, compulsive, ritualistic, sameness, and restricted range (Bodfish, Symons, Parker, & Lewis, 2000). Items from scales are summed to obtain a measure of severity of repetitive behavior.
Statistics
Statistical analyses were performed on subject-averaged EEG and motor response data with the subject averages being the observations. The primary analysis model was the repeated measures ANOVA, with dependent variables being relative gamma power at the 11 selected EEG channels described above, as well as reaction time, and response accuracy to target stimuli (i.e., motor responses). Relative gamma power at the selected EEG channels was analyzed using ANOVA with Stimulus (Target, Non-target, Standard) and Hemisphere (Left, Right) as factors (all within participants); differences in anterior and posterior relative gamma power were also analyzed. For hemispheric differences the following channel combinations were compared: left and right lateral frontal (F7, F8); left and right medial frontal (F1, F2); left and right lateral parietal (P7, P8); left and right medial parietal (P3, P4). For anterior and posterior differences the following channel combinations were compared: lateral left anterior and posterior (F7, P7); medial left anterior and posterior (F1, P3); lateral right anterior and posterior (F8, P8); medial right anterior and posterior (F2, P4). The between subject factors included the following group comparisons: Baseline (ASD vs. controls), Treatment (ASD pre-TMS vs. ASD post-TMS), and Wait-list (ASD Pre-WTL vs. ASD Post-WTL) (i.e., no TMS). For behavioral questionnaire data a Group (waiting-list vs. treatment) × Time (pre- vs. post-TMS) ANOVA was completed to determine changes associated with TMS. For all ANOVAs, Greenhouse-Geisser corrected p-values were employed where appropriate. SPSS v.14 and Sigma Stat 3.1 packages were used for statistical analysis.
Results
Subjects Characteristics
We enrolled 25 autistic patients (ASD group), 21 males and 4 females, with a mean age of 13.8 ± 4.3 years. Sixteen of them were randomly assigned to active 1.0 Hz TMS treatment (TMS group), while nine were randomly assigned to the waiting-list group (WTL group). Mean age of subjects in the TMS group was 13.9 ± 5.3 years and 13.5 ± 2.0 years in the waiting-list group. Mean full-scale IQ score for children with ASD was 86.0 ± 24.7. Two of the subjects in the ASD group had been previously diagnosed as mentally retarded; however, they were successful in completing all required tasks. The mean full-scale IQ of the active TMS group was not significantly different from the randomly assigned waiting-list group. We recruited 20 control subjects (CNT group), 12 males and 8 females, mean age 15.3 ± 5.1 years. There were no statistically significant age differences between the groups.
Baseline (Pre-TMS) Group Differences
Evoked EEG Activity
One-way ANOVA analysis revealed that evoked gamma power was significantly higher to target Kanizsa stimuli at all channels in the control group compared to the ASD group (p < .001). A Stimulus (Target, Non-target) × Group (ASD, Control) interaction was significant at all channels (p < .001) indicating significantly higher evoked gamma power to target Kanizsa stimuli compared to non-target Kanizsa stimuli in controls; while the ASD group had a minimal difference in evoked gamma power between target and non-target Kanizsa stimuli actually demonstrating more gamma power to non-targets (Figure 3). An analysis of differences in evoked gamma power between anterior and posterior regions revealed a Topography (Anterior, Posterior) × Group (ASD, Control) interaction over the left hemisphere to all stimuli where controls had higher evoked gamma power over frontal (F7) compared to posterior (P7) regions (F=5.4891, p = 0.024); while the ASD group showed a negligible difference with slightly higher evoked gamma power over posterior (P7) regions. There were no significant hemispheric differences elucidated between ASD and control groups in evoked gamma power during baseline analysis.
Figure 3.
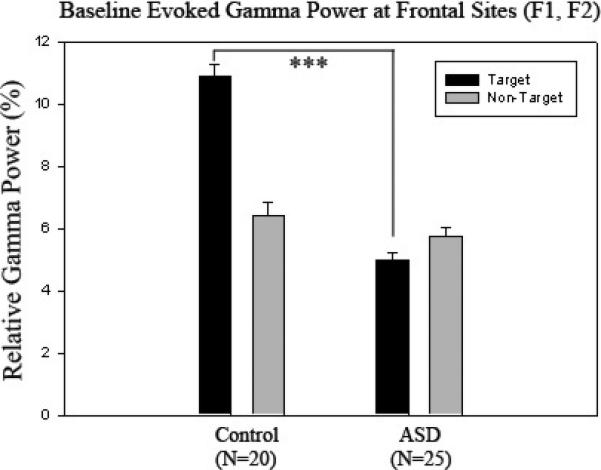
Relative evoked gamma power at frontal sites (F1, F2) in control (N=20) and ASD groups (N=25) to target and non-target stimuli. Controls have significantly higher evoked gamma power to target Kanizsa stimuli compared to the ASD group (p < .001) with more of a pronounced difference between target and non-target stimuli.
Motor Responses
Reaction time (RT) between the ASD (N = 25) and control (N = 20) groups was not significantly different (Mean 498.3 ± SD 105.7 ms in ASD vs. 478.3 ± 89.1 ms in controls). However, the ASD group made significantly more errors compared to controls (13.52 ± 15.91% vs. 2.18 ± 4.26%, F = 9.56, p = 0.003). The difference in response accuracy was better exposed for commission errors (11.06 ± 13.9% in ASD vs. 1.29 ± 3.38% in controls, F = 9.28, p = 0.004).
Post-TMS Group Differences
Evoked EEG Activity
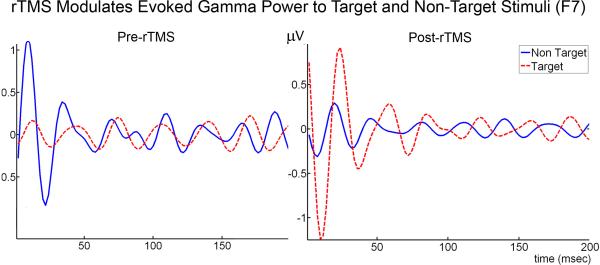
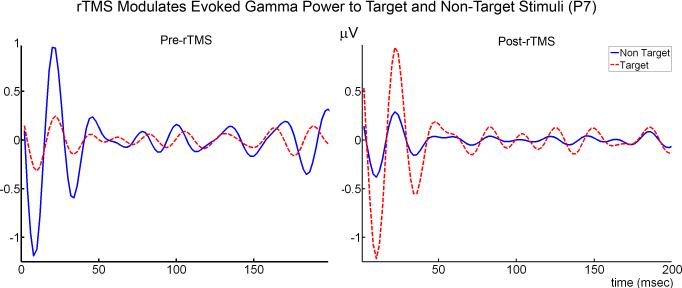
One-way ANOVA analysis revealed that evoked gamma power significantly increased to target Kanizsa stimuli at all channels as a result of rTMS treatment (p < .001). A Stimulus (Target, Non-target) × Group (Pre-rTMS, Post-rTMS) interaction was significant at all channels (p < 0.001) indicating increases in evoked gamma power to target stimuli with a decrease to non-targets following treatment (Figures 4-6). There were no significant, topographic (hemisphere, anterior vs. posterior) differences revealed following rTMS treatment. The waiting-list group (i.e., ASD patients with an 8 week interval between Kanizsa, illusory figure tests with no rTMS treatment) did not show significant evoked gamma power increases to target Kanizsa stimuli at any channels following the waiting period. In fact, they showed the opposite effect at two posterior EEG channels: Evoked-gamma power decreased to targets following the waiting period at P4 (F=9.455, p = 0.008) and P7 (F=5.862, p = 0 .029). Additionally, repeated measures analysis revealed significant Stimulus (Target, Non-target) × Group (Pre-wait, Post-wait) interactions at F1, F2, P3, P4 (all p < 0.05) indicating a significant increase in evoked gamma power to non-targets with a slight decrease to targets following the waiting period.
Figure 4.
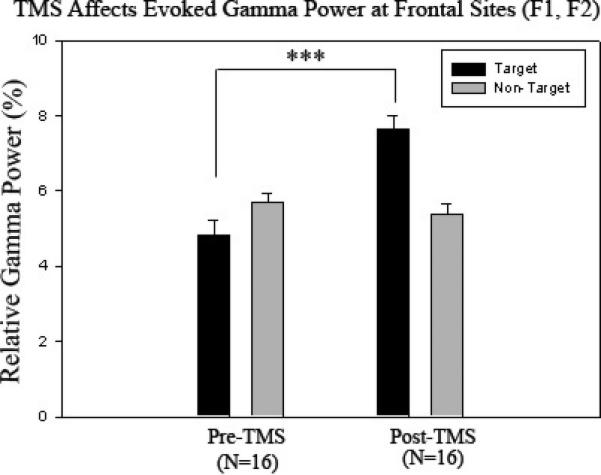
Relative evoked gamma power at frontal sites (F1, F2) in Pre-TMS (N=16) and Post-TMS groups (N=16) to target and non-target stimuli. Relative evoked gamma power significantly increases to target stimuli (p < .001) with more of a pronounced difference between target and non-target stimuli as a result of rTMS.
Figure 6.
Average amplitude of evoked gamma oscillations in response to non-target and target Kanizsa stimuli in subjects with ASD before (N = 16) and after rTMS (N = 16) at left lateral frontal (F7) and parietal (P7) EEG recording sites. Single trial EEG was averaged across 30 trials in each condition (non-target, target).
Motor Responses
Reaction time (RT) between the ASD Pre-TMS (N = 16) and ASD Post-TMS (N = 16) groups was not significantly different following rTMS (502.1 ± 99.2 ms in Pre-ASD vs. 517.5 ± 109.4 ms in Post-ASD, F = 0.17, p = .681). There was an improvement in response accuracy following rTMS treatment, but the difference did not reach significance (14.9 ± 17.9% before rTMS vs. 6.2 ± 8.5% after rTMS, F = 3.04, p = 0.091). The waiting list group did not show any differences in RT and accuracy with repeated Kanizsa tests (489.9 ± 123.9 ms in ASD Pre-WTL vs. 452.3 ± 122.8 ms in ASD Post-WTL, F = 0.367, p = 0.554, 10.9 ± 12.2% in ASD Pre-WTL vs. 8.4 ± 12.9% in ASD Post-WTL, F = 0.166, p = 0.690).
Clinical Evaluations after TMS
Results of the clinical evaluations are presented in Table 1. Following 12 sessions of bilateral rTMS subjects were reported to have reduced repetitive and restricted behavior patterns as measured by the Repetitive Behavior Scale (RBS). Additionally subjects showed a statistically significant reduction in irritability as measured by the Aberrant Behavior Checklist (ABC). No changes in social awareness or hyperactivity reached significance as a result of rTMS.
Table 1.
Pre- and Post- TMS Treatment Measures (N=16)
| Scale | Pre-Treatment Mean (SD) | Post-Treatment Mean (SD) | t-test |
|---|---|---|---|
| Repetitive Behavior1 | 30.8 (15.4) | 18.5 (12.8) | t=6.08, p =0.02 |
| Social Awareness2 | 82.0 (10.1) | 78.5 (9.3) | n.s. |
| Irritability3 | 10.3 (5.7) | 4.3 (4.2) | t=11.18, p =0.002 |
| Hyperactivity4 | 14.8 (7.3) | 10.8 (7.1) | n.s |
Raw score for Repetitive Behavior Scale – Revised, higher score indicates more impairment.
T-score for Social Awareness subscale of Social Responsiveness Scale, higher score indicates more impairment.
Raw score for Irritability subscale of the Aberrant Behavior Checklist, higher score indicates more impairment.
Raw score for Hyperactivity subscale of the Aberrant Behavior Checklist, higher score indicates more impairment.
Reported Side Effects of TMS
Before each session subjects were asked if they experienced any side effects as a result of their previous TMS session. The most commonly (i.e., 5 of 16 in active TMS group) reported side effect was an ‘itching’ sensation around the nose during stimulation. One subject reported a mild, transient tension-type headache on the day of stimulation. There was no discomfort reported due to the sound of the pulses. Overall, no subjects reported any lasting side effects.
Discussion
Our hypothesis was that individuals with ASD would show amplified and indiscriminative evoked gamma power in response to illusory figures at early stages of visual processing, and that 12 sessions of bilateral, ‘slow’ rTMS would attenuate amplified, early gamma activity and improve discriminatory gamma activity between relevant and irrelevant visual stimuli. Our results indicate that prior to rTMS individuals with ASD had a minimal difference in evoked gamma power between target and non-target Kanizsa stimuli at all channels. In fact, evoked gamma power responses were slightly larger in response to non-target Kanizsa stimuli relative to targets. In contrast the control group had a significantly higher evoked gamma power to target Kanizsa stimuli compared to non-target Kanizsa stimuli showing clear differences in visual stimulus discrimination. Additionally the control group showed a greater difference in evoked gamma power between frontal and parietal regions to all stimuli over the left hemisphere: Controls had more frontal as compared to parietal gamma activity, while the ASD group showed negligible topographic differences. These baseline findings are similar to the findings of Grice et al. (2001) where individuals with Autism did not show significant differences in frontal gamma activity during the processing of upright and inverted faces, whereas control subjects showed clear discriminative increases in frontal gamma activity when the faces where presented upright compared to inverted. These findings also correspond to our previous investigation (Sokhadze et al., 2009b) where we found positive differences in gamma oscillation power (i.e., 30-80 Hz, 0-800 msec) between target and non-target Kanizsa stimuli where decreased, especially over the lateral frontal (F7, F8) and parietal (P7, P8) EEG sites, in adolescents and young adults with ASD; this was mainly due to significant increases in gamma power at all recording sites, especially evoked gamma (i.e., ~100 ms) over frontal channels, to non-target Kanizsa stimuli compared to controls.
It has been argued that evoked gamma band activity reflects the effect of attention on early visual processing (Herrmann & Mecklinger, 2000) and sensory-memory matching processes (Herrmann, Munck, & Engel, 2004). Additionally evoked gamma activity has been associated with the binding of perceptual information within the same cortical area, as compared to the feed-forward and feed-back processing (i.e., over a whole network of cortical areas) associated with induced gamma oscillations. Our baseline results indicate that in ASD evoked gamma activity is not discriminative of stimulus type, whereas in controls early gamma power differences between target and non-target stimuli are highly significant.
There are a few plausible explanations as to why the gamma response does not allow for discrimination between stimuli in ASD. It is well known that ASD is associated with amplified responses to incoming sensory information. Studies suggest that the neural systems of individuals with ASD are over-activated (Belmonte & Yurgelin-Todd, 2003), and there is a lack of cortical inhibitory tone (Casanova, Buxhoeveden, & Brown, 2002a; Casanova, Buxhoeveden, Switala, & Roy, 2002b; Casanova et al., 2006b; Rubenstein & Merzenich, 2003). Deficits in cortical inhibitory processes and poor signal-to-noise ratios may result in increased simultaneous activity of competing local networks where no pattern can emerge to dominate and constrain perceptual processing. In a network that is over-activated and ‘noisy,’ local cortical connectivity may be enhanced at the expense of long-range cortical connections and individuals with ASD may have difficulty directing attention: It may not be possible for them to selectively activate specific perceptual systems based on the relevance of a stimulus (i.e., target vs. non-target).
Our previous findings investigating event-related potentials (ERP) during a novelty processing task further supports the idea of difficulty discriminating task-relevant from irrelevant stimuli in ASD (see Sokhadze et al., 2009a). Briefly, we found that subjects with ASD showed a lack of stimulus discrimination between target and non-target stimuli compared to controls, and this was mainly due to significantly prolonged and augmented ERP components to irrelevant distracter stimuli over frontal and parietal recording sites. Early ERP components (e.g., P100, N100) were especially increased to irrelevant distracter stimuli in the ASD group indicating augmented responses at early stages of visual processing (i.e., ~ 100 msec). Early gamma components (i.e., evoked) are measured at the same time over the same cortical regions as these early ERP components. The very early burst of gamma activity between 80 and 120 msec found by Brown et al. (2005) and our findings of augmented evoked gamma (Sokhdze et al., 2009b) and early ERP responses (Sokhadze et al., 2009a) to task irrelevant stimuli support the idea of disturbances in the activation task-relevant neuronal assemblies and the perceptual control of attention in ASD. Although we found significant group differences in relative evoked gamma power in processing relevant and irrelevant visual stimuli in this study, it is important to mention why we did not find significantly amplified relative evoked gamma power in the ASD group compared to controls. We attribute this to the fact that relative gamma band power is calculated in reference to the entire EEG spectrum, and in ASD it has previously been shown that other frequency ranges are augmented as well (e.g., Stroganova et al., 2007; Dawson et al., 1995).
Our findings after 12 sessions of bilateral rTMS to the DLPFC showed that evoked gamma power significantly increased to target Kanizsa stimuli in the ASD group at all channels. Furthermore, repeated measures analysis revealed highly significant increases in evoked gamma power to target stimuli with a slight decrease to non-targets following treatment. Individuals with ASD showed significant improvement in discriminatory gamma activity between relevant and irrelevant visual stimuli following rTMS treatment. These findings corroborate with our previous study (Sokhadze et al., 2009b) where we found that six sessions of ‘slow’ (i.e., 0.5 Hz) rTMS significantly reduced gamma power (i.e., 30-80 Hz, 0-800 msec) to non-target stimuli thereby improving discriminatory gamma activity. As mentioned earlier in non-impaired individuals, gamma activity has been shown to increase during ‘target-present’ compared to ‘target-absent’ trials (Tallon-Baundry et al., 1996; Müller et al., 1996; Brown et al., 2005). Our findings show that before rTMS individuals with ASD are unable to selectively activate evoked gamma activity based on the relevance of a stimulus which may reflect ‘noisy’ perceptual processing and a reduction in cortical inhibitory tone; this may be related to the strong aversive reactions to sensory stimuli commonly recorded in autistic individuals. Twelve sessions of bilateral, ‘slow’ rTMS applied to the DLPFC significantly improved differences in discriminatory gamma activity at early stages of visual perception. We hypothesize that ‘slow’ rTMS increased inhibitory tone by selectively activating double-bouquet cells at the periphery of cortical minicolumns, and over a course of treatment-attenuated ‘noisy’ and amplified cortical activity improving discriminatory gamma activity.
The randomly assigned waiting-list group (i.e., ASD patients with an 8 week interval between Kanizsa, illusory figure tests and no rTMS treatment) did not show significant improvement in discriminatory gamma activity. In fact, at two posterior EEG channels (i.e., P4, P7) evoked gamma power significantly decreased to target stimuli following the waiting period and repeated measures analysis revealed significant increases in evoked gamma power to non-targets with a slight decrease to targets at frontal and parietal channels (i.e., F1, F2, P3, P4). Moreover, the waiting-list group showed the opposite effect as compared to the active rTMS group validating the effect of rTMS and discrediting any effect of habituation.
Methodologically speaking, the 30-45Hz portion of the gamma band has been especially associated with visual information processing and attentional perceptual mechanisms (e.g., Müller et al., 2000). Refining our method of analysis to isolate this portion of the gamma band relative to the entire EEG spectrum (i.e., percentage of relative gamma power) proved to be a useful approach in isolating this activity and avoided any complications due to power line interference. Additionally limiting our analysis to evoked gamma activity further confirmed abnormalities during early stages of visual processing (i.e., 100 ms) corroborating with our earlier findings (Sokhadze et al., 2009a ). This methodological approach is in contrast to our previous study (Sokhadze et al., 2009b) where we calculated both evoked and induced gamma band power (i.e., 0-800 ms) between 30-80Hz. Overall our updated method of analyzing gamma band activity is better defined and adjusted to effectively assess group differences in discriminatory gamma activity.
Behaviorally speaking, while there were no significant baseline differences in reaction time between the ASD group and controls, the ASD group did have significantly more errors in response to target stimuli; these results indicate compromised selective attention and executive function in the ASD group compared to controls. After rTMS the ASD group did show improvement in response accuracy, however the results did not quite reach statistical significance. Analysis of behavioral questionnaires showed statistically significant reductions in repetitive behavior and irritability as a result of rTMS. Considering caregivers of individuals with ASD often find repetitive behaviors (i.e., stereotyped, ritualistic, restricted range) and irritability to be particularly challenging, rTMS may prove to be a valuable treatment option in addressing these behaviors.
Our study had some limitations that should be addressed. We included 3 subjects over the age of 17 which increased the standard deviation of age for our ASD subjects. For future studies we are limiting our enrollment to include only children and young adults between the ages of 8 and 17. Additionally, we enrolled two subjects who were previously diagnosed as mentally retarded, and this increased the standard deviation of IQ for the ASD group. Despite these limitations all subjects were able to perform the required tasks.
In conclusion, there is surmounting evidence of augmented and indiscriminative cortical activity at early-stages of visual processing in individuals with ASD. In this study we showed that in ASD evoked gamma activity is not discriminative of stimulus type, whereas in controls early gamma power differences between target and non-target stimuli were highly significant. In a network that is over-activated and ‘noisy’ it may not be possible for individuals with ASD to selectively activate specific perceptual systems based on the relevance of a stimulus (i.e., target vs. non-target). Following 12 sessions of bilateral ‘slow’ rTMS treatment individuals with ASD showed significant improvement in discriminatory gamma activity between relevant and irrelevant visual stimuli; ‘slow’ rTMS may have increased cortical inhibitory tone and improved differences in evoked gamma activity between stimuli by attenuating amplified cortical activity. Our preliminary findings suggest rTMS has the potential to become a unique therapeutic tool capable of addressing some of the core symptoms of ASD. Considering the few therapeutic options currently available for ASD, TMS is a welcome option capable of playing an important role in improving the quality of life for many with the disorder.
Figure 5.
Average amplitude of evoked gamma oscillations in response to non-target and target Kanizsa stimuli in subjects with ASD before (N = 16) and after rTMS (N = 16) at left lateral frontal (F7) and parietal (P7) EEG recording sites. Single trial EEG was averaged across 30 trials in each condition (non-target, target).
Acknowledgments
Funding for this work was provided by NIH grants R01 MH086784 (MFC and ES) and R01 MH088893 (MFC).
References
- Aman MG. Management of hyperactivity and other acting out problems in patients with autism spectrum disorder. Seminars in Pediatric Neurology. 2004;11:225–228. doi: 10.1016/j.spen.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Aman MG, Singh NN. Aberrant behavior checklist—Community. Slosson Educational Publications; East Aurora, NY: 1994. Supplementary manual. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM-IV TR) 4th ed. American Psychiatric Association; Washington, DC: 2000. (text revised) [Google Scholar]
- Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Cognitive Brain Research. 2003;17:651–664. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- Bertrand O, Tallon-Baudry C. Oscillatory gamma activity in humans: A possible role for object representation. International Journal of Psychophysiology. 2000;38:211–223. doi: 10.1016/s0167-8760(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Lewis MH. Repetitive behavior scale. Western Carolina Center Research Reports; Morganton, NC: 1999. [Google Scholar]
- Bodfish JW, Symons FS, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Prager A, Muellbacher W, et al. Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology. 2000;54:1529–1531. doi: 10.1212/wnl.54.7.1529. [DOI] [PubMed] [Google Scholar]
- Brown C, Gruber T, Boucher J, Rippon G, Brock J. Gamma abnormalities during perception of illusory figures in autism. Cortex. 2005;41:364–376. doi: 10.1016/s0010-9452(08)70273-9. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. Journal of Child Neurology. 2002a;17:692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002b;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten I, Switala AE, van England H, Heinsen H, Steinbuch HWM, et al. Abnormalities of cortical minicolumnar organization in the prefrontal lobes of autistic patients. Clinical Neuroscience Research. 2006b;6:127–133. [Google Scholar]
- Casanova MF, van Kooten I, van Engeland H, Heinsen H, Steinbursch HWM, Hof PR, et al. Minicolumnar abnormalities in autism II. Neuronal size and number. Acta Neuropathologica. 2006c;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Casanova MF. The neuropathology of autism. Brain Pathology. 2007;17:422–433. doi: 10.1111/j.1750-3639.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF. Neuropathological and genetic findings in autism: The significance of a putative minicolumnopathy. The Neuroscientist. 2006a;12:435–441. doi: 10.1177/1073858406290375. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autism. The Neuroscientist. 2003;9:496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- Charman T. Autism spectrum disorders. Psychiatry. 2008;7(8):331–334. [Google Scholar]
- Constantino JN, Gruber CP. The social responsiveness scale (SRS) manual. Western Psychological Services; Los Angeles, CA: 2005. [Google Scholar]
- Dawson G, Klinger-Grofer L, Panagiotides H, et al. Subgroups of autistic children based on social behavior display distinct patterns of brain activity. Journal of Abnormal Child Psychology. 1995;23:569–583. doi: 10.1007/BF01447662. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Transcranial magnetic stimulation: A new investigational and treatment tool in psychiatry. Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:406–415. doi: 10.1176/jnp.14.4.406. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KAC. Neuronal circuits of the neocortex. Annual Review of Neuroscience. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Faraday M. Effects on the production of electricity from magnetism. In: Williams LP, editor. Michael Faraday. Basic Books; New York: 1831. p. 531. 1965. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders—non-patient edition (SCID-NP) New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. American Journal of Psychiatry. 2003;160:835–845. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Billstedt E. Autism and Asperger syndrome: coexistence with other clinical disorders. Acta Psychiatrica Scandinavica. 2000;102:321–330. doi: 10.1034/j.1600-0447.2000.102005321.x. [DOI] [PubMed] [Google Scholar]
- Greenberg BD. Transcranial magnetic stimulation in anxiety disorders. In: George MS, Belmaker RH, editors. Transcranial magnetic stimulation in clinical psychiatry. American Psychiatric Publishing, Inc.; Washington, DC: 2007. pp. 165–178. [Google Scholar]
- Grothe B, Klump GM. Temporal processing in sensory systems. Current Opinion in Neurobiology. 2000;10:467–73. doi: 10.1016/s0959-4388(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Gomes E, Pedroso FS, Wagner MB. Auditory hypersensitivity in the autistic spectrum disorder. Pró-fono: revista de atualização científica. 2008;20:279–84. doi: 10.1590/s0104-56872008000400013. [DOI] [PubMed] [Google Scholar]
- Goupillaud P, Grossman A, Morlet J. Cycle-octave and related transforms in seismic signal analysis. Geoexploration. 1984;23:85–102. [Google Scholar]
- Grice SJ, Spratling MW, Karmiloff-Smith A, Halit H, Csibra G, De Haan M, Johnson MH. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. NeuroReport. 2001;12:2697–2700. doi: 10.1097/00001756-200108280-00021. [DOI] [PubMed] [Google Scholar]
- Happé FGE. Autism: Cognitive deficit or cognitive style? Trends in Cognitive Sciences. 1999;3:216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Siebner HR, Bakker M, Munchau A, Bloem BR. Repetitive transcranial magnetic stimulation to improve mood and motor function in Parkinson's disease. Journal of Neurological Sciences. 2006;248:84–96. doi: 10.1016/j.jns.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Munck MHJ, Engel AK. Cognitive functions of gamma-band activity: memory match and utilisation. Trends in Cognitive Sciences. 2004;88:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Mecklinger A. Magnetoencephalographic responses to illusory figures: early evoked gamma is affected by processing of stimulus features. International Journal of Psychophysiology. 2000;38:265–81. doi: 10.1016/s0167-8760(00)00170-7. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Mecklinger A, Pfeifer E. Gamma responses and ERPs in a visual classification task. Clinical Neurophysiology. 1999;110:636–42. doi: 10.1016/s1388-2457(99)00002-4. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Cavus I. Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. American Journal of Psychiatry. 2002;159:1093–1102. doi: 10.1176/appi.ajp.159.7.1093. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Russo J, Avery DH. A meta-analysis of repetitive transcranial magnetic stimulation in the treatment of depression. Psychopharmacology Bulletin. 2001;35:149–169. [PubMed] [Google Scholar]
- Kanizsa G. Subjective contours. Scientific American. 1976;235:48–52. doi: 10.1038/scientificamerican0476-48. [DOI] [PubMed] [Google Scholar]
- Keil A, Gruber T, Müller MM. Functional correlates of macroscopic high-frequency brain activity in the human visual system. Neuroscience and Biobehavioral Reviews. 2001;25:527–534. doi: 10.1016/s0149-7634(01)00031-8. [DOI] [PubMed] [Google Scholar]
- Le Couteur A, Lord C, Rutter M. The autism diagnostic interview—Revised (ADI-R) Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- Lewine JD, Andrews R, Chez M, Patil AA, Devinsky O, Smith M, Kanner A, Davis JT, Funke M, Jones G, Chong B, Provencal S, Weisend M, Lee RR, Orrison WW. Magnetoencephalographic patterns of epileptiform activity in children with regressive autism spectrum disorders. Pediatrics. 1999;104:405–418. doi: 10.1542/peds.104.3.405. [DOI] [PubMed] [Google Scholar]
- Loo C, Mitchell P. A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. Journal of Affective Disorders. 2005;88:255–267. doi: 10.1016/j.jad.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event related brain dynamics. Trends in Cognitive Sciences. 2004;85:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Introduction. Computation in cortical columns. Cerebral Cortex. 2003;13:2–4. doi: 10.1093/cercor/13.1.2. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Müller MM, Bosch J, Elbert T, Kreiter A, Sosa MV, Sosa PV, Rockstroh B. Visually induced gamma-based responses in human electroencephalographic activity – a link to animal studies. Experimental Brain Research. 1996;112:96–102. doi: 10.1007/BF00227182. [DOI] [PubMed] [Google Scholar]
- Müller MM, Gruber T. Induced gamma-band responses in the human EEG are related to attentional information processing. Visual Cognition. 2001;8:579–592. [Google Scholar]
- Müller MM, Gruber T, Keil A. Modulation of induced gamma band activity in the human EEG by attention and visual information processing. International Journal of Psychophysiology. 2000;38:283–299. doi: 10.1016/s0167-8760(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wasserman EM, et al. Responses to rapid-rate transcranial magnetic stimulation of the human cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience--virtual lesion, chronometry, and functional connectivity. Current Opinion in Neurobiology. 2000;10:232–7. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related cortical desynchronisation detected by power measurements of scalp EEG. Electroencephalography and Clinical Neurophysiology. 1977;42:817–826. doi: 10.1016/0013-4694(77)90235-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronisation and desynchronisation: basic principles. Clinical Neurophysiology. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Ratey JJ. Shadow Syndroms: The Mild Forms of Major Mental Disorders that Sabotage Us. Bantam Books; New York: 1998. [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the “new psychophysiology”. International Journal of Psychophysiology. 2007;63:164–72. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception's shadow: Long distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Rosenberg PB, Mehndiratta RB, Mehndiratta YP, Wamer A, Rosse RB, Balish M. Repetitive magnetic stimulation treatment of comorbid posttraumatic stress disorder and major depression. Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:270–276. doi: 10.1176/jnp.14.3.270. [DOI] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain, and Behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Movshon JA. Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron. 1999;24:67–77. doi: 10.1016/s0896-6273(00)80822-3. [DOI] [PubMed] [Google Scholar]
- Shibata T, Shimoyama I, Ito T, Abla D, Iwasa H, Koseki K, Yamanouchi N, Sato T, Nakajima Y. Attention changes the peak latency of the visual gamma-band oscillation of the EEG. Neuroreport. 1999;10:1167–1170. doi: 10.1097/00001756-199904260-00002. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray C. Visual feature integration and the temporal correlation hypothesis. Annual Review of Neuroscience. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Sokhadze E, Baruth J, Tasman A, Sears L, Mathai G, El-Baz A, et al. Event-related potential study of novelty processing abnormalities in autism. Applied Psychophysiology and Biofeedback. 2009a;34:37–51. doi: 10.1007/s10484-009-9074-5. [DOI] [PubMed] [Google Scholar]
- Sokhadze E, El-Baz A, Baruth J, Mathai G, Sears L, Casanova M. Effects of low frequency repetitive transcranial magnetic stimulation (rTMS) on gamma frequency oscillations and event-related potentials during processing of illusory figures in autism. Journal of Autism and Developmental Disorders. 2009b;39:619–34. doi: 10.1007/s10803-008-0662-7. [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, Elam M, et al. Abnormal EEG lateralization in boys with autism. Clinical Neurophysiology. 2007;118:1842–54. doi: 10.1016/j.clinph.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40Hz visual responses in human. Journal of Neuroscience. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Treisman A. Solutions to the binding problem: progress through controversy and convergence. Neuron. 1999;24:105–110. doi: 10.1016/s0896-6273(00)80826-0. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: A review. Clinical Neurophysiology. 2001;112:1367–1377. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 4th ed. Harcourt Assessment, Inc.; San Antonio, TX: 2003. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale for intelligence. Harcourt Assessment, Inc.; San Antonio, TX: 2004. [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. International Journal of Psychophysiology. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]