Abstract
Controlled matrix interactions were presented to pancreatic β-cells in three-dimensional culture within poly(ethylene glycol) hydrogels. Dispersed MIN6 β-cells were encapsulated in gel environments containing the following entrapped extracellular matrix (ECM) proteins: collagen type I, collagen type IV, fibrinogen, fibronectin, laminin, and vitronectin. In ECM-containing gels, β-cell survival was significantly better than in gels without ECM over 10 days. Correspondingly, apoptosis in encapsulated β-cells was less in the presence of each matrix protein, suggesting the ability of individual matrix interactions to prevent matrix signaling-related apoptosis (anoikis). MIN6 β-cells cultured in gels containing collagen type IV or laminin secreted more insulin in response to glucose stimulation than β-cells in all other experimental conditions. Variations in collagen type IV or laminin concentration between 10 μg/mL and 250 μg/mL did not affect insulin secretion. Finally, β-cell function in hydrogels presenting both collagen type IV and laminin revealed synergistic interactions. With a total protein concentration of 100 μg/mL, three gel compositions of varying ratios of collagen type IV to laminin (25:75, 50:50, and 75:25) were tested. In the presence of 25 μg/mL of collagen type IV and 75 μg/mL of laminin, β-cell insulin secretion was greater than with laminin or collagen type IV individually. These results demonstrate that specific, rationally designed extracellular environments promote isolated β-cell survival and function.
Introduction
Abetter understanding of the interactions between pancreatic β-cells and elements of their local microenvironment will contribute to advances in cell replacement therapies for treating insulin-dependent diabetes mellitus. The ability to reestablish critical extracellular matrix (ECM)–β-cell signaling may improve current islet culture techniques used between islet isolation and transplantation, as well as the design of an artificial, immunoprotective islet carrier for transplantation. Several reports have demonstrated better in vitro survival and function of islets or individual β-cells cultured on ECM-derived substrates, both cell-secreted matrices,1-8 and individual purified ECM proteins.6,9,10-12 Islet–matrix interactions have most often been studied with insulin-producing cells cultured two-dimensionally on ECM-coated tissue culture surfaces; however, under the proper conditions, some ECM analogs, such as collagens and Matrigel, form three-dimensional (3D) gels, allowing for the entrapment of islets or individual β-cells and the study of cell–matrix interactions in three dimensions. There is emerging interest in differences that may exist in the survival and activity of cells cultured in two- versus 3D environments.13
Several cell-secreted matrices have been used to improve islet culture and study interactions between insulin-producing cells and matrix molecules. Matrix secreted by bovine corneal endothelial cells improved islet survival14 and insulin secretion15 and induced adult β-cell proliferation.1 Studies of rat β-cells cultured on matrix produced using a rat bladder carcinoma line (804G) focused on specific integrin interactions and their effect on cell survival, spreading, and insulin secretion.3,16 The integrin α6β1 interacted with laminin in the 804G-secreted matrix and influenced β-cell function.3 Similar to studies with cell-derived matrices, culture experiments with purified individual ECM proteins resulted in better islet survival and function. Collagen type IV11 and laminin,6 both components of the basement membrane, contributed to greater insulin release. Islets cultured on collagen type I–coated surfaces and those treated with soluble fibronectin exhibited less apoptosis and greater insulin secretion.12 Vitronectin influenced β-cell adhesion and migration via αv integrin interactions.10
Three-dimensional islet culture experiments have been performed with ECM-based gels, which at the proper concentrations, form in culture medium at 37°C. Islet–matrix interactions were studied in collagen-based hydrogels,9 small intestinal submucosa,7 and Matrigel.2,4,8 In each study, islet survival and function were better than that under control culture conditions (non-treated tissue culture plates). In collagen type I hydrogels, the addition of collagen type IV and laminin increased islet insulin secretion.9 Studies of islet–matrix interactions in a 3D culture environment more closely mimic native islet conditions than 2D culture conditions. However, the conditions required for ECM gelation, including the limited number of matrix proteins that will form 3D gels and the concentration ranges required for gel formation limit experiments in ECM-based gels.
Poly(ethylene glycol) (PEG) hydrogels provide a blank 3D extracellular environment for testing microenvironmental culture parameters. Cells do not interact directly with the highly hydrated gels because of minimal protein adsorption to the PEG network, thus allowing for the controlled introduction of specific cell–matrix interactions in a 3D culture platform. Specifically, matrix proteins can be added to the gel environment during gel formation, individually or in combination at specified concentrations, independent of the ranges required for gelation of ECM-based 3D culture systems. Although the lack of extracellular interactions in the unmodified, hydrated hydrogel system is more similar to a suspension culture environment than to a 3D tissue structure, the structural properties of the network support the rounded morphology of isolated islets and individual β-cells and allow for the controlled presentation of specific extracellular interactions to the entire cell surface.
Previous research has demonstrated the cytocompatibility of PEG hydrogels formed via photoinitiated polymerization for encapsulation of MIN6 β-cells.17 Here we exploit this platform to examine the effects of matrix proteins on promoting β-cell survival and function. Pancreatic islets consist of approximately 70% insulin-producing β-cells. Unlike primary β-cells, which have limited capacity for in vitro regeneration and even survival after dispersion from isolated islets, the immortalized MIN6 β-cell line offers a model insulin-producing cell type for in vitro experimentation,18 including, in this case, the screening of various ECM proteins for extracellular interactions that support β-cell survival and function. Several reports have studied apoptosis related to extracellular interactions in the immortalized MIN6 β-cell line.19,20 MIN6 β-cells have also been investigated in the form of multicellular aggregates termed “pseudoislets.”19 In this work, MIN6 β-cells were encapsulated as singly dispersed cells to allow for the introduction of individual cell–matrix interactions in the absence of the confounding effects of cell–cell contacts found in pseudoislets and native islet structures. Specifically, MIN6 β-cells were encapsulated with each of the following ECM proteins: collagen type I, collagen type IV, fibrinogen, fibronectin, laminin, and vitronectin. Cell survival, apoptosis, and glucose-stimulated insulin secretion were assessed for β-cells cultured with each matrix protein. ECM protein concentration and combinations of matrix proteins were also investigated within the 3D PEG culture platform.
Experimental Procedures
PEG macromer and hydrogel synthesis
Methacrylate end groups were added to PEG by reacting linear PEG  (Sigma, St. Louis, MO) with methacrylic anhydride (Sigma) at a molar ratio of 1:10 using microwave irradiation under solvent-free conditions.21 The dimethacrylated PEG (PEGDM) product was collected by precipitation into chilled (4°C) ethyl ether (Sigma). Macromer purification was achieved using dialysis in deionized water using cellulose ester dialysis tubing with a molecular-weight cutoff of 1000 g/mol (Spectrum Laboratories, Rancho Dominguez, CA). Purified PEGDM was collected by lyophilization and stored at 4°C under nitrogen. Consistency in PEGDM preparations was ensured using 1H nuclear magnetic resonance to determine percentage methacrylation by comparing the area under the integrals for the vinyl resonances (δ = 5.7 ppm, δ = 6.1 ppm) with that for the PEG backbone (methylene protons, δ = 4.4 ppm). Percentage methacrylation was 95 ± 5%.
(Sigma, St. Louis, MO) with methacrylic anhydride (Sigma) at a molar ratio of 1:10 using microwave irradiation under solvent-free conditions.21 The dimethacrylated PEG (PEGDM) product was collected by precipitation into chilled (4°C) ethyl ether (Sigma). Macromer purification was achieved using dialysis in deionized water using cellulose ester dialysis tubing with a molecular-weight cutoff of 1000 g/mol (Spectrum Laboratories, Rancho Dominguez, CA). Purified PEGDM was collected by lyophilization and stored at 4°C under nitrogen. Consistency in PEGDM preparations was ensured using 1H nuclear magnetic resonance to determine percentage methacrylation by comparing the area under the integrals for the vinyl resonances (δ = 5.7 ppm, δ = 6.1 ppm) with that for the PEG backbone (methylene protons, δ = 4.4 ppm). Percentage methacrylation was 95 ± 5%.
Hydrogel precursor solution was prepared with 10 wt % PEGDM in Hanks balanced salt solution (Gibco, Carlsbad, CA) and 0.025 wt % of the photoinitiator Darocur 2-hydroxy-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone(Ciba-Geigy, Basel, Switzerland). This solution was filter-sterilized, and hydrogels formed when 30-μL disk-shaped aliquots were exposed to 365-nm ultraviolet light at an intensity of approximately 7mW cm2 for 10 min. The volumetric swelling ratio of polymerized gels was calculated from the mass swelling ratio (swollen mass/dry mass) using density conversion factors to ensure consistency in PEG hydrogel structure.
Matrix protein entrapment in hydrogels
Collagen type I, collagen type IV, and entactin-free laminin isolated from mouse sarcoma were purchased from BD Biosciences (San Jose, CA), and fibrinogen, fibronectin, and vitronectin were purchase from Sigma. Matrix proteins were dissolved at specified concentrations in the 10 wt % PEGDM hydrogel precursor solution and entrapped throughout the gel network structure upon photoinitiated polymerization of the PEG hydrogel.
Laminin-containing PEG hydrogels and gels without matrix protein were prepared for immunostaining using cryosectioning. Sections were blocked with bovine serum albumin (BSA), incubated with a solution of rabbit anti-mouse laminin primary antibody (Sigma), and incubated with donkey anti-rabbit secondary antibody (Jackson Immunologics, West Grove, PA). Gel sections blocked and incubated with secondary antibody served as staining controls to test for nonspecific binding of the secondary antibody.
MIN6 β-cell culture and encapsulation
Insulin-producing cells from the murine pancreatic β-cell line MIN6 were used as a primary β-cell model to screen potentially influential cell–matrix interactions on cell function (e.g., survival, insulin secretion). These cells have been previously employed to study homotypic islet cell interactions,22,23 as well as β-cell–matrix interactions in 2D culture.20 MIN6 β-cells were cultured in RPMI 1640 (Gibco) supplemented with 1% penicillin-streptomycin (Gibco), 0.5 μg/mL fungizone (Gibco), and 10% fetal bovine serum (Gibco). Cells were plated in 75-cm2 treated tissue culture flasks and incubated at 37°C in humid conditions with 5% carbon dioxide. Cells were passaged approximately once per week and fed by medium exchange every 3 days.
MIN6 β-cells were encapsulated by suspension in the PEGDM macromer solution before ultraviolet light exposure. The cytocompatibility of the photoinitiator system and polymerization conditions used has been previously demonstrated with several mammalian cell lines, including MIN6 β-cells.17,24 Approximately 50,000 MIN6 cells were entrapped in each 30-μL hydrogel sample. At this concentration, β-cells were singly dispersed throughout the samples to test the effects of cell–matrix interactions alone, in the absence of cell–cell contacts. Immediately after hydrogel polymerization, encapsulation samples were transferred to culture medium–containing 6-well tissue culture plates. Samples were cultured on an orbital shaker (∼40 rpm), and culture medium exchanges occurred every 3 days.
Encapsulated β-cell viability and apoptosis
MIN6 β-cell survival within PEG hydrogels was observed using a membrane integrity assay, LIVE/DEAD, from Molecular Probes, Inc. (Eugene, OR). Encapsulation samples were submersed in staining solution for 10 min at 37°C, and rinsed in phosphate buffered saline (PBS;Gibco) before imaging (n = 3). Live cells were identified by green fluorescence with the reduction of calcein AM by esterase activity in the cytoplasm, and dead cells were observed by red fluorescence upon binding of ethidium homodimer to exposed DNA.
The Vybrant Apoptosis Assay Kit #3 (Molecular Probes) was used to identify apoptotic cells within 3D culture samples (n = 3). Cell-laden hydrogels were rinsed in the supplied binding buffer, incubated in annexin V staining solution for 15 min at room temperature, and rinsed before imaging. Cell-impermeant, fluorescein isothiocyanate–conjugated annexin V binds to phosphatidylserine, a protein located on the inner leaflet of the plasma membrane in healthy cells, which is found in the outer leaflet during the early stages of apoptosis. Propidium iodide binding to exposed DNA counterstained for necrotic cells. Double staining is observed in necrotic cells because the compromised cell membrane allows for annexin V staining of phosphatidylserine within the cell membrane, as well as propidium iodide binding to DNA. Non-apoptotic, live cells remain unstained using the Vybrant assay.
Laser scanning confocal microscopy was used to image β-cells stained with either assay within PEG hydrogels (LSM 5 Pascal, Zeiss, Germany). Image stacks approximately 200 μm thick were acquired at 5-μm intervals and projected into single-plane images. Stained β-cells were manually counted in images (n ≥ 3) of each sample, and percentages of cells identified in either assay were calculated.
Glucose-stimulated insulin secretion of encapsulated β-cells
Three-dimensionally cultured MIN6 β-cells were exposed to static glucose stimulation at specified time points (n = 3). Cell-containing gels were placed in a low-glucose-concentration solution (1.1 mM) for 45 min followed by incubation in a highglucose-concentration buffer (16.7 mM) for 1 h. Preliminary experiments confirmed glucose-dependent insulin secretion from encapsulated β-cells, and insulin released in basal glucose levels confirmed the absence of non-glucose-dependent stimulation by the polymer network or entrapped matrix proteins (data not shown). High-glucose buffer solutions were collected for insulin measurement using enzyme-linked immunosorbent assay. An insulin sandwich ELISA was constructed using two monoclonal insulin antibodies (Biodesign International, Saco, ME), streptavidin horse radish peroxidase (HRP; Jackson Immunoresearch Laboratories Inc., West Grove, PA), and 3,3',5,5'-tetramethylbenzidine (TMB) ultra as the HRP substrate (Pierce, Rockford, IL). Briefly, high-binding, clear, 96-well plates were coated with monoclonal anti-insulin antibody (1:1000 dilution) overnight at 4°C. After three washes with PBS-Triton (0.1%), standard insulin solutions and experimental samples were added to antibody-coated wells and incubated at 37°C for 1 h. Next, biotin-conjugated monoclonal anti-insulin antibody was added to each well (1:8000 dilution, per manufacturer instructions) and incubated for an additional hour at room temperature. After three wash cycles, HRP-conjugated streptavidin (1:1000 dilution) was incubated in sample wells for 1 h at 37°C. After five washes, bound antibody complexes were developed with TMB ultra for 15 min protected from light, and the absorbance of each sample solution was measured (Wallac Victor2 1420 Multilabel Counter, Perkin Elmer, Waltham, MA). Average error values for standard solutions were within 5% of absorbance values.
The CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI) was used to measure the total adenosine triphosphate (ATP) content of each hydrogel sample. Samples were incubated in a 1:1 solution of culture medium and CellTiter-Glo reagent for 30 min on an orbital shaker (∼200 rpm). Sample solutions were transferred from the wells containing hydrogel samples to fresh wells of white 96-well plates. The luminescence of sample solutions was measured using a microplate reader (Wallac Victor2 1420 Multilabel Counter) after solutions were transferred in 200-μL aliquots to opaque 96-well plates. The measured insulin release values for each β-cell-hydrogel sample were normalized according to the measured amount of ATP in the respective sample to eliminate differences in observed insulin secretion due to variations in the number of cells within each sample. Because small ATP molecules readily pass through the hydrogel network, the measurement of ATP does not require the physical destruction of the hydrogel network, eliminating the experimental error introduced by this process. In control experiments (data not shown), ATP measured from cultured MIN6 was found to be proportional to cell number.
Statistical analysis
Results are presented as average values ± standard deviations. Data corresponding to each experimental condition were compared with control values in a two-tailed, unpaired Student t-test (p < 0.05), and data were further analyzed using analysis of variance (ANOVA) and Tukey's and Newman-Keuls secondary tests for significance. Additionally, Pearson correlation coefficients were calculated for each experimental curve to observe relationships between experimental conditions.
Results
A 3D cell culture platform with controlled matrix interactions
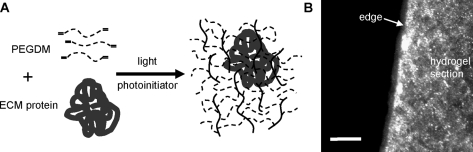
Synthetic hydrogel environments for 3D, encapsulated β-cell culture were synthesized using photoinitiated polymerization of dimethacrylated PEG. To test the effects of cell–matrix interactions on encapsulated β-cell survival and function, ECM proteins were incorporated into the hydrogel culture environment by physical entrapment. Matrix proteins were homogeneously entrapped in PEG hydrogels, individually and in combination, at desired concentrations. Retention of matrix proteins within the hydrogel is possible because the molecular dimensions of the proteins are larger than the mesh size of the gel network. Figure 1A portrays a simplified schematic of a PEG hydrogel network with entrapped ECM protein, and a list of the ECM proteins used in this study with their respective molecular weights and known structural dimensions is given in Table 1.
FIG. 1.
(A) Simple schematic of poly(ethylene glycol) (PEG) hydrogel formation and extracellular matrix (ECM) protein entrapment. (B) Fluorescent antibody staining of laminin entrapped throughout a sectioned PEG hydrogel sample. Scale bar is 200 μm. PEGDM, dimethacrylated PEG.
Table 1.
| Matrix Protein | Approximate Molecular Weight (kDa) | Structural Details |
|---|---|---|
| Collagen, type I | 450 | Triple helical fibrils, 300 nm long |
| Collagen, type IV | 180 | 400nm long chain with C-terminal globular domain, nonfibrillar |
| Fibrinogen | 340 | Trinodular rod, approximately 48 nm long and 7 nm wide |
| Fibronectin | 440 | Two subunits with an extended length 120-160 nm, each 60-70 nm long and 2-3 nm thick |
| Laminin | 900 | Cross-shaped, with long arm (80 nm in length) and three short arms (25-40nm in length) |
| Vitronectin | 75 | Globular shape ∼20nm, greater for multimeric units |
To observe the homogeneous distribution of entrapped matrix protein, hydrogels containing laminin were examined using immunohistochemical staining. The fluorescent staining observed in Figure 1B indicates the presence of laminin within a sectioned hydrogel layer. The entrapped laminin is distributed throughout the hydrogel sample, available for interaction with β-cells entrapped anywhere in the gel. Also, the conformation of the entrapped laminin was sufficiently retained to enable recognition using anti-laminin antibody staining. No staining was observed in control samples that did not contain laminin.
Individual matrix interactions and β-cell survival
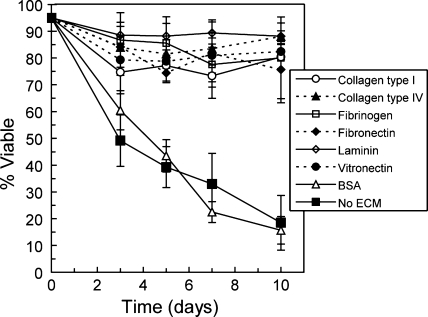
The influence of individual β-cell–matrix interactions on cell survival in 3D culture was investigated within a model PEG hydrogel environment. Singly dispersed MIN6 β-cells were encapsulated in gels containing the following individual ECM proteins: collagen type I, collagen type IV, fibrinogen, fibronectin, laminin, and vitronectin. Protein-free hydrogels and gels containing BSA served as non-matrix-presenting controls. All proteins were entrapped at 100 μg/mL, a concentration within the range of those tested in previous studies of islet-matrix interactions in 2D culture3,5,6,10–12 and in collagen gels.9 Encapsulated cell viability was monitored with culture time by staining with the LIVE/DEAD cytotoxicity kit (Molecular Probes). The percentage of live β-cells encapsulated with each matrix protein over 10 days in culture is shown in Figure 2. The viability of cells encapsulated in the presence of ECM proteins was significantly higher than that of controls beyond 5 days in culture (p < 0.05), with no statistical differences in β-cell survival observed between the various matrix proteins over the entire experimental time course as determined according to secondary significance tests. Calculated Pearson correlation coefficients indicate a negative linear trend in β-cell survival with time in control samples with no ECM and with BSA (r = −0.74 and r = −0.97, respectively). Linear trends were not observed in samples containing matrix protein.
FIG. 2.
MIN6 β-cell survival as a percentage of live cells stained with the LIVE/DEAD Cytotoxicity Assay (Molecular Probes) over 10 days in three-dimensional culture with and without ECM protein interactions (n = 3). BSA, bovine serum albumin; ECM, extracellular matrix.
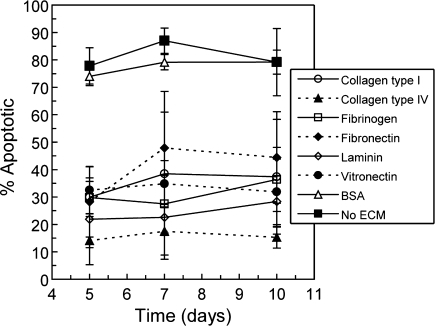
Apoptosis of β-cells in 3D culture with specific matrix interactions was also observed over 10 days in culture. Annexin V staining was used to identify apoptotic β-cells in gel constructs containing individual ECM proteins. This assay did not stain non-apoptotic live cells. Confirmed according to ANOVA analysis, a significantly lower level of apoptosis than in controls was observed in all 3D β-cell culture samples presenting matrix interactions (Fig. 3). As with β-cell survival in 3D culture, statistical differences were not observed over the entire time course between the apoptosis percentages in samples containing any of the tested matrix proteins as determined according to secondary significance tests. Calculated Pearson correlation coefficients did not indicate the presence of any linear trends (−0.50 < r < 0.50).
FIG. 3.
Percentage of apoptotic β-cells relative to necrotic cells identified using annexin V staining in three-dimensional culture with and without matrix protein interactions over 10 days in culture (n = 3). The Vybrant Apoptosis (Molecular Probes) assay labels only apoptotic and necrotic cells; therefore, live, non-apoptotic β-cells are not represented in this data. BSA, bovine serum albumin, ECM, extracellular matrix.
Individual matrix interactions and β-cell insulin secretion
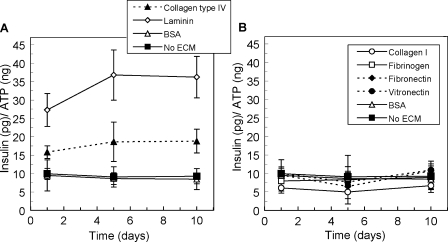
The effects of cell–matrix interactions on β-cell insulin secretion in response to glucose stimulation were investigated by exposing three-dimensionally cultured MIN6 β-cells in PEG hydrogels with and without matrix proteins to high glucose at specified time points over 10 days. To avoid disparities in insulin secretion due to differences in the number of cells in each sample, the amount of insulin released from each encapsulation sample was normalized according to the ATP content of the respective sample. Variations in sample cell number resulted primarily from cell death in control samples over the experimental time course, whereas minor sample-to-sample variations also occurred at the time of gel formation. Given the differences in the molecular dimensions of insulin (radius in solution (rs) = 1.47 nm) and the network structure (mesh size ∼25 nm), insulin diffusion through the hydrogel network was not a limitation in these experiments. The graphs in Figure 4 present normalized insulin release data for MIN6 β-cells cultured in the presence of each matrix protein over 10 days in culture. The inclusion of collagen type IV and laminin in the 3D β-cell culture environment contributed to significantly greater glucose-stimulated insulin secretion (Fig. 4A, p < 0.01). For example, after 10 days in culture, β-cells in collagen type IV–containing gels secreted more than 50% more insulin, and insulin secretion was approximately 350% greater than from those in laminin-presenting gels. In contrast, the presence of collagen type I, fibrinogen, fibronectin, and vitronectin did not result in insulin secretion values significantly greater than those of control samples, as determined by secondary significance tests (Fig. 4B). Pearson correlation coefficients suggested no linear trends for insulin release in any experimental condition (−0.50 <r < 0.50).
FIG. 4.
Glucose stimulated insulin secretion with culture time from MIN6 β-cells cultured in three-dimensional poly(ethylene glycol) gels presenting (A) collagen type IV and laminin and (B) collagen type I, fibrinogen, fibronectin, and vitronectin, relative to control samples containing bovine serum albumin (BSA) or no matrix protein (n = 3). ECM, extracellular matrix; ATP, adenosine triphosphate.
Matrix protein concentration and β-cell insulin secretion
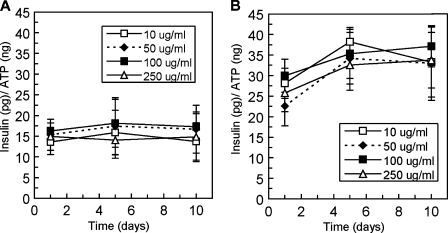
Previously reported investigations of β-cell–matrix interactions not only employed a variety of matrix substrates in 2D and 3D culture, but also varied matrix protein concentrations ranging from 10 μg/mL12 to 250 μg/mL.9 To investigate the effects of matrix concentrations within this range on β-cell insulin secretion, four concentrations of collagen type IV and laminin (10, 50, 100, and 250 μg/mL) were introduced to the β-cell culture environment. Static glucose-stimulated insulin secretion experiments were performed as previously described, and again released insulin values were normalized according to sample ATP content. No statistical differences in insulin secretion were observed with respect to collagen type IV concentration (Fig. 5A) or laminin concentration (Fig. 5B) at any point in the experimental time course (p > 0.05). Pearson correlation coefficients calculated for each condition did not confirm any linear trends (−0.50 <r < 0.50).
FIG. 5.
Glucose-stimulated insulin secretion over 10 days in culture from β-cells in three-dimensional poly(ethylene glycol) gels containing varied (A) collagen type IV concentration and (B) laminin concentration (n = 3). ATP, adenosine triphosphate.
Synergistic effects of ECM protein combinations and β-cell insulin secretion
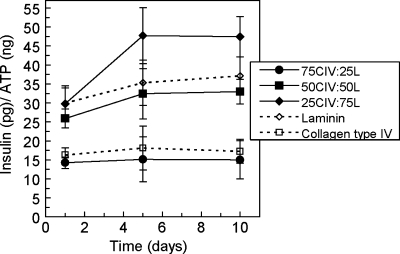
The highly controlled 3D hydrogel culture environment allows for the presentation of not only individual matrix protein interactions at varying concentrations, but also the introduction of specific combinations of ECM proteins. Based on the functional results for β-cells encapsulated with collagen type IV and laminin and the localization of these two proteins in the basement membrane, MIN6 β-cells were encapsulated with combinations of collagen type IV and laminin. With a total entrapped protein concentration of 100 μg/mL, three ratios of collagen type IV to laminin were tested: 25:75, 50:50, and 75:25. With this notation, the ratio also corresponds to individual protein concentration (i.e., 50% collagen type IV is also 50 μg/mL). Insulin released in response to glucose stimulation from β-cells in each condition was normalized according to ATP content, plotted over culture time, and compared with that from β-cells cultured with collagen type IV or laminin individually (Fig. 6). Insulin secretion from β-cells entrapped with an initial 50:50 combination of the matrix proteins was similar to that from cells exposed to laminin alone, but samples initially containing a 25:75 ratio of collagen type IV to laminin secreted more insulin than those with only laminin at days 5 and 10, as confirmed by ANOVA and secondary significance tests (p < 0.05). Insulin release from samples formed with a 75:25 mixture of collagen type IV and laminin was similar to that from those containing only collagen type IV. Calculated Pearson correlation coefficients were not indicative of linear trends for any condition (−0.50 < r < 0.50).
FIG. 6.
Insulin release from β-cells cultured in poly(ethylene glycol) gels with varied relative initial compositions of collagen type IV and laminin over 10 days in culture (n = 3). (CIV, collagen type IV; L, laminin).
Discussion
ECM signaling influences cellular differentiation, maintenance, and function. In mature tissues, cell–matrix interactions can activate intracellular signaling pathways that regulate cell proliferation, survival, and many other cell processes. Anoikis is the name given to programmed cell death resulting from inadequate or inappropriate matrix signaling.30 In vivo, anoikis is essential for tissue development and for the maintenance of high-turnover tissues, and resistance to anoikis has been implicated in the malignancy of certain types of cancer.31 However, anoikis after tissue isolation can limit the study of certain cells in vitro and limit the success of cell transplantation therapies. Anoikis has been shown to reduce functional islet mass after islet isolation.32,33 Several intracellular signaling pathways have been implicated in the regulation of apoptosis by cell–matrix binding events, including the phosphoinositide-3-kinase/protein kinase B pathway via activation by the integrin-signaling molecules focal adhesion kinase (FAK), Shc, and intregrin-linked kinase.31,34
Low survival of MIN6 β-cells was observed after 5 days of 3D culture in PEG gels in the absence of cell–cell and cell-matrix interactions. Better β-cell survival occurred when the following ECM proteins were introduced into the 3D PEG environment: collagen type I, collagen type IV, fibrinogen, fibronectin, laminin, and vitronectin. In addition to better survival, less β-cell apoptosis was observed in culture environments that provided cell–matrix interactions. Together these results suggest that anoikis in three-dimensionally cultured β-cells may be suppressed by the presence of ECM components. However, because anoikis occurs in response to not only inadequate but also inappropriate matrix signaling, the rescue effect observed with all of the tested matrix proteins may be unexpected. Table 2 lists each matrix protein with its respective major localization and the cell surface receptors known to bind them. Receptors listed in bold have been identified on β-cells.3,5,10,11,35 One commonality among the tested proteins, with the exception of the collagens, is the RGD binding sequence,39 which has recently been shown to reduce islet cell apoptosis when delivered in soluble form to two-dimensionally cultured islets.40 Additionally, Hammar et al. demonstrated the phosphorylation of FAK upon binding of β-cells to matrix secreted by 804G cells,41 and more recently, their group has shown that blocking β1 integrin binding to this matrix inhibited the phosphorylation of FAK.42
Table 2.
Extracellular Matrix (ECM) Proteins and Their Respective Primary Localizations and Known Cell Surface Receptors25–28,36–38
| Matrix Protein | Localization | Receptors |
|---|---|---|
| Collagen, type I | ECM of most tissues | αvβ3, α2β1, 30-, 70-, and 250-kDa receptors |
| Collagen, type IV | Basement membrane | α1β1, α1β2 |
| Fibrinogen | Plasma | αvβ3, αIIbβ3, α5β1 |
| Fibronectin | Plasma, ECM of most tissues | αvβ1, αvβ3, αvβ6, α4β1, α5β1, α4β7, αIIbβ3, |
| Laminin | Basement membrane | α1β1, α2β1, α3β1, α5β1, α6β1, α6β4, α7β1, α9β1, 67- and 110-kDa laminin receptors, α-dystroglycan |
| Vitronectin | Plasma, ECM of most tissue | αvβ1, αvβ3, αvβ5, αvβ8, αIIbβ3, α8β1 |
Insulin secretion in response to high glucose concentration (16.7 mM) was measured from MIN6 β-cells cultured in PEG hydrogels presenting matrix interactions. The amount of insulin released per cell was approximately 350% greater in samples containing laminin and 75% greater in those with collagen type IV than that released from β-cells cultured without matrix interactions and cells cultured with the other tested matrix proteins over the 10-day experimental time course (Fig. 4). As listed in Table 2, collagen type IV and laminin are associated with basement membranes. Nikolova et al. recently demonstrated the localization of these matrix proteins in the vascular basement membrane surrounding intraislet capillaries in vivo and provided evidence that β-cell contact with collagen type IV and laminin resulted in greater insulin gene expression both on matrix-coated culture substrates and when soluble matrix protein was added to the culture medium.20 As mentioned previously, several investigations have reported better β-cell function in culture on collagen type IV– and laminin-coated surfaces6,11 and in collagen type I hydrogels that also contained these proteins.9 The results presented in this work confirm that the individual effects of these specific cell–matrix interactions on β-cell function are preserved in a 3D hydrogel culture environment. The flexibility of the hydrogel culture environment also allowed us to observe statistically similar effects of collagen type IV and laminin on β-cell function at various concentrations used in previous investigations.
The ability of cell–matrix interactions to influence β-cell insulin secretion in 2D and 3D culture and as individual, purified proteins or components of cell-secreted matrices is well established. However, the exact signaling events that translate matrix protein–receptor binding into better insulin production and glucose-responsive secretion are not fully understood. Cell–matrix interactions directly influence cytoskeletal organization, and changes in the β-cell cytoskeleton are likely to influence glucose-stimulated insulin secretion. Disruption of actin filaments in β-cells by Clostridium botulinum C2 toxin resulted in impaired insulin secretion believed to be the result of limited access of insulin granules to the plasma membrane.43 More recently, F-actin remodeling has been shown to influence insulin secretion from MIN6 β-cells and primary rat β-cells, specifically through association with the target membrane-associated soluble N-ethylmaleimide-sensitive factor-attachment protein receptor complex responsible for insulin granule plasma membrane docking and fusion.44 Tomas et al. also observed F-actin remodeling upon glucose stimulation and found that the calcium-dependent F-actin severing protein gelsolin is required for proper cytoskeletal organization.45 Additionally, the activation of nuclear factor-κB in β-cells attached to matrix substrates contributes to actin re-organization in the interior of β-cells and the expression of genes implicated in glucose-stimulated insulin secretion.46
The controlled 3D culture environment of PEG hydrogels allows not only for the presentation of individual matrix proteins at specified concentrations, but also for combinations of matrix proteins in varying relative concentrations. MIN6 β-cells were cultured with three combinations of laminin and collagen type IV, and the pairing that presented more laminin (75 μg/mL) than collagen type IV (25 μg/mL) contributed to the highest average insulin secretion values. This combination is most similar to the relative amounts of these proteins in normal basement membrane ratios, where collagen type IV and laminin are found in an approximately 1:1 molar ratio.47 Matrigel also contains more laminin than collagen type IV (56% laminin vs 31% collagen type IV),48 and the 804G cell-secreted matrix used for islet culture is rich in the laminin V isoform.3 The laminin and collagen type IV combination with the highest amount of collagen type IV resulted in insulin secretion similar to that in the presence of collagen type IV alone, even though laminin is present at 25 μg/mL, a concentration within the range shown not to alter laminin-associated insulin secretion (Fig. 5B). These results suggest that an excess of collagen type IV relative to laminin may interfere with β-cell–laminin interactions, possibly by cell receptor blocking or direct binding of collagen to laminin to mask laminin binding sequences. Binding between laminin and collagen type IV is primarily facilitated by nidogen, which is not present in these cultures, but direct binding between the two matrix proteins has been observed.49-51
Encapsulated MIN6 β-cells were cultured in the absence of cell–cell contacts to study the effects of specific cell–ECM interactions individually and in combination. Cell–cell contacts rescued encapsulated MIN6 survival in previous experiments,17 and the resulting cell survival would not have allowed for the identification of cell–matrix interactions also able to preserve MIN6 survival if cell–cell contacts were present in the current investigation. When covalently tethered to the PEG hydrogel network, peptide sequences including RGD and several others found in laminin also influenced encapsulated MIN6 β-cell survival and function52 but not to the extent observed when whole matrix proteins were entrapped in the β-cell extracellular environment. It is likely that the greater complexity of potential interactions available between the whole protein structure and multiple cell-surface receptors causes better cell survival and function in the presence of complete proteins. In the present study, interactions with laminin and collagen type IV were identified as beneficial to β-cell function, and based on these results, the effects of laminin and collagen type IV on the survival and function of primary islets in 3D hydrogel culture should be explored. Future investigations into these interactions should test specific receptor–protein interactions with the use of functional blocking antibodies for cell-surface receptors known to bind to these proteins and to be expressed by the islet cell types.
In conclusion, the work presented here demonstrates the utility of PEG hydrogels as a 3D culture platform for studying controlled extracellular interactions. In an encapsulation environment devoid of cell–cell and cell–matrix interactions, individual MIN6 β-cells exhibited low survival rates in less than 1 week, but in the presence of matrix interactions, β-cell survival was improved through a reduction in apoptosis. Although all of the tested proteins improved β-cell survival, only collagen type IV and laminin, both basement membrane proteins, contributed to better β-cell function. In addition to identifying these influential interactions in 3D culture, the PEG culture platform allowed us to test the effects of varying matrix protein concentrations and combinations on β-cell function. The ability to present specific extracellular interactions within a rationally designed 3D culture platform will be useful in future investigations of isolated islets and the complex cell–cell and cell–matrix interactions that influence their coordinated function.
Acknowledgments
The authors gratefully acknowledge research funding from the National Science Foundation (NSF Grant EEC044771), the American Diabetes Association (Grant 7-04-RA-38 to KH), and the Howard Hughes Medical Institute. Funding for LMW was provided by a NSF Graduate Research Fellowship and a Department of Education Graduate Assistantships in Areas of National Need fellowship.
References
- 1.Schuppin G.T. Bonner-Weir S. Montana E. Kaiser N. Weir G.C. Replication of adult pancreatic-beta cells cultured on bovine corneal endothelial cell extracellular matrix. In Vitro Cell Dev Biol Anim. 1993;29A:339. doi: 10.1007/BF02633963. [DOI] [PubMed] [Google Scholar]
- 2.Perfetti R. Henderson T.E. Wang Y. Montrose-Rafizadeh C. Egan J.M. Insulin release and insulin mRNA levels in rat islets of Langerhans cultured on extracellular matrix. Pancreas. 1996;13:47. doi: 10.1097/00006676-199607000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosco D. Meda P. Halban P.A. Rouiller D.G. Importance of cell-matrix interactions in rat islet β-cell secretion in vitro: role of α6β1 integrin. Diabetes. 2000;49:233. doi: 10.2337/diabetes.49.2.233. [DOI] [PubMed] [Google Scholar]
- 4.Oberg-Welsh C. Long-term culture in Matrigel enhances the insulin secretion of fetal porcine islet-like cell clusters in vitro. Pancreas. 2001;22:157. doi: 10.1097/00006676-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Ris F. Hammar E. Bosco D. Pilloud C. Maedler K. Donath M.Y. Oberholzer J. Zeender E. Morel P. Rouiller D. Halban P.A. Impact of integrin-matrix matching and inhibition of apoptosis on the survival of purified human beta-cells in vitro. Diabetologia. 2002;45:841. doi: 10.1007/s00125-002-0840-7. [DOI] [PubMed] [Google Scholar]
- 6.Edamura K. Nasu K. Iwami Y. Ogawa H. Sasaki N. Ohgawara H. Effect of adhesion of collagen molecules on cell attachment, insulin secretion, and glucose responsiveness in the cultured adult porcine endocrine pancreas: a preliminary study. Cell Transplant. 2003;12:439. doi: 10.3727/000000003108746867. [DOI] [PubMed] [Google Scholar]
- 7.Woods E.J. Walsh C.M. Sidner R.A. Zieger M.A.J. Lakey J.R.T. Ricordi C. Critser J.K. Improved in vitro function of islets using small intestinal submucosa. Transplant Proc. 2004;36:1175. doi: 10.1016/j.transproceed.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Knight K.R. Uda Y. Findlay M.W. Brown D.L. Cronin K.J. Jamieson E. Tai T. Keramidaris E. Penington A.J. Rophael J. Harrison L.C. Morrison W.A. Vascularized tissue-engineered chambers promote survival and function of transplanted islets and improve glycemic control. FASEB J. 2006;20:565. doi: 10.1096/fj.05-4879fje. [DOI] [PubMed] [Google Scholar]
- 9.Nagata N. Iwanaga A. Inoue K. Tabata Y. Co-culture of extracellular matrix suppresses the cell death of rat pancreatic islets. J. Biomater. Sci. Polymer Edn. 2002;13:579. doi: 10.1163/15685620260178418. [DOI] [PubMed] [Google Scholar]
- 10.Kaido T. Perez B. Yebra M. Hill J. Cirulli V. Hayek A. Montgomery A.M. αv-integrin utilization in human β-cell adhesion, spreading, and motility. J. Biol. Chem. 2004;279:17731. doi: 10.1074/jbc.M308425200. [DOI] [PubMed] [Google Scholar]
- 11.Kaido T. Yebra M. Cirulli V. Montgomery A.M. Regulation of human β-cell adhesion, motility, and insulin secretion by collagen IV and its receptor α1β1. J. Biol. Chem. 2004;279:53762. doi: 10.1074/jbc.M411202200. [DOI] [PubMed] [Google Scholar]
- 12.Wang R.N. Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J. Endocrinol. 1999;163:181. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 13.Abbott A. Biology's new dimension. Nature. 2003;424:870. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser N. Corcos A.P. Tur-Sinai A. Ariav Y. Cerasi E. Monolayer culture of adult rat pancreatic islets on extracellular matrix: long term maintenance of differentiated β-cell function. Endocrinology. 1988;123:834. doi: 10.1210/endo-123-2-834. [DOI] [PubMed] [Google Scholar]
- 15.Beattie G.M. Lappi D.A. Baird A. Hayek A. Functional impact of attachment and purification in the short term culture of pancreatic islets. J. Clin. Endocrinol. Metab. 1991;73:93. doi: 10.1210/jcem-73-1-93. [DOI] [PubMed] [Google Scholar]
- 16.Bosco D. Gonelle-Gispert C. Wollheim C.B. Halban P.A. Rouiller D.G. Increased intracellular calcium is required for spreading of rat islet β-cells on extracellular matrix. Diabetes. 2001;50:1039. doi: 10.2337/diabetes.50.5.1039. [DOI] [PubMed] [Google Scholar]
- 17.Weber L.M. He J. Bradley B. Haskins K. Anseth K.S. PEG-based hydrogels as an in vitro encapsulation platform for testing controlled β-cell microenvironments. Acta Biomater. 2006;2:1. doi: 10.1016/j.actbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Miyazaki J.I. Araki K. Yamato E. Ikegami H. Asano T. Shibasaki Y. Oka Y. Yamamura K.I. Establishment of a pancreatic β-cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 19.Luther M.J. Davies E. Muller D. Harrison M. Bone A.J. Persaud S.J. Jones P.M. Cell-to-cell contact influences proliferative marker expression and apoptosis in MIN6 cells grown in islet-like structures. Am. J. Physiol. Endocrinol. Metab. 2005;288:502. doi: 10.1152/ajpendo.00424.2004. [DOI] [PubMed] [Google Scholar]
- 20.Nikolova G. Jabs N. Konstantinova I. Domogatskaya A. Tryggvason K. Sorokin L. Fassler R. Gu G. Gerber H. Ferrar N. Melton D.A. Lammert E. The vascular basement membrane: a niche for insulin gene expression and β cell proliferation. Dev. Cell. 2006;10:397. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Lin-Gibson S. Bencherif S. Cooper J.A. Wetzel S.J. Antonucci J.M. Vogel B.M. Horkay F. Washburn N. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004;5:1280. doi: 10.1021/bm0498777. [DOI] [PubMed] [Google Scholar]
- 22.Brereton H.C. Carvell M.J. Asare-Anane H. Roberts G. Christie M.R. Persaud S.J. Jones P.M. Homotypic cell contact enhances insulin but not glucagon secretion. Biochem. Biophys. Res. Commun. 2006;344:995. doi: 10.1016/j.bbrc.2006.03.214. [DOI] [PubMed] [Google Scholar]
- 23.Luther M.J. Hauge-Evans A. Souza K.L. Jorns A. Lenzen S. Persaud S.J. Jones P.M. MIN6 β-cell-β-cell interactions influence insulin secretory responses to nutrients and non-nutrients. Biochem. Biophys. Res. Commun. 2006;343:99. doi: 10.1016/j.bbrc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Bryant S.J. Nuttelman C.R. Anseth K.S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polymer Ed. 2000;11:439. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 25.Olsen B.R. Ninomiya Y. Collagens. In: Kreis T., editor; Vale R., editor. Guidebook to the Extracellular Matrix, Anchor, and Adhesion Proteins. New York: Oxford University Press; 1999. pp. 380–408. [Google Scholar]
- 26.Ruggeri Z.M. Fibrinogen/fibrin. In: Kreis T., editor; Vale R., editor. Guidebook to the Extracellular Matrix, Anchor, and Adhesion Proteins. New York: Oxford University Press; 1999. pp. 417–421. [Google Scholar]
- 27.Sasaki T. Timpl R. Laminins. In: Kreis T., editor; Vale R., editor. Guidebook to the Extracellular Matrix, Anchor, and Adhesion Proteins. New York: Oxford University Press; 1999. pp. 434–443. [Google Scholar]
- 28.Hall D. Vitronectin. In: Kreis T., editor; Vale R., editor. Guidebook to the Extracellular Matrix, Anchor, and Adhesion Proteins. New York: Oxford University Press; 1999. pp. 496–498. [Google Scholar]
- 29.Hynes R. Fibronectins. In: Kreis T., editor; Vale R., editor. Guidebook to the Extracellular Matrix, Anchor, and Adhesion Proteins. New York: Oxford University Press; 1999. pp. 422–425. [Google Scholar]
- 30.Frisch S.M. Hunter F. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994;124:619. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frisch S.M. Screaton R.A. Anoikis mechanisms. Curr. Opin. Cell Biol. 2001;13:555. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 32.Thomas F.T. Contreras J.L. Bilbao G. Ricordi C. Curiel D. Thomas J.M. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery. 1999;126:299. [PubMed] [Google Scholar]
- 33.Thomas F. Wu J. Contreras J.L. Smyth C. Bilbao G. He J. Thomas J. A tripartite anoikis-like mechanism causes early isolated islet apoptosis. Surgery. 2001;130:333. doi: 10.1067/msy.2001.116413. [DOI] [PubMed] [Google Scholar]
- 34.Frisch S.M. Ruoslahti E. Integrins and anoikis. Curr. Opin. Cell Biol. 1997;9:701. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 35.Meda P. Bosco D. Communication of islet cells: molecules and functions. In: Habener J.F., editor; Hussain M., editor. Molecular Basis of Pancreas Development and function. Norwell, MA: Kluwer Academic Publishers; 2001. pp. 143–163. [Google Scholar]
- 36.Masters K.S. Anseth K.S. Cell-material interactions. In: Peppas N., editor; Sefton M., editor. Molecular and Cellular Foundations of Biomaterials: Advances in Chemical Engineering. Vol 29. New York: Academic Press; 2004. pp. 38–74. [Google Scholar]
- 37.Yamada K.M. Adhesive recognition sequences. J. Biol. Chem. 1991;266:12809. [PubMed] [Google Scholar]
- 38.Tashiro K. Monji A. Yoshida I. Hayashi Y. Matsuda K. Tashiro N. Mitsuyama Y. An IKLLI-containing peptide derived from the laminin α1 chain mediating heparin-binding, cell adhesion, neurite outgrowth and proliferation, represents a binding site for integrin α3β1 and heparin sulphate proteoglycan. Biochem. J. 1999;340:119. [PMC free article] [PubMed] [Google Scholar]
- 39.Ruoslahti E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Devl. Biol. 1996;12:697. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 40.Pinkse G.G.M. Bouwman W.P. Jiawan-Lalai R. Terpstra O.T. Bruijn J.A. de Heer E. Integrin signaling via RGD peptides and anti-β1 antibodies confers resistance to apoptosis in islets of langerhans. Diabetes. 2006;55:312. doi: 10.2337/diabetes.55.02.06.db04-0195. [DOI] [PubMed] [Google Scholar]
- 41.Hammar E. Parnaud G. Bosco D. Perriraz N. Maedler K. Donath M. Rouiller D.G. Halban P.A. Extracellular matrix protects pancreatic β-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 2004;53:2034. doi: 10.2337/diabetes.53.8.2034. [DOI] [PubMed] [Google Scholar]
- 42.Parnaud G. Hammar E. Rouiller D.G. Armanet M. Halban P.A. Bosco D. Blockade of β1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat β-cells attached on extracellular matrix. Diabetes. 2006;55:1413. doi: 10.2337/db05-1388. [DOI] [PubMed] [Google Scholar]
- 43.Li G. Rungger-Brandle E. Just I. Jonas J.C. Aktories K. Wollheim C.B. Effect of disruption of actin filaments by Clostridium botulinum C2 toxin on insulin secretion in HIT-T15 cells and pancreatic islets. Molec. Biol. Cell. 1994;5:1199. doi: 10.1091/mbc.5.11.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thurmond D.C. Gonelle-Gispert C. Furukawa M. Halban P.A. Pessin J.E. Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein) complex. Molec. Endocrinol. 2003;17:732. doi: 10.1210/me.2002-0333. [DOI] [PubMed] [Google Scholar]
- 45.Tomas A. Yermen B. Min L. Pessin J.E. Halban P.A. Regulation of pancreatic beta-cell insulin secretion by actin cytoskeleton remodeling: role of gelsolin and cooperation with the MAPK signaling pathway. J. Cell Sci. 2006;15:2156. doi: 10.1242/jcs.02942. [DOI] [PubMed] [Google Scholar]
- 46.Hammar E.B. Irminger J.C. Rickenbach K. Parnaud G. Ribaux P. Bosco D. Rouiller D. Halban P.A. Activation of NF-κB by extracellular matrix is involved in spreading and glucose-stimulated insulin secretion of pancreatic beta cells. J. Biol. Chem. 2005;280:30630. doi: 10.1074/jbc.M502493200. [DOI] [PubMed] [Google Scholar]
- 47.Kleinman H.K. McGarvey M.L. Hassell J.R. Star V.L. Cannon F.B. Laurie G.W. Martin G.R. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 48.BD Biosciences. BD MatrigelTM Basement Membrane Matrix and BD MatrigelTM Basement Membrane Matrix High Concentration (HC) [on-line] www.bdbiosciences.com/discovery_labware/products/display_product.php?keyID = 230. [Accessed May 1 2006]. www.bdbiosciences.com/discovery_labware/products/display_product.php?keyID = 230
- 49.Charonis A.S. Tsilibary E.C. Yurchenco P.D. Furthmayr H. Binding of laminin to type IV collagen: a morphological study. J. Cell Biol. 1985;100:1848. doi: 10.1083/jcb.100.6.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laurie G.W. Bing J.T. Kleinman H.K. Hassell J.R. Aumailley M. Martin G.R. Feldmann R.J. Localization of binding sites for laminin, heparin sulfate proteoglycan and fibronectin on basement membrane (type IV) collagen. J. Mol. Biol. 1986;189:205. doi: 10.1016/0022-2836(86)90391-8. [DOI] [PubMed] [Google Scholar]
- 51.Ancsin J.B. Kisilevsky R. Laminin interactions important for basement membrane assembly are promoted by zinc and implicate laminin zinc finger-like sequences. J. Biol. Chem. 1996;271:6845. doi: 10.1074/jbc.271.12.6845. [DOI] [PubMed] [Google Scholar]
- 52.Weber L.M. Hayda K.N. Haskins K. Anseth K.S. The effects of cell-matrix interactions on encapsulated β-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]