Abstract
Stem and progenitor cells are emerging as a potential source for cell-based therapies, in which large homogenous populations of differentiated cells are frequently deemed necessary for efficacy. Methods focused on biochemical cues have not yet yielded the numbers of endothelial cells thought necessary for cardiovascular applications. Interest in alternate methods has prompted the study of physical cues on stem and progenitor cell differentiation. In this study, fluid-based shear stress, at levels comparable to those experienced by endothelial cells in large vessels, was applied during the first few days of mouse embryonic stem cell differentiation. After 2 days of applied shear stress, there were increases in cell proliferation and in protein expression of endothelial markers (FLK1, VECAD, and PECAM). Further, treatment increased the number of FLK1+ cells from 1% to 40%, which were then capable of forming vessel-like structures in vitro. Thus, shear stress may be used to direct differentiation of embryonic stem cells toward an endothelial-like phenotype, helping to address the cell sourcing issue in cardiovascular regenerative medicine and tissue engineering.
Introduction
Stem and progenitor cells are emerging as a potential cell source for regenerative medicine and tissue engineering, in which large homogenous populations of differentiated cells are frequently deemed necessary for cell-based therapies. In cardiovascular applications, the availability of large numbers of endothelial cells will likely have the greatest impact (1) as an anti-thrombogenic layer in the endothelialization of vascular grafts, and (2) in promoting vascularization, that is, formation of new vessel structures, in injured or engineered tissues. Unfortunately, neither primary nor culture-expanded sources of mature endothelial cells adequately meet these needs. Stem and progenitor cells, including bone marrow-derived stem cells,1,2 circulating progenitor cells,3 umbilical cord blood cells,4 and embryonic stem cells (ESCs),5,6 have been shown to differentiate into endothelial cells and are potential cell sources for regenerative medicine.
Endothelial cells have been derived from mouse ESCs through both embryoid body (EB) formation and monolayer differentiation. When ESCs are removed from feeder layers and grown in the absence of leukemia inhibitory factor in suspension culture, cells aggregate to form EBs and differentiate into cells of all three germ lineages, including mesodermal endothelial cells.5 In a more directed approach to endothelial differentiation using two-dimensional monolayers, mouse ESCs are cultured on a collagen type IV substrate with medium supplemented with vascular endothelial growth factor (VEGF).7,8 In both of these approaches, the number of endothelial-like cells is low or involves extensive and diverse cultures conditions with periodic cell sorting. Thus, methods using only biochemical cues to influence cell fate decisions may not address the demand for large cell numbers for clinical therapies. To explore a wider range of cues to direct differentiation, recent studies have focused on the use of physical signals during stem cell culture.9
Some efforts to generate large homogenous populations of cells have focused on controlling the mechanical microenvironment. Application of fluid-based shear stress on cells grown on a flat surface promotes an endothelial phenotype in multiple progenitor cells, such as murine embryonic mesenchymal10 and ESC-derived FLK1+11,12 cells, as well as human blood-derived endothelial13–16 cells. Those studies utilized cells that already had been sorted and selected from a mesodermal lineage. It has yet to be determined whether shear stress can promote an endothelial-like phenotype from pluripotent cells using a single-step process without presorting for specific subpopulations. Therefore, the objective of this study was to determine the effect of fluid-based shear stress on promoting an endothelial phenotype during the early differentiation of murine ESCs.
Materials and Methods
Materials
Knockout Dulbecco's modified Eagle's medium (DMEM), knockout serum replacement, ESC-qualified fetal bovine serum (ESQ-FBS), nonessential amino acids, trypsin-ethylenediaminetetraacetic acid, cell dissociation buffer, and phosphate-buffered saline with calcium and magnesium (PBS++) were purchased from Invitrogen. Beta-mercaptoethanol, gelatin, donkey serum, bovine albumin, ribonuclease A, triton-X, ethanol, polyoxyethylenesorbitan monolaurate, mitomycin C, and dimethyl sulphoxide were purchased from Sigma-Aldrich. Alpha modification of Eagle's medium (αMEM), FBS, L-glutamine, penicillin/streptomycin solution (PS) and PBS were purchased from Mediatech, Inc. Mouse ESD3 ESCs, STO cells, and DMEM were purchased form ATCC. ESGRO® was purchased from Chemicon International and collagen type IV was from BD Biosciences. Endothelial medium and cells were purchased from Lonza. The peristaltic pump, tubing, pulse dampener, and media bottles were all from Cole Parmer and plastic spacers for the flow chamber were customized using material from Precision Brand. Anti-OCT4, anti-PECAM1, anti-VECAD, and IgG control unconjugated antibodies were from Santa Cruz Biotechnology, Inc. Preconjugated anti-vimentin and anti-FLK1, as well as all FITC- and PE-conjugated secondary antibodies, were from Research Diagnostics, Inc. Low retention microcentrifuge tubes were from Fisher Scientific International. Propidium iodide, phalloidin, Hoechst 33258, and LinearFlow™ calibration beads were from Molecular Probes.
Expansion of mouse ESCs
Mouse ESD3 ESCs were initially expanded on a mitotically inactivated STO feeder layer. STO fibroblasts were expanded in fibroblast medium (αMEM supplemented with 10% FBS and PS) and at confluence treated with mitomycin C to inhibit further proliferation. After overnight acclimation, the ESCs were plated onto the feeder layer in the medium consisting of DMEM supplemented with 10% ESQ-FBS, 1000 U/mL ESGRO (leukemia inhibitory factor), 0.1 mM beta-mercaptoethanol, and PS. Before the fusion of adjacent colonies, cells were frozen down and stored in LN2. To prepare the ESCs for use and markedly reduce (routinely to <5%) the presence of the mitotically inactive feeder cells, vial contents were thawed and expanded on gelatin-coated dishes in the culture medium (knockout DMEM supplemented with 15% ESQ-FBS, 5% knockout serum replacement, 4 mM L-glutamine, 0.1 mM nonessential amino acids, 1000 U/mL ESGRO, 0.1 mM beta-mercaptoethanol, and PS) for 3 days.
Initial differentiation of ESCs (pretreatment)
For all samples, mouse ESCs were differentiated for 2 days before treatment. Glass slides were coated with collagen type IV at 3.5 μg/cm2 for 2 h. Expanded ESCs (as above) were then seeded at 10,000 cells/cm2 in 1 mL and allowed to adhere for 1 h. At the end of seeding, 25 mL of differentiation medium (αMEM supplemented with 10% FBS, 0.1 mM beta-mercaptoethanol, and PS) was added and the cell-seeded slides were cultured (37°C and 5% CO2) for a period of 48 h to allow cell attachment.
Applied fluid shear stress
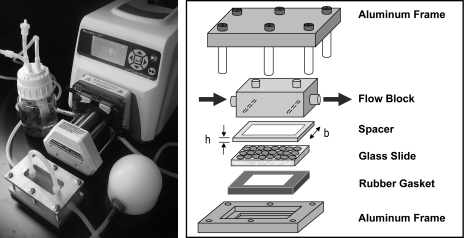
Fluid shear stress was applied using a parallel plate apparatus and a peristaltic pump, as described previously.17–19 Briefly, a plastic shim was placed atop a glass slide to create a channel 0.5 mm high by 26.5 mm wide. A custom-designed flow block was then placed flat on the shim, with inlet and outlet ports that directed fluid flow through the channel. An aluminum frame and rubber gasket were used to clamp the pieces together and prevent fluid leaks (Fig. 1).
FIG. 1.
Fluid shear stress system. A picture of the peristaltic pump, dampener, parallel plate chamber, and medium reservoir in a closed loop system (left, appearing clockwise). A schematic of the parallel plate chamber: cells are seeded onto a glass slide, a plastic spacer creates a flow channel with a specified height (h) and width (b), a flow block directs medium from the inlet down through the channel and then out, and an aluminum frame with rubber gasket clamps the pieces together and prevent leaks (right). Shear stress on the cell monolayer is related to the flow rate, viscosity of the fluid, and dimensions of the channel (refer to Materials and Methods for equation).
The parallel plate chamber was placed in a closed series with a medium reservoir, peristaltic pump, and dampener. The reservoir was filled with 125 mL of differentiation medium that recirculated independently for each sample through the system. A peristaltic pump was used to positively displace the medium through the dampener, resulting in a steady flow of medium into the flow block. In this configuration, τ = 6Qμ/(bh2), such that the shear stress (τ) is dependent on the flow rate (Q), viscosity of the medium (μ), and the width and height of the channel (b and h, respectively). Using this system, we applied a steady laminar shear stress of 15 dyn/cm2 (level experienced by endothelial cells in large vessels) for 48 h. As in previous studies, control samples were statically cultured in Petri dishes with 125 mL of differentiation medium.
Cell number and flow cytometry
At the end of treatment, experimental (glass slides removed from the parallel plate apparatus) and control samples were prepared for analysis. Cells were trypsinized off the glass slides and assessed for number using a coulter counter (Multisizer 3 from Beckman Coulter, Inc.).
Cells were assessed for cell cycle and protein expression using flow cytometry. Cells were removed from the glass slides for both experimental and control samples using either trypsin or cell dissociation buffer. Cell suspensions for cycle analysis were fixed by the drop-wise addition of 70% ethanol and stored in 4°C. These resuspended samples were stained with 50 μg/mL propidium iodide and 100 μg/mL ribonuclease A for 1 h at 37°C. A cytometer (BD Vantage from BD Biosciences) was used to assess fluorescence; histograms were analyzed to determine the percentage of cells in the G0/G1, S, and G2/M phases. For protein expression, separate cell suspensions were fixed with 4% formaldehyde for 15 min at 4°C and then stored in buffer solution (0.3% bovine serum albumin and 0.001% polyoxyethylenesorbitan monolaurate in PBS++) at 4°C. Samples assessed for intracellular markers were permeabilized with 0.5% triton-X. All samples were blocked with 10% donkey serum and incubated with a primary and then a secondary antibody (except for the PE-conjugated anti-FLK1 antibody). Markers assessed included OCT4 (a transcription factor related to pluripotency), vimentin (an intermediate filament associated with the mesodermal lineage), FLK1 (a VEGF receptor), VECAD and PECAM1 (both markers of mature endothelial cells), as well as actin found in smooth muscle cells (α-SMA). Primary and secondary antibodies were used at a dilution of 1:100 and 1:200, respectively. Species-appropriate IgG controls and marker-appropriate cell lines (such as D1 marrow and brain endothelial cells from ATCC) were used as negative and positive staining controls, respectively.
Quantification of population expression for each protein marker utilized multiple replicates from three to four separate experiments. In parallel to each sample set, fluorescence was also measured of LinearFlow Calibration kits, composed of sets of stable fluorescently labeled polystyrene beads, generating a reference calibration curve of fluorescence (range of 1%–100%). For each sample, histograms generated from the cytometer were analyzed to compute a median fluorescence, which was converted to standardized percentages using the calibration curve.
Correlation of quantitative values with observation of fluorescence was performed using ImageStream (Amnis Corporation), a technology combining flow cytometry and imaging. Preparation was similar to that described above, except that samples were dual-labeled for OCT4 (FITC fluorochrome) and FLK1 (PE fluorochrome). Before analysis, samples were stored in 1% formaldehyde. Cell nuclei were subsequently stained with DRAQ5. For each registered event on the cytometer, fluorescence levels and corresponding images were recorded. Single cells were selected by gating for events with intermediate DRAQ5 intensity and high nuclear aspect ratio, to distinguish from anucleate (low DRAQ5 intensity) and multi-cellular (high DRAQ5 intensity and low aspect ratio) events. Apoptotic cells were distinguished based on DNA condensation and nuclear fragmentation.20 Using these selection criteria, protein expression was assessed on intact, nonapoptotic single cells.
Structure formation
A functional assay for the endothelial phenotype is in vitro chord-like structure formation.21 Tissue culture plates (48 well) were coated with 110 μL/well of cold BD Matrigel™ and then kept at 37°C for 30 min for polymerization. Cells from static and shear samples, as well as ESCs, were trypsinized to generate single-cell solutions. Human aortic endothelial cells, cultured per vendor instructions, were used as a positive control for chord formation. Cells of each type (28,000) were seeded into separate wells and imaged 24 h later. Evaluation was repeated for three independent experiments.
Statistical analysis
Data are presented as mean ± standard error of the mean. Experimental and control samples were compared using a t-test, where p-values <0.05 were considered significant.
Results
Cell number and cycle
In samples exposed to SHEAR stress compared to the STATIC controls, there was an increase in cell number and percentage of proliferating cells (Table 1). There was a more than twofold increase in cell number in SHEAR samples compared to STATIC controls (88 ± 15 × 105 vs. 43 ± 6 × 105 cells; p < 0.05). Additionally, the percentage of cells undergoing DNA replication (S phase) was also significantly (p < 0.001) increased in the SHEAR samples (21.5% ± 0.4% vs. 17.7% ± 0.2%). Thus, applied shear stress can increase cell proliferation during early differentiation of ESCs.
Table 1.
Summary of Cell Number and Cycle Percentages for Samples That Were Cultured Under STATIC or SHEAR Conditions
| STATIC | SHEAR | p-Value | |
|---|---|---|---|
| Number (106 cells) | 43 ± 6 | 88 ± 15 | <0.05 |
| Cycle (% of total) | |||
| G0/G1 | 60.7 ± 0.6 | 56.1 ± 1.0 | <0.01 |
| S | 17.7 ± 0.2 | 21.5 ± 0.4 | <0.01 |
| G2/M | 21.9 ± 0.5 | 22.8 ± 0.7 | 0.33 |
p-Values were determined by t-test.
Protein expression
Stem cell differentiation was characterized by quantifying levels of protein expression. Markers of mesoderm (vimentin), endothelial (FLK1, VECAD, and PECAM1), and smooth muscle phenotype (α-SMA) were assessed using flow cytometry (Fig. 2). While there was likely differentiation to all three germ lineages, the significant (p < 0.05) increase in vimentin expression in SHEAR samples compared to STATIC controls indicates a promotion toward the mesodermal lineage, which includes the vascular endothelial and smooth muscle cells. α-SMA expression, however, remained unchanged (p = 0.67), whereas expression levels of the endothelial markers FLK1, VECAD, and PECAM were all significantly (p < 0.05) increased in SHEAR samples.
FIG. 2.
Protein expression. Using flow cytometry, STATIC and SHEAR samples were analyzed for smooth muscle cell actin (A, C), vimentin (B), FLK1 (D), PECAM1 (E), and VECAD (F) expression levels. (A) is a representative cytometry assessment, showing an overlay of histograms for the IgG control (filled), a STATIC and a SHEAR sample (indicated by arrows). For each STATIC and SHEAR sample, the median of each histogram was determined and normalized using calibration beads. Graphs are averages (mean ± standard error of the mean) of normalized values from different experiments (3–4 replicates were averaged per experiment; n = 3–4 experiments). Asterisks indicate significant changes compared to static controls (p < 0.05).
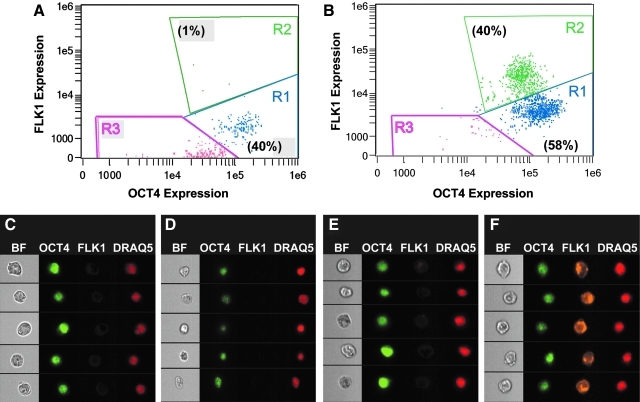
Combined imaging and flow cytometry allowed for correlation of quantitated fluorescence levels with fluorescent images for each cell (Fig. 3). Four replicates were analyzed for both SHEAR and STATIC samples, and the values, plots, and images of a typical sample for each group are presented. For STATIC and SHEAR samples, single nonapoptotic cells were assessed for dual expression of OCT4 and FLK1 and found to cluster in different regions of bivariate plots of fluorescence. In samples exposed to shear, 40% of the cells were highly expressing FLK1 (Fig. 3B). In control samples, only 1% of the cells was in the same region and had similarly high FLK1 expression levels (Fig. 3A). For five representative cells from different regions, brightfield images, as well as staining for OCT4, FLK1, and DRAQ5 are shown (Fig. 3C–F). For all cells, the transcription factor OCT4 co-localized with the nuclear stain DRAQ5. In Regions 1 and 3 (Fig. 3C–E), only background levels of FLK1 staining are visible. The cells in Region 2 (shown for SHEAR sample in Fig. 3F), however, expressed FLK1, indicated by punctuated staining. Thus, flow cytometry alone indicated that the overall population expression of FLK1 increased in the experimental group, whereas the combined technology of imaging with flow cytometry showed that the number of FLK1-positive cells had increased in response to fluid shear stress.
FIG. 3.
Co-expression of OCT4 and FLK1 in cells for a representative STATIC and SHEAR sample. Samples were dual labeled with anti-OCT4 (AF488) and anti-FLK1 (PE) antibodies and analyzed on an imaging flow cytometer (A: STATIC, B: SHEAR). Cells were categorized into three subpopulations: OCT4 + FLK1−(R1), OCT4+FLK1+(R2), and OCT4−FLK1−(R3). Values within parentheses indicate the percentage of cells within the region. Five representative cells are displayed from different regions: STATIC R1 (C), STATIC R3 (D), SHEAR R1 (E), and SHEAR R2 (F). Columns, from left to right, consist of brightfield (BF), OCT4, FLK1, and DRAQ5 images of a given cell in each row.
Structure formation
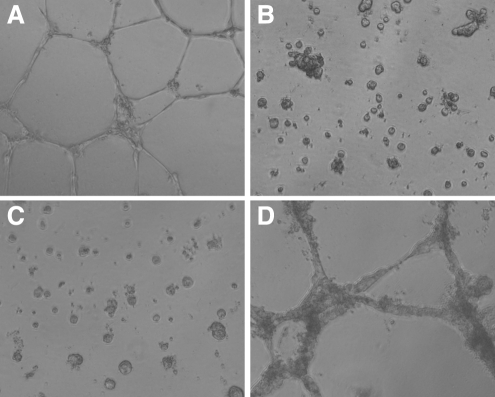
The ability of cells to form vessel-like structures in vitro is considered a functional property of endothelial cells. When expanded endothelial cells were seeded onto Matrigel-coated surfaces, structures formed within 24 h (Fig. 4A). Under those same conditions, both undifferentiated ESCs as well as after 4 days of differentiation under static conditions (STATIC samples) only formed cell clumps (Fig. 4B and C, respectively). Cells exposed to 2 days of shear during differentiation (SHEAR samples) formed structures similar to that of the endothelial cells. Failure of the STATIC samples to form structures is likely due to an insufficient number of endothelial-like cells, as dilution of endothelial cells with other phenotypes has been shown to inhibit structure formation in this assay.22 Further, the apparent differences in structure thickness between the SHEAR samples and the endothelial cell controls are consistent with the presence of a limited population of nonendothelial cells.22 Overall, it was found that during the first 4 days of ESC differentiation, 2 days of fluid shear stress promoted structure formation in vitro.
FIG. 4.
Structure formation in vitro. Cells were seeded onto BD Matrigel™-coated surfaces. Images were taken at 24 h of endothelial cells (A), undifferentiated mouse embryonic stem cells (B), cells cultured under STATIC conditions (C), and cells exposed to SHEAR (D).
Discussion
The application of 2 days of shear stress during early differentiation of ESCs promotes an endothelial-like phenotype. Fluid flow induces an increased percentage of proliferating cells and a net increase in cell number. As assessed by protein expression, differentiation is promoted along a mesodermal lineage toward a vascular phenotype. Shear stress, comparable to physiologic levels imposed by blood flow on endothelial cells in large vessels, specifically promotes differentiation toward an endothelial-like, as opposed to a smooth muscle, phenotype. Further, cells exposed to shear stress during culture form chord-like structures in an in vitro Matrigel assay, similar to primary endothelial cells. Overall, the application of 2 days of fluid shear stress during the early differentiation of mouse ESCs promotes protein expression and functional capabilities similar to primary endothelial cells.
In these studies a single-step process without any cell sorting or additional growth factors markedly increased the number of endothelial-like cells. Growth conditions included 2 days of culture on collagen type IV-coated glass slides before treatment for 2 days of fluid shear stress at 15 dyn/cm2. While the pretreatment was necessary to establish cellular adhesion before the application of fluid flow, further testing is necessary to determine if alterations in the experimental parameters, including surface substrate and shear stress magnitude and duration, might be more favorable for differentiation toward a mature endothelial phenotype.
Endothelial cells, which see a range of shear stresses due to blood flow through the vasculature, are often studied in fluid-based shear studies in vitro. The influence of laminar shear stress on adult endothelial cells has been well studied and documented.23,24 More recently, studies have investigated the effects of shear stress on cell populations derived from stem and progenitor cells. Mature endothelial cells differentiated from ESCs have been shown to align parallel to the direction of laminar shear stress,8,25 as is known for differentiated adult endothelial cells. For less differentiated cells, laminar shear stress has promoted an endothelial-like phenotype in ESC-derived FLK1+ cells,11,12 endothelial progenitors,13–16 and mesenchymal progenitors.10 In the case of those cells, however, shear stress had been applied after cell sorting or more extensive differentiation than in these studies. These differences in approach are meaningful, as studies have shown that when sorted cells are cultured separately, the differentiation patterns are markedly altered.6 Further, some studies investigated the effects of shear after longer durations of pretreatment than was used in these studies. Changes in pretreatment from 2 to 4 days, however, can increase brachyury gene expression (indicative of mesodermal lineage) almost fivefold (7.9 ± 0.6 vs. 38.3 ± 4.6 mM/M GAPDH, p < 0.001; unpublished data), indicating markedly different populations. In allowing ESCs to differentiate for only 2 days before treatment, our in vitro studies currently report the effect of shear stress on the most unspecialized cells, at the earliest stages of differentiation. Future studies can use this model system with ESCs to compare directly the effect of shear stress at different stages of differentiation.
Fluid flow can differentially influence cell fate decisions of ESCs and its derivatives. ESCs grown on Petri dishes in a simple continuous fluid perfusion system tripled in number over 6 days while retaining pluripotency.26 Recently Adamo et al.27 used a rotating cone to apply fluid shear stress to cells adhered to dishes and found differentiation toward a hematopoetic phenotype. Stir-based bioreactors, in which cells are in suspension in continuously moving fluid, have been found to increase the quality of differentiating EBs by preventing agglomeration.28,29 Under specific conditions, these same bioreactors increase the cell number of specific phenotypes, such as hematopoeitic precursors30 and cardiomyocytes.31,32 These systems are convenient formats for scaling up cell production, but create inhomogeneous shear stress fields that complicate study of smaller subpopulations of cells and intracellular signaling pathways. The parallel plate configuration used in these studies, however, creates a well-defined steady laminar shear stress profile.17 Further work in this system to study the basic response of stem cells to shear stress may help define design specification and culture conditions for the optimization of scaleable bioreactor systems for research and clinical cell production.
Study into the effects of the physical microenvironment on pluripotent cells has been limited. Results indicate, however, that specific mechanical cues may interact synergistically with chemical cues, potentially even replacing certain costly growth factors. A study that applied mechanical strain in addition to feeder conditioned medium found elevated expression of pluripotency markers in human ESCs.33 In another study, it was found that the addition of VEGF was necessary to promote an endothelial phenotype,7 while these studies used similar conditions and were able to considerably increase the percentage of endothelial-like cells in the presence of serum without supplemental growth factors. Other studies have also found that the application of fluid shear stress to progenitor cells promotes endothelial differentiation to levels commensurate to that achieved with VEGF stimulation alone11 or can accelerate the kinetics of the process.13 It remains unknown, however, whether the signaling pathways governing differentiation due to applied physical forces are the same in response to chemical cues. This question is of increasing relevance with the emergence of induced pluripotent stem cells for personalized regenerative medicine applications.
These studies showed that applied shear stress can be used to promote an endothelial-like phenotype during the early differentiation of ESCs without the addition of cytokines. Future studies are needed, however, to optimize treatment conditions, such as stress magnitude and duration, for differentiation toward a mature endothelial phenotype, as well as characterize effects on other cellular phenotypes. Overall, these studies help in understanding the influence of the physical microenvironment on cell fate decisions for the rational design of regenerative medicine applications.
Acknowledgments
The authors thank the Georgia Tech/Emory Center for the Engineering of Living Tissues (National Science Foundation Engineering Research Center: NSF EEC-9731643) and the Ruth L. Kirschstein National Research Service Award (1F32HL076978-01A1) for financial support. All work for this article was done at the Parker H. Petit Institute for Bioengineering and Bioscience at the Georgia Institute of Technology. The only exception is the analysis of samples using ImageStream technology, for which the authors would like to thank Philip J. Morrissey, Sherree Lee Friend, and Thaddeus C. George of Amnis Corporation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kocher A.A. Schuster M.D. Szabolcs M.J. Takuma S. Burkhoff D. Wang J., et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 2.Oswald J. Boxberger S. Jorgensen B. Feldmann S. Ehninger G. Bornhauser M., et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T. Murohara T. Sullivan A. Silver M. van der Zee R. Li T., et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 4.Chiu B. Wan J.Z. Abley D. Akabutu J. Induction of vascular endothelial phenotype and cellular proliferation from human cord blood stem cells cultured in simulated microgravity. Acta Astronaut. 2005;56:918. doi: 10.1016/j.actaastro.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Levenberg S. Golub J.S. Amit M. Itskovitz-Eldor J. Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:4391. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamashita J. Itoh H. Hirashima M. Ogawa M. Nishikawa S. Yurugi T., et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa S.I. Nishikawa S. Hirashima M. Matsuyoshi N. Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 8.McCloskey K.E. Lyons I. Rao R.R. Stice S.L. Nerem R.M. Purified and proliferating endothelial cells derived and expanded in vitro from embryonic stem cells. Endothelium. 2003;10:329. doi: 10.1080/10623320390272325. [DOI] [PubMed] [Google Scholar]
- 9.Heng B.C. Cao T. Haider H.K. Wang D.Z. Sim E.K. Ng S.C. An overview and synopsis of techniques for directing stem cell differentiation in vitro. Cell Tissue Res. 2004;315:291. doi: 10.1007/s00441-003-0847-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang H. Riha G.M. Yan S. Li M. Chai H. Yang H., et al. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto K. Sokabe T. Watabe T. Miyazono K. Yamashita J.K. Obi S., et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 12.Masumura T. Yamamoto K. Shimizu N. Obi S. Ando J. Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arterioscler Thromb Vasc Biol. 2009;29:2125. doi: 10.1161/ATVBAHA.109.193185. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto K. Takahashi T. Asahara T. Ohura N. Sokabe T. Kamiya A., et al. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress. J Appl Physiol. 2003;95:2081. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 14.Tao J. Yang Z. Wang J.M. Wang L.C. Luo C.F. Tang A.L., et al. Shear stress increases Cu/Zn SOD activity and mRNA expression in human endothelial progenitor cells. J Hum Hypertens. 2007;21:353. doi: 10.1038/sj.jhh.1002147. [DOI] [PubMed] [Google Scholar]
- 15.Wu C.C. Chao Y.C. Chen C.N. Chien S. Chen Y.C. Chien C.C., et al. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J Biomech. 2008;41:813. doi: 10.1016/j.jbiomech.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Ye C. Bai L. Yan Z.Q. Wang Y.H. Jiang Z.L. Shear stress and vascular smooth muscle cells promote endothelial differentiation of endothelial progenitor cells via activation of Akt. Clin Biomech. 2008;23(Suppl 1):S118. doi: 10.1016/j.clinbiomech.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Levesque M.J. Nerem R.M. The elongation and orientation of cultured endothelial cells in response to shear stress. J Biomech Eng. 1985;107:341. doi: 10.1115/1.3138567. [DOI] [PubMed] [Google Scholar]
- 18.Butcher J.T. Penrod A.M. Garcia A.J. Nerem R.M. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler Thromb Vasc Biol. 2004;24:1429. doi: 10.1161/01.ATV.0000130462.50769.5a. [DOI] [PubMed] [Google Scholar]
- 19.Butcher J.T. Nerem R.M. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng. 2006;12:905. doi: 10.1089/ten.2006.12.905. [DOI] [PubMed] [Google Scholar]
- 20.George T.C. Basiji D.A. Hall B.E. Lynch D.H. Ortyn W.E. Perry D.J., et al. Distinguishing modes of cell death using the ImageStream multispectral imaging flow cytometer. Cytometry A. 2004;59:237. doi: 10.1002/cyto.a.20048. [DOI] [PubMed] [Google Scholar]
- 21.Liu J. Wang X.B. Park D.S. Lisanti M.P. Caveolin-1 expression enhances endothelial capillary tubule formation. J Biol Chem. 2002;277:10661. doi: 10.1074/jbc.M110354200. [DOI] [PubMed] [Google Scholar]
- 22.Duffy G.P. Ahsan T. O'Brien T. Barry F. Nerem R.M. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng Part A. 2009;15:2459. doi: 10.1089/ten.TEA.2008.0341. [DOI] [PubMed] [Google Scholar]
- 23.Chien S. Li S. Shyy Y.J. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 24.Fisher A.B. Chien S. Barakat A.I. Nerem R.M. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol. 2001;281:L529. doi: 10.1152/ajplung.2001.281.3.L529. [DOI] [PubMed] [Google Scholar]
- 25.Metallo C.M. Vodyanik M.A. de Pablo J.J. Slukvin I.I. Palecek S.P. The response of human embryonic stem cell-derived endothelial cells to shear stress. Biotechnol Bioeng. 2008;100:830. doi: 10.1002/bit.21809. [DOI] [PubMed] [Google Scholar]
- 26.Oh S.K. Fong W.J. Teo Y. Tan H.L. Padmanabhan J. Chin A.C., et al. High density cultures of embryonic stem cells. Biotechnol Bioeng. 2005;91:523. doi: 10.1002/bit.20650. [DOI] [PubMed] [Google Scholar]
- 27.Adamo L. Naveiras O. Wenzel P.L. McKinney-Freeman S. Mack P.J. Gracia-Sancho J., et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpenedo R. Sargent C. McDevitt T. Rotary suspension culture enhances the efficiency, yield and homogeneity of embryoid body differentiation. Stem Cells. 2007;25:2224. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 29.Gerecht-Nir S. Cohen S. Itskovitz-Eldor J. Bioreactor cultivation enhances the efficiency of human embryoid body (hEB) formation and differentiation. Biotechnol Bioeng. 2004;86:493. doi: 10.1002/bit.20045. [DOI] [PubMed] [Google Scholar]
- 30.Dang S.M. Kyba M. Perlingeiro R. Daley G.Q. Zandstra P.W. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78:442. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 31.Bauwens C. Yin T. Dang S. Peerani R. Zandstra P.W. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: oxygen-mediated enhancement of cardiomyocyte output. Biotechnol Bioeng. 2005;90:452. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- 32.Sargent C.Y. Berguig G.Y. McDevitt T.C. Cardiomyogenic differentiation of embryoid bodies is promoted by rotary orbital suspension culture. Tissue Eng Part A. 2009;15:331. doi: 10.1089/ten.tea.2008.0145. [DOI] [PubMed] [Google Scholar]
- 33.Saha S. Ji L. de Pablo J.J. Palecek S.P. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]