Abstract
Cajal-Retzius (CR) cells are the most significant source of reelin, an extracellular matrix glycoprotein essential for cortical development. Strategically located in the marginal zone, CR cells control radial migration and laminar positioning of pyramidal neurons of the cortical plate. They degenerate and undergo cell death when cortical migration is completed. In human cortex development, reelin-expressing CR cells are already present in the early preplate, and continue to increase in number after the appearance of the cortical plate. In the course of the first half of gestation, the reelin signal in the marginal zone undergoes a huge amplification in parallel with the growth of the cortical plate and the expansion of the cortical surface. A significant source of CR cells is the cortical hem, a putative signalling centre at the interface of the prospective hippocampus and the choroid plexus. Hem-derived CR cells co-express reelin and p73, a transcription factor of the p53-family. They form the predominant CR cell population of the human neocortex. Characteristically, CR cells express the anti-apoptotic isoform DeltaNp73 which may be responsible for the protracted lifespan of human CR cells and the morphological differentiation of their axonal plexus. This dense fibre plexus, absent in lower mammals, amplifies the reelin-signal and establishes a physical boundary between the cortical plate and the marginal zone. In this review, we analyze the multiple sources of reelin/p73 positive CR cells at the interface of various telencephalic centres and the choroid plexus of the lateral ventricles. Additional populations of CR cells may derive from the thalamic eminence in the ventral thalamus and from the strionuclear neuroepithelium, or ‘amygdalar hem’. Comparative studies in a variety of species indicate that the cortical hem is the main origin of CR cells destined for the neocortex, and is most highly developed in the human brain. The close association between cortical hem and choroid plexus suggests a concerted role in the evolutionary increase of CR cells, amplification of the reelin signal in the marginal zone, and cortical expansion.
Keywords: cerebral cortex, choroid plexus, development, p73, reelin
Introduction
Cajal-Retzius (CR) cells have attracted the attention of developmental neuroscientists for more than one century. First described by Retzius (1883) and Ramón y Cajal (1899) in the marginal zone of the fetal and early postnatal human neocortex (reviewed by Meyer et al. 1999), over the last 50 years they have been documented in a variety of mammalian and nonmammalian vertebrates. While the classical Golgi studies were centred mostly on the conspicuous morphological features of CR cells, more recent work has focused on their molecular signature and their developmental origins. Most importantly, CR cells secrete the extracellular matrix protein reelin (D’Arcangelo et al. 1995; Ogawa et al. 1995; Meyer & Goffinet, 1998), which is essential for cortical development. Furthermore, CR cells express the transcription factor Tbr1, a marker of pallial neurons (Hevner et al. 2003), and the p53 homologue p73 (Yang et al. 2000; Meyer et al. 2002). In addition, human CR cells stand out by specifically expressing the RNA gene HAR1F (human accelerated regions), part of a region of the human genome that has shown a significant evolutionary acceleration since the divergence of humans and chimpanzees (Pollard et al. 2006).
Although, initially, CR cells were believed to derive from the pallial ventricular zone, it is now accepted that they are generated in discrete domains at the borders of the pallium, from where they migrate tangentially all over the cortical marginal zone. The most prominent source of CR cells in the neocortex is the cortical hem, a putative signalling centre at the interface of the hippocampal primordium and the choroid plexus anlage (Grove et al. 1998; Meyer et al. 2002; Bielle et al. 2005; Yoshida et al. 2006). In this review, we focus on the association between reelin-expression in CR cells, the cortical hem, and the choroid plexus, and discuss the evolutionary changes in these structures that may have contributed to the unique features of the human neocortex.
The importance of Cajal-Retzius cells and reelin for cortical development
Cajal-Retzius (CR) cells secrete reelin, a glycoprotein that is crucial for the radial migration of excitatory neurons from the ventricular zone into the cortical plate (D’Arcangelo et al. 1995, 1997; reviewed by Tissir & Goffinet, 2003; Meyer, 2007). During the early stages of corticogenesis, CR cells are the only source of reelin, with reelin-positive interneurons appearing later in development (Schiffmann et al. 1997; Alcantara et al. 1998; Meyer & Goffinet, 1998). CR cells, restricted to the marginal zone, release high amounts of reelin into the extracellular matrix. The reelin signal is taken up by the radial glia and radially migrating neurons, which express the reelin-receptors ApoER2 and VLDLR and the intracellular adapter protein disabled 1 (Dab1) (Rice et al. 1998; D’Arcangelo et al. 1999; Benhayon et al. 2003). Tyrosine phosphorylation of Dab1 is necessary for signal transduction (Hiesberger et al. 1999; Bock et al. 2003). The integrity of this signalling pathway is essential for correct positioning of cortical-plate neurons, and disruption of any of its components leads to a failure of radial migration (Sheldon et al. 1997; Trommsdorff et al. 1999; Howell et al. 2000; Kuo et al. 2005). In rodents, mutations of the genes involved in the reelin-Dab1 pathway lead to a common ‘reeler-like’ phenotype, in which the cortical layers are grossly inverted and layer 1 is missing. It is interesting to note, however, that despite the distortion of cortical architecture, the basic connectivity of the reeler cortex is maintained, whereas the most severe manifestations of reelin-deficiency – ataxia, tremor, and dystonia – are caused by the hypoplasia of the cerebellum (Lambert & Goffinet, 1998; Molnar et al. 1998). In turn, mutations of the human REELIN gene lead to lissencephaly associated with cerebellar hypoplasia, epilepsy and severe mental retardation (Hong et al. 2000), with a critical impairment of the development of cognitive functions. This indicates that reelin signalling has a higher impact on cortical development in human than in mouse.
The presence of reelin-expressing CR neurons in the outer layer of the developing pallium is evolutionarily conserved, and they have been reported in many amniote species, from turtles, crocodiles, lizards and rodents to primates, including human (Goffinet et al. 1999; Perez-Garcia et al. 2001; Tissir et al. 2003; Molnar et al. 2006). The increase in reelin levels in CR cells of the marginal zone has been suggested to be a driving factor in the progressive enlargement and differentiation of the pallium in evolution, with the highest reelin expression taking place in the human cortex (Tissir & Goffinet, 2003). In fact, the developing human cortex is the best example of the importance of reelin in CR cells. They first appear during the earliest preplate stage and increase in numbers during the early formation of the cortical plate (Fig. 1A); they differentiate during the stage of maximum migration into the cortical plate (Fig. 1B), and eventually disappear when migration is over (Fig. 1C) (Meyer & Goffinet, 1998; Meyer et al. 2000, 2002). In the following paragraphs we address the mechanisms that may lead to the specific prominence of CR cells in the human cortex.
Fig. 1.
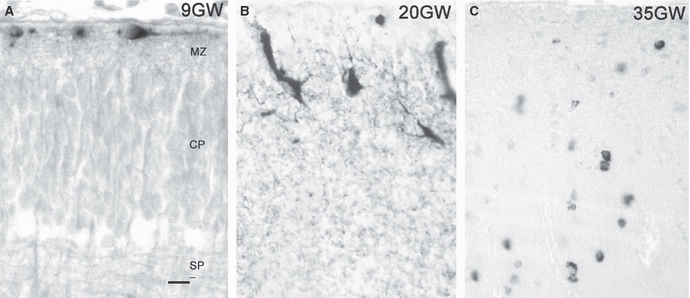
Reelin expression in the developing human cortex. (A) In the early marginal zone (9 GW), reelin-immunoreactive (ir) CR cells are immature and located just below the pial surface. MZ, marginal zone; CP, cortical plate; SP, subplate. (B) High-power view of the marginal zone at 20 GW. CR cells have changed their morphology and orientation, and extend numerous reelin-immunoreactive (ir) processes. (C) At 35 GW, most CR cells have disappeared, and the marginal zone is occupied by many small reelin-ir interneurons. Scale in (A) for A–C: 15 μm.
What makes human Cajal-Retzius cells so special?
The prototypic CR cell is a bipolar neuron with a poorly branched dendritic tree and a horizontal orientation close to the pial surface. This morphology is clearly the prevalent one in nonmammalian vertebrates and rodents, and even in human it correctly describes the CR morphology in the preplate and early cortical plate stages (Fig. 1A). However, with the emergence of the subpial granular layer (SGL) in the marginal zone at around 13 gestational weeks (GW) (Brun, 1965; Gadisseux et al. 1992; Meyer & Gonzalez-Hernandez, 1993), the morphology of human CR cells changes dramatically. At 12–15 GW, they lie within the compact, cell-dense SGL (Fig. 2A), but around 15/16 GW they descend to a deeper position within the MZ (Fig. 2B), perhaps displaced by the stream of tangentially migrating subpial granule cells. Around 18 GW, human CR cells display the bizarre morphologies depicted by Retzius (Fig. 2C), often adopting a vertical orientation (Fig. 1B) and extending long processes, most prominently an axonal plexus at the interface of cortical plate and marginal zone. Figure 2 illustrates the morphology of this plexus at 14 GW (Fig. 2A), 16 GW (Fig. 2B), and 23 GW (Fig. 2C), visualized with DiI-tracing, photoconversion and counterstaining with cresyl-violet (Meyer & Gonzalez-Hernandez, 1993).
Fig. 2.
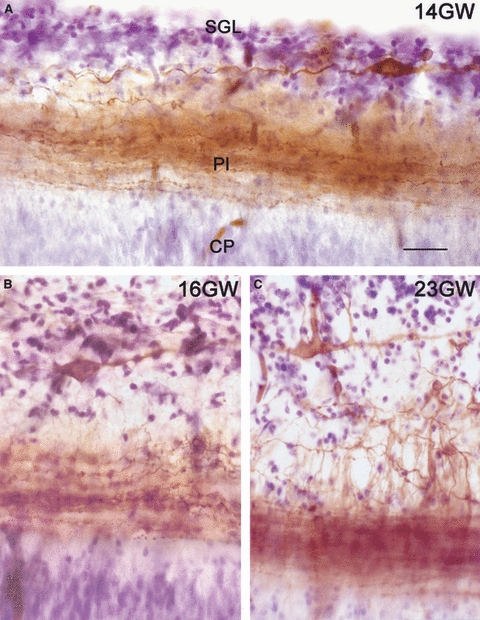
Morphology of human CR cells and their axonal plexus, visualized with DiI tracing followed by photoconversion and Nissl-staining. (A) At 14 GW, CR cells within the subpial granular layer (SGL) extend the dense axonal plexus (Pl) at the interface of marginal zone (MZ) and cortical plate (CP). (B) At 16 GW, CR cells change to a deeper position in the MZ, below the SGL. (C) At 23 GW. CR cells display bizarre morphologies, whereas the SGL cells disperse throughout the MZ. Scale in (A) for A–C: 20 μm.
The plexus may fulfil a double role: first, it establishes a clear-cut anatomical boundary between cortical plate and MZ that may prevent migration from one compartment into the other; second, it increases the surface of reelin-expressing structures, and leads to an exponential increase of reelin levels just above the upper border of the cortical plate. At the end of gestation, most CR cells have disappeared and are replaced by small reelin-expressing interneurons of the molecular layer (Fig. 1C). The fibre plexus disintegrates as well, and subpial granule cells distribute all over the marginal zone and even descend into the superficial cortical plate. The distinct axonal plexus of CR cells has been described so far only in human cortex. Although rodent CR cells extend axons that project into the marginal zone and secrete reelin (Derer et al. 2001), they do not form a compact fibre tract.
Cajal-Retzius cells provide an interesting example for programmed cell death. Although it has sometimes been argued that CR cells do not die but become diluted in an expanding cortex, there is direct evidence for degeneration and death of CR cells in human and rodents (Derer & Derer, 1990; Meyer & Gonzalez-Hernandez, 1993; Meyer et al. 2002; Tissir et al. 2009). In human, they disappear from 23 GW onward, in mice by the end of the first postnatal week. The tumour protein p73, widely used as a marker molecule for CR cells, plays a pivotal role in the life and death of a neuron (Kaghad et al. 1997; Pozniak et al. 2002; Jacobs et al. 2006). In the developing brain, expression of p73 is restricted to CR cells, the cortical hem and the choroid plexuses (Yang et al. 2000; Meyer et al. 2004; Tissir et al. 2009).
Co-expression of p73 and reelin in CR cells may be more pronounced in human than in mouse. In the mouse, the majority (98%) of p73-positive CR cells co-express reelin, whereas only 75% of reelin-positive CR cells co-express p73 (Tissir et al. 2009). By contrast, in human fetuses, virtually all cells in the neocortical MZ with the typical CR cell morphology co-express reelin and p73 (Meyer et al. 2002; Cabrera-Socorro et al. 2007). p73 is a complex protein that appears in two main isoforms with opposite functions. The transactivation-competent TA-isoforms have pro-apoptotic activities and predominate in the cortical hem (Yang et al. 2000; Meyer et al. 2004). In turn, the N-truncated (DeltaNp73) isoforms are able to bind to DNA and to form dimers with TAp73, as well as with p53 and p63, and behave as dominant negative, anti-apoptotic factors (Yang et al. 2000; Grob et al. 2001; Pozniak et al. 2002; Jacobs et al. 2006). Expression of DeltaNp73 is characteristic of the CR cell population derived from the cortical hem (Yang et al. 2000; Meyer et al. 2002, 2004; Tissir et al. 2009).
How important is p73 for the developing cortex? Complete inactivation of the p73 gene in mutant mice leads to the absence of all reelin/p73-expressing CR cells from the earliest stages on (Yang et al. 2000; Meyer et al. 2002). Nevertheless, there are still a few residual reelin-expressing neurons in the marginal zone that are able to compensate the loss of the hem-population and to prevent a reeler phenotype. Selective inactivation of DeltaNp73 leads to premature death of CR cells and to a dramatic decrease of reelin levels in the marginal zone without, however, producing apparent cortical abnormalities (Tissir et al. 2009). These studies show that the production and maintenance of the main CR cell population depend on p73, but also demonstrate that the mouse cortex tolerates the loss of its main source of reelin surprisingly well. The lissencephaly cases with mutations of the human REELIN gene suggest that the human cortex may be more sensitive to a loss or decrease in number of CR cells.
It is thus tempting to attribute the protracted life span of human CR cells (approximately 20 weeks vs. 20 days in the mouse) to the presence of anti-apoptotic DeltaNp73. The expression in CR cells of a protein that may cause survival or death according to the molecular context must be taken into account when we consider the reasons for their disappearance after midgestation. Upregulation of TAp73 expression and/or change of the ratio TAp73/DeltaNp73 may be involved in this process. The co-expression of reelin and anti-apoptotic DeltaNp73 during the main migration period of the human neocortex may provide an evolutionary advantage for a longer lifetime of CR cells and the outgrowth and maintenance of the axonal plexus.
Multiple sources of Cajal-Retzius cells exist at the interface of the forebrain with the telencephalic choroid plexus
Although the origin of CR cells has long been disputed, it is now accepted that they have multiple origins located at the borders of the pallium. The most prominent source is the cortical hem, a putative signalling centre at the medial edge of the cortex characterized by the combined expression of several Wnt and Bmp genes (Grove et al. 1998; Meyer et al. 2002; Abu-Khalil et al. 2004; Yoshida et al. 2006). Other sources of CR cells have been identified on the basis of reelin- and/or p73 expression: the septum, the retrobulbar area, the ventral hem (at the site of the future ventral hippocampus), the caudomedial telencephalon and, more recently, the thalamic eminence (Meyer & Wahle, 1999; Meyer et al. 2002; Abraham et al. 2004; Takiguchi-Hayashi et al. 2004; Cabrera-Socorro et al. 2007; Tissir et al. 2009). In addition, the mouse subpallial/pallial boundary and the septum give rise to CR cells that are reelin- and Dbx1-positive but p73-negative (Bielle et al. 2005). We would like to point out that with the exception of the Dbx1-positive pallial-subpallial boundary, all the other sources of CR cells are connected with the choroid plexus, at least at an early stage of development.
We systematically examined the possible relationship between the origin of CR cells and the choroid plexus in human embryos, where both structures are highly developed and their anatomical interfaces easier to detect (Cabrera-Socorro et al. 2007). We observed that the calcium-binding protein calbindin is a reliable marker for both the human choroid plexus anlage and the cortical hem (Figs 3 and 6). The section in Fig. 3A shows a horizontal view of telencephalon and diencephalon of an embryo at Carnegie stage (CS) 18 (6.5 GW). It illustrates the ring-like arrangement of the cortical hem from rostral to caudal, and its direct continuity with the future choroid plexus. In more ventral sections, the choroid plexus is continuous rostrally with the septum through the taenia tecta or rostral hippocampal rudiment, also positive for calbindin (represented in the diagram in Fig. 6A). In addition, the telencephalic choroid plexus connects with the thalamic eminence (TE), another calbindin-positive structure. Calbindin-expression is still prominent throughout the cortical hem and choroid plexus at 9 GW (Fig. 3B), shortly before the emergence of the dorsal hippocampus (Abraham et al. 2004), and concurrent with the highest production of CR cells in the cortical hem, as visualized with p73-immunoreacivity (compare Figs 3B and 5A). However, after the overt appearance of the hippocampus at 13 GW (Fig. 3C), positivity for calbindin disappears from the hem and remains only in the stalk of the choroid plexus. Calbindin is thus a marker for the transient human hem and choroid plexus anlage and indicates that both structures share common features. We found that all the areas at the interface of the telencephalon and the choroid plexus (plus the thalamic eminence) give rise to streams of p73-positive cells that migrate into neighbouring pallial and subpallial regions (summarized in Fig. 6). Most of these cells are also reelin-positive, which, as mentioned above, is the distinctive molecule of a CR cell (Meyer et al. 2002).
Fig. 3.
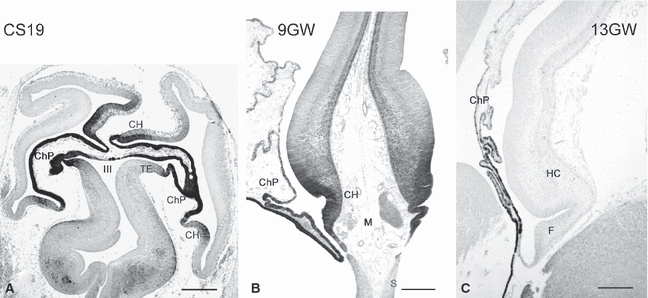
Calbindin marks the human cortical hem and choroid plexus anlage. (A) Human embryo at Carnegie stage 19 (6.5 GW), horizontal section. CH, cortical hem; ChP, choroid plexus; TE, Thalamic eminence; III, third ventricle. (B) The cortical hem at 9 GW, coronal section. At the moment of highest production of CR cells, the hem and choroid plexus are strongly ir for calbindin (compare with Fig. 5A). M, head mesenchyme; S, septum. (C) At 13 GW the cortical hem has disappeared, and its site is now occupied by the fimbria (F). Only the stalk of the choroid plexus is calbindin-ir. HC, hippocampus. Scales: (A) 500 μm, (B) 250 μm, (C) 300 μm.
Fig. 6.
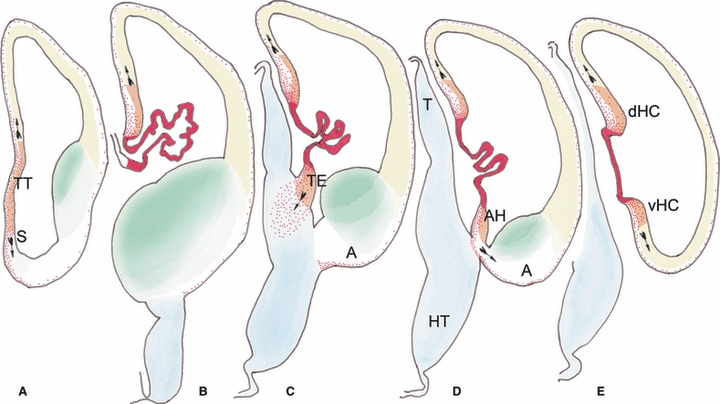
Diagram summarizing the origins of p73-expressing Cajal-Retzius cells in a human embryo at Carnegie stage 20 (7 GW). For this figure, representative sections were immunostained for calbindin and p73, drawn with a camera lucida, scanned and coloured using Adobe photoshop software. A is the most rostral section at the level of the taenia tecta (TT) or rostral hippocampus, and E shows the most caudal section, near the junction of the hems of the future dorsal hippocampus (dHC) and ventral hippocampus (vHC) (the hems precede the ouvert appearance of the hippocampus which develops later). From B to E, the choroid plexus forms the centre of the hem system. The taenia tecta (in A) lies rostral to the choroid plexus and can thus be included in the peri-choroidal hem system. Colour codes: The choroid plexus/hem core areas are in red, representing the highest intensity of calbindin staining. The neuroepithelium of the hem areas, displaying moderate calbindin positivity, is in orange. Cajal-Retzius cells, positive for p73, are represented as red dots. The cortical neuroepithelium is in yellow, the diencephalon in blue, and the ganglionic eminences are in green. The arrows, taken from a drawing by Cajal, indicate the proposed migratory direction of Cajal-Retzius cells. Notice that at this stage the production of Cajal-Retzius is lower than at 9 GW, shown in Fig. 5, whereas the thalamic eminence (TE in C) has already attained its maximum size. A, amygdale; AH, amygdalar hem; HT, hypothalamus; S, septum; T, thalamus.
Fig. 5.
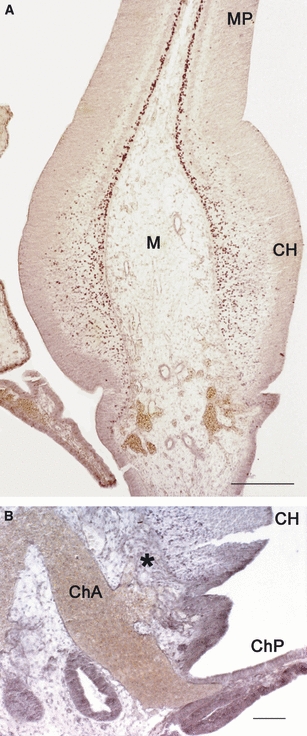
p73 in the human cortical hem and choroid plexus at 9 GW. (A) Section parallel to that shown in Fig. 3B, showing the intense production of CR cells at this stage. In (B) a large blood vessel, presumably the future anterior choroid artery (ChA), enters the choroid plexus (ChP). It also provides numerous small branches (marked with an asterisk) for the cortical hem (CH), suggesting a concerted development of both structures. M, Midline mesenchyme; MP, medial pallium. Scales: (A) 250 μm, (B) 30 μm.
The relative prominence of each CR cell source is species-dependent. In human and mouse, the septum gives rise to both p73/reelin-positive and p73-negative/reelin-positive CR cells which seem to migrate into the piriform cortex and adjacent medial and dorsal pallium (Meyer et al. 2002; Bielle et al. 2005). Also in lizard embryos (lacerta Gallotti), the septum is the origin of numerous reelin/p73-expressing CR cells that spread over the olfactory telencephalon (Cabrera-Socorro et al. 2007). Another prominent source of p73-positive cells in lizard is more caudal, in the TE at the junction of telencephalic choroid plexus and diencephalon from where numerous cells migrate ventrally toward the surface of the cortical amygdala and into the ventral thalamus. By contrast, the cortical hem adjacent to the dorsal pallium is poorly developed, and there is no migration of p73-expressing CR cells into the cortex. CR cells in the lizard cortex are thus reelin-positive but p73-negative and apparently do not derive from the cortical hem.
Our data in lizard, mouse and human suggest that of the several sources of CR cells, the cortical hem is the one that undergoes the most dramatic evolutionary changes, probably related to the expansion of the neocortex in mammals, and particularly, in human.
So far, we have related the cortical hem with the production of CR cells destined for the neocortex. The situation in the human brain is more complex because here the cortical hem can be subdivided into a dorsal hem, adjacent to the dorsal hippocampus, and a ventral hem, located in the temporal horn of the lateral ventricle and connected to the ventral hippocampus. The dorsal hippocampus disappears while the corpus callosum develops, and only the ventral hippocampus gives rise to the definitive hippocampal formation. In previous work (Abraham et al. 2004), we described the ventral hem as the origin of the reelin/p73-expressing CR cells of the human hippocampus and suggested the deep hippocampal fissure as their main destination. It remains an open question to what extent the ventral hem may give rise also to CR cells of the temporal and occipital neocortex. As the dorsal hippocampus persists in the rodent brain, the cortical hem may be differently patterned in human and mouse. Certainly, these important species differences have to be taken into account when the regional specification of CR is studied with genetic markers.
The thalamic eminence and the ‘amygdalar hem’ are putative sources of Cajal-Retzius cells
The TE is the most recent addition to the list of putative CR cell origins (Cabrera-Socorro et al. 2007; Tissir et al. 2009). The rodent TE is characterized by the expression of calretinin (Abbott & Jacobowitz, 1999) and Tbr1, a pallial marker, despite its location in the diencephalons (in human, the TE is positive for calbindin but not for calretinin). The expression of DeltaNp73 in the TE has been reported recently (Tissir et al. 2009). The sequence of mouse brain sections in Fig. 4A–D illustrates the development of the p73-immunoreactive cells at the TE/choroid interface, using a polyclonal antibody against p73 (Yang et al. 2000). The TE is well developed at embryonic day (E) 12, when numerous p73-positive cells originate from the TE/choroid boundary and migrate into the ventral thalamus (Fig. 4A). At E14.5 (Fig. 4B), after the entrance of thalamocortical fibers in the internal capsule, the TE is reduced in size and p73-positive cells are now distributed in a ring-like fashion around the internal capsule. They express only low levels of reelin (data not shown), and their final destination is unknown, although they may reach the di-telencephalic sulcus and amygdala. Taking into account the quite specific expression pattern of DeltaNp73 in the embryonic brain (CR cells and choroid plexuses), we suggest that the TE-derived neurons represent a subtype of CR cells, perhaps functioning as guidepost neurons for the internal capsule.
Fig. 4.
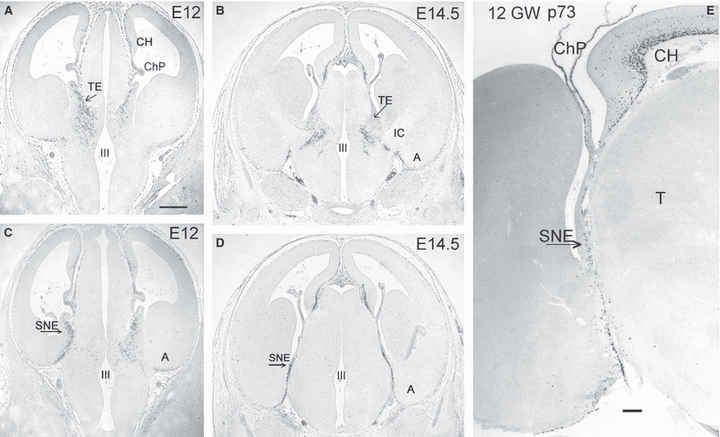
(A–D) Cortical hem (CH), thalamic eminence (TE) and strionuclear neuroepithelium (SNE), or ‘amygdalar hem’, are origins of p73-immunoreactive (ir) neurons in mouse embryos. (A) A stream of p73-ir cells arises in the TE at E12 and distributes throughout the ventral thalamus. (B) At E14.5, the TE is reduced in size, and the p73-ir cells are curved around the internal capsule (IC). (C) At E12 the SNE/choroid plexus interface (arrow) is the origin of p73-ir cells that migrate ventrally into the amygdala (A). The left side is more caudal than the right, showing the SNE. (D) At E14.5, the SNE is reduced in size. (E) Human cortical hem and SNE at 12 GW. p73-ir cells arise from the cortical hem (CH) dorsally, and the SNE ventrally, with the choroid plexus (ChP) in the centre. T, Thalamus. Scales in (A) for A–D: 200 μm, in (E) 50 μm.
At a level immediately caudal to the TE, we define another putative source of CR cells that has so far gone unnoticed. The mouse brain section in Fig. 4C corresponds to the level of the corticomedial amygdala at E12, which is connected to the choroid plexus by means of a small triangular area of neuroepithelium, or ‘amygdalar hem’. It is also known as the ‘strionuclear neuroepithelium’ (Altman & Bayer, 1995), a term that indicates its closeness to and possible relationship with the bed nucleus of the stria terminalis. At E12, a narrow stream of p73-positive cells migrates from the ‘amygdalar hem’ to the basal surface of the telencephalon. These cells begin to express high levels of reelin once they are close to the pial surface, and might represent the CR cells destined for the amygdala and entorhinal cortex. At E14.5 (Fig. 4D), the ‘amygdalar hem’ is reduced in size, and p73-positive cells along the diencephalic/telencephalic boundary are less numerous.
We would like to point out that the TE is small and very short-lived in the human brain, which is reflected in a less intense production of p73-expressing cells. We identified the TE in embryos from CS18 to CS20 (6.5–7 GW) on the basis of calbindin- and p73-expression (Fig. 6C), but already at CS23 (8 GW) it was reduced in size and displaced dorsally by the growing internal capsule (data not shown). The ‘amygdalar hem’, visible at CS20 (Fig. 6D), persists for some more time, as we identified it in fetuses of 12 GW (Fig. 4E) and even in older fetal stages.
We propose that there is a clear evolutionary trend towards an increased prominence of the cortical hem and, in parallel, towards an increased proportion of reelin/p73-expressing CR cells destined for the neocortex and, in human, also for the hippocampal formation in the temporal lobe. The other sources (septum, TE, ‘amygdalar hem’) may be less important for human neocortical development, and destined rather for paleocortical and corticomedial amygdalar areas, which in human – a microsmatic species – are less highly differentiated.
The various choroid plexus-related sources of p73-positive CR cells share a common fate: they undergo regression and form part of the taenia system, i.e. the fine membranes that attach the telencephalic choroid plexus to the main fibre tracts near the midline. The cortical hem adjacent to the hippocampus gives rise to the taenia of the fimbria/fornix. The interface of the amygdalar hem with the choroid plexus will be occupied by the stria terminalis, and the TE will transform into the lamina affixa at the dorsolateral surface of the thalamus (Letinic & Kostovic, 1997).
Cajal-Retzius cells, the cortical hem and the telencephalic choroid plexus evolve in concert
The dorsal midline of the developing telencephalon is divided into a most medial part (the choroid plexus), an intermediate part (the cortical hem), and a lateral part (formed by the hippocampal primordium and medial cortex) (Furuta et al. 1997; Currle et al. 2005; Cheng et al. 2006). The molecular interdependency between the cortical hem and the developing choroid plexus has been analyzed intensively. The cortical hem as a putative signalling centre was initially defined as a tissue rich in Wnt genes (Wnt2b, Wnt3a and Wnt5a), adjacent to the choroid plexus. Several genes (Msx2, Wnt5a and Bmp7) are expressed in both cortical hem and choroid plexus epithelium. Wnt genes in the cortical hem are upregulated as the choroid plexus begins to form, suggesting inductive interactions between the two structures (Grove et al. 1998). Bmp signalling is essential for the development of the choroid plexus and patterning of the dorsal midline (Furuta et al. 1997; Hebert et al. 2002; Cheng et al. 2006). Furthermore, the close relationship between CR cells and the choroid plexus is reflected in the expression by the proneural bHLH gene neurogenin 2 (Ngn2) and the repressor genes Hes1 and Hes5 in the prospective choroid plexus anlage. Hes and Ngn2 would antagonistically regulate the specification of choroid plexus epithelium vs. CR cell fates (Imayoshi et al. 2008), to the point that inactivation of Hes1, Hes3 and Hes5 leads to the enhanced formation of CR cells and to complete loss of choroid plexus epithelium cells. The expression of the hem-characteristic WNT2b gene has also been documented in the human brain (Abu-Khalil et al. 2004), suggesting that the basic molecular regulators of the dorsal midline are evolutionary conserved.
In addition to the well known function of the choroid plexuses in CSF secretion, the telencephalic plexus may provide an expanding force for shaping the growing cortex from within (Desmond & Jacobsen, 1977; Dziegielewska et al. 2001). During early fetal life, the choroid plexuses of the human lateral ventricles display a unique phenomenon that has been described as ‘colloidal distension’ (Dziegielewska et al. 2001) or ‘blooming stage’ (Bayer & Altman, 2006) and which can also be observed in Fig. 3B. As discussed by Dziegielewska et al. (2001), the transient distension of the choroid plexus, sometimes considered pathological, may represent a driving force for expanding the large ventricular system. Human choroid plexus development may thus have features that are not readily apparent in species with a smaller ventricular system.
The cerebral vasculature and its capacity to shape the size and differentiation of the brain is usually not addressed in the current literature on cortex development. However, as pointed out by Striedter (2005), the modification of one component of a complex system leads to concurrent modifications of related components, and the increase in size of the head is one of the determining factors in the evolution of the brain. In the course of our studies of brain material of a variety of species, it caught our attention that the blood vessels perfusing the human choroid plexus increased in size just before 9 GW, the moment of maximum production of CR cells in the cortical hem (Fig. 5A). We also noticed that branches of the main vessel (possibly the anterior choroid artery) ramified along the surface of the cortical hem (Fig. 5B). The age of 8/9 GW corresponds approximately to CS 23 and represents a crucial step in cortical development, because the cortical plate has just formed and the massive migration into the cortex has started (O’Rahilly & Müller, 1994; Meyer et al. 2000).
The concerted development of the telencephalic choroid plexuses, cortical hem and CR cells, summarized in Fig. 6, may represent a mechanism by which the ventricular surface of the telencephalic vesicles expands at the same pace as the MZ. The number of available reelin/p73-producing CR cells would thus be coupled to the size of the proliferative ventricular zone, which in turn would be regulated by the expanding force of the choroid plexus and its vascular support. From this point of view, the multiple origins of CR cells at the interface of the telencephalon and ventral thalamus with the telencephalic choroid plexuses might reflect the evolutionary history of the brain and the patterning role of the dorsal midline in shaping the growing cortex.
References
- Abbott LC, Jacobowitz DM. Developmental expression of calretinin-immunoreactivity in the thalamic eminence of the fetal mouse. Int J Dev Neurosci. 1999;17:331–345. doi: 10.1016/s0736-5748(99)00037-4. [DOI] [PubMed] [Google Scholar]
- Abraham H, Perez-Garcia CG, Meyer G. p73 and reelin in Cajal-Retzius cells of the developing human hippocampal formation. Cereb Cortex. 2004;14:484–495. doi: 10.1093/cercor/bhh010. [DOI] [PubMed] [Google Scholar]
- Abu-Khalil A, Fu L, Grove EA, et al. Wnt genes define distinct boundaries in the developing human brain: implications for human forebrain patterning. J Comp Neurol. 2004;474:276–288. doi: 10.1002/cne.20112. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Ruiz M, D’Arcangelo G, et al. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Atlas of Prenatal Rat Brain Development. Boca Raton, FL: CRC Press; 1995. [Google Scholar]
- Bayer SA, Altman J. The Human Brain during the Late First Trimester. Boca Raton: CRC Press, Taylor & Francis group; 2006. [Google Scholar]
- Benhayon D, Magdaleno S, Curran T. Binding of purified reelin to ApoER2 and VLDLR mediates tyrosine phosphorylation of Disabled-1. Brain Res Mol Brain Res. 2003;112:33–45. doi: 10.1016/s0169-328x(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, et al. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Bock HH, Jossin Y, Liu P, et al. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to reelin signaling and is required for normal cortical lamination. J Biol Chem. 2003;278:38772–38779. doi: 10.1074/jbc.M306416200. [DOI] [PubMed] [Google Scholar]
- Brun A. The subpial granular layer of the foetal cerebral cortex in man. Its ontogeny and significance in congenital cortical malformations. Acta Pathol Microbiol Scand Suppl. 1965;179:3–98. [PubMed] [Google Scholar]
- Cabrera-Socorro A, Hernandez-Acosta NC, Gonzalez-Gomez M, et al. Comparative aspects of p73 and reelin expression in Cajal-Retzius cells and the cortical hem in lizard, mouse and human. Brain Res. 2007;1132:59–70. doi: 10.1016/j.brainres.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Cheng X, Hsu CM, Currle DS, et al. Central roles of the roof plate in telencephalic development and holoprosencephaly. J Neurosci. 2006;26:7640–7649. doi: 10.1523/JNEUROSCI.0714-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currle DS, Cheng X, Hsu CM, et al. Direct and indirect roles of CNS dorsal midline cells in choroid plexus epithelia formation. Development. 2005;132:3549–3559. doi: 10.1242/dev.01915. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Nakajima K, Miyata T, et al. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo G, Homayouni R, Keshvara L, et al. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Derer P, Derer M. Cajal-Retzius cell ontogenesis and death in mouse brain visualized with horseradish peroxidase and electron microscopy. Neuroscience. 1990;36:839–856. doi: 10.1016/0306-4522(90)90027-2. [DOI] [PubMed] [Google Scholar]
- Derer P, Derer M, Goffinet A. Axonal secretion of reelin by Cajal-Retzius cells: evidence from comparison of normal and Reln(Orl) mutant mice. J Comp Neurol. 2001;440:136–143. doi: 10.1002/cne.1375. [DOI] [PubMed] [Google Scholar]
- Desmond ME, Jacobsen AG. Embryonic brain enlargement requires cerebrospinal fluid pressure. Dev Biol. 1977;55:188–198. doi: 10.1016/0012-1606(77)90364-5. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Ek J, Habgood MD, et al. Development of the choroid plexus. Microsc Res Tech. 2001;52:5–20. doi: 10.1002/1097-0029(20010101)52:1<5::AID-JEMT3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Gadisseux JF, Goffinet AM, Lyon G, et al. The human transient subpial granular layer: an optical, immunohistochemical, and ultrastructural analysis. J Comp Neurol. 1992;324:94–114. doi: 10.1002/cne.903240108. [DOI] [PubMed] [Google Scholar]
- Goffinet AM, Bar I, Bernier B, et al. Reelin expression during embryonic brain development in lacertilian lizards. J Comp Neurol. 1999;414:533–550. doi: 10.1002/(sici)1096-9861(19991129)414:4<533::aid-cne8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Grob TJ, Novak U, Maisse C, et al. Human delta Np73 regulates a dominant negative feedback loop for TAp73 and p53. Cell Death Differ. 2001;8:1213–1223. doi: 10.1038/sj.cdd.4400962. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, et al. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Hebert JM, Mishina Y, McConnell SK. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron. 2002;35:1029–1041. doi: 10.1016/s0896-6273(02)00900-5. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Neogi T, Englund C, et al. Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin. Brain Res Dev Brain Res. 2003;141:39–53. doi: 10.1016/s0165-3806(02)00641-7. [DOI] [PubMed] [Google Scholar]
- Hiesberger T, Trommsdorff M, Howell BW, et al. Direct binding of reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet. 2000;26:93–96. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Hildebrand JD, et al. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol. 2000;10:877–885. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Shimogori T, Ohtsuka T, et al. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development. 2008;135:2531–2541. doi: 10.1242/dev.021535. [DOI] [PubMed] [Google Scholar]
- Jacobs WB, Kaplan DR, Miller FD. The p53 family in nervous system development and disease. J Neurochem. 2006;97:1571–1584. doi: 10.1111/j.1471-4159.2006.03980.x. [DOI] [PubMed] [Google Scholar]
- Kaghad M, Bonnet H, Yang A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- Kuo G, Arnaud L, Kronstad-O’Brien P, et al. Absence of Fyn and Src causes a reeler-like phenotype. J Neurosci. 2005;25:8578–8586. doi: 10.1523/JNEUROSCI.1656-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert de Rouvroit C, Goffinet AM. The reeler mouse as a model of brain development. Adv Anat Embryol Cell Biol. 1998;150:1–106. [PubMed] [Google Scholar]
- Letinic K, Kostovic I. Transient fetal structure, the gangliothalamic body, connects telencephalic germinal zone with all thalamic regions in the developing human brain. J Comp Neurol. 1997;384:373–395. doi: 10.1002/(sici)1096-9861(19970804)384:3<373::aid-cne5>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- Meyer G. Genetic Control of Neuronal Migrations in Human Cortical Development. Berlin: Springer-Verlag; 2007. [PubMed] [Google Scholar]
- Meyer G, Goffinet AM. Prenatal development of reelin-immunoreactive neurons in the human neocortex. J Comp Neurol. 1998;397:29–40. [PubMed] [Google Scholar]
- Meyer G, Gonzalez-Hernandez T. Developmental changes in layer I of the human neocortex during prenatal life: a DiI-tracing and AChE and NADPH-d histochemistry study. J Comp Neurol. 1993;338:317–336. doi: 10.1002/cne.903380302. [DOI] [PubMed] [Google Scholar]
- Meyer G, Wahle P. The paleocortical ventricle is the origin of reelin-expressing neurons in the marginal zone of the foetal human neocortex. Eur J Neurosci. 1999;11:3937–3944. doi: 10.1046/j.1460-9568.1999.00818.x. [DOI] [PubMed] [Google Scholar]
- Meyer G, Goffinet AM, Fairen A. What is a Cajal-Retzius cell? A reassessment of a classical cell type based on recent observations in the developing neocortex. Cereb Cortex. 1999;9:765–775. doi: 10.1093/cercor/9.8.765. [DOI] [PubMed] [Google Scholar]
- Meyer G, Schaaps JP, Moreau L, et al. Embryonic and early fetal development of the human neocortex. J Neurosci. 2000;20:1858–1868. doi: 10.1523/JNEUROSCI.20-05-01858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Perez-Garcia CG, Abraham H, et al. Expression of p73 and Reelin in the developing human cortex. J Neurosci. 2002;22:4973–4986. doi: 10.1523/JNEUROSCI.22-12-04973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Cabrera Socorro A, Perez Garcia CG, et al. Developmental roles of p73 in Cajal-Retzius cells and cortical patterning. J Neurosci. 2004;24:9878–9887. doi: 10.1523/JNEUROSCI.3060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Adams R, Goffinet AM, et al. The role of the first postmitotic cortical cells in the development of thalamocortical innervation in the reeler mouse. J Neurosci. 1998;18:5746–5765. doi: 10.1523/JNEUROSCI.18-15-05746.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Metin C, Stoykova A, et al. Comparative aspects of cerebral cortical development. Eur J Neurosci. 2006;23:921–934. doi: 10.1111/j.1460-9568.2006.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, et al. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. The Embryonic Human Brain. An Atlas of Developmental Stages. New York: Wiley-Liss; 1994. [Google Scholar]
- Perez-Garcia CG, Gonzalez-Delgado FJ, Suarez-Sola ML, et al. Reelin-immunoreactive neurons in the adult vertebrate pallium. J Chem Neuroanat. 2001;21:41–51. doi: 10.1016/s0891-0618(00)00104-6. [DOI] [PubMed] [Google Scholar]
- Pollard KS, Salama SR, Lambert N, et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443:167–172. doi: 10.1038/nature05113. [DOI] [PubMed] [Google Scholar]
- Pozniak CD, Barnabe-Heider F, Rymar VV, et al. p73 is required for survival and maintenance of CNS neurons. J Neurosci. 2002;22:9800–9809. doi: 10.1523/JNEUROSCI.22-22-09800.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramón y Cajal S. Estudios sobre la corteza cerebral humana. I Corteza visual. Rev Trimestral Micrográfica. 1899;4:1–63. [Google Scholar]
- Retzius G. Die Cajal′schen Zellen der Grosshirnrinde beim Menschen und bei Säugetieren. Biol Untersuch Neue Folge. 1883;5:1–8. [Google Scholar]
- Rice DS, Sheldon M, D’Arcangelo G, et al. Disabled-1 acts downstream of reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development. 1998;125:3719–3729. doi: 10.1242/dev.125.18.3719. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Bernier B, Goffinet AM. Reelin mRNA expression during mouse brain development. Eur J Neurosci. 1997;9:1055–1071. doi: 10.1111/j.1460-9568.1997.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Sheldon M, Rice DS, D’Arcangelo G, et al. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature. 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- Striedter GF. Principles of Brain Evolution. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Takiguchi-Hayashi K, Sekiguchi M, Ashigaki S, et al. Generation of reelin-positive marginal zone cells from the caudomedial wall of telencephalic vesicles. J Neurosci. 2004;24:2286–2295. doi: 10.1523/JNEUROSCI.4671-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Tissir F, Lambert DeRouvroitC, Sire JY, et al. Reelin expression during embryonic brain development in Crocodylus niloticus. J Comp Neurol. 2003;457:250–262. doi: 10.1002/cne.10573. [DOI] [PubMed] [Google Scholar]
- Tissir F, Ravni A, Achouri Y, et al. DeltaNp73 regulates neuronal survival in vivo. Proc Natl Acad Sci U S A. 2009;106:16871–16876. doi: 10.1073/pnas.0903191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Yang A, Walker N, Bronson R, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, et al. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]


