Abstract
In this historical review, we trace the early history of research on the fetal subplate zone, subplate neurons and interstitial neurons in the white matter of the adult nervous system. We arrive at several general conclusions. First, a century of research clearly testifies that interstitial neurons, subplate neurons and the subplate zone were first observed and variously described in the human brain – or, in more general terms, in large brains of gyrencephalic mammals, characterized by an abundant white matter and slow and protracted prenatal and postnatal development. Secondly, the subplate zone cannot be meaningfully defined using a single criterion – be it a specific population of cells, fibres or a specific molecular or genetic marker. The subplate zone is a highly dynamic architectonic compartment and its size and cellular composition do not remain constant during development. Thirdly, it is important to make a clear distinction between the subplate zone and the subplate (and interstitial) neurons. The transient existence of the subplate zone (as a specific architectonic compartment of the fetal telencephalic wall) should not be equated with the putative transient existence of subplate neurons. It is clear that in rodents, and to an even greater extent in humans and monkeys, a significant number of subplate cells survive and remain functional throughout life.
Keywords: epilepsy, human cortical development, interstitial neurons, schizophrenia, subplate neurons, subplate zone
Introduction
Neurons present in various white matter compartments of the adult nervous system have historically been designated by various names, such as out-lying, displaced, aberrant, heterotopic, paragriseal, intramedullary, intercalated and interstitial (Das & Kreutzberg, 1968). It is widely accepted that they were first described in the spinal cord and the spinal and cranial nerves (Sosa & Andrew, 1947; Das & Kreutzberg, 1968), and that they were regarded as part of the adjacent neural structures displaced into the white matter by mechanical forces operating during the growth and development of the nervous system (Das & Kreutzberg, 1968, p. 9). It is also usually stated that the term ‘interstitial neurons’ was coined by Ramón y Cajal in 1911, either because in his monograph he described ‘interstitial nerve cells’ in various fibre bundles (Das & Kreutzberg, 1968) or because the name interstitial neurons ‘used originally by Ramón y Cajal (1911) for the cells situated within cerebellar white matter, is appropriate for the cells in the other regions as well’ (Kostović & Rakic, 1980, p. 220). Finally, it is generally accepted that interstitial neurons of the cerebral white matter represent a postnatally surviving subset (or ‘remnant’ or even ‘vestige’) of transient fetal subplate neurons (Kostović & Rakic, 1980, 1990; Luskin & Shatz, 1985; Valverde & Facal-Valverde, 1988; Allendoerfer & Shatz, 1994; Kostović & Judaš, 2002, 2006, 2007, 2010; Molnár et al. 2006; Clancy et al. 2009; Friedlander & Torres-Reveron, 2009; Luhmann et al. 2009; Suárez-Solá et al. 2009; Kanold & Luhmann, 2010; Kostović et al. 2010).
However, it has not escaped our attention that the real history of discovery of interstitial neurons, subplate neurons and the subplate zone has been much more complex than implied in above-mentioned reviews and slowly enfolded along a century-old and often tortuous path. Thus, an in-depth historical review would represent a good introduction to our accompanying study (Judaš et al. 2010) and several other papers in this special issue. It may also shed new light on several current ‘bones of contention’ in analyzing the developmental origin and species-specific fates of the transient fetal subplate zone, fetal subplate neurons, and postnatal interstitial neurons in the mammalian cerebral white matter. We find it useful to organize this review by first providing answers to the following questions:
Did Ramón y Cajal really introduce the term interstitial neurons and, if so, when and for which cells?
Were neurons of the white matter really first observed in the adult spinal cord?
Who was the first to observe fetal cells which we at present describe as subplate neurons?
Who was the first to describe and/or illustrate a transient cytoarchitectonic compartment of the fetal telencephalic wall which we at present designate as the subplate zone?
If the subplate zone is a transient fetal structure, does this really mean that subplate neurons are a transient fetal type of cell, too?
The first use of the term ‘interstitial neurons’
So, was it Ramón y Cajal? Yes – however, not in 1911, but much earlier; and not for interstitial neurons of the cerebral (or even cerebellar) white matter, but for all kinds of other cells – starting with inconspicious sympathetic neurons of guts and pancreas. If one seeks to establish priority, one should quote original publications, which in this case means hardly available and sometimes almost forgoten studies published in Spanish, German or French. However, most of these data can be also retrieved and traced to the original by careful browsing of two recent collections translated into English (DeFelipe & Jones, 1988; Ramón y Cajal, 1995a,b;).
First, it should be noted that the technical terms ‘interstitium’ (literally: ‘between-space’, or Zwischenraum) and the derived adjective ‘interstitial’ were commonplace in histology textbooks and monographs during the second half of the 19th century (e.g. Boll, 1874, p. 21; Stricker, 1871, p. 57; Henle, 1879, pp. 242, 246). Secondly, it is important to realize that although Santiago Ramón y Cajal (who was an avid reader of contemporary histology textbooks and monographs) was probably the first to apply this term systematically in the field of neurohistology, in the long series of his publications he used the adjective ‘interstitial’ in three different ways: (i) to describe a special population of cells (sympathetic neurons in gut and pancreas) as interstitial cells; (ii) to describe a number of specific groups of neurons located within axonal fibre tracts as interstitial nuclei; and (iii) to describe isolated cells displaced from the grey matter to the adjacent white matter as interstital neurons. Curiously, he never applied the term ‘interstitial neurons’ to neurons situated in the subcortical white matter of the telencephalon (see below).
Cajal's interstitial cells
In his initial publications on the peripheral nervous system, published alone (Ramón y Cajal, 1891a, 1892, 1893) or in collaboration with Claudio Sala y Pons (Ramón y Cajal & Sala y Pons, 1891), Cajal described neurons and axon terminations in the pancreas and various intestinal organs and pointed out the existence of special sympathetic interstitial cells (Fig. 1C–E), which were also described in detail in the second volume of his great monograph (Ramón y Cajal, 1911, pp. 923–938) as well as in his posthumously published contribution to the neuron theory (Ramón y Cajal, 1935, p. 973). The clear proof that he regarded these cells as a special type of neuron is given in the first volume of his monograph (Ramón y Cajal, 1909, p. 55) where he offers a general classification of neuronal types and describes these interstitial cells in glands and the enteric system of the gut (corpuscules interstitiels des glandes et du grand sympathique intestinal) as a special type of the first major class of neurons comparable to amacrine cells of the retina. These cells were described as ‘Zellen Ramón y Cajal's’ by Alexander S. Dogiel (Dogiel, 1895, 1896) and the nature and significance of these ‘Cajalschen interstitielle Zellen’ was hotly debated in leading histological handbooks of the early 20th century (Stöhr, 1928, pp. 246, 362, 369).
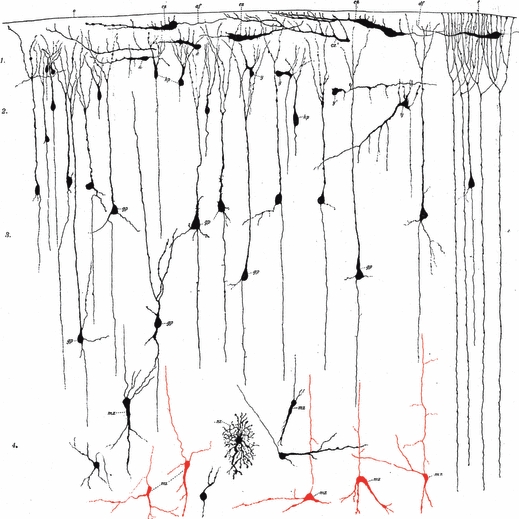
Fig. 1.
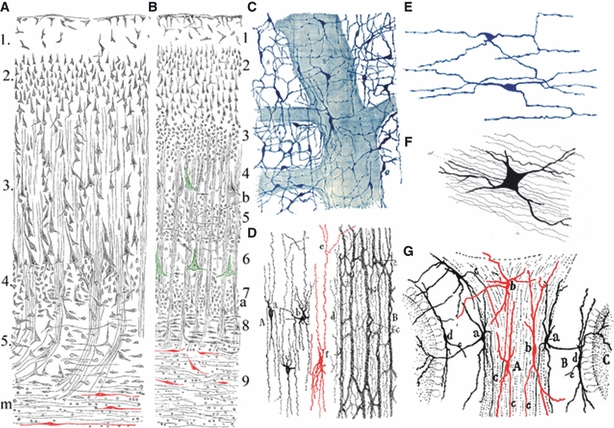
Meynert was first to describe neurons in the adult subcortical white matter, and Ramón y Cajal introduced the term interstitial cells in neurohistology. Neurons (red) in the subcortical white matter of the human brain (A – frontal, B – primary visual cortex) were first described by Theodor Meynert in 1867 (here reproduced from Meynert, 1884, pp. 53 and 63). However, the term interstitial cells was initially (1891–93) applied by Ramón y Cajal to describe a special type of visceral sympathetic neurons in glands and the enteric system of the gut (C–,E – here reproduced from Ramón y Cajal, 1911, pp. 924–927; (C,D) interstitial cells in Auerbach's plexus and muscular layer of the rabbit intestine, stained by methylene blue; (E) interstitial cells among muscle fibres of the guinea-pig intestine, stained by rapid Golgi method). Cajal in 1896 also described neurons in the cerebellar white matter as interstitial cells (G –Ramón y Cajal, 1896, p. 24, fig. 5b), but it should be noted that these cells were first described by Gustav Retzius (F –Retzius, 1892, fig. 4 of Plate XIX). For details, see text.
Cajal's interstitial nuclei
Ramón y Cajal described as interstitial nuclei a number of specific groups of neurons embedded within bundles of axons. For example, in the first volume of his Histology (Ramón y Cajal, 1909) he described the processus reticularis of the spinal cord as interstitial nucleus (noyau gris interstitiel– p. 291) and upon commenting on Sherrington's out-lying cells he calls them interstitial or displaced cells (les cellules interstitielles ou déplacées– p. 404) and collectively describes them as intersitial nucleus (noyau interstitiel– p. 404). He also variably described one of three nuclei related to the solitary tract as interstial nucleus (noyau interstitiel– p. 685), interstitial focus (foyer interstitiel– p. 733), interstitial ganglion or external grey column (le ganglion interstitiel ou colonne grise externe– p. 737). In the second volume of his Histology (Ramón y Cajal, 1911) he described interstitial nucleus situated at the superior end of the posterior longitudinal fascicle (noyau interstitiel placé a l'extrémité supérieure du faisceau longitudinal postérieur– pp. 261–264), an interstitial nucleus of the lateral lemniscus (noyau interstitiel du ruban de Reil latéral– p. 289), interstitial nucleus of the Forel's field (noyau interstitiel du champ de Forel– p. 453), and interstitial nucleus of the stria terminalis (noyau interstitiel de la voie de projection de l’écorce temporale– p. 723; namely, Cajal regarded stria terminalis –strie cornée, bandelette semi-circulaire ou strie terminale– as a projection pathway of the temporal cortex).
Cajal's interstital cells in cerebellar white matter
In his initial descriptions of the cerebellum, first published in Spanish (Ramón y Cajal, 1888, 1889a) and subsequently translated into French (Ramón y Cajal, 1889b, 1890a,b) Cajal did not mention or illustrate interstitial cells in the cerebellar white matter. In the subsequent extensive study of medulla oblongata, cerebellum and cranial nerves (Ramón y Cajal, 1895), Cajal for the first time added a short note concerning the presence of special large stellate cells (células estrelladas) in the granular layer (in addition to already-described Golgi cells). Here he just mentioned in passing that they are very rare and that some are located within the granular layer, whereas others can reside ‘within the axis of cerebellar lamellae, in the midst of the white matter’ (Alguna de ellas residía en el eje de una laminilla, casi en plena substancia blanca–Ramón y Cajal, 1895, p. 25). However, in the subsequent translation of this study into German (Ramón y Cajal, 1896) Cajal updated their description and here for the first time published an illustration (Fig. 1G). He then stated that these large stellate cells can be divided in two classes: marginal (located at the border of cerebellar cortex and the white matter) and interstital (located within the white matter), and that some are even present as horizontally oriented spindle cells below the layer of Purkinje cells (Ramón y Cajal, 1896, pp. 23–24 and Fig. 5b in which letter b denotes ‘interstitielle Zellen der weissen Substanz’). Finally, in his Histology (Ramón y Cajal, 1911, pp. 50–53 and Figs 36 and 39) he offered a final description and classification of these ‘fusiform or stellate cells with long axons’ (cellules fusiformes ou étoilées a cylindre-axe long) which are present as three varieties: (i) external or intragriseal (externes ou intra-grises), (ii) marginal (marginales), i.e. placed at the border of cortex and white matter, and (iii) interstitial or deep (interstitielles ou profondes) and situated within the white axis of the cerebellar folium, more or less distant from the cortical grey substance. In text and figure legends they were variably described as ‘cellules interstitielles’, ‘cellules interstitielles ou profondes’ or ‘grosses cellules interstitielles de la substance blanche’. However, it should be noted that the first to describe and illustrate these cells was not Cajal, but Gustaf Retzius (see Fig. 1F), as acknowledged by Cajal himself. In 1892, Retzius published a description of Golgi cells and climbing fibres and stated (Retzius, 1892, p. 58):‘With respect to these cells I have to mention that I observed solitary large multipolar neurons here and then also within the cerebellar white matter, more or less deep below the granular layer and completely surrounded by myelinated white matter axons. One such cell with its stained axon is reproduced in the figure 4 of Table XIX...’ (Fig. 1F).
Fig. 5.
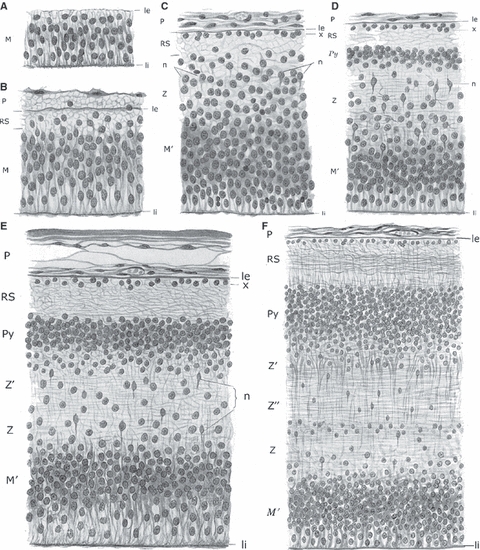
Von Economo & Koskinas realized that the upper intermediate zone gives rise to layer VIb and interstitial neurons in the adult human brain. Laminar development of the telencephalic wall from the stage of ependymal primordium (A, ependymären Anlage, 5th fetal week) to the completed development of the cortical plate (Pyramidenschicht) during the 5th fetal month (F), according to Von Economo & Koskinas (1925, pp. 88–89, Figs 47–52). (A–C) Sequential development of ventricular zone (M), marginal zone (RS) and intermediate zone (Z) from 5–7 weeks of gestation. (D) The appearance of the cortical plate (Py) during the 8th week of gestation. (E) During the 3rd fetal month, the cortical plate (Py) is well developed, and the intermediate zone becomes divided into an inner part (Z – fetal white matter) and an outer or upper part (Z’), which represents the transition towards the cortical plate and itself contributes to the formation of the future cortex (i.e. Z’ is future layer VIb). (F) All layers of the fetal telencephalic wall are much thicker and better developed during the 5th fetal month, but the basic arrangement is the same as in 3rd month. Z and Z’’ will transform into the deep white matter, and Z’ will transform into layer VIb and gyral white matter. le, li, external and internal limiting membrane; M, Keimschicht (ventricular zone); M’, Matrix developed from the previous Keimschicht (did they want to differentiate ventricular zone from the ependymal lining?); n, Neuroblasten (migrating neurons); P, pial membrane; Py, Pyramidenschicht, i.e. Rindenschicht (cortical plate) from which the cortex proper (die eigentliche zellführende Rinde) develops; RS, Randschleier (marginal zone), i.e. future molecular layer; x, Keimschicht (germinal layer) within the marginal zone, from which glial and Cajal-Retzius cells of the future molecular layer develop (they probably ment the subpial granular layer of Ranke); Z, Z’’, Zwischenschicht (intermediate zone) from which the future white matter develops; Z’, the upper part of the intermediate zone at its border with the cortical plate – this is a future layer VIb.
Cajal's displaced subcortical white matter neurons in the developing cerebral cortex of rodents and humans
Ramón y Cajal was also the first to describe systematically the presence of well differentiated neurons in the subcortical white matter of early postnatal rodents and humans (according to the age of specimens, these cells should be regarded as interstitial neurons and not as subplate neurons –Ramón y Cajal, 1891b, 1899a,b,c). Curiously, he never used the term interstitial cells to describe these neurons because he obviously regarded them as deep cortical neurons simply displaced in the subjacent white matter. The first description in rodents (Fig. 2A) was published in French in the journal La Céllule (Ramón y Cajal, 1891a,b, p. 174 and Planche II, Fig. 7: h, cellules siégeant dans la substance blanche– cells situated in the white matter). Soon after that, Gustav Retzius described similar deep neurons with ascending axons (Fig. 3) in the neocortex of the fetal dog (Retzius, 1893, plate I, Fig. 1).
Fig. 2.
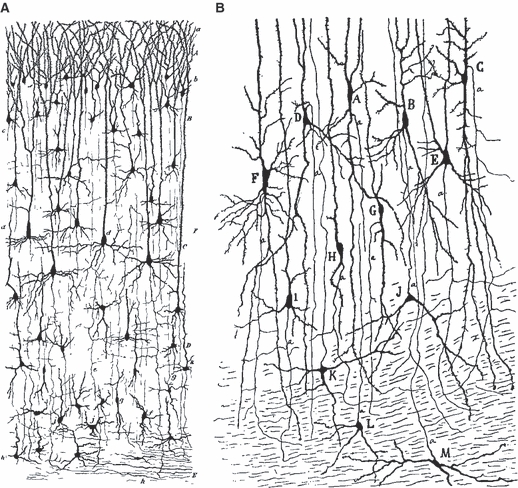
Ramón y Cajal (in 1891 for rodents and 1899–1902 for humans) was the first to describe neurons situated in the developing subcortical white mater of fetal and early postnatal mammals. However, Cajal regarded them simply as displaced from the adjacent cortex and called them white matter cells. (A) Golgi-stained neurons of supraventricular cortex of 1-month-old mouse: h, cells situated in the white matter (Ramón y Cajal, 1891a,b, p. 174;, plate II, fig. 7 – h, cellules siéegeant dans la substance blanche). (B) Rapid Golgi-staining of lateral entorhinal cortex (la corteza esfenoidal in Cajal's original) of 1-month-old human infant. (K–M) White matter cells with ascending axons (Ramón y Cajal, 1901, p. 50, Fig. 23: K–M, células de la substancia blanca provistas de axon ascendente; reproduced in German translation as Ramón y Cajal 1903, p. 61, Fig. 23: K–M, Zellen der weissen Substanz mit aufsteigendem Axencylinder; also reproduced in French translation as Ramón y Cajal, 1911, p. 696: K–M, cellules de la substance blanche munies d'un cylindre-axe ascendant).
Fig. 7.
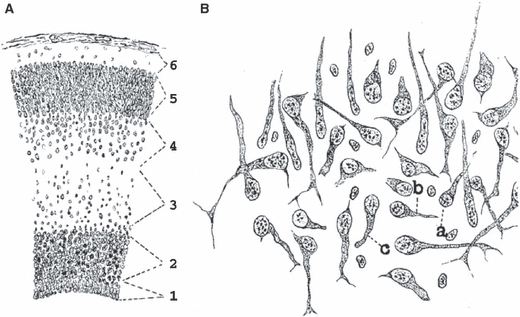
Hatai in 1902 described the subplate zone in the fetal cat. Schematic representation (A) of six layers of the telencephalic wall in the fetal cat as described by Hatai (1902). Note that it was probably the first description of the subventricular zone (layer 2) as well as the subplate zone (layer 4) with variously oriented and better differentiated cells (B) in comparison to strictly radially oriented cells in the cortical plate (5) and the intermediate zone (3). For details, see text.
Fig. 3.
Retzius published an early description of subplate neurons in the fetal dog. Gustav Retzius was among the first to described deep neurons with ascending axons (red –mz) located at the border of cortical plate and subjacent ‘fetal white matter’ in the neocortex of the fetal dog (Retzius, 1893, plate I, fig. 1). This figure also gives the first illustration of Cajal-Retzius cells (cz) in the marginal zone of the fetal dog.
In his series of studies on the human cerebral cortex (Ramón y Cajal, 1899a,b,c, 1900a,b, 1901, 1902a), which were soon translated into German (Ramón y Cajal, 1900c,d, 1902b, 1903), Cajal repeatedly described these cells, especially in visual, insular, cingulate and entorhinal cortex (Fig. 2B), usually in newborn infants aged 25–30 days. He reproduced all these findings in the second volume of his Histology (Ramón y Cajal, 1911). However, in none of these publications did he designate them as interstitial neurons, but as ‘white matter cells’ or ‘white matter cells with ascending axons’. Obviously, he regarded them as neurons of the deep cortical layer, displaced in the immediately adjacent white matter.
Interstitial neurons in the white matter of the adult nervous system
Interstitial neurons in the adult cerebral white matter
The presence of neurons in the subcortical white matter of the human brain was first described and illustrated by Theodor Meynert in 1867, and additionally commented on in his subsequent publications (Meynert, 1867, 1872, 1884). Meynert illustrated these cells in both superior frontal (Fig. 1A) and primary visual (Fig. 1B) human cortex and pointed out that these are spindle-shaped (fusiform) neurons which are oriented vertically to the pial surface within the gyral crowns, but horizontally at the bottom of sulci. He also suggested that they have a special functional relationship to short corticocortical association fibres (fibrae arcuatae, or Meynert's U-fibres) and that these fusiform cells may therefore be regarded as intercalated cells of his Associationssystem of short corticocortical fibres (Meynert, 1872). Franz Boll from Berlin also described small multipolar neurons as a normal component of the cerebral white matter, along with glial cells (Boll, 1874, pp. 67–69 plus a footnote on p. 26) and their existence was confirmed in an influential textbook of histology published by Henle (1879, p. 22). Thus, interstitial neurons were first described in the cerebral white matter and they were regarded as a normal finding. This view received its final confirmation in two major classics of human cortical architectonics.
First, Cécile and Oskar Vogt (Vogt & Vogt, 1919, Textfigure 10 and pp. 299–304) stated that Brodmann's six-layered ontogenetic Grundtypus of cortical lamination has to be modified because (p. 299, our translation): ‘below the cell-dense layer VI of Brodmann there is another, seventh layer of significantly decreased cell-packing density, which Brodmann observed only in Area striata and wrongly interpreted as a part of layer VI of the neighbouring Area occipitalis. Thus, in fact one has to admit the existence of seven-layered ontogenetic Grundtypus.’ And little later (p. 300): ‘Our Textfigure 10 (see p. 304) illustrates that Grundtypus in a schema derived from a combination of different cortical localities of the frontal cortex of one newborn... Sublayer VIa contains small cells of different forms, but triangular cells are prevalent. In contrast, sublayer VIb is composed of a smaller number of predominantly fusiform cells. In that sublayer one can easily distinguish cell-denser VIbα and cell-poorer VIbβ. However, for historical reasons we did not introduce this seventh layer in our basic lamination schema.’ In other words, Vogts retained the original Brodmann–Vogt description of layer VI as Lamina multiformis, and its division in Sublamina triangularis (VIa) and Sublamina fusiformis (VIb). In their Textfigure 10 they in fact illustrate subcortical interstitial neurons as sublayer VIbβ of their seventh cortical layer. Finally, in describing general architectonic features of sublayer VIb, they state (p. 312): ‘Everywhere in the adult cortex we can distinguish the principal part of VIb (Pars principalis, VIbα) from the deeper, limiting part of VIb (Pars limitans, VIbβ), which is characterized not only by decrease in number of fusiform cells – as in fetus (Fig. 10) – but also by the appearance of numerous nuclei of glial cells.’
Fig. 10.
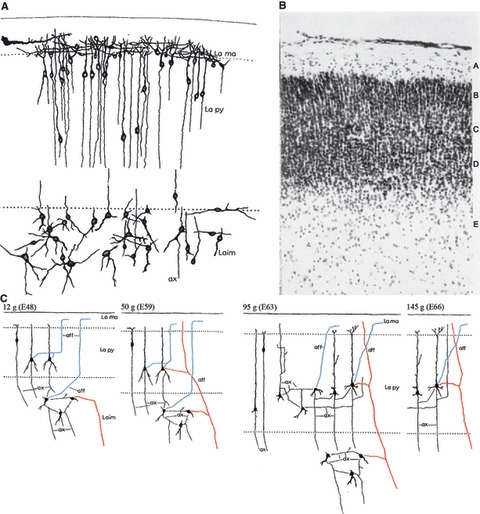
Karl-Erik Aström was at the brink of discovering the subplate zone in the fetal sheep (Aström, 1967, composed from his slightly modified figs 9, 13 and 25, with permission of Elsevier). (A) Well-developed stellate neurons form a separate (subpyramidal) stratum below the cortical plate in 25 g fetus (embryonic day E53). (B) The same cells can be seen as a transitional zone (E) in the Nissl-stained section of 39 g (E57) fetus. (C) Diagram summarizing connectivity of the early marginal zone, cortical plate and subpyramidal zone (subplate) between E48 and E66. Note that subpyramidal stellate cells receive afferents both from the marginal zone (blue) and from subcortical structures (red) and establish local circuit connections. Note also that these cells are absent at E66, when according to Aström all input–output and local circuitry moved within the cortical plate (because he was convinced that stellate cells become incorporated into the cortical plate as the future layer VIb). Gestation in sheep lasts 140–150 days.
Secondly, in probably the best and most detailed published account of the architectonics of the adult human cerebral cortex, Von Economo & Koskinas (1925, pp. 169–178) offered a clear-cut description of sublayer VIb and its transition to the white matter, the presence of interstitial neurons as well the interpretation of their developmental origin. Their findings can be summarized as follows. Layer VI should be properly named Lamina fusiformis (and not multiformis) because it is predominantly composed of spindle cells in both its upper (VIa) and lower (VIb) part. Although triangular and stellate cells are also present, the main difference between VIa and VIb is in cell size and cell-packing density, not in the cell types. The layer VI is best developed within the gyral crowns, where its cells are oriented perpendicular to the pia, but it is extremely thin at the bottom of cortical sulci, where its small fusiform cells are oriented horizontally. Regional differences in the thickness of layer VI are predominantly caused by differences in layer VIb, depending on whether it is sharply delimited from the underlying white matter or there is a very gradual transition. The layer VI is the thickest (1–1.5 mm) in precentral gyrus, temporal pole and gyrus transversus insulae, whereas it is the thinnest of sensory koniocortices (0.3–0.6 mm). The sublayer VIb (p. 170, our translation) ‘is that part of the layer VI which with its small cells stretches out into the subcortical white matter and gradually disappears in the depth at the point, where suddenly there appear a greatly increased number of rows of glial cells characteristic for the white matter’. That border is especially visible in overly stained Nissl sections, in which one can better see a bluish background neuropil between spindle cells of the cortex proper, which disappears concomitantly with the appearance of rows of glial cells. The cells of VIb are frequently outposted between myelinated bundles of white matter axons, and in some regions (such as the precentral gyrus) these interstitial cells can cover the region several times thicker than the cortex itself and may reach the vicinity of the centrum semiovale. All these interstitial cells represent a normal finding, i.e. a normal architectonic feature of the cerebral cortex; only when they are present deep within the corona radiata and centrum semiovale may there be a sign of pathological changes. Interstitial neurons are especially numerous and prominent in the insular region within the external and the extreme capsule. Finally, Von Economo & Koskinas (1925, p. 171) concluded that sublayer VIb does not develop from the embryonic cortical plate, but from those neuroblasts which were arrested in their migration just below the cortical plate, i.e. in the so-called upper intermediate zone (oberen Zwischenschicht); this could also explain individual differences in the frequency of fusiform cells within the subcortical white matter – see explanation in the section on the subplate zone.
Von Economo's student José Aldama performed a detailed cytoarchitectonic analysis of the entire cerebral cortex of an 11-month-old child and a 5.5-year-old child (Aldama, 1930). He found that at 11 months the cortex was still quite immature, and gyral white matter was filled with numerous fusiform neurons which could occasionally be observed even within the centrum semiovale. He also found that in a 5.5-year-old child the cortex/white matter border was still far more blurred in comparison with adult brain, mostly because numerous fusiform neurons stretch from layer VI deep into the subcortical white matter (especially in the frontal lobe). He also noted numerous interstitial neurons in the external and extreme capsule below the insular cortex.
Interstitial neurons in the spinal cord
It is usually stated that neurons in the spinal cord white matter were first noted by Gaskell in alligator (Gaskell, 1886) and in birds (Gaskell, 1889), but described in detail as out-lying cells a few years later by Charles Scott Sherrington (Sherrington, 1890; Cooper & Sherrington, 1940; for review, see Molnár & Brown, 2010). However, we learn from Sherrington himself that the first to note such cells in the spinal cord of an ox was Torquato Beisso of Torino in 1873. In addition, they were described in the spinal cord of dog by Schiefferdecker (1874) and Von Lenhossek (1889). Furthermore, the father of psychoanalysis, Sigmund Freud, while he was still a medical student in Vienna, published two studies on the spinal cord of ammocoetes (Petromyzon Planeri) in the proceedings of the Vienna Academy of Sciences (Freud, 1877, 1879). In these studies he described innere Hinterzellen as outlying cells of Clarke's columns within the dorsal funiculi. These findings were confirmed by Von Lenhossek (1889) and by Sherrington (1890). The most detailed analysis of interstitial neurons in all parts of the human spinal cord white matter was published by Poljak (1922) who was at that time an assistant at the University of Zagreb School of Medicine in Croatia. Soon after that, he settled in Chicago (where he remained until his death in 1955), changed his name in Stephen Polyak and published his famous and often cited monographs on the retina (Polyak, 1941) and vertebrate visual system (Polyak, 1957). It should be also noted that in the roof plate of the spinal cord of human embryos and young fetuses, sensory-type neurons were found which in the later stages were situated in the dorsal funiculus and after that disappeared as the fetus grew (Youngstrom, 1944; Humphrey, 1950). That was the first description of a transient population of spinal cord neurons which was later described and analyzed in detail as ‘borderline cells’ (Knyihar et al. 1978). This example of a transient fetal neuronal population which disappears presumably after fulfilling some special developmental role was frequently quoted as an example in studies on the subplate zone in 1980s (which assumed that subplate neurons similarly disappear after fulfilling their developmental role).
Early history of subplate and interstitial neurons in developmental brain disorders in the human
In the opening decades of the 20th century, the view that interstitial neurons represent normal components of the adult subcortical white matter was seriously challenged by a number of neuropathologists. At that time an increasing number of neuropathologists became deeply interested in the pathogenesis of developmental brain disorders and these studies enfolded along two main lines of research.
Von Monakow introduces the concept of heterotopic neurons in developmental brain disorders
The first conceptual approach was developed by Constantin von Monakow and his students in Zurich, who subsumed the majority of cortical migration disorders under the umbrella term ‘heterotopia’. Von Monakow (1901) developed a classification of heterotopia consisting of six groups (of increasing severity), but Vogt (1905), who claimed that he personally analyzed practically all existing types of heterotopia in collections from the Von Monakow Institute, simplified the classification into five groups. What is of interest here is the definition of the first (least severe) group of heterotopias in both classifications. According to Von Monakow (1901, p. 567), the first group consists of solitary neurons or small groups of neurons which were arrested (or misrouted) during migration from the ventricular zone and settled at the wrong destinations (usually somewhere within the cerebral white matter). The typical example would consist of interstitial neurons of the cerebral white matter (which can also be observed in normal brains) or spinal cord neurons displaced into the adjacent spinal white matter. Vogt (1905, p. 146) offered basically the same definition of the first group, but pointed out that individually misplaced neurons in the cerebral white matter can develop further, survive and even establish connections – so that a transitional (migratory) stage becomes transformed into a permanent (fixed) condition, which he interpreted as a ‘fixation of a certain developmental phase’. He also stated that this type of heterotopy is not necessarily connected with architectonic alterations of the overlying cortex. His final important comment was that interstitial neurons can indeed be observed in the normal brain, but always within the gyral white matter, whereas in pathological cases they were much more numerous and present also in the deep white matter.
Ranke introduces the concept of pathological persistence of transient fetal neurons
The second, and much more important, conceptual approach was developed by Ranke (1910). In his extensive study (the same in which he clearly described and named the subpial granular layer for the first time), he analyzed various types of malformations in human fetal brains and in concluding paragraphs offered a specific interpretation of the presence and subsequent fate of fetal interstitial (subplate) neurons (Ranke, 1910, pp. 99–100). He first pointed out that malformed fetal brains (with his Status verrucosus deformis) display a great number of cells within the cerebral white matter (Marklager). Whereas Wilhelm His described such cells in a 4-month-old fetus as migrating neurons, Ranke concluded that this could hardly be the case, because he observed them also in the 5th and 7th fetal months. Moreover, these neurons were also present in normal fetuses during the 6th fetal month, and they were much better differentiated than cells of the cortical plate – they were large, voluminous, with big processes and in fact displayed the level of maturation which cortical pyramidal neurons would attain only at birth. Therefore, Ranke concluded that such well differentiated neurons cannot possibly be migratory cells and designated them as (Ranke, 1910, p. 100; our translation): ‘neurons in the white matter (Nervenzellen des Markes) that display a complex morphology during the period in which the cortical plate consists predominantly of undifferentiated elements, and most probably have a specific function, i.e. already represent developmentally fixed elements. I cannot as yet say anything firm concerning their survival during normal development. However, I think it is possible that they without exception transform into those infrequent and solitary neurons present in the gyral white matter of the normal brain, and that their seemingly large number in the fetal brain is only apparent because they are much more tightly packed before the development of myelinated axons.’ Ranke then pointed out that the number of these cells is greatly increased in disordered brains (in brains of idiots as well as in some cases of juvenile paralysis –‘in Idiotengehirnen, auch gelegentlich bei juveniler Paralyse’) and finally concluded (Ranke, 1910, p. 100): ‘It should be pointed out that these elements – like Cajal's fetal cells – already before the end of fetal development display certain symptoms of regressive changes in nuclei and protoplasma, which clearly suggests that they – or at least the majority of them – represent fetal elements which during the normal development lose their cellular structure and can survive postnatally only in pathological circumstances – as an expression of some developmental brain disorder.’
Thus, Ranke introduced two important concepts: (i) that these neurons represent a specific and functional, but transient, fetal population of neurons, which disappear and die after fulfilling their (unknown) developmental role, and (ii) that their persistence in postnatal or adult brain represents an obvious sign of pathology.
Interstitial neurons in early studies of epilepsy
Ranke's view was widely accepted by a large majority of neuropathologists, especially those who studied the pathogenesis of so-called genuine epilepsy (for review, see Rondoni, 1909; Jakob, 1914; Wohlwill, 1916; Pollak, 1922 and numerous references therein). It took almost two decades to realize again that interstitial neurons were not just signs of pathology, but normal constituents of the adult cerebral white matter. This was slowly achieved through repeated studies of completely normal brains, initially by Wohlwill (1916), Pollak (1922), Neubürger (1922) and, finally and most decisively, Oppermann (1929). However, the legacy remained – in the closing section of this review we will see that both key concepts of Ranke were forcefully revived in 1980s, in interpretations of the developmental role and fate of subplate neurons, as well as in neuropathological studies of epilepsy and schizophrenia.
Early history of the subplate zone
Wilhelm His and Hans Jacob correctly described the fetal white matter, but not the subplate zone
In retrospect, it is interesting to note that the subplate zone was in fact clearly illustrated in a number of classical publications on human cortical development, starting with the seminal monograph of His (1904). However, its significance was not understood, and it was usually simply regarded as a part of the intermediate zone, i.e. future white matter. For example, His (1904, p. 106) stated that during the 3rd fetal month, the human telencephalic wall consists of four main layers (Fig. 4A), which he described as (i) ventricular zone (his Matrix or Innenplatte), (ii) intermediate zone (variably designated as Zwischenschicht, Zwischenplatte, Mantelschicht, Intermediärschicht), (iii) cortical plate (his Rindenschicht), and (iv) marginal zone (his Randschicht or Randschleier). He repeatedly stated that the intermediate zone consists exclusively of glial cells, migratory neurons (Fig. 4A) and some ingrowing fibres. However, he also distinguished between the primary intermediate zone (primäre Zwischenschicht) characteristic for an early telencephalic wall, and during the subsequent development (especially during the 4th fetal month) he described the division of IZ into a deep zone (Innenzone, rich in cells and fibres) and a superficial zone (Aussenzone, with migratory neurons and glial cells). During the 4th fetal month (Fig. 4B,C) this outer part was described as a ‘pale, wide zone, the intermediate zone’ (blasse, breite Zone, die Zwischenschicht), whereas the inner part was collectively described as the fetal white matter (Markschichten) and subdivided in four sublayers (layers 2–5 in Fig. 4B) as follows: inner striped layer (layer 2 –innere streifige Schicht), inner transitional layer (layer 3 –innere Uebergangsschicht), outer striped layer (layer 4 –äussere streifige Schicht), and outer transitional layer (layer 5 –äussere Uebergangsschicht); Thus, what we today describe as fetal white matter (intermediate/subventricular zone), His also declared to be the primordium of the fetal white matter (Markschichten), and what we today describe as the subplate zone, His simply designated it as a secondary intermediate zone, maintaining that it contains only glial cells and migratory neurons, and did not comment further on its fate or significance.
Fig. 4.
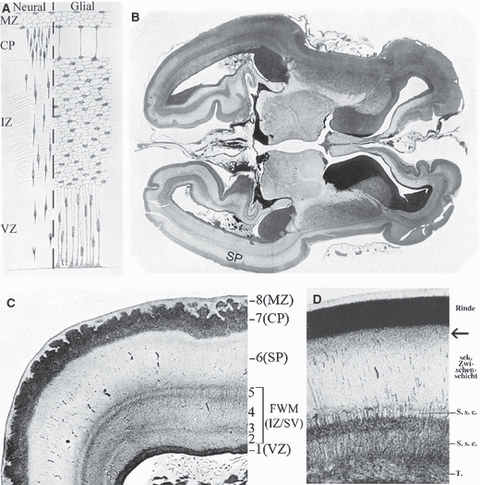
Wilhelm His and Hans Jacob correctly described human fetal white matter but not the subplate zone. Wilhelm His (His, 1904; Figs 73, 75 and 79) published a classical description of layers of the human telencephalic wall during the 3rd (A) and 4th (B,C) fetal months. These layers are here denoted using modern terminology (for original terms of His, see text). Note that His described his layers 2–5 (C) as fetal white matter, and he described the subplate zone (his layer 6) as ‘secondary intermediate zone’. MZ, marginal zone; CP, cortical plate; IZ, intermediate zone; VZ, ventricular zone; SP, subplate zone; FWM (IZ/SV), fetal white matter, i.e. intermediate/subventricular zone. A few decades later, Hans Jacob (Jacob, 1936, p. 3, Fig. 1) confirmed His's findings in a 3-month-old human fetus (D), explicitly described the subplate zone as a secondary intermediate zone (sekundäre Zwischenschicht), and pointed out the transient presence of the Unterschicht z of Filimonov (D, arrow). For details, see text.
A very similar interpretation was published in a later study of Jacob (1936) who accepted His's subdivision of the deep intermediate zone (Fig. 4D) but tried to identify the specific fibre content of its sublayers and claimed that layer 2 is occupied by Tapetum (callosal projections), layer 3 represented the internal sagittal stratum, and layer 5 represented the external sagittal stratum, just as in the adult brain. He explicitly described the subplate as ‘the secondary intermediate zone’ (sekundäre Zwischenschicht) but in distinction to His he depicted its most superficial part (Fig. 4D, arrow) as a transient and transitional ‘Unterschicht z’ as previously described by Filimonov (see below).
Von Economo and Koskinas realized that the upper intermediate zone gives rise to layer Ib and interstitial neurons in the adult human brain
Von Economo & Koskinas (1925, pp. 87–93) published a description of the developing human telencephalic wall from 5 weeks of gestation until the 5th fetal month (Fig. 5), which can be summarized as follows. From the 5th to the 7th week of gestation, there is sequential development of ventricular, marginal and intermediate zones (Fig. 5A–C). The cortical plate appears during the 8th week of gestation (Fig. 5D) and continues to increase in thickness (through the arrival of migrating neuroblasts) until the 5th month. However, during the 3rd month (Fig. 5E) the initially simple intermediate zone (Z) becomes subdivided in two parts (Z and Z’). The inner part (Z) is situated above the ventricular zone and represents the fetal white matter. The outer (upper) part (Z’) represents a zone of transition towards the cortical plate and in fact, together with the cortical plate, contributes to the formation of the adult neocortex because it will transform into layer VIb. During the 5th fetal month (Fig. 5F), the marginal zone starts to transform into the molecular layer, the cortical plate will soon enter the Brodmann's six-layered Grundtypus and transform into the cortex proper, the most superficial part of the intermediate zone (Z’–der oberste Lage der Zwischenschicht) transforms into the gyral white matter and the layer VIb, whereas the remaining parts of the intermediate zone (Z’’ and Z) transform into the deep white matter. As already mentioned, Von Economo & Koskinas regarded interstitial neurons in the adult brain as VIb cells dispersed throughout the subcortical white matter. Thus, one may be tempted to conclude that they in fact were the first to note the human subplate zone. However, they did not elaborate on this concise description, never explained the reasons which led to such an interpretation, and subsequently published exclusively on the adult human cerebral cortex. Furthermore, their description and interpretation remained ignored or forgotten for the next 50 years – obviously, few people with an interest in developing cortex thought that they would be able to find anything useful in the huge and scarcely available text and atlas of the adult human cortical cytoarchitectonics.
Filimonov's Unterschicht z in fact corresponds to the subplate formation stage in the human fetal cortex
In 1929, Ivan N. Filimonov published a very detailed study of prenatal and postnatal cytoarchitectonic development of the human cerebral cortex (Filimonoff, 1929). He analyzed primary motor, primary somatosensory and primary and associational visual cortex in 20 brains of fetuses, newborns, children and adults. His analysis included eight fetal brains (age range: 6 months to term newborn), and his primary focus was on prenatal areal and laminar differentiation of the six-layered ontogenetic cortical Grundtypus as defined by Brodmann. Thus, he completely omitted to mention fetal or adult interstitial neurons in any specimens from the 6th fetal month onwards. However, he also analyzed one human fetus aged 3.5 fetal months and noted that even at that early period, the fetal telencephalic wall displays clear regional differences in lamination (Fig. 6), although at that time the cortical plate proper appeared to have uniform structure in all cortical regions. In the occipital lobe, Filimonov described an elaborate lamination of the fetal white matter (Markschichten of His) and its regional differences (Fig. 6B,C). However, his key novel observation was that the outer part of the ‘secondary intermediate zone’ (outer pale layer –äussere, helle Schicht, i.e. die Zwischenschicht–Filimonoff, 1929, p. 332) consists of two sublayers (Fig. 6–Zsch and z). The most superficial part of the intermediate zone (Zsch) displays an increased cell-packing density and gradually merges with the cortical plate (Filimonoff, 1929, p. 332; our translation): ‘This sublayer, which displays a transitional increase in cell-packing density and is situated between the cortical plate and the intermediate zone sensu strictiori, we conventionally designated with the letter z, i.e. as Unterschicht z.’ Its cells are more rounded or polygonal, and its appearance displays pronounced regional variations with respect to whether it is sharply delimited from the cortical plate or whether their border is almost completely blurred – compare its appearance in lateral occipital (Fig. 6A), rostrolateral occipital (Fig. 6B) and rostrolateral ventral occipital cortex (Fig. 6C) in a 3.5-month-old fetus. Filimonov did not offer any further comments or explanations concerning the developmental origin or subsequent fate of his Unterschicht z; he was satisfied to point out that it is a transitory feature which clearly shows that there are regional differences in laminar development of the fetal telencephalic wall even during the 3rd and 4th fetal montha. Jacob (1936) reiterated this point and confirmed that Unterschicht z is no longer visible in a 5-month-old fetus.
Fig. 6.
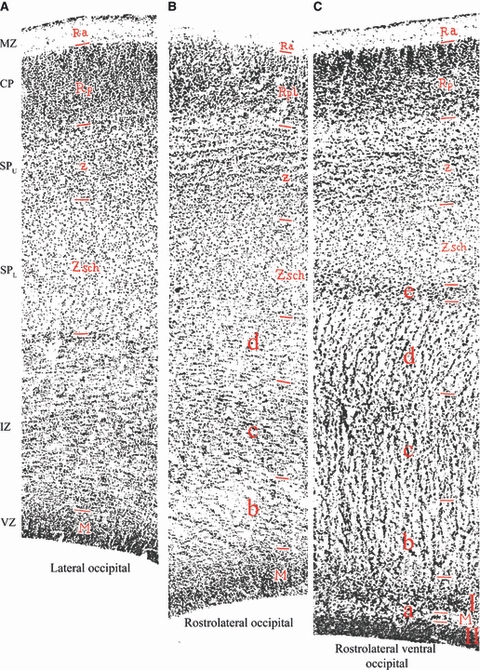
Filimonov describes his ‘Unterschicht z’ which corresponds to the upper subplate zone in humans. Regional differences in the lamination of the telencephalic wall in the occipital lobe of 3.5-month-old human fetus (Filimonoff, 1929, Plate 35, Figs 1, 2 and 3 – A, B and C, respectively). Modern designations are given at the left margin, and original Filimonov's as red letters within the sections, as follows: MZ, marginal zone (Ra –Randschleier); CP, cortical plate (Rpl –Rindenplatte); SPU, upper subplate zone (Unterschicht z); SPL, lower subplate zone (Zsch, i.e. secondary intermediate zone sensu strictiori); IZ, intermediate and subventricular zone, which Filimonov did not designate as a single zone but divided into several sublayers as follows: a = pale stripe (heller Streifen, which divides ventricular zone into Matrix I and Matrix II); b = inner pale layer (innere helle Schicht); c = dark layer (dunkle Schicht); d = middle pale layer (mittlere helle Schicht); e = outer dark layer (äussere dunkle Schicht, which corresponds to the position of the external capsule); VZ, ventricular zone (M –Matrix, which in ventral rostrolateral occipital wall is further divided in Matrix I and Matrix II, separated by a thin pale stripe of fibres –a). For additional explanation, see text.
Filimonov's discovery of the Unterschicht z served as basis for the introduction of a highly influential staging system of the early human fetal cortex
The Unterschicht z described by Filimonov was put in a proper context in subsequent studies of G. I. Poljakov (Poliakov, 1949, 1959, 1961, 1965; Poljakow, 1979) who developed a staging system which was adopted and slightly modified by Richard L. Sidman and Pasko Rakic in their highly influential reviews (Sidman & Rakic, 1973, 1982). Poljakov divided the entire human prenatal cortical development into three major periods: (i) an early period of migration and consolidation (2nd to 4th fetal month), (ii) a middle or transitional period of pre-differentiation of cortical layers (4th to 6th fetal month), and (iii) a late period of final differentiation of cortical layers (6th month to birth). He furthermore divided his early period into four stages of cortical plate development, which he described as: (i) initial formation, (ii) primary consolidation, (iii) migratory-consolidating differentiation, and (iv) secondary consolidation. Sidman & Rakic (1973, 1982) adopted that scheme as verified and supplemented it with additional data from their own material. They changed Poljakov's original term ‘consolidation’ into ‘condensation’ (although consolidation seems to be more appropriate), and described these four stages as follows (Sidman & Rakic, 1973, p. 5):
Stage I: Initial formation of the cortical plate (7–8 PCW).
Stage II: Primary condensation of the cortical plate (10–11 PCW) – the cortical plate increases in thickness, becomes more compact, and is clearly demarcated from the fibre-rich part of the intermediate zone. Stage III: Bilaminate cortical plate (11–13 PCW) – the uniform and compact cortical plate of the second stage becomes subdivided into an inner zone occupied mainly by cells with relatively large, somewhat widely spaced nuclei and an outer zone of cells with densely packed, oval nuclei elongated in the axis perpendicular to the cortical surface. Stage IV: Secondary condensation (13–15 PCW) – the cortical plate again becomes homogeneous in appearance and in a sense resembles a thickened version of stage II.
From this description, it is obvious that what Filimonoff (1929) described as Unterschicht z in fact corresponds to the stage III of Poljakov and Sidman-Rakic (bilaminate cortical plate, i.e. the stage between primary and secondary consolidation of the cortical plate). However, after the discovery of the subplate zone (Kostović & Molliver, 1974), it soon became obvious that stage III in fact corresponds to the subplate formation stage or stage of the ‘second’ cortical plate (Kostović & Rakic, 1990, pp. 443–444) and that Filimonov's Unterschicht z in fact corresponds to the upper subplate, SPU (see Fig. 4 in Kostović & Rakic, 1990). A corresponding stage, i.e. the division of incipient subplate in its upper and lower part, was also noted in the first study of the subplate zone in the fetal cat on the basis of autoradiographic findings (Luskin & Shatz, 1985). These findings clearly show that an initial description of the subplate as layer VIb in the fetal cat (Marin-Padilla, 1971, 1972, 1978) was overly simplified and that it at best can only be applied to the first (earliest generated) wave of subplate neurons. For a detailed criticism of this original concept of the primordial plexiform layer (or preplate) see a recent review (Bystron et al. 2008) as well as a number of recent studies which pointed out a previously unsuspected complexity of the early neocortical anlage in humans (Zecevic et al. 1999; Meyer et al. 2000; Zecevic & Rakic, 2001; Rakic & Zecevic, 2003; Bystron et al. 2005, 2006) as well as in rodents (Rickmann et al. 1977; Hevner et al. 2003; Jiménez et al. 2003; Bielle et al. 2005; García-Moreno et al. 2007).
Early descriptions of the fetal subplate zone in non-primate mammals
Finally, it should be noted that prescient descriptions of the fetal subplate zone were published even for cats (Hatai, 1902), rats (Sugita, 1917) and sheep (Godina, 1951).
Hatai (1902) performed a detailed study of orientation of migratory neuroblasts in the cerebral cortex of fetal cats and described the fetal telencephalic wall as consisting of six layers, starting from the ventricular surface and simply designated as first, second, etc. (Fig. 7A). Layers 1, 3, 5 and 6 are obviously ventricular zone, intermediate zone, cortical plate and marginal zone, respectively. However, it seems that Hatai was the first to describe and illustrate the subventricular zone (layer 2) because he stated (p. 200) that: ‘The second layer is composed of spherical or oblong cells. Numerous mitotic figures are noticeable in this layer.’ Finally, he noticed that the majority of cells within the intermediate zone and the cortical plate are oriented perpendicular to the pial surface, but that there is a distinct transitional zone in between (his layer 4) and that, in this zone, the cell-bodies are crowded more densely than in the third layer and that the majority of them show quite a different arrangement as well as a more complex structure (see Fig. 7B). It seems that he in fact described the subplate zone of the fetal cat – especially because he also stated (p. 203): ‘This fourth layer in the adult human cortex stained with Golgi's method reveals many cells (cells of Martinotti), the main dendrites of which run towards the ventricles, while the axone goes towards the surface of the cortex, thus exhibiting the arrangement at maturity which this method of development would lead us to expect.’
Sugita (1917) published an excellent description of all essential features of both subplate and layer VII (Fig. 8) in newborn and adult rat so that it is probably the best and simplest solution to quote key passages from the original text. ‘The lamina multiformis, in the albino rat, is distinctly separated into two sublayers by a narrow, light band very poor in cells. The broader (ectal) sublayer which lies immediately below the lam. gang. is rich in cells, about equal in size to the cells of the lam. pyr., but slightly less stained. The narrower (ental) sublayer which lies under the band poor in cells forms the boundary to the white substance, and consists of polymorphous cells, somewhat larger in size than the small pyramids and tinted a little more deeply’ (Sugita, 1917, p. 526–527; see our Fig. 8B,C). In the newborn rat (Fig. 8A):‘The lamina multiformis (VI) is divided into two sublayers by a band, poor in cells, as is seen in the adult, but at this phase the number of the cells in the ental sublayer is very much greater and they are larger and better stained than the cells in the ectal sublayer. Their orientation is irregular, some having the apical process vertical, some oblique and some in an inverted direction (Hatai, 1902)... But we cannot regard this sublayer as purely temporary, for it remains all through life persisting as a special thin layer containing large, polymorphous, deeply-staining cells, that is the ental sublayer of the lamina multiformis. However, in this earlier phase it contains transitional elements, since the number of cells is greater here in younger than in the older brains, so that in the newborn we see even seven or more rows of cells in this sublayer, while in the adult only three or four rows appear’ (Sugita, 1917, pp. 539–540).
Fig. 8.
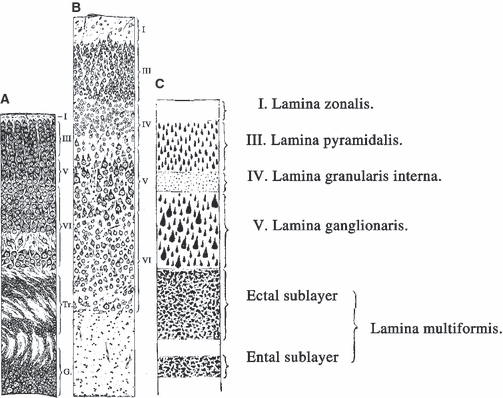
Sugita in 1917 described the rodent subplate and layer VII. The first description of the rodent subplate and layer VII in the prematurely born albino rat (A) and the adult rat (B,C) published by Naoki Sugita (Sugita, 1917, Figs 3, 8, and 9, pp. 526 and 538). Note that his ental layer corresponds to the subplate (in newborn), i.e. to layer VIb or layer VII (in the adult), which are in both cases separated from the ectal layer (VIa) by a thin cell-poor band. However, the ental layer decreases in size postnatally to approximately 50% of its newborn size. Tr. = transitional layers in the newborn (corresponding to intermediate/subventricular zone) which disappear 3 or 4 days after birth. For details, see text.
Finally, Giovanni Godina from Torino clearly described interstitial (subplate) neurons (Fig. 9D,E) in the cortex of fetal sheep (Godina, 1951). He noted that the deep two-thirds of the intermediate zone contain migratory neurons regularly arranged and oriented perpendicular to the pial surface, but that the outer third of the intermediate zone (below the cortical plate) contains variously oriented large cells with pale, vesicular nuclei, abundant cytoplasm and usually three to four radiating dendrites, and that the number of these well differentiated neurons increases with fetal age. He also noted that these neurons were first present below the cortical plate (midfetal period), then become numerous in the white matter of developing cortical gyri (late fetal period – see Fig. 9D,E), remain numerous (and predominantly fusiform) in the gyral white matter of the newborn, and largely disappear by the 4th postnatal month. However, he was convinced that these were just migratory neurons which were slowed-down in their migration to the cortical plate because (i) an increasing amount of developing fibre tract presented an obstacle to their migration, and (ii) they started to differentiate already during migration through the intermediate zone (Godina, 1951, p. 427), so that in the end they have to travel by means of ‘ameboid movement of their dendrites’ (Godina, 1951, p. 429–per movimento ameboide dei dendriti) to settle finally within the deep cortical layer VI. Thus, he did not recognize the real significance of the fetal subplate zone or postnatal interstitial neurons.
Fig. 9.
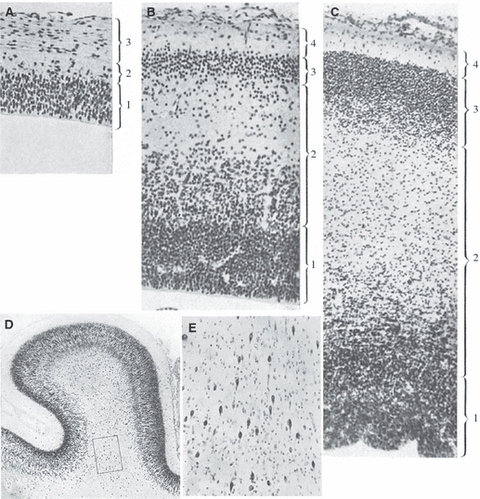
Early description of interstitial cells in the cortex of the fetal sheep (Godina, 1951, composed and slightly modified from his figures 1, 2 and 3– A-C, respectively – and figures 15 and 16 – D,E; reproduced with permission of Springer). 1 = ventricular zone (strato germinativo, strato della matrice); 2 (in A) and 4 (in B, C) = marginal zone (velo marginale, strato marginale); 2 (in B,C) = intermediate zone (strato intermedio, zona intermedia); 3 (in B,C) = cortical plate (strato neuroblastico, strato formativo). (A–C) fetal telencephalic wall in embryos of 18 mm, 43 mm (= 45 embryonic days), and 75 mm (= 63 embryonic days), respectively. (D,E) Nissl stained section through the motor cortex of the sheep fetus 235 mm long (5th fetal month); rectangle encompases numerous interstitial cells in the gyral white matter (magnified in E). However, Godina concluded that these young fusiform neurons present in the gyral white matter are ‘in the process of migration toward the cortex’ (essi sono in migrazione verso la corteccia). Note also that he simply designated as the intermediate zone everything situated between the ventricular zone and the cortical plate. For explanation, see text.
Discovery of the subplate zone
The final impetus for the discovery of the subplate zone as a specific, and functionally important, architectonic compartment of the fetal telencephalic did not come from descriptive histological studies but from neurophysiological studies of the development of spontaneous and evoked cortical activity, coupled with electron-microscopic studies of synaptogenesis and, soon after that, the application of axonal tracing techniques. In other words, decisive for the emergence of the concept of the subplate zone as a transient, dynamically changing and functional compartment was the combined application of functional and structural criteria and approaches. To understand this, a brief introduction to the state of functional studies of cortical development during 1960s and early 1970s would seem to be required.
Early neurophysiological studies of prenatal cortex paved the way for discovery of the subplate zone
Early studies in which the changes in electrophysiological responses were correlated to developmental changes in the cortical morphology have been done on newborn rabbits (Hunt & Goldring, 1951) and newborn cats (Purpura et al. 1960; Noback & Purpura, 1961; Purpura, 1961a,b,c; Voeller et al. 1963). On the basis of these studies, it was suggested that the cortical surface negativity of the evoked response arises from postsynaptic activity in the apical dendrites, and the surface positivity arises from the later developing basilar dendrites (Purpura, 1961a,b,c;).
However, all these investigations were made exclusively during the postnatal period. Therefore, a group of investigators at the Karolinska Institute in Stockholm initiated a series of investigations on the prenatal development of cortical mechanisms in the somatosensory cortex of sheep (Bernhard et al. 1959, 1967; Eidelberg et al. 1965; for review see Bernhard et al. 1967; Molliver, 1967). Results of that research were summarized in three related studies published jointly in 1967 in a special issue of Progress in Brain Research (Vol. 26) entitled ‘Developmental Neurology’ (Aström, 1967; Bernhard et al. 1967; Molliver, 1967). In these studies, Karl-Erik Aström performed Nissl and Golgi analysis of the same sheep fetuses used in physiological experiments, to provide anatomical data for the interpretation of physiological phenomena. Aström found that in the early fetal period (between embryonic days E48 and E66 – gestation in sheep lasts 140–150 days) there is a special population of early developing stellate cells at the interface between the developing cortical plate and the uppermost part of the intermediate zone (Fig. 10). At the same time, basal dendrites of the deep pyramidal neurons of the cortical plate penetrated into that zone and intermingled with dendrites and axons of stellate cells. Thus, Aström (1967, p. 18) concluded that although ‘stellate cells are situated in intermediate layer (La im), they should be considered as part of the primordial isocortex’ and that stellate cells (p. 22) ‘should be considered as deepest stratum of primordial isocortex although by definition they are situated in the intermediate zone.’ He also stressed (p. 22): ‘Larger stellate cells are seen especially in the outer part of the intermediate zone and particularly in the subpyramidal region, where they form a stratum, which should be considered to be a stratum of the primitive cortex’ and designated this transitional zone between pyramidal layer proper and intermediate zone as a subpyramidal zone or ‘a stratum in the subpyramidal area’ (cortical plate being lamina pyramidalis or pyramidal layer). However, Aström concluded that these stellate cells (p. 44) ‘will later become incorporated into the pyramidal layer. They should, therefore, be considered as parts of the primordial isocortex even if they appear to be situated in the intermediate layer, which is conventionally regarded as forthcoming white matter.’ His final verdict on these (obviously subplate) neurons was (Aström, 1967, p. 48): ‘It is remarkable that, as early as at the 12-g stage (E48), well developed stellate cells were observed in the subpyramidal regions. Nissl-stained sections indicate that such cells are present even in the 6-g fetus (E43). They are first scattered in the outer part of the intermediate zone, where they form a stratum. During the subsequent development, cell bodies of prospective pyramidal and other cortical neurons, which have developed and migrated later than the stellate cells in question, will occupy positions in the wide extracellular regions of the outer part of the intermediate zone. In other words, the pyramidal zone will extend downwards and incorporate the stellate cells) [our emphasis]. The wide spaces referred to will also receive association and projection fibers as well as glia cells.’
Thus, Aström in fact described the early subplate zone in the fetal sheep, only to conclude that it suddenly disappears at midgestation (around E65) and becomes ‘swallowed’ by the growing cortical plate. He conspiciously did not mention stellate cells in any described stage after E65 and did not refer to the possible existence of interstitial neurons in the gyral white matter of newborn or postnatal sheep. However, one of his conclusions is worth quoting because it clearly demonstrates that Aström proposed the same concept as Marin-Padilla (1971)– but 3 years earlier:‘No further comments are required when stating that the marginal zone corresponds to the first cortical stratum, and that the stellate cells in the outer part of the intermediate zone will become parts of the 6th stratum (layer VIb according to Lorente de Nó, 1938, or layer 7 according to Ramón y Cajal, 1911). These strata could already be recognized in the 6.5-g specimen... The close proximity of the marginal and the stellate cell layers in the very young fetuses, the early maturation of these cells and their connection through afferent fibers point to a relationship between them which may or may not disappear during their subsequent evolution’ (Aström, 1967, pp. 52–53; see also our Fig. 10C). As a 6.5-g fetus is E43 days, it is soon after the cortical plate separated Cajal-Retzius cells and his stellate cells.
From physiological experiments to electron microscopy – the discovery of the subplate zone
However, Molliver (1967) found this finding of early developing stellate cells more interesting. In his investigation of the ontogenetic development of the evoked cortical response to tactile stimulation of the nose in unanaesthetized sheep fetuses (kept in placental contact with the decerebrate ewe) he found that the first detectable evoked cortical response was obtained at E55 and that it was a surface positive potential. Comparing his findings with data of Aström, Molliver hypothesized that this first surface positive response may reflect postsynaptic potentials in stellate cells and basal dendrites residing at the junction of pyramidal and intermediate zones. Thus, he concluded (Molliver, 1967, p. 88): ‘This is an hypothesis which will be investigated in fetal animals by deep microelectrode penetrations and by a microscopic search for functional contacts near the junction of the pyramidal and intermediate zones’ [our emphasis].
Indeed, in collaboration with Hendrik Van der Loos at The Johns Hopkins University, Molliver initiated electron-microscopic studies of fetal cerebral cortex in dogs, cats and rodents (Molliver & Van der Loos, 1970). Ivica Kostović from Zagreb joined the laboratory as a postdoctoral fellow in 1972, with the specific aim to study synaptogenesis in the human fetal neocortex. This collaboration soon led to the discovery of early bilaminar synaptogenesis in the human fetal somatosensory cortex (Molliver et al. 1973) and finally, in 1974, to the discovery of the subplate zone (Kostović & Molliver, 1974). As this seminal discovery was published as a brief report in the Anatomical Record, it seems best to quote the key message (Kostović & Molliver, 1974, p. 395):
‘By correlating the distribution of synapses with Nissl- and Golgi-architectonics we have arrived at a new interpretation of the morphologic characteristics and the extent (in depth) of fetal cortex... Deep to the cortical plate is the pale, less dense intermediate zone, which increases vastly in thickness in later stages and can be divided into two parts, both containing radially elongated migrating neuroblasts. The inner part contains large bundles of axons and may be regarded as fetal white matter. The outer part (4 mm thick at 24 weeks gest.), containing relatively mature pyramids and multipolar neurons with long (ca. 1 mm) dendrites radiating in all directions, is deemed part of fetal cortex and is designated SUBPLATE LAYER. Synapses were found in the marginal zone and throughout the subplate layer (to 5 mm below pia at 24 weeks). From 15–24 weeks there was a major increase in the density of synapses in the subplate layer and at progressively more superficial depths within the cortical plate. In a given cylinder of cortex, the majority of synapses is in the subplate layer. The subplate layer is the thickest part of human fetal cortex and is the major site of early synaptogenesis and neuron maturation. This layer – also observed by us in carnivores and rodents – reaches its greatest prominence in man. The fate of the subplate layer (i.e. its adult counterpart) and its role in cortical circuitry are unknown and will be the subject of the further study.’
The subplate zone as waiting compartment for ingrowing cortical afferents and the site of early synaptic interactions
This novel concept was substantially strengthened by subsequent experimental demonstration that in fetal rhesus monkey visual cortex, the subplate zone represents a ‘waiting compartment’ for ingrowing thalamocortical (geniculate) afferents (Rakic, 1976, 1977). Over the next decade, the concept of the subplate as the waiting compartment for growing cortical afferents was amply documented in comparative studies on human and monkey brain conducted by Ivica Kostovic (at Zagreb University School of Medicine) and Pasko Rakic and Patricia Shoer Goldman-Rakic (at Yale University School of Medicine). Thus, Kostovic and Rakic published key studies on postnatal interstitial neurons (Kostović & Rakic, 1980) and on the comparative developmental history of the subplate zone (Kostović & Rakic, 1990). Early synaptogenesis and a waiting compartment for thalamocortical afferents were demonstrated in the human cingulate cortex (Kostović & Krmpotić, 1976), the human auditory cortex (Krmpotić-Nemanić et al. 1983), human and monkey prefrontal cortex (Kostović & Goldman-Rakic, 1983), human and monkey visual cortex (Kostović & Rakic, 1984), and human and monkey somatosensory and visual cortex (Kostović & Rakic, 1990). It was also demonstrated that the subplate in humans serves as a waiting compartment for basal forebrain cholinergic afferents (Kostović, 1986), and in rhesus monkeys for callosal and ipsilateral corticocortical afferents (Goldman-Rakic, 1982; Schwartz & Goldman-Rakic, 1991; Schwartz et al. 1991), which was later confirmed for human brain, too (DeAzevedo et al. 1997). The subplate zone was also incorporated in the first revision (Rakic, 1982) of the original Boulder Committee (1970) scheme, as well as in its recent revision (Bystron et al. 2008).
Starting from 1985, the concept of the subplate zone has been further elaborated in a series of important experimental studies performed on fetal cats by the research groups of Carla Shatz (Luskin & Shatz, 1985; Chun et al. 1987; Chun & Shatz, 1988a,b, 1989a,b; Allendoerfer & Shatz, 1994), Facundo Valverde (Valverde & Facal-Valverde, 1987, 1988) and Gundela Meyer (Wahle & Meyer, 1987, 1989; Wahle et al. 1987; Meyer & Wahle, 1988).
Lessons from history – new trends in subplate research
Hopefully, this historical review has brought forward several useful take-home messages.
First, a century of research clearly testifies that interstitial neurons, subplate neurons and the subplate zone were first observed and variously described in the human brain – or, in more general terms, in large brains of gyrencephalic mammals, characterized by an abundant white matter and slow and protracted prenatal and postnatal development. Although, at present, rodents are the most frequently used model for studying developmental and functional roles of the subplate by means of a panoply of molecular biological and genetic tools, it is reasonable to assume that study of rodents alone probably would never have led to the discovery of the subplate. Initial studies (Sugita, 1917) had already demonstrated that the rodent subplate is not really a transient structure, but a well defined layer (VIb or VII) which persists as a part of the ‘traditional’ cortex throughout the rodent lifespan. In recent years, the rodent subplate/layer VII has been increasingly recognized as a ‘special case’ probably characteristic for this specific group of mammals and with significantly different developmental history and postnatal roles in comparison with primates and humans (Reep & Goodwin, 1988; Reep, 2000; Valverde et al. 1989, 1995; Robertson et al. 2000; Clancy & Cauller, 1999; Clancy et al. 2001, 2009;Arias et al. 2002; Tomioka et al. 2005).
The subplate zone cannot be meaningfully defined using a single criterion
The second historical lesson to be taken from a centry of repeated failures to grasp the proper nature and significance of the subplate in descriptive histological studies is that subplate cannot be meaningfully defined using a single criterion – a single anatomical feature, the extent of distribution of a single class of neuron, or expression of a single molecule or gene are not sufficiently precise markers, as already pointed out in the seminal study of Kostovic & Rakic (1990, p. 463).
The subplate zone is a highly dynamic architectonic compartment, and its size and cellular composition do not remain constant as development progresses. The subplate contains elements in common with other fetal zones (e.g. radial glial cells, radially migrating neurons, tangentially migrating neurons, early developing astrocytes, precursors of oligodendrocytes), but it also (at least at the peak of its development) contains certain elements sui generis: subplate neurons with morphologically, chemically and connectionally diverse phenotypes, heterogeneous contingents of ‘waiting’ cortical afferents and transient synapses, an enlarged extracellular space filled with an abundant, hydrophyllic extracellular matrix of specific composition which enables its easy visualization on MR images (Kostović et al. 2002), a highly developed but transient microvascular network, etc. On the other hand, single molecular or genetic markers may be useful for tracing the developmental origin and fate of fetal subplate neurons and surviving postnatal interstitial neurons (Dunn et al. 1995; Ishii et al. 2001; Kolk et al. 2001; Heuer et al. 2003). Moreover, the powerful cell separation and gene profiling methods recently opened the way for selective recognition of different subpopulations of subplate neurons and for tracing their fate in normal development as well as in brain disorders (Hoerder-Suabedissen et al. 2009).
Although the subplate zone is transient, many subplate neurons are not
The third lesson is that a clear distinction should be made between the subplate zone and subplate (and interstitial) neurons. The zone appears at the beginning of early fetal period, and disappears during the early postnatal life. Its dissolution is caused by a host of interrelated factors: the relocation of waiting afferents to the cortical plate (with concomitant loss of numerous synapses and great decrease in the extracellular matrix); the disappearance of previously present radial glial cells and migratory neurons; the simplification of previously highly elaborated microvascular network – and, probably, by cell death of at least some subplate neurons. However, the transient existence of the subplate zone (as a specific architectonic compartment of the fetal telencephalic wall) should not be equated with the putative transient existence of subplate neurons. It is clear that both in rodents and, even more, in humans and monkeys, a significant number of subplate cells survive and remain well and functioning throughout life (see below).
Revival of early concepts in current developmental and neuropathological studies
It is also interesting to note the revival of key concepts of Ranke (1910) after the final discovery of the subplate zone. Ranke proposed that subplate neurons (his Nervenzellen in Mark) are transient fetal neurons with a special developmental function which subsequently disappear. This idea is still alive and well, being initially promoted especially by the study of Luskin & Shatz (1985) on ‘earliest generated and co-generated Cajal-Retzius and subplate cells’ who stated: ‘These observations suggest that both sets of early generated cells may provide a framework for the development of the cortical plate that largely disappears when the process is complete’ (p. 1063). ‘Their disappearance in conjunction with these events may indicate that these cells play a unique role in the establishment of the cerebral cortex, and, once this role is complete, they are eliminated by cell death’ (p. 1074).
Ranke's second proposal, that postnatal persistence of subplate (interstitial) neurons represents a sign of pathology, has been abundantly exploited over the past few decades in neuropathological research on developmental origins of epilepsy and schizophrenia.
In the context of temporal lobe epilepsy, interstitial neurons within white matter are usually described as heterotopic (Meencke & Janz, 1984; Hardiman et al. 1988; Armstrong, 1993; Wolf & Weistler, 1993; Mischel et al. 1995) and thought to represent one element of a group of developmental disorders frequently referred to as cortical dysplasia (Taylor et al. 1971), glioneuronal hamartia (Wolf & Weistler, 1993), microdysgenesis (Meencke & Janz, 1984; Hardiman et al. 1988; Armstrong, 1993) or heterotopia (Wolf et al. 1993). Rojiani et al. (1996) found that these ‘residual/heterotopic neurons’ were significantly more numerous in temporal than in frontal or occipital cortex, and concluded that they represent interstitial remnants of the subplate which have failed to undergo programmed cell death. Others suggested that these neurons represent arrested migration of neuroblasts along radial glia, possibly secondary to injury to the radial glia (Sarnat, 1992). Rojiani et al. (1996) concluded (p. 181): ‘whatever the mechanism involved in generating this heterotopia, it clearly displays some regional variability and is a fairly consistent phenomenon in apparently ‘normal’ individuals.’ Similarly, as classical German authors did almost a century ago, Meencke (1983) described an increase in the number of ‘heterotopic’ interstitial neurons in the inferior frontal gyrus white matter of epilepsy patients in comparison with normal controls. It has been also proposed that subplate-like neurons may persist in cortical dysplasia and contribute to the manifestation of pharmacoresistant epilepsy in adults (Cepeda et al. 2007).
With respect to the developmental hypothesis of schizophrenia, after the initial report that in schizophrenia there is an increased number of deep interstitial neurons (Akbarian et al. 1993a,b, 1996), a number of studies pointed out that, in fact, if the number of interstitial neurons is indeed increased in schizophrenia, the increase is prominent in a subpopulation of superficial interstitial neurons (within 1 mm from cortex), at least in a subset of schizophrenic patients and in different cortical regions such as the frontal and temporal regions (Anderson et al. 1996; Beasley et al. 2002; Kirkpatrick et al. 2003; Ikeda et al. 2004; Eastwood & Harrison, 2005; Bertram et al. 2007), inferior parietal (Kirkpatrick et al. 1999), entorhinal (Rioux et al. 2003; Eastwood & Harrison, 2005) and cingulate cortex (Connor et al. 2009). For recent reviews, see Eastwood & Harrison (2003) and Connor et al. (2009).
Interstitial neurons as a normal and functionally important component of the adult cerebrum
In contrast, and in full agreement with our data and interpretation (see Judaš et al., 2010), several research groups recently pointed out that interstitial neurons should be regarded as normal and important components of the human and monkey adult white matter and not just as ‘a relict of the subplate, a transient compartment proper of development and without a known function in the adult brain’ (Suárez-Solá et al. 2009). Suárez-Solá et al. (2009) justly conclude (p. 2): ‘primate interstitial neurons are not just incidental remnants of early-born neurons, but rather seem to belong to a distinct neuronal system that is intimately connected to the white matter and may carry out activities pertinent to this location’– such as coordinating inter-areal connectivity and regulation of blood flow.
In fact, it has been repeatedly pointed out that the developmental history of the subplate is very different in humans and other primates than in carnivores and rodents (Kostović & Rakic, 1980, 1990; Meyer et al. 2000; Smart et al. 2002; Lukaszewicz et al. 2005; Molnár et al. 2006; Meyer, 2007; Bayatti et al. 2008; Bystron et al. 2008; Suárez-Solá et al. 2009). For example, in contrast to the situation in rodents, neurons are continuously added to the subplate in primates during the later stages of corticogenesis (Kostović & Rakic, 1990; Smart et al. 2002; Meyer, 2007; Bystron et al. 2008). A similar difference between rodents and primates was also noted in the development of layer I neurons (Zecevic & Rakic, 2001).
In a recent review (Friedlander & Torres-Reveron, 2009) it has been proposed that subplate neurons undergo a temporal re-specification of function throughout life. Indeed, it is logical to assume on the basis of available evidence that neurons in general may serve different functions over the course of an organism's life – the phenomenon aptly described as ‘a pleiotropy of cellular function in the temporal domain’ (Friedlander & Torres-Reveron, 2009).
An overall postnatal decrease in the number of surviving subplate neurons does not necessarily mean that they become functionally less important – and millions of adult interstitial neurons in monkey and human brain (Kostović & Rakic, 1980) certainly do not represent a ‘minor neuronal population’. The potential significance of interstitial neurons for pathogenesis of various neurological and mental disorders should not be searched for in their existence per se, but in potential alterations of their developmental fate, their altered position in cortical circuitry and thus their altered functioning (Friedlander & Torres-Reveron, 2009; Kostović et al. 2010).
On the basis of physiological data obtained almost exclusively in rodents, Friedlander & Torres-Reveron (2009) assumed that the majority of interstitial neurons are glutamatergic and therefore proposed that the surviving group of subplate neurons may function as a sort of cortical gatekeeper, modulating information flow into and out of modules of overlying cortex to other cortical sites, as their excitatory synaptic output to the cortical layers above could provide either feed-forward (for the thalamocortical inputs) and/or feedback (for the cortical efferents) information.
On the other hand, in humans and monkeys a substantial portion of both subplate and interstitial neurons are probably GABAergic inhibitory interneurons (Kostović & Rakic, 1990; Meinecke & Rakic, 1992; for review see Judaš et al., 2010). Therefore, we recently proposed (Kostović et al. 2010) that GABAergic interstitial neurons serve as important modulators of normal cortical functions and that this inhibitory action is facilitated by their strategic position at the cortical/white matter interface where limbic and modulatory afferent pathways enter the prefrontal cortex. Thus, an enlarged population of inhibitory interstitial neurons may lead to the increased inhibition of prefrontal cortical neurons because it may alter the differential ‘gating’ of limbic and modulatory inputs (as well as other cortical and subcortical inputs) and cause a functional disconnectivity between the prefrontal and limbic cortex in the adolescent brain in the pathogenesis of schizophrenia (Kostović et al. 2010).
Acknowledgments
This work was supported by the Croatian Ministry of Science, Education and Sport grant No. 108-1081870-1878 (to M.J.) and Unity through Knowledge Fund (UKF) grant (director: Ivica Kostović).
References
- Akbarian S, Bunney WE, Potkin SG, et al. Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry. 1993a;50:169–177. doi: 10.1001/archpsyc.1993.01820150007001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Vinuela A, Kim JJ, et al. Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry. 1993b;50:178–187. doi: 10.1001/archpsyc.1993.01820150016002. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, et al. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53:425–436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- Aldama J. Cytoarchitektonik der Grosshirnrinde eines 5 jährigen und eines 1 jährigen Kindes. Z ges Neurol Psychiat. 1930;130:532–626. [Google Scholar]
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Volk DW, Lewis DA. Increased density of microtubule associated protein 2-immunoreactive neurons in the prefrontal white matter of schizophrenic subjects. Schizophr Res. 1996;19:111–119. doi: 10.1016/0920-9964(96)88521-5. [DOI] [PubMed] [Google Scholar]
- Arias MS, Baratta J, Yu J, et al. Absence of selectivity in the loss of neurons from the developing cortical subplate of the rat. Brain Res Dev Brain Res. 2002;139:331–335. doi: 10.1016/s0165-3806(02)00582-5. [DOI] [PubMed] [Google Scholar]
- Armstrong DD. The neuropathology of temporal lobe epilepsy. J Neuropathol Exp Neurol. 1993;52:433–443. doi: 10.1097/00005072-199309000-00001. [DOI] [PubMed] [Google Scholar]
- Aström KE. On the early development of the isocortex in fetal sheep. Prog Brain Res. 1967;26:1–59. [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, et al. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex. 2008;18:1536–1548. doi: 10.1093/cercor/bhm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley CL, Cotter DR, Everall IP. Density and distribution of white matter neurons in schizophrenia, bipolar disorder and major depressive disorder: no evidence for abnormalities of neuronal migration. Mol Psychiatry. 2002;7:564–570. doi: 10.1038/sj.mp.4001038. [DOI] [PubMed] [Google Scholar]
- Bernhard CG, Kaiser IH, Kolmodin GM. On the development of cortical activity in fetal sheep. Acta Physiol Scand. 1959;47:333–349. [PubMed] [Google Scholar]
- Bernhard CG, Kolmodin GM, Meyerson BA. On the prenatal development of function and structure in the somesthetic cortex of the sheep. Prog Brain Res. 1967;26:60–77. doi: 10.1016/S0079-6123(08)61419-3. [DOI] [PubMed] [Google Scholar]
- Bertram I, Bernstein HG, Lendeckel U, et al. Immunohistochemical evidence for impaired neuregulin-1 signalling in the prefrontal cortex in schizophrenia and in unipolar depression. Ann N Y Acad Sci. 2007;1096:147–156. doi: 10.1196/annals.1397.080. [DOI] [PubMed] [Google Scholar]
- Bielle F, Griveau A, Narboux-Neme N, et al. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- Boll F. Die Histiologie und Histiogenese der nervösen Centralorgane. Arch Psychiatr Nervenkr. 1874;4:1–138. [Google Scholar]
- Boulder Committee. Embryonic vertebrate central nervous system. Revised terminology. Anat Rec. 1970;166:257–261. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- Bystron I, Molnár Z, Otellin V, et al. Tangential networks of precocious neurons and early axonal outgrowth in the embryonic human forebrain. J Neurosci. 2005;25:2781–2792. doi: 10.1523/JNEUROSCI.4770-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Rakic P, Molnár Z, et al. The first neurons of the human cerebral cortex. Nat Neurosci. 2006;9:880–886. doi: 10.1038/nn1726. [DOI] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Wu N, et al. Immature neurons and GABA networks may contribute to epileptogenesis in pediatric cortical dysplasia. Epilepsia. 2007;48(Suppl 5):79–85. doi: 10.1111/j.1528-1167.2007.01293.x. [DOI] [PubMed] [Google Scholar]
- Chun JM, Shatz CJ. A fibronectin-like molecule is present in the developing cat cerebral cortex and is correlated with subplate neurons. J Cell Biol. 1988a;106:857–872. doi: 10.1083/jcb.106.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JM, Shatz CJ. Distribution of synaptic vesicle antigens is correlated with the disappearance of a transient synaptic zone in the developing cerebral cortex. Neuron. 1988b;1:297–310. doi: 10.1016/0896-6273(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Chun JM, Shatz CJ. Interstitial cells of the adult neocortical white matter are the remnant of the early-generated subplate neuron population. J Comp Neurol. 1989a;282:555–569. doi: 10.1002/cne.902820407. [DOI] [PubMed] [Google Scholar]
- Chun JM, Shatz CJ. The earliest generated neurons of the cat cerebral cortex: characterization by MAP2 and neurotransmitter immunocytochemistry during fetal life. J Neurosci. 1989b;9:1648–1667. doi: 10.1523/JNEUROSCI.09-05-01648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JM, Nakamura MJ, Shatz CJ. Transient cells of the developing mammalian telencephalon are peptide-immunoreactive neurons. Nature. 1987;325:617–620. doi: 10.1038/325617a0. [DOI] [PubMed] [Google Scholar]
- Clancy B, Cauller LJ. Widespread projections from subgriseal neurons (layer VII) to layer I in adult rat cortex. J Comp Neurol. 1999;407:275–286. [PubMed] [Google Scholar]
- Clancy B, Silva-Filho M, Friedlander MJ. Structure and projections of white matter neurons in the postnatal rat visual cortex. J Comp Neurol. 2001;434:233–252. doi: 10.1002/cne.1174. [DOI] [PubMed] [Google Scholar]
- Clancy B, Teague-Ross TJ, Nagarajan R. Cross-species analyses of the cortical GABAergic and subplate neural populations. Front Neuroanat. 2009;3:1–14. doi: 10.3389/neuro.05.020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CM, Guo Y, Akbarian S. Cingulate white matter neurons in schizophrenia and bipolar disorder. Biol Psychiatry. 2009;66:486–493. doi: 10.1016/j.biopsych.2009.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Sherrington CS. Gower's tract and spinal border cells. Brain. 1940;6(Part 2):123–134. [Google Scholar]
- Das GD, Kreutzberg GW. Evaluation of interstitial nerve cells in the central nervous system: a correlative study using acetylcholinesterase and Golgi techniques. Ergeb Anat. 1968;41:1–58. [PubMed] [Google Scholar]
- DeAzevedo LC, Hedin-Pereira C, Lent R. Callosal neurons in the cingulate cortical plate and subplate of human fetuses. J Comp Neurol. 1997;386:60–70. doi: 10.1002/(sici)1096-9861(19970915)386:1<60::aid-cne7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. Cajal on the Cerebral Cortex. An Annotated Translation of the Complete Writings. New York & Oxford: Oxford University Press; 1988. [Google Scholar]
- Dogiel AS. Zur Frage über die Ganglien der Darmgeflechte bei den Säugetieren. Anat Anz. 1895;10:517–524. [Google Scholar]
- Dogiel AS. Zwei Arten sympatischer Nervenzellen. Anat Anz. 1896;11:679–687. [Google Scholar]
- Dunn JA, Kirsch JD, Naegele JR. Transient immuno-globulin-like molecules are present in the subplate zone and cerebral cortex during postnatal development. Cereb Cortex. 1995;5:494–505. doi: 10.1093/cercor/5.6.494. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry. 2003;8:821–831. doi: 10.1038/sj.mp.4001399. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neuron density in the dorsolateral prefrontal cortex and parahippocampal gyrus in schizophrenia. Schizophr Res. 2005;79:181–188. doi: 10.1016/j.schres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Kolmodin GM, Meyerson BA. Ontogenesis of the steady potential and the direct cortical response in fetal sheep brain. Exp Neurol. 1965;12:198–214. [Google Scholar]
- Filimonoff IN. Zur embryonalen und postembryonalen Entwicklung der Grosshirnrinde des Menschen. J Psychol Neurol (Leipzig) 1929;39:323–389. [Google Scholar]
- Freud S. Vienna:Sitzungsberichte der Mathe-matisch-naturwissenschaftlichen Classe der Kaiserlichen Akade-mie der Wissenschaften Wien. 1877. Ueber den Ursprung der hinteren Nervenwurzeln im Rückenmark von Ammocoetes (Petromyzon Planeri). Von Sigmund Freud, stud. med. (Aus dem physiologischen Institute der Wiener Universität) pp. 15–27. LXXV. Band. III. Abteilung, Jahrgang 1877. – Heft I bis V. [Google Scholar]
- Freud S. Vienna:Sitzung-sberichte der Mathematisch-naturwissenschaftliche Classe der Kaiserlichen Akademie der Wissenschaften Wien, vol. LXXVIII. pp. 1879. Ueber Spinalganglien und Rückenmark des Petromyzon. Von stud. med. Sigm. Freud (Aus dem physio-logischen Institute der Wiener Universität) pp. 81–167. 3rd edn, 1878. – Notebook I–V (June–December. [Google Scholar]
- Friedlander MJ, Torres-Reveron J. The changing roles of neurons in the cortical subplate. Front Neuroanat. 2009;3:1–8. doi: 10.3389/neuro.05.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Moreno F, López-Mascaraque L, De Carlos JA. Origins and migratory routes of murine Cajal-Retzius cells. J Comp Neurol. 2007;500:419–432. doi: 10.1002/cne.21128. [DOI] [PubMed] [Google Scholar]
- Gaskell WH. On a segmental group of ganglion cells in the spinal cord of the alligator (Proceedings of the Physiological Society 1885, No. IV) J Physiol (Lond) 1886;7:xxix–xxx. [Google Scholar]
- Gaskell WH. On the relation between the structure, function, distribution and origin of the cranial nerves; together with a theory of the origin of the nervous system of Vertebrata. J Physiol (Lond) 1889;10:153–211. doi: 10.1113/jphysiol.1889.sp000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godina G. Istogenesi e differenziazione dei neuroni e degli elementi gliali della corteccia cerebrale. Z Zellforschung. 1951;36:401–435. [PubMed] [Google Scholar]
- Goldman-Rakic PS. Neuronal development and plasticity of association cortex in primates. Neurosci Res Program Bull. 1982;20:520–532. [PubMed] [Google Scholar]
- Hardiman O, Burke T, Phillips J, et al. Microdysgenesis in resected temporal neocortex: incidence and clinical significance in focal epilepsy. Neurology. 1988;38:1041–1047. doi: 10.1212/wnl.38.7.1041. [DOI] [PubMed] [Google Scholar]
- Hatai S. Observations on the developing neurones of the cerebral cortex of foetal cats. J Comp Neurol. 1902;12:199–204. [Google Scholar]
- Henle J. Braunschweig: Druck und Verlag von Friedrich Vieweg und Sohn; 1879. Handbuch der Nervenlehre des Menschen. Mit zahlreichen in den Text eingedruckten Holzstichen. Zweite verbesserte Auflage. [Google Scholar]
- Heuer J, Christ S, Friedrichsen S, et al. Connective tissue growth factor: a novel marker of layer VII neurons in the rat cerebral cortex. Neuroscience. 2003;119:43–52. doi: 10.1016/s0306-4522(03)00100-3. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Neogi T, Englund C, et al. Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin. Brain Res Dev Brain Res. 2003;141:39–53. doi: 10.1016/s0165-3806(02)00641-7. [DOI] [PubMed] [Google Scholar]
- His W. Die Entwickelung des menschlichen Gehirns während der ersten Monate. Leipzig: S. Hirzel; 1904. [Google Scholar]
- Hoerder-Suabedissen A, Wang WZ, Lee S, et al. Novel marker reveals subpopulation of subplate neurons in the murine cerebral cortex. Cereb Cortex. 2009;19:1738–1750. doi: 10.1093/cercor/bhn195. [DOI] [PubMed] [Google Scholar]
- Humphrey T. Intramedullary sensory ganglion cells in the roof plate area of the embryonic human spinal cord. J Comp Neurol. 1950;92:333–400. doi: 10.1002/cne.900920304. [DOI] [PubMed] [Google Scholar]
- Hunt WE, Goldring S. Maturation of evoked response of the visual cortex in the postnatal rabbit. Electroencephalogr Clin Neurophysiol. 1951;3:465–471. doi: 10.1016/0013-4694(51)90034-x. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ikeda K, Iritani S, et al. Distribution of neuropeptide Y interneurons in the dorsal prefrontal cortex of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:379–383. doi: 10.1016/j.pnpbp.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Ishii K, Murata A, Yoshimura K, et al. A novel monoclonal antibody K1 recognises early neurones in the rat cortex. Neurosci Res. 2001;39:31–37. doi: 10.1016/s0168-0102(00)00197-8. [DOI] [PubMed] [Google Scholar]
- Jacob H. Faktoren bei der Entstehung der normalen und der entwicklungsgestörten Hirnrinde. Z ges Neurol Psychiat. 1936;155:1–39. [Google Scholar]
- Jakob A. Zur Pathologie der Epilepsie. Z ges Neurol Psychiat. 1914;23:1–65. [Google Scholar]
- Jiménez D, Rivera R, López-Mascaraque L, et al. Origin of the cortical layer I in rodents. Dev Neurosci. 2003;25:105–115. doi: 10.1159/000072260. [DOI] [PubMed] [Google Scholar]
- Judaš M, Sedmak G, Pletikos M, et al. Populations of subplate and interstitial neurons in fetal and adult human telencephalon. J Anat. 2010;217:381–399. doi: 10.1111/j.1469-7580.2010.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Conley RC, Kakoyannis A, et al. Interstitial cells of the white matter in the inferior parietal cortex in schizophrenia: an unbiased cell-counting study. Synapse. 1999;34:95–102. doi: 10.1002/(SICI)1098-2396(199911)34:2<95::AID-SYN2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias NC, Conley RR, et al. Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. J Nerv Ment Dis. 2003;191:563–567. doi: 10.1097/01.nmd.0000087181.61164.e1. [DOI] [PubMed] [Google Scholar]
- Knyihar E, Csillik B, Rakic P. Transient synapses in the embryonic primate spinal cord. Science. 1978;202:1206–1209. doi: 10.1126/science.103200. [DOI] [PubMed] [Google Scholar]
- Kolk SM, Whitman MC, Yun ME, et al. A unique subpopulation of Tbr1-expressing deep layer neurons in the developing cerebral cortex. Mol Cell Neurosci. 2001;30:538–551. doi: 10.1016/j.mcn.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Kostović I. Prenatal development of nucleus basalis complex and related fibre systems in man: a histochemical study. Neuroscience. 1986;17:1047–1077. doi: 10.1016/0306-4522(86)90077-1. [DOI] [PubMed] [Google Scholar]
- Kostović I, Goldman-Rakic PS. Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol. 1983;219:431–447. doi: 10.1002/cne.902190405. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. Correlation between sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002;267:1–6. doi: 10.1002/ar.10069. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. Prolonged coexistence of transient and permanent circuitry elements in the developing cerebral cortex of fetuses and preterm infants. Dev Med Child Neurol. 2006;48:388–393. doi: 10.1017/S0012162206000831. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev. 2007;31:1157–1168. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr. 2010;99:1119–1127. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- Kostović I, Krmpotić J. Early prenatal ontogenesis of the neuronal connections in the interhemispheric cortex of the human gyrus cinguli. Verh Anat Ges. 1976;70:305–316. [PubMed] [Google Scholar]
- Kostović I, Molliver ME. A new interpretation of the laminar development of cerebral cortex: synaptogenesis in different layers of neopallium in the human fetus. Anat Rec. 1974;178:395. [Google Scholar]
- Kostović I, Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9:219–242. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Kostović I, Rakic P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient acetylcholinesterase staining. J Neurosci. 1984;4:25–42. doi: 10.1523/JNEUROSCI.04-01-00025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M, Radoš M, et al. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M, Sedmak G. Developmental history of the subplate zone, subplate neurons and interstitial white matter neurons: relevance for schizophrenia. Int J Dev Neurosci. 2010 doi: 10.1016/j.ijdevneu.2010.09.005. in press. [DOI] [PubMed] [Google Scholar]
- Krmpotić-Nemanić J, Kostović I, Kelović Z, et al. Development of the human fetal auditory cortex: growth of afferent fibers. Acta Anat. 1983;116:69–73. [PubMed] [Google Scholar]
- Luhmann HJ, Kilb W, Hanganu-Opatz I. Subplate cells: amplifiers of neuronal activity in the developing cerebral cortex. Front Neuroanat. 2009 doi: 10.3389/neuro.05.019.2009. 31–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, et al. G1 phase regulation, area-specific cell cycle control, and cyto-architectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ. Studies of the earliest generated cells of the cat's visual cortex: cogeneration of subplate and marginal zones. J Neurosci. 1985;5:1062–1075. doi: 10.1523/JNEUROSCI.05-04-01062.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. Early prenatal ontogenesis of the cerebral cortex (neocortex) of the cat (Felis domestica). A Golgi study. I. The primordial neocortical organization. Z Anat Entwicklungsgesch. 1971;134:117–145. doi: 10.1007/BF00519296. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Prenatal ontogenetic history of the principal neurons of the neocortex of the cat (Felis domestica). A Golgi study. II. Developmental differences and their significances. Z Anat Entwicklungsgesch. 1972;136:125–142. doi: 10.1007/BF00519174. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Dual origin of the mammalian neocortex and evolution of the cortical plate. Anat Embryol. 1978;152:109–126. doi: 10.1007/BF00315920. [DOI] [PubMed] [Google Scholar]
- Meencke HJ. The density of dystopic neurons in the white matter of the gyrus frontalis inferior in epilepsies. J Neurol. 1983;230:171–181. doi: 10.1007/BF00313628. [DOI] [PubMed] [Google Scholar]
- Meencke HJ, Janz D. Neuropathological findings in primary generalized epilepsy: a study of eight cases. Epilepsia. 1984;25:8–21. doi: 10.1111/j.1528-1157.1984.tb04149.x. [DOI] [PubMed] [Google Scholar]
- Meinecke DL, Rakic P. Expression of GABA and GABA-A receptors by neurons of the subplate zone in developing primate occipital cortex: evidence for transient local circuits. J Comp Neurol. 1992;317:91–101. doi: 10.1002/cne.903170107. [DOI] [PubMed] [Google Scholar]
- Meyer G. Genetic control of neuronal migrations in human cortical development. Adv Anat Embryol Cell Biol. 2007;189:1–111. [PubMed] [Google Scholar]
- Meyer G, Wahle P. Early postnatal development of cholecystokinin-immunoreactive structures in the visual cortex of the cat. J Comp Neurol. 1988;276:360–386. doi: 10.1002/cne.902760304. [DOI] [PubMed] [Google Scholar]
- Meyer G, Schaaps JP, Moreau L, et al. Embryonic and early fetal development of the human neocortex. J Neurosci. 2000;20:1858–1868. doi: 10.1523/JNEUROSCI.20-05-01858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynert T. Der Bau der Grosshirnrinde und seine örtlichen Verschiedenheiten, nebst einem pathologisch-anatomischen Corollarium. Vierteljschr Psychiat. 1867;1:77–93, 126–170, 198–217. [Google Scholar]
- Meynert T. Vom Gehirn der Säugetiere. In: Stricker S, editor. Handbuch der Lehre von den Geweben des Meenschen und der Thiere. Leipzig: Wilhelm Engelmann; 1872. pp. 694–808. Bd. 2. [Google Scholar]
- Meynert T. Klinik der Erkrankungen des Vorderhirns begründet auf dessen Bau, Leistungen und Ernährung. Vienna: Wilhelm Braumüller; 1884. Psychiatrie. [Google Scholar]
- Mischel PS, Nguyen LP, Vinters HV. Cerebral cortical dysplasia associated with pediatric epilepsy. Review of neuropathologic features and proposal for a grading system. J Neuropathol Exp Neurol. 1995;54:137–153. doi: 10.1097/00005072-199503000-00001. [DOI] [PubMed] [Google Scholar]
- Molliver ME. An ontogenetic study of evoked somesthetic cortical responses in the sheep. Prog Brain Res. 1967;26:78–91. doi: 10.1016/S0079-6123(08)61420-X. [DOI] [PubMed] [Google Scholar]
- Molliver ME, Van der Loos H. The ontogenesis of cortical circuitry: the spatial distribution of synapses in somesthetic cortex of newborn dog. Ergeb Anat Entwicklungsgesch. 1970;42:1–53. [PubMed] [Google Scholar]
- Molliver ME, Kostović I, Van der Loos H. The development of synapses in cerebral cortex of the human fetus. Brain Res. 1973;50:403–407. doi: 10.1016/0006-8993(73)90741-5. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Brown RE. Insights into the life and work of Sir Charles Sherrington. Nat Rev Neurosci. 2010;11:429–436. doi: 10.1038/nrn2835. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Métin C, Stoykova A, et al. Comparative aspects of cerebral cortical development. Eur J Neurosci. 2006;23:921–934. doi: 10.1111/j.1460-9568.2006.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubürger K. Zur Histopathologie der multiplen Sklerose im Kindesalter. Z ges Neurol Psychiat. 1922;76:384–414. [Google Scholar]
- Noback CR, Purpura DP. Postnatal ontogenesis of neurons in cat neocortex. J Comp Neurol. 1961;117:291–307. doi: 10.1002/cne.901170303. [DOI] [PubMed] [Google Scholar]
- Oppermann K. Cajalsche Horizontalzellen und Gang-lienzellen des Marks. Z ges Neurol Psychiat. 1929;120:121–137. [Google Scholar]
- Poliakov GI. Structural organization of the human cerebral cortex during ontogenetic development. In: Sarkisov SA, Filimonov IN, Preobrazenskaya NS, editors. Cytorchitectonics of the Cerebral Cortex in Man [in Russian] Moscow: Medgiz; 1949. pp. 33–92. [Google Scholar]
- Poliakov GI. Progressive neuron differentiation of the human cerebral cortex in ontogenesis. In: Sarkisov SA, Preobrazenskaya NS, editors. Development of the Central Nervous System [in Russian] Moscow: Medgiz; 1959. pp. 11–26. [Google Scholar]
- Poliakov GI. Some results of research into the development of the neuronal structure of the cortical ends of the analyzers in man. J Comp Neurol. 1961;117:197–212. doi: 10.1002/cne.901170206. [DOI] [PubMed] [Google Scholar]
- Poliakov GI. Development of the cerebral neocortex during first half of intrauterine life. In: Sarkisov SA, editor. Development of the Child's Brain. Leningrad: Meditsina; 1965. pp. 22–52. [in Russian] [Google Scholar]
- Poljak S. Ueber die sogenannten versprengten Gang-lienzellen in der weissen Substanz des menschlichen Rückenmarkes (Mit 13 Abbildungen im Text) Arb Neurol Inst Wiener Univ. 1922;23:1–20. [Google Scholar]
- Poljakow GI. Schönheit. Leipzig: VEB Georg Thieme; 1979. Entwicklung der Neuronen der menschlichen Grosshirnrinde (Herausgegeben von B. [Google Scholar]
- Pollak E. Anlage und Epilepsie (Mit 5 Abbildungen im Text) Arb Neurol Inst Wiener Univ. 1922;23:119–147. [Google Scholar]
- Polyak S. The Retina. Chicago: The University of Chicago Press; 1941. [Google Scholar]
- Polyak S. The Vertebrate Visual System. Chicago: The University of Chicago Press; 1957. [Google Scholar]
- Purpura DP. Structure and function of cortical synaptic organizations activated by corticopetal afferents in newborn cat. Brain Behav. 1961a;1:95–138. [Google Scholar]
- Purpura DP. Morphological basis of elementary evoked response patterns in the neocortex of the newborn cat. Ann NY Acad Sci. 1961b;92:840–859. doi: 10.1111/j.1749-6632.1961.tb40961.x. [DOI] [PubMed] [Google Scholar]
- Purpura DP. Analysis of axodendritic synaptic organizations in immature cerebral cortex. Ann NY Acad Sci. 1961c;94:604–654. doi: 10.1111/j.1749-6632.1961.tb35561.x. [DOI] [PubMed] [Google Scholar]
- Purpura DP, Carmichael MW, Housepian EM. Physiological and anatomical studies of development of superficial axodendritic synaptic pathways in neocortex. Exp Neurol. 1960;2:324–347. doi: 10.1016/0014-4886(60)90019-4. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in the rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977;278:245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Rakic P. Early developmental events: cell lineages, acquisition of neuronal positions, and areal and laminar development. Neurosci Res Program Bull. 1982;20:439–451. [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Emerging complexity of layer I in human cerebral cortex. Cereb Cortex. 2003;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Sobre las fibras nerviosas de la capa molecular del cerebelo. Rev Trimestral Histol Norm Patol. 1888;1:33–49. [Google Scholar]
- Ramón y Cajal S. Sobre las fibras nerviosas de la capa granulosa del cerebelo. Rev Trimestral Histol Norm Patol. 1889a;1:107–118. [Google Scholar]
- Ramón y Cajal S. Sur l'origine et la direction des prolongations nerveuses de la couche moléculaire du cervelet (Avec pl. XVIII et XIX.) Internat Mschr Anat Physiol. 1889b;6:158–174. [Google Scholar]
- Ramón y Cajal S. Sur les fibres nerveuses de la couche granuleuse du cervelet et sur l’évolution des éléments cérébelleux. Internat Mschr Anat Physiol. 1890a;7:12–31. [Google Scholar]
- Ramón y Cajal S. A propos de certains éléments bipolaires du cervelet avec quelques détails nouveaux sur l’évolution des fibres cérébelleuses. Internat Mschr Anat Physiol. 1890b;7:447–468. [Google Scholar]
- Ramón y Cajal S. Barcelona: Gaceta Sanitaria; 1891a. Notas preventivas sobre la retina y gran simpatico de los mamíferos (extracts) [Google Scholar]
- Ramón y Cajal S. Sur la structure de l’écorce cérébrale de quelques mammiferes. Cellule. 1891b;7:125–176. [Google Scholar]
- Ramón y Cajal S. El plexo de Auerbach de los Batracios. 1892. pp. 23–28. Trabajos del laboratorio de histologia de la Facultad de medicina de Barcelona.
- Ramón y Cajal S. Madrid: 1893. Los ganglios y plexos nerviosos del intestino de los mamíferos y pequenas adiciones a nuestros trabajos sobre la medula y gran simpatico general; p. 45. 1893. [Google Scholar]
- Ramón y Cajal S. Madrid: Don I. Bolívar, Tesorero; 1895. Apuntes para el estudio del bulbo raquídeo, cerebelo y orígen de los nervios encefálicos (Memorias de historia natural – Sesión del 6 de Febrero de 1895). Anal Soc Espan Histor NatSerie II. Tomo Cuarto (XXIV). pp, 5–118, el Cuaderno 1.°. (III. Corteza del cerebelo, pp. 25–26) [Google Scholar]
- Ramón y Cajal S. Beitrag zum Studium der Medulla oblongata, des Kleinhirns und des Ursprungs der Gehirnnerven. Deutsche vom Verfasser erweiterte Ausgabe besorgt von Johannes Bresler, mit einem Vorwort von E. Mendel. ch. III: Kleinhirnrinde. Leipzig: Verlag von Johann Ambrosius Barth; 1896. [Google Scholar]
- Ramón y Cajal S. Apuntes para el estudio estructural de la corteza visual del cerebro humano. Rev Ibero-Americana Cienc Méd. 1899a;1:146–157. [Google Scholar]
- Ramón y Cajal S. Estudios sobre la corteza cerebral humana I: corteza visual. Rev Trimestral Micrográf Madrid. 1899b;4:1–63. [Google Scholar]
- Ramón y Cajal S. Estudios sobre la corteza cerebral humana II: estructura de la corteza motriz del hombre y mamiferos superiores. Rev Trimestral Micrográf Madrid. 1899c;4:117–200. [Google Scholar]
- Ramón y Cajal S. Estudios sobre la corteza cerebral humana II: corteza motriz (conclusión) Rev Trimestral Micrográf Madrid. 1900a;5:1–11. [Google Scholar]
- Ramón y Cajal S. Estudios sobre la corteza cerebral humana III: Corteza acustica. Rev Trimestral Micrográf Madrid. 1900b;5:129–183. [Google Scholar]
- Ramón y Cajal S. In: Studien über die Hirnrinde des Menschen. I. Die Sehrinde. Bresler JA, translator. Leipzig: Barth; 1900c. [Google Scholar]
- Ramón y Cajal S. In: Studien über die Hirnrinde des Menschen. II. Die Bewegungsrinde. Bresler JA, translator. Leipzig: Barth; 1900d. [Google Scholar]
- Ramón y Cajal S. Estudios sobre la corteza cerebral humana. IV. Estructura de la corteza cerebral olfativa del hombre y mamíferos. Trab Lab Invest biol Univ Madrid. 1901;1:1–140. [Google Scholar]
- Ramón y Cajal S. Sobre un ganglio especial de la corteza esfeno-occipital. Trab Lab Invest biol Univ Madrid. 1902a;1:189–206. [Google Scholar]
- Ramón y Cajal S. In: Studien über die Hirnrinde des Menschen. III. Die Hörrinde. Bresler JA, translator. Leipzig: Barth; 1902b. [Google Scholar]
- Ramón y Cajal S. In: Studien über die Hirnrinde des Menschen. IV. Die Riechrinde beim Menschen und Säugetiere. Bresler JA, translator. Leipzig: Barth; 1903. [Google Scholar]
- Ramón y Cajal S. Histologie du Systeme Nerveux de l’Homme & des Vertébrés. Edition francaise revue & mise a jour par l’Auteur, traduite de l’Espagnol par le Dr. L. Azoulay. Tome I. Généralités, Moelle, Ganglions rachidiens, Bulbe & Protubérance. Paris: A. Maloine; 1909. [Google Scholar]
- Ramón y Cajal S. Histologie du Systeme Nerveux de l’Homme & des Vertébrés. Edition francaise revue & mise a jour par l’Auteur, traduite de l’Espagnol par le Dr. L. Azoulay. Tome II. Cervelet, Cerveau moyen, Rétine, Couche optique, Corps strié, Ecorce cérébrale générale & régionale, Grand sympathique. Paris: A. Maloine; 1911. [Google Scholar]
- Ramón y Cajal S. Die Neuronenlehre. In: Bumke O, Foerster O, editors. Handbuch der Neurologie. Erster Band. Allgemeine Neurologie I: Anatomie. Berlin: Verlag von Julius Springer; 1935. pp. 887–994. [Google Scholar]
- Ramón y Cajal S. Histology of the nervous system of man and vertebrates. In: Swanson Neely, Swanson Larry W., translators. General Principles, Spinal Cord, Spinal Ganglia, Medulla & Pons. New York: Oxford University Press; 1995a. Volume One. [Google Scholar]
- Ramón y Cajal S. Histology of the Nervous System of Man and Vertebrates. In: Swanson Neely, Swanson Larry W., translators. Cerebellum, Midbrain, Retina, Diencephalon, Corpus Striatum, Cerebral Cortex – in General and Regional, Autonomic System. New York: Oxford University Press; 1995b. Volume Two. [Google Scholar]
- Ramón y Cajal S, Sala y Pons C. Terminación de los nervios y tubas glandulares del pancreas de los vertebrados. Barcelona: 1891. [Google Scholar]
- Ranke O. Beiträge zur Kenntnis der normalen und pathologischen Hirnrindenbildung. Beitr Pathol Anat. 1910;47:51–125. [Google Scholar]
- Reep RL. Cortical layer VII and persistent subplate cells in mammalian brains. Brain Behav Evol. 2000;56:212–234. doi: 10.1159/000047206. [DOI] [PubMed] [Google Scholar]
- Reep RL, Goodwin GS. Layer VII of rodent cerebral cortex. Neurosci Lett. 1988;90:15–20. doi: 10.1016/0304-3940(88)90779-3. [DOI] [PubMed] [Google Scholar]
- Retzius G. Kleinere Mittheilungen von dem Gebiete der Nervenhistologie. I. Ueber die Golgi'schen Zellen und die Kletterfasern Ramón y Cajal's in der Kleinhirnrinde. Biol Unters Neue Folge. 1892;4:57–59. [Google Scholar]
- Retzius G. Die Cajal'schen Zellen der Grosshirnrinde beim Menschen und bei Säugethieren. Biol Unters Neue Folge. 1893;5:1–8. [Google Scholar]
- Rickmann M, Chronwall BM, Wolff JR. On the development of non-pyramidal neurons and axons outside the cortical plate: the early marginal zone as a pallial anlage. Anat Embryol. 1977;151:285–307. doi: 10.1007/BF00318931. [DOI] [PubMed] [Google Scholar]
- Rioux L, Nissanov J, Lauber K, et al. Distribution of microtubule-associated protein MAP2-immunoreactive inter-stitial neurons in the parahippocampal white matter in subjects with schizophrenia. Am J Psychiatry. 2003;160:149–155. doi: 10.1176/appi.ajp.160.1.149. [DOI] [PubMed] [Google Scholar]
- Robertson RT, Annis CM, Baratta J, et al. Do subplate neurons comprise a transient population of cells in developing neocortex of rats? J Comp Neurol. 2000;426:632–650. doi: 10.1002/1096-9861(20001030)426:4<632::aid-cne10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Rojiani AM, Emery JA, Anderson KJ, et al. Distribution of heterotopic neurons in normal hemispheric white matter: a morphometric analysis. J Neuropathol Exp Neurol. 1996;55:178–183. doi: 10.1097/00005072-199602000-00006. [DOI] [PubMed] [Google Scholar]
- Rondoni P. Beiträge zum Studium der Entwickelungsk-rankheiten des Gehirns (Hierzu 14 Abbildungen im Text und Tafel VII–IX.). Erster Teil. Ueber hereditäre Hirnlues und juvenile progressive Paralyse. Part 2. Ueber die Bioldung der Strata der Hirnrinde unter normalen und pathologischen Verhältnissen. Arch Psychiatr Nervenkr. 1909;45:1004–1096. [Google Scholar]
- Sarnat HB. Cerebral Dysgenesis: Embryology and Clinical Expression. New York: Oxford University Press; 1992. [Google Scholar]
- Schiefferdecker P. Beiträge zur Kenntnis des Faserverlaufs im Rückenmark. Arch Mikr Anat. 1874;10:471–494. [Google Scholar]
- Schwartz ML, Goldman-Rakic PS. Prenatal specification of callosal connections in rhesus monkey. J Comp Neurol. 1991;307:144–162. doi: 10.1002/cne.903070113. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Rakic P, Goldman-Rakic PS. Early phenotype expression of cortical neurons: evidence that a subclass of migrating neurons have callosal axons. Proc Natl Acad Sci U S A. 1991;88:1354–1358. doi: 10.1073/pnas.88.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. On out-lying nerve cells in the mammalian spinal cord. Philos Trans R Soc Lond B Biol Sci. 1890;181:33–48. [Google Scholar]
- Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res. 1973;62:1–35. doi: 10.1016/0006-8993(73)90617-3. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Development of the human central nervous system. In: Haymaker W, Adams RD, editors. Histology and Histopathology of the Nervous System. Springfield: Thomas; 1982. pp. 3–145. [Google Scholar]
- Smart IHM, Dehay C, Giroud P, et al. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa JM, Andrew W. Heterotopic neurons of the sensory type in the intramedullary portion of the motor roots. J Comp Neurol. 1947;86:167–182. doi: 10.1002/cne.900860203. [DOI] [PubMed] [Google Scholar]
- Stöhr P., Jr . Die Anteile des cerebrospinalen Nervensystems. Die peripherischen Anteile des vegetativen Nervensystems. In: Von Möllendorff W, editor. Handbuch der mikroskopischen Anatomie des Menschen. Vol. 4. Nervensystem. Part 1. Nervengewebe – Das peripherische Nervensystem – Das Zentralnervensystem. Berlin: Verlag von Julius Springer; 1928. pp. 202–447. [Google Scholar]
- Stricker S. Part 1. Leipzig: Verlag von Wilhelm Engelmann; 1871. Handbuch der Lehre von den Geweben des Menschen und der Thiere. [Google Scholar]
- Suárez-Solá ML, González-Delgado FJ, Pueyo-Morlans M, et al. Neurons in the white matter of the adult human neocortex. Front Neuroanat. 2009;3:1–6. doi: 10.3389/neuro.05.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita N. Comparative studies on the growth of the cerebral cortex. II. On the increase in the thickness of the cerebral cortex during the postnatal growth of the brain. Albino rat. J Comp Neurol. 1917;28:511–591. [Google Scholar]
- Taylor DC, Falconer MA, Bruton CJ, et al. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry. 1971;34:541–552. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka R, Okamoto K, Furuta T, et al. Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur J Neurosci. 2005;21:1587–1600. doi: 10.1111/j.1460-9568.2005.03989.x. [DOI] [PubMed] [Google Scholar]
- Valverde F, Facal-Valverde MV. Transitory population of cells in the temporal cortex of kittens. Brain Res Dev Brain Res. 1987;32:283–288. doi: 10.1016/0165-3806(87)90108-8. [DOI] [PubMed] [Google Scholar]
- Valverde F, Facal-Valverde MV. Postnatal development of interstitial (subplate) cells in the white matter of the temporal cortex of kittens: a correlated Golgi and electron microscopic study. J Comp Neurol. 1988;269:168–192. doi: 10.1002/cne.902690203. [DOI] [PubMed] [Google Scholar]
- Valverde F, Facal-Valverde MV, Santacana M, et al. Development and differentiation of early generated cells of sublayer VIb in the somatosensory cortex of the rat: a correlated Golgi and autoradiographic study. J Comp Neurol. 1989;290:118–140. doi: 10.1002/cne.902900108. [DOI] [PubMed] [Google Scholar]
- Valverde F, López-Mascaraque L, Santacana M, et al. Persistence of early-generated neurons in the rodent subplate: assessment of cell death in neocortex during the early postnatal period. J Neurosci. 1995;15:5014–5024. doi: 10.1523/JNEUROSCI.15-07-05014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voeller K, Pappas GD, Purpura DP. Electron microscope study of development of cat superficial neocortex. Exp Neurol. 1963;7:107–130. doi: 10.1016/s0014-4886(63)80003-5. [DOI] [PubMed] [Google Scholar]
- Vogt H. Ueber die Anatomie, das Wesen und die Entstehung mikrocephaler Missbildungen nebst Beiträgen über die Entwickelungsstörungen der Architektonik des Zentralnervensystems. Arb Hirnanat Inst Zürich. 1905;1:1–203. [Google Scholar]
- Vogt C, Vogt O. Allgemeinere Ergebnisse unserer Hirnforschung. J Psychol Neurol (Leipzig) 1919;25(Suppl 1):5–462. [Google Scholar]
- Von Economo C, Koskinas GN. Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. Vienna: Verlag von Julius Springer; 1925. [Google Scholar]
- Von Lenhossek M. Ueber den Verlauf der Hinterwurzeln im Rückenmark. Arch Mikr Anat. 1889;34:157–197. [Google Scholar]
- Von Monakow C. Ergebnisse der Allgemeinen Pathologie und Pathologischen Anatomie des Menschen und der Tiere (von Lubarsch & Ostertag), 6th year: 1899. Wiesbaden: Verlag von J.F. Bergmann; 1901. Ueber die Missbildungen des Centralnervensystems; pp. 513–582. [Google Scholar]
- Wahle P, Meyer G. Morphology and quantitative changes of transient NPY-ir neuronal populations during early postnatal development of the cat visual cortex. J Comp Neurol. 1987;261:165–192. doi: 10.1002/cne.902610202. [DOI] [PubMed] [Google Scholar]
- Wahle P, Meyer G. Early postnatal development of vasoactive intestinal polypeptide- and peptide histidine isoleucine-immunoreactive structures in the cat visual cortex. J Comp Neurol. 1989;282:215–248. doi: 10.1002/cne.902820206. [DOI] [PubMed] [Google Scholar]
- Wahle P, Meyer G, Wu JY, et al. Morphology and axon terminal pattern of glutamate decarboxylase-immunoreactive cell types in the white matter of the cat occipital cortex during early postnatal development. Brain Res Dev Brain Res. 1987;36:53–61. doi: 10.1016/0165-3806(87)90064-2. [DOI] [PubMed] [Google Scholar]
- Wohlwill F. Entwicklungsstörungen des Gehirns und Epilepsie. Zugleich ein Beitrag zur pathologischen Anatomie und genese der Heterotopien grauer Substanz. Z ges Neurol Psychiat. 1916;33:261–293. [Google Scholar]
- Wolf HK, Weistler OD. Surgical pathology of chronic epileptic disorders. Brain Pathol. 1993;3:371–380. doi: 10.1111/j.1750-3639.1993.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Zenter J, Hufnagel A, et al. Surgical pathology of chronic epileptic seizure disorders: experience with 63 specimens with extra-temporal corticectomies, lobectomies and functional hemispherectomies. Acta Neuropathol. 1993;86:466–472. doi: 10.1007/BF00228581. [DOI] [PubMed] [Google Scholar]
- Youngstrom KA. Intramedullary sensory type ganglion cells in the spinal cord of human embryos. J Comp Neurol. 1944;81:47–53. [Google Scholar]
- Zecevic N, Rakic P. Development of layer I neurons in the primate cerebral cortex. J Neurosci. 2001;21:5607–5619. doi: 10.1523/JNEUROSCI.21-15-05607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Milosevic A, Rakic S, et al. Early development and composition of the human primordial plexiform layer: an immunohistochemical study. J Comp Neurol. 1999;412:241–254. [PubMed] [Google Scholar]


