Abstract
The subplate is a largely transient zone containing precocious neurons involved in several key steps of cortical development. The majority of subplate neurons form a compact layer in mouse, but are dispersed throughout a much larger zone in the human. In rodent, subplate neurons are among the earliest born neocortical cells, whereas in primate, neurons are added to the subplate throughout cortical neurogenesis. Magnetic resonance imaging and histochemical studies show that the human subplate grows in size until the end of the second trimester. Previous microarray experiments in mice have shown several genes that are specifically expressed in the subplate layer of the rodent dorsal cortex. Here we examined the human subplate for some of these markers. In the human dorsal cortex, connective tissue growth factor-positive neurons can be seen in the ventricular zone at 15–22 postconceptional weeks (PCW) (most at 17 PCW) and are present in the subplate at 22 PCW. The nuclear receptor-related 1 protein is mostly expressed in the subplate in the dorsal cortex, but also in lower layer 6 in the lateral and perirhinal cortex, and can be detected from 12 PCW. Our results suggest that connective tissue growth factor- and nuclear receptor-related 1-positive cells are two distinct cell populations of the human subplate. Furthermore, our microarray analysis in rodent suggested that subplate neurons produce plasma proteins. Here we demonstrate that the human subplate also expresses α2zinc-binding globulin and Alpha-2-Heremans-Schmid glycoprotein/human fetuin. In addition, the established subplate neuron marker neuropeptide Y is expressed superficially, whereas potassium/chloride co-transporter (KCC2)-positive neurons are localized in the deep subplate at 16 PCW. These observations imply that the human subplate shares gene expression patterns with rodent, but is more compartmentalized into superficial and deep sublayers. This increased complexity of the human subplate may contribute to differential vulnerability in response to hypoxia/ischaemia across the depth of the cortex. Combining knowledge of cell-type specific subplate gene expression with modern imaging methods will enable a better understanding of neuropathologies involving the subplate.
Keywords: cerebral cortex, connective tissue growth factor, magnetic resonance imaging, nuclear receptor-related 1, plasma proteins, subplate, thalamocortical projections
Introduction
The subplate is defined as a transient zone below the cortical plate and above the intermediate zone in the developing cortex (Bystron et al. 2008). It was first described in the human cortex (Kostović & Molliver, 1974) and then in the fetal macaque (Rakic, 1977), carnivores (Luskin & Shatz, 1985) and rat (Rickmann et al. 1977). Marin-Padilla (1971) described the splitting of the earliest postmitotic cortical layer, the primordial plexiform zone, into the marginal zone and subplate in carnivores, a finding that was subsequently confirmed with birthdating studies by Luskin & Shatz (1985). In human, the subplate starts to appear as a more superficial compartment of the intermediate zone termed the presubplate at 7–8 postconceptional weeks (PCW) (Carnegie stages 20–22) at the ventrolateral level of the telencephalic wall (Zecevic, 1993; Bystron et al. 2008), whereas the deeper part of the intermediate zone forms the future white matter. In rodents, the subplate layer is much smaller but nevertheless clearly defined (Lund & Mustari, 1977; Molnár, 2000; Hoerder-Suabedissen et al. 2009). In species investigated so far, subplate neurons are among the first-born neurons in the telencephalic wall and start establishing the first synapses by midgestation (Molliver & Van der Loos, 1970). The first description of early synaptogenesis in the rat subplate below the cortical plate was by König et al. (1975) and the description of the waiting period of the thalamocortical projections in rat was published by Lund & Mustari (1977). The earliest functional circuit of the neocortex forms in the subplate zone by midgestation in both humans and rodents (Allendoerfer & Shatz, 1994; Kanold & Luhmann, 2010).
Multiple functions of subplate neurons during cortical development
Subplate neurons play an important role in thalamocortical axon pathfinding at the level of the initial areal targeting (Ghosh et al. 1990; Molnár & Blakemore, 1995; Catalano & Shatz, 1998; López-Bendito & Molnár, 2003) as well as the eventual innervation of cortical layer 4 by thalamic afferents and establishment of optical orientation columns (Kanold et al. 2003). They are also necessary for the maturation of inhibition in cortical layer 4 in areas innervated by the thalamus (Kanold & Shatz, 2006), and drive oscillations in the gap junction-coupled early cortical syncytium (Dupont et al. 2006). During development, subplate neurons are electrically active and capable of firing action potentials (Hanganu et al. 2001; Moore et al. 2009) while incorporating, at least transiently, into the cortical and subcortical circuitry (Friauf & Shatz, 1991; Higashi et al. 2002, 2005; Kanold et al. 2003; Piñon et al. 2009). Subplate neurons develop both intracortical and subcortical projections (Allendoerfer & Shatz, 1994). Both electrophysiological properties and cell morphology point to a high degree of underlying diversity among subplate neurons (Antonini & Shatz, 1990; Hanganu et al. 2001; Hoerder, 2007). There are two basic classes of neuronal phenotypes in the subplate, glutamatergic and γ-aminobutyric acid (GABA)ergic (Antonini & Shatz, 1990; Meinecke & Rakic, 1992), with each class expressing heterogeneous molecular markers (Allendoerfer & Shatz, 1994; Hevner & Zecevic, 2006; Hoerder-Suabedissen et al. 2009). It is not yet clear whether the same types of subplate neurons possess intracortical and extracortical projections and how the diverse somatodendritic morphologies relate to the equally diverse neurochemical properties and physiological fingerprints.
Species differences in subplate size, subplate neurogenesis and adult survival
In any species, the subplate is a highly dynamic compartment in the developing cortex containing both stationary and radially and tangentially migrating glutamatergic and GABAergic neurons, various corticopetal and corticofugal projections, glial cells and blood vessels (Hevner & Zecevic, 2006; Kanold & Luhmann, 2010). There are huge differences between the sizes of the human and mouse cerebral cortex, with corresponding differences in the duration of cortical neurogenesis (Takahashi et al. 1995; Kornack & Rakic, 1998). Therefore, it is not a surprise that there are differences in the relative proportions of the subplate to the cortical plate and in the transient nature between the rodent (mouse and rat) and primate (macaque and human) subplate.
In rodents, most of the subplate cells are in a thin band of cells separating the white matter from layer 6, but occasionally some scattered cells in the upper intermediate zone are also considered as part of the rodent subplate (Zhao et al. 2009). In rat, layer 6b becomes distinct from the rest of the cortical plate around embryonic day (E)16–18 (Lund & Mustari, 1977). Birthdating studies in rodent revealed that the subplate is among the earliest generated and earliest mature cortical neuron population (Bayer & Altman, 1990). Murine subplate cells are born around E11 (Price et al. 1997), just past midway through the mouse gestational period.
The proportion of the subplate in relation to the rest of the cortical compartments is much greater in the macaque or human cortex (Kostović & Rakic, 1990) than in the rodent cortex. In human, the subplate zone proper becomes visible as a cell-poor/fibre-rich layer situated between the intermediate zone and cortical plate (Kostović & Rakic, 1990; Meyer, 2007) at around 14/15 PCW, which is the beginning of the second trimester of human gestation. It forms from the merging of the deepest layer of the cortical plate, analogous to layer 6b of the rodent, with an already formed presubplate that contains few neurons but a differentiated neuropil featuring dendritic arborizations (Mrzljak et al. 1988) and synapses (Kostović & Rakic, 1990), which include GABAergic elements (Bayatti et al. 2008) and monoaminergic innervation from the brainstem (Zecevic & Verney, 1995). This coincides with the invasion of the subplate region by thalamocortical afferents (Kostović & Goldman-Rakic, 1983; Kostović & Rakic, 1984) as well as basal forebrain afferents (Kostović, 1986) leading to rapid expansion of the subplate such that it comprises 35% of the cerebral wall by 16 PCW. In contrast to the rodent subplate, birthdating studies in primates revealed that neurons are continuously added to the subplate until relatively late stages of corticogenesis (Smart et al. 2002; Lukaszewicz et al. 2005; Molnár et al. 2006). From 14 to 25 PCW in human a large number of T-box brain gene 1 (TBR1)-positive neurons are continuously added to the subplate compartment, which increases in width concurrent with the growth of the cortical plate, with the highest density of cells always found at the border between the cortical plate and subplate (Meyer, 2007). The subplate reaches its maximum thickness at the late second and early third trimester, and thereafter the subplate gradually decreases in size and becomes unrecognizable around the sixth postnatal month (Kostović & Rakic, 1990). Some of the interstitial white matter cells found in the adult cortex are considered as remnants of the subplate cells (Kostović & Rakic, 1990), whereas others arise in the intermediate zone (see Judas et al., this issue). A comparative database of neurodevelopment suggests that the timing of early events of cortical development is remarkably conserved between rodent and primate development (Finlay & Darlington, 1995; Finlay et al. 1998, 2001; http://www.translatingtime.net/). The beginning of subplate neurogenesis and the arrival of the first GABAergic neurons in the subplate occur at similar stages in mouse and human. The early pattern of cortical neurogenesis is also very similar (Carney et al. 2007). Thus, the continued addition of neurons to the primate subplate and the relatively larger proportions represent major differences at later stages.
Plasma proteins in the developing brain
Plasma proteins are generally thought of as rather worthy but dull molecules, circulating in the blood and having functions to do with the carriage of biologically much more interesting material, such as hormones, vitamins, growth factors and trace metals. However, a number of immunocytochemical and in-situ hybridization studies show that several plasma proteins, or at least macromolecules that are immunocytochemically indistinguishable from them, are present within many cell types in different tissues of the body, including neurons in the developing brain. It has generally been assumed that these proteins, e.g. α-fetoprotein, transferrin and albumin, are found in the fetal brain because they penetrate a supposedly immature blood–brain barrier. However, there is now very good evidence that the blood–brain barrier is well formed even at the earliest stages of embryonic development (Saunders et al. 2008), and immunocytochemical findings together with identification of mRNAs for a large number of plasma proteins in the developing human brain indicate in-situ synthesis (Møllgård et al. 1988). We have previously observed that subplate neurons specifically synthesize certain plasma proteins and in the current study we give further evidence for this.
Magnetic resonance imaging of the human subplate in utero
Histologically, the subplate is composed of numerous cells (neurons, glia, endothelial cells of blood vessels), surrounded by an abundance of highly hydrophilic extracellular matrix, rich in glycosaminoglycans, chondroitin aminoglycans, laminin, fibronectin and adhesion molecules (Allendoerfer & Shatz, 1994). The presence of this extracellular matrix, stained with periodic acid Schiff–Alcian blue, has recently been correlated with neuroimaging of human postmortem specimens (Judas et al. 2003; Kostović & Juda , 2007; Kostović & Vasung, 2009). On T1-weighted postmortem magnetic resonance (MR) images, the subplate is a low-intensity layer despite its highly cellular nature, being governed by the highly hydrophilic extracellular matrix. This produces a high contrast between the subplate and the high signal intensity cortical plate on conventional MR image sequences. There is little information on the in-vivo human subplate because of the difficulties in studying the extremely preterm infant whilst the subplate remains MR visible (< 28 postmenstrual weeks, equivalent to < 26 PCW). However, fetal MR imaging is becoming an increasingly used technique from approximately 19 postmenstrual weeks, complementing antenatal ultrasound in clinical practice (Perkins et al. 2008).
, 2007; Kostović & Vasung, 2009). On T1-weighted postmortem magnetic resonance (MR) images, the subplate is a low-intensity layer despite its highly cellular nature, being governed by the highly hydrophilic extracellular matrix. This produces a high contrast between the subplate and the high signal intensity cortical plate on conventional MR image sequences. There is little information on the in-vivo human subplate because of the difficulties in studying the extremely preterm infant whilst the subplate remains MR visible (< 28 postmenstrual weeks, equivalent to < 26 PCW). However, fetal MR imaging is becoming an increasingly used technique from approximately 19 postmenstrual weeks, complementing antenatal ultrasound in clinical practice (Perkins et al. 2008).
New tools for the systematic analysis of gene expression in the murine subplate
Recent advances in gene expression analyses, cell separation and transgenic models have revolutionized the identification of molecular tags for a multitude of neuronal subtypes (Toledo-Rodriguez et al. 2004; Molnár & Cheung, 2006; Molyneaux et al. 2007). Classical anatomical and physiological methods are now complemented by optogenetic and molecular approaches (Thomson & Bannister, 2003; Nelson et al. 2006), so that very specific classes of neurons can be functionally modulated or eliminated with precise temporal control (Miesenböck & Kevrekidis, 2005; Miyoshi & Fishell, 2006; Luo et al. 2008). The knowledge of subplate-specific molecular tags for the structural and functional dissection of the subplate and thalamocortical circuits will be invaluable. The markers that have been identified in rodent need extensive validation in human before they can be fully interpreted in human histopathology. We initiated such validation, and the preliminary results indicate interesting similarities but also considerable differences.
Methods and materials
Tissue samples
Sections from human embryos and fetuses ranging in age from 12 to 22 PCW were obtained from three sources. (i) MRC–Wellcome Trust Human Developmental Biology Resource at Newcastle University (http://www.hdbr.org; Lindsay & Copp, 2005) and (ii) MRC Clinical Sciences Centre, Hammersmith Hospital, London, UK. The use of the specimens was approved by the Newcastle and North Tyneside NHS Health Authority Joint Ethics Committee and the Research Ethics Committee of the Hammersmith Hospital and permitted with appropriate maternal written consent. (iii) An additional series of paraffin sections of human fetuses, also ranging from 12 to 22 PCW, was obtained from the Developmental Biology Unit–ICMM Human Embryonic/Fetal Biobank, University of Copenhagen, Denmark. Informed consent was obtained according to the guidelines of the Helsinki Declaration II. Age was estimated from measurements of foot length and heel to knee length compared with a standard growth chart (Hern, 1984). Consecutive serial sections (5–8 μm) from each brain were selected, deparaffinized in xylene and rehydrated in graded alcohols followed by immunohistochemistry or in-situ hybridization.
Immunohistochemistry
Fixation, paraffin embedding and general immunoperoxidase histochemistry including controls have been described in detail previously (Møllgård & Jacobsen, 1984; Møllgård et al. 1988; Bayatti et al. 2008). Microwave-mediated antigen retrieval was performed on the deparaffinized sections in antigen unmasking solution (Vector Laboratories Ltd, Peterborough, UK) following the manufacturer's instructions. The sections were then rinsed in phosphate-buffered saline (PBS), quenched in 1.5% hydrogen peroxide (Sigma-Aldrich, UK) and blocked for 2 h at room temperature (RT) with 4% donkey/goat serum (Sigma, UK).
The following primary antibodies were used: anti-neuropeptide Y (NPY) (Sigma-Aldrich, N9528, rabbit polyclonal, 1 : 8000), KCC2 (Upstate Biotech, AB3264, rabbit polyclonal, 1 : 500), calretinin (CR) (Swant, 6B3, mouse monoclonal, 1 : 2000), TBR1 (Abcam, ab31940, rabbit polyclonal, 1 : 500), growth-associated protein 43 kilodalton (GAP43) (Sigma-Aldrich, G9264, mouse monoclonal, 1 : 1000), synaptophysin (Sigma-Aldrich, S5768, mouse monoclonal, 1 : 1000), nuclear receptor-related 1 (NURR1) (Research and Diagnostics, AF2156, goat polyclonal, 1 : 200), forkhead box P2 (FOXP2) (Abcam, ab16046, rabbit polyclonal, 1 : 500), rabbit anti-human Alpha-2-Heremans-Schmid glycoprotein (α2HS) (Behringwerke AG, Germany, 1 : 500) and rabbit anti-human α2zinc-binding globulin (RAHu/Apl, Nordic, Netherlands, 1 : 200–1 : 1000). All antibodies were diluted in 0.1 m PBS with 0.1% Triton.
Streptavidin horseradish peroxidase and biotinylated secondary antibodies were diluted 1 : 200 in 0.1 m PBS with 0.1% Triton (Vector Laboratories Ltd). Paraffin sections of forebrain were immunostained on slide and frozen sections of medulla were immunostained free-floating. Sections were colour reacted with 3,3′-Diaminobenzidine (Vector Laboratories Ltd) according to the manufacturer's instructions. Sections were dehydrated through serial alcohol, cleared in xylene (BDH) and coverslipped using DePeX (BDH).
In-situ hybridization
A DNA fragment (506 bp), corresponding to the region of the human connective tissue growth factor (CTGF) mRNA 1022–1527 bases, was generated from human genomic DNA (Promega UK Ltd) using the forward and reverse primers: forward = 5′-CTGCACCAGCATGAAGACAT-3′; reverse = 5′ TGCTCCTAAAGCCACACCTT-3′. The resulting polymerase chain reaction product was ligated into the pST-Blue 1 plasmid (Novagen, Nottingham, UK). The antisense and sense (a negative control) cRNA probes were transcribed using either SP6 or T7 RNA polymerase with digoxigenin-labelled RNA mixture, respectively (Roche, Penzberg, Germany). Deparaffinized sections were washed in 0.1 m PBS twice and treated with proteinase K (20 μg mL−1, Roche) in PBS for 8 min at RT. After two washes in PBS, the sections were postfixed in 4% paraformaldehyde (in PBS) at RT for 20 min. They were then deproteinized with 0.1 N HCl for 5 min, acetylated with acetic anhydride (0.25% in 0.1 m triethanolamine hydrochloride) and prehybridized at RT for at least 1 h in a solution containing 50% formamide, 10 mm Tris (pH 7.6), 200 μg mL−1Escherichia coli tRNA, 1× Denhardt's solution, 10% dextran sulphate, 600 mm NaCl, 0.25% sodium dodecyl sulphate and 1 mm EDTA. The sections were hybridized in the same buffer with the digoxigenin-labelled probes (3 ng μL−1) overnight at 66 °C. After hybridization, sections were washed to a final stringency of 30 mm NaCl 3 mm sodium citrate−1 at 50 °C and detected by anti-digoxigenin-alkaline phosphatase antibody in conjunction with a mixture of nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate (Roche). The details of the mouse probes, including Ctgf (shown in Fig. 1), have been described in Hoerder-Suabedissen et al. (2009).
Fig. 1.

Nuclear receptor-related 1 (Nurr1) and connective tissue growth factor (Ctgf) expression in the mouse subplate revealed with immunohistochemistry and in-situ hybridization on coronal sections at embryonic day (E)15 and 17 and postnatal day (P)8. (a) At E15, Nurr1-immunoreactive cells are most apparent in the ventral pallium but they are also beginning to appear in the subplate. (b) At the same stage, no Ctgf-expressing cells are detected in the subplate, whereas Ctgf is expressed in the ventricular and subventricular zones. (c) By E17, Nurr1 expression is stronger in the subplate. (d) By E17, subplate cells have started to express Ctgf along the narrow layer of 6b. The Nurr1 and Ctgf expression reach a peak at early P8. (e) Nurr1 expression is restricted to the subplate in the dorsal cortex, whereas there is broader expression, extending to layers 6 and even 5, in addition to the subplate in the lateral cortex. There is additional extracortical expression in the claustrum, endopyriform nucleus and lateral amygdala (not shown). (f) Ctgf expression was very specific to the subplate in both the dorsal and lateral cortex throughout development and in the adult. (g and h) High magnification of subplate cells in the boxed regions in e and f. Scale bars: 200 μm (a–d); 500 μm (e and f); 250 μm (g and h).
Imaging and image analysis
Sections were imaged and analysed for cell distribution using a transmission light microscope (DMR, Leica Microsystems, Milton Keynes, UK). Images for publication were contrast adjusted and compiled using Adobe Photoshop CS3. All cerebral cortical images from 15, 17 and 21.8 PCW displayed in this study were taken from the dorsal cortex.
In-utero magnetic resonance imaging
The MR scanning was performed with ethical approval and parental permission on normal volunteer women with uneventful pregnancies at the Perinatal Imaging, MRC Clinical Sciences Centre, Hammersmith Hospital, London, UK. No sedation was used and examinations took up to 60 min. Women were imaged in a lateral tilt position to avoid compression of the vena cava from the pregnant uterus. Single-shot T2-weighted sequences represent the mainstay of the examination; an image slice can be acquired in < 1 s, thereby ‘freezing’ fetal motion.
Results
Overview of Nurr1 and Ctgf expression in the developing mouse brain
We have previously studied the expression of selected murine subplate markers identified by our microarray analysis and we described the detailed expression pattern of nuclear receptor-related 1/Nr4a2 (Nurr1), monooxygenase Dbh-like 1, transmembrane protein 163 and Ctgf (Hoerder-Suabedissen et al. 2009). Figure 1 gives an overview of Nurr1 and Ctgf expression at three developmental stages (E15, 17 and postnatal day 8) to facilitate future comparisons with human. Nurr1 immunoreactivity appears earlier in the subplate than Ctgf expression, which is localized to ventricular and subventricular zones at E15 (Fig. 1a,b). By E17, both Nurr1 and Ctgf are strongly expressed in the narrow layer of the subplate in layer 6b (Fig. 1c,d). The Nurr1 and Ctgf expression both become stronger by early postnatal day 8 (Fig. 1e–h). Nurr1 expression is restricted to the subplate in the dorsal cortex, whereas there is broader expression, extending to layers 6 and even 5 in addition to the subplate in the lateral cortex. Ctgf expression is specific to the subplate in both the dorsal and lateral cortex throughout development and in adult. Both Nurr1 and Ctgf demonstrate additional extracortical expression in the claustrum, endopyriform nucleus and lateral amygdala.
NURR1 and CTGF expression in the human developing cortex
To investigate the gene expression patterns of the human subplate, we examined the CTGF mRNA and NURR1 protein expression in the developing human cortex between 12 and 22 PCW. Studies in our laboratory and those of other investigators have shown that these two genes are preferentially expressed in the subplate in rodents and non-human primates (Arimatsu et al. 2003; Watakabe et al. 2007; Hoerder-Suabedissen et al. 2009).
As shown in Fig. 2a, NURR1 expression can be observed in the cortical plate at the beginning of the second trimester (12 PCW). NURR1-positive cells are predominantly localized in layer 6 and only sparsely in the subplate at this stage. As development proceeds, the subplate increases in thickness, and at 15 PCW a stronger NURR1 signal is detected in the subplate. The expression of NURR1 in the cortical plate remains in the lower part of layer 6 when compared with the expression of GAP43 (which labels axons in the subplate) and FOXP2 (a marker of layer 6) (Fig. 2b,c, respectively). During 17–22 PCW, NURR1 labelling remains along the border between lower layer 6 and the subplate zone with the majority of cells present in the superficial subplate (Fig. 3d,e,j–l). NURR1 is not expressed in the ventricular zone (VZ) at the examined ages (12–22 PCW) (Fig 3d,f).
Fig. 2.

Comparisons of TBR1, NURR1, GAP43, synaptophysin and FOXP2 expression patterns on serial paraffin sections from the dorsolateral part of the frontal cortex at (a) 12 and (b and c) 15 postconceptional weeks (PCW). (a) A distinct subplate (SP) at 12 PCW that is moderately immunostained for GAP43 and strongly immunopositive for synaptophysin, whereas the cortical plate (CP) shows no expression of these markers. At 12 PCW, TBR1 is expressed throughout layer 6 and the SP. NURR1 is confined to the lower portion of the CP corresponding to layer 6. Scale bar: 200 μm. (b) TBR1, NURR1 and GAP43 expression at 15 PCW. By this stage, NURR1-immunoreactive cells are more numerous in the SP zone labelled with GAP43 and the lower part of the GAP43-negative CP. TBR1 is expressed throughout the SP and CP. The bulk of the NURR1 expression starts in putative layer 6 of the CP and is increasing in the upper SP at later developmental stages. Scale bar: 200 μm. (c) NURR1 and FOXP2 expression in the human cerebral cortex at 15 PCW. At 15 PCW, immunohistochemistry for FOXP2 and NURR1 on adjacent sections demonstrated partial overlap in the expression pattern. FOXP2, a marker associated with layer 6 cortical neurons, is expressed more superficially than NURR1, but there is a clear overlap between the two zones. NURR1 is expressed in both lower layer 6 and the upper SP. Scale bar: 200 μm. CP-VI, cortical plate-layer VI; CP-V, cortical plate-layer V; FOXP2, forkhead box P2; GAP43, growth-associated protein 43 kilodalton; IZ, intermediate zone; MZ, marginal zone; NURR1, nuclear receptor-related 1; TBR1, T-box brain gene 1.
Fig. 3.
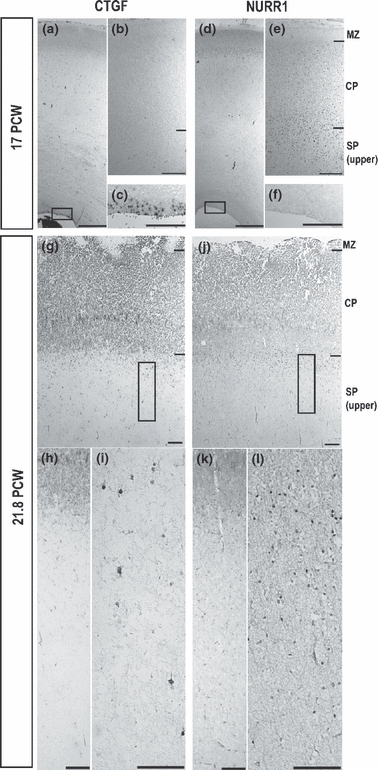
Connective tissue growth factor (CTGF) and nuclear receptor-related 1 (NURR1) expression in the 17 and 21.8 postconceptional weeks (PCW) human cortex revealed with in-situ hybridization and immunoreactivity. (a–c) At 17 PCW, in-situ hybridization of CTGF expression showed no CTGF-positive cells in the subplate (SP) (a and b), but the presence of numerous CTGF-positive cells in the ventricular zone (c). (d–f) At 17 PCW, NURR1 immunoreactivity was located around the border between layer 6 and the SP zone (e), but there was none in the ventricular zone (f). Scale bars: 1 mm (a and d) and 200 μm (b, c, e and f). In-situ hybridization for CTGF and immunohistochemistry for NURR1 revealed SP populations with a slightly different distribution at 21.8 PCW in the human parietal cerebral cortex. (g–i) CTGF-positive cells were sparsely scattered throughout the SP zone (g–i). (j–l) NURR1-immunoreactive cells were located around the border between layer 6 and the SP zone, with the majority of cells present in a superficial subcompartment of the SP. Scale bars: 200 μm (g, h, j and k); 100 μm (i and l). CP, cortical plate; MZ, marginal zone.
In contrast, a few CTGF-positive cells can be identified in the VZ from as early as 15 PCW (data not shown) and are abundant in the VZ at 17 PCW (Fig. 3a,c). No CTGF-positive cells were found in the subplate (Fig. 3b) between 15 and 17 PCW. By 21.8 PCW, sparse labelling of CTGF-positive cells has appeared across the subplate with no labelling in the cortical plate. At the same time, fewer CTGF-positive cells appear to be present in the VZ (Fig. 3g–i).
Comparing expression patterns of CTGF, NURR1, NPY and KCC2 in the human subplate
During the second trimester, the subplate increases significantly in thickness and complexity. In order to identify different subpopulations of subplate cells at this stage, we compared expression profiles of NPY, KCC2, the calcium binding protein CR, NURR1 and CTGF.
Figure 4 shows immunostaining for NPY, KCC2 and CR at 16 PCW. Although NPY-immunoreactive neurons can be seen throughout the subplate as well as in the cortical plate up to the border with the marginal zone, they are present in the greatest density in the upper subplate. NPY-positive neurons appear to be either bipolar, orientated tangentially or radially, or multipolar. KCC2-positive neurons, however, are absent from the cortical plate, rare in the upper subplate and intermediate zone, but clustered in the lower subplate. The morphology of these cells varies from multipolar to sometimes pyramidal with short immunoreactive processes. CR-positive neurons are mostly small and distributed evenly throughout the subplate. They are also present in the cortical plate where they are more usually located in the upper half. There is a dense localization of CR-positive neurons and neurites in the marginal zone.
Fig. 4.

Neuropeptide Y (NPY) and potassium/chloride co-transporter (KCC2) are expressed at distinct depths of the human subplate (SP) in the dorsolateral part of the posterior frontal cortex at 16 postconceptional weeks (PCW). The figure shows the immunostaining for NPY, KCC2 and the calcium-binding protein calretinin (CR) alongside adjacent sections stained for cresyl violet. Although NPY neurones can be seen throughout the SP as well as in the cortical plate (CP) up to the border with the marginal zone (MZ), they are clearly present in the greatest density in the upper SP (delineated with a box). A high-power image of these NPY neurons shows them to be either bipolar, orientated tangentially or radially, or multipolar, giving off varicose processes. KCC2-positive neurons, however, are absent from the CP, rare in the upper SP or intermediate zone, but clustered in the lower SP (delineated with a box) and at high power appear multipolar, sometimes pyramidal with short immunoreactive processes. CR-positive neurones are mostly small and distributed evenly throughout the SP. They are also present in the CP where they are more usually located in the upper layer. There is a dense distribution of CR-positive neurons and neurites in the MZ. Scale bars: 500 μm (low power); 100 μm (high power). IZ, intermediate zone; MZ, marginal zone.
The CTGF-positive and NURR1-positive subpopulations of subplate neurons show differential distribution patterns in the human subplate. From 15 PCW, NURR1-positive cells are located around the border between layer 6 and the subplate zone, forming a discernible band in the superficial layer of the subplate (Figs 2b,c and 3e,j–l). The CTGF-positive cells only appear in the subplate at 21.8 PCW and are widespread throughout the whole subplate zone (Fig. 3g–i).
Specific expression of plasma proteins in the human subplate
Our recent microarray screen of the postnatal murine subplate and histological observations suggest that subplate neurons are highly secretive (Kondo S and Molnár Z, unpublished data). Previously, we have also shown that neurons in the developing human cortex synthesize and secrete a range of plasma proteins (Møllgård et al. 1988). We therefore analysed the expression patterns of two plasma proteins –α2zinc-binding globulin and α2HS glycoprotein (human fetuin) – in a 14 PCW human cortex. The α2zinc-binding globulin immunoreactivity is strongest in the outer half of the marginal zone and in the subplate (Fig. 5a). Similarly, the immunoreactivity for α2HS glycoprotein is strongest in the subplate (Fig. 5b,c). The labelling is restricted to the cytoplasm and strongest in large ovoid subplate neurons with round, slightly eccentric cell nuclei (Fig. 5d,e).
Fig. 5.

Expression of α2zinc-binding globulin (a) and α2HS glycoprotein (human fetuin, b–e) in the subplate (SP) in a 14 postconceptional weeks (PCW) human cortex. (a) Coronal section through the forebrain of a 14 PCW fetus stained for α2zinc-binding globulin. There is a strong reactivity of incoming fibres particularly in the outer half of the marginal zone (MZ) and weaker staining of the SP where both radial and tangential fibre bundles are present. Note the lack of staining in the ventricular zone (VZ) and subventricular zone (SVZ). Scale bar: 500 μm. (b–e) Coronal section of the dorsolateral neocortex from a 14 PCW human fetus stained for α2HS glycoprotein (human fetuin) showing a strong reactivity in the SP (b). The framed area, which contains the large strongly positive neurons, is shown in higher magnification in c. The two encircled cells are enlarged in d and e. These SP neurons appear ovoid in shape and show strongly stained cytoplasm and round, slightly eccentric cell nuclei. Scale bars: 500 μm (b), 100 μm (c–e). CP, cortical plate; IZ, intermediate zone; MZ, marginal zone.
Fetal magnetic resonance imaging of the subplate in utero
Using T2-weighted images, the subplate can be well defined as the layer of high signal intensity (Fig. 6) immediately beneath the low signal intensity cortex, distinguishable from the subjacent fetal white matter, which shows slightly less high signal intensity. At early gestation (23 PCW), the subplate is visible as a continuous band (Fig. 6a–c). At later gestations (27 PCW), the subplate becomes more ‘patchy’ in its distribution, both regional and in relation to the cortical folds (Fig. 6d,e). From 26 PCW, the subplate is only visualized at the tops of gyri in many brain regions. This development can be quantified with simple 2D measures (Perkins et al. 2008).
Fig. 6.

In-utero magnetic resonance imaging (MRI) of the fetal subplate. T2-weighted single-shot images in axial (a and d), coronal (b and e) and sagittal (c and f) planes acquired in a fetus at 23 postconceptional weeks (PCW) (a–c) and at 27 PCW (d–f) using a 1.5 Tesla scanner. The layers of the hemisphere are clearly seen at 23 PCW (a–c). The unfolded cortical ribbon is seen as low signal intensity (top arrow). Cortical thickness averages approximately 1.5 mm. The subplate is seen as high signal intensity, reflecting its hydrophilic extracellular matrix (middle arrow). At this gestation the subplate is thicker than the cortex. The developing white matter has a low signal intensity band reflecting an increased cellular content (bottom arrow). At 27 PCW the cortex now shows some folding with obvious sulci and gyri. The cortical thickness remains at an average of approximately 1.5 mm. The subplate, however, has involuted on MRI and is only visible as foci of high signal intensity within the gyri [arrows (e and f)].
Discussion
We studied the expression of selected genes in the human cortex during development between 12 and 22 PCW and relate these expression data to previous studies in various animal models and to observations by MR in-utero imaging in human. Our current study in human demonstrated that CTGF mRNA, NURR1, NPY, KCC2, α2zinc-binding globulin and α2HS glycoprotein (human fetuin) are all expressed in the subplate but not always in overlapping patterns. Due to limited availability of human tissues, the data that we collected in this study can only give preliminary information about the developing human cortex at each time-point.
Subplate subpopulations in the developing human cortex
The CTGF and NURR1 show different expression patterns in the developing cortex. NURR1-positive cells form a dense band along the border of lower layer 6 and the upper subplate, whereas CTGF-positive cells are scattered throughout the subplate. This suggests that NURR1 and CTGF label at least two distinct populations in human; however, the number of cells in the human subplate that co-express CTGF and NURR1 is currently unknown. In addition, CTGF-positive cells appear relatively later in the subplate zone than NURR1-positive cells but they are expressed in the VZ at early gestation ages (15–17 PCW).
The strong expression of CTGF mRNA in the human cortical VZ at 17 PCW was an interesting finding that is in agreement with previous observations in mouse (Friedrichsen et al. 2003; Wang WZ and Oeschger FM, unpublished data). Moderate expression of CTGF mRNA in the VZ appeared as early as E12 in mouse and is maintained until E18, whereas its expression in the subplate can only be identified from E16 onwards.
The fate of these germinal zone cells expressing CTGF mRNA is not yet established. We hypothesize that, in human, many cells born between 13 and 17 PCW in the VZ could be considered as subplate neurons, as the timing coincides with the fastest expansion period of the subplate. CTGF-positive cells generated in the VZ during this period could therefore be part of the late-born subplate. This could also explain why CTGF is expressed later compared with NURR1 in the subplate.
The other possible explanation is that NURR1 is expressed first, followed by CTGF. As in human, in mouse Nurr1 expression precedes Ctgf expression in the subplate – Nurr1 appears by E14 and Ctgf by E16 (Fig. 1) (Wang WZ, Oeschger FM and Molnár Z, unpublished data). Watakabe et al. (2007) show that the majority of Nurr1-positive cells co-express Ctgf indicating that, at least in rodents, the two markers are expressed sequentially and overlapping in these cells. However, in adult mouse, there are more Ctgf-positive cells than Nurr1-positive cells in the subplate in the dorsal cortex (Watakabe et al. 2007; Wang WZ, Oeschger FM and Molnár Z, unpublished data) indicating that there are at least two populations.
There are several questions to be resolved. Does the primate cortex contain similar subplate cells as rodents, or does it contain additional cell types? Are there late-born subplate cells in rodents as has been proposed for primate? If not, what is the relation between the mouse and human VZ cells expressing CTGF mRNA? What are the roles of CTGF in the progenitors and subplate? In fibroblasts and osteoblasts, it has been suggested that CTGF can regulate the cell cycle and thus proliferation (Frazier et al. 1996; Safadi et al. 2003). Could it play a similar role in VZ progenitors during neurogenesis? It is not known if developmental cell death affects specific cell classes within the subplate, or whether all subpopulations are equally reduced in human as they seem to be reduced without selectivity in rat (Arias et al. 2002). In humans, it would be particularly important to differentiate between early-born components, probably related to transient roles of the subplate, and later-appearing resident cells, which may not be important for early development but rather involved in activities proper to the adult white matter. More generally, are there neurochemical differences between the early- and late-born primate subplate cells?
In mouse, Foxp2 is mostly expressed in neurons of layer 6, whereas Nurr1 is localized to the subplate in the dorsal cortex (Ferland et al. 2003; Hoerder-Suabedissen et al. 2009). In the lateral/ventral cortex Nurr1 is additionally expressed in layer 6 and 5 neurons. We related the position of NURR1-positive cells to the FOXP2-positive cells in the dorsal cortex of human and demonstrated considerable early overlap at 15 PCW. This could indicate that NURR1 expression starts off in layer 6 before 15 PCW, but some of the NURR1-positive cells later translocate into the upper subplate around the time of thalamocortical afferent invasion. This suggestion is in line with Kostović and Rakic's original proposal on the condensation of elements of layer 6 with the presubplate (Kostović & Rakic, 1990; Kostović & Judaš, 2007).
Compartmentalization in the human subplate
A possible explanation for the enlargement of the subplate in primate brain may be the more prolonged period of neurogenesis, or the longer waiting period of thalamic afferents during the assembly of the much more elaborate and diverse cortical connectivity. In proportion, the human subplate is much larger than in mouse and it seems to have superficial and deep compartments with differential neurogenesis, cell death and vulnerability (Ayoub & Kostović, 2009; Suárez-Solá et al. 2009). In addition, different densities of innervation can be observed in separate compartments at particular stages of development; for instance, there is a massive accumulation of thalamocortical fibres and synapses in the superficial compartment at 21–23 PCW just prior to the end of the waiting period and invasion of the cortical plate (Kostović & Rakic, 1990; Kostović & Judaš, 2007), an event that is even visible by in-utero imaging (Kostović & Vasung, 2009; see also GAP43 expression in Fig. 2a,b of the present study).
The NPY-positive subplate neurons are found superficially near to the cortical plate in human and could form part of the local GABAergic circuitry (Delalle et al. 1997; Bayatti et al. 2008), whereas KCC2-positive neurons localize to the border with the intermediate zone (Bayatti et al. 2008; Fig. 4 of the present study). In primates, NOS-positive/NPY-positive persisting subplate neurons have also been proposed to form part of the neural system involved in the coupling of cortical microvessels to neuronal activity (Cauli et al. 2004; Friedlander & Torres-Reveron, 2009). Expression of the KCC2 chloride transporter changes the equilibrium potential for chloride ions, permitting neurons to respond to GABA by hyperpolarization, the normal mature response (Rivera et al. 2005). However, other subplate and cortical plate neurons do not express KCC2 at this stage of development, suggesting that most neurons would still be under the immature, depolarizing influence of GABA. Indeed, the subplate and cortical plate continue to generate spontaneous network activity dependent on the depolarizing action of GABA until shortly after birth in the human (Vanhatalo et al. 2005). The significance of this precocious development amongst a group of neurons deep in the subplate remains obscure at present. Further investigations of the gene expression, cell types and connectivity of the subcompartments within the large human subplate are required.
Plasma proteins are produced in the human subplate
The distribution of a number of plasma proteins in relation to the overall histogenesis of the human and sheep cerebral cortex was described previously (Møllgård & Jacobsen, 1984; Møllgård et al. 1984; Reynolds & Møllgård, 1985). We show here that two plasma proteins (α2HS glycoprotein and α2zinc-binding globulin) are predominantly expressed by large, ovoid neurons in the developing 14 PCW subplate. This morphology is often associated with cells involved in secretive roles (Kondo S and Molnár Z, unpublished data). Similarly, in our previous studies a distinct territorial staining of various cells types and fibre systems within the subplate for different plasma proteins was noted. Thus, many of the large well-differentiated neurons as well as a few spindle-shaped neurons distributed throughout the entire subplate were positive for α-fetoprotein, albumin, prealbumin and transferrin (Møllgård & Jacobsen, 1984). Some loosely packed tangential fibres in the border of the intermediate and subplate zones were positive for α-fetoprotein, albumin and transferrin. These stained tangential fibres crossed a radially oriented plasma protein-positive fibre system that spanned the entire subplate zone (Møllgård & Jacobsen, 1984). Incoming fibres stained for the plasma protein Zn-binding globulin terminated on both sides of the cortical plate and delineated the subplate zone (Dziegielewska et al. 1993a and Fig. 5 in the present study).
A similar distribution of plasma proteins including Zn-binding globulin was present in the subplate of the developing sheep neocortex, and a positive staining for fetuin, in particular, characterized many neurons of the upper subplate, which also showed a positive signal for fetuin mRNA (data not shown). In the lower subplate, some very large neurons were strongly stained and labelled indicating local in-situ synthesis (Dziegielewska et al. 1993b). The functional significance of plasma proteins synthesized by subplate neurons is not known but the general properties of plasma proteins fall into three main categories: (i) they may act as carrier proteins (metal, hormone and growth factor binding); (ii) they may have growth-promoting and cell adhesion properties, possibly associated with cortical plasticity; and (iii) they may be involved in apoptosis. Thus, plasma proteins synthesized by subplate neurons may be involved in some of these processes during the formation and modulation of the developing neocortex. It will be important to establish the co-expression of these plasma proteins with additional subplate markers and correlate the expression with short- and long-range connections, as well as determining whether the subplate cells are the only cortical source of these plasma proteins within the central nervous system.
Combining imaging with histopathology
Cerebral cortical developmental disorders (schizophrenia, autism, attention deficit/hyperactivity disorder, dyslexia) and preterm hypoxic injuries such as periventricular leucomalacia have been linked to abnormalities of the subplate (Volpe, 2001; McQuillen et al. 2003). The idea of selective vulnerability of the subplate has been reinforced by experiments where excitatory amino acid lesions elicited selective subplate damage (Ghosh et al. 1990; Innocenti & Berbel, 1991). In a neonatal rat model of hypoxia–ischaemia, selective vulnerability of the subplate has been suggested using bromodeoxyuridine birthdating methods (McQuillen et al. 2003; Failor et al. 2010). The possibility that certain subplate subpopulations could be more susceptible to hypoxia–ischaemia than others should be systematically examined with the new set of subplate markers in human pathological cases and in relevant animal models (Oeschger et al. 2010).
The MR imaging also suggests that there is a regional difference in the loss of the subplate during normal development as it appears ‘patchy’ in late gestation, both regionally and in relation to the cortical folds, and later can only be seen at the tops of gyri in many brain regions. It would be interesting to investigate if there is a link between early gene expression (e.g. KCC2 expression) and future cell death in the deeper compartments of the subplate and to determine the cell types that increase in number in cognitive disorders (Eastwood & Harrison, 2003).
Understanding of cell-type-specific subplate gene expression, and development of early connections together with modern imaging methods will enable the more selective investigation of various neuropathologies involving the subplate. Some of these pathologies can be revealed by in-utero imaging in human following the advent of novel image-processing algorithms (Rutherford, 2002). T2-weighted single-shot fast-acquisition techniques allow excellent anatomical definition within a single imaging slice even in the presence of fetal motion (Perkins et al. 2008). Fetal MR imaging allows the detection of all areas of the brain regardless of fetal position or maternal habitus. It increases the detection of new abnormalities and improves the characterization of abnormalities already identified on ultrasound. In addition, it provides an ideal technique for the objective study of the development of the brain, both in the normally developing fetus and in those with congenital or acquired abnormalities. It is desirable to continue the correlation between the improving imaging and more diverse histological studies to aid clinicopathological diagnosis (Kostović & Vasung, 2009; Vasung & Kostović, in this issue).
Conclusions
We believe that it is impossible to understand the normal and pathological circuit formation without comprehending the transient structures contributing to its normal (and presumably abnormal) development, just as it is not possible to understand how a complex building or a bridge was constructed without detailed knowledge of the actual scaffolding utilized during the time of the building. The description of subtype-specific markers can help with the monitoring and modulation of these cells selectively in animal models and in histopathological analysis in human. Our preliminary analysis in human suggests increased complexity in both subplate cell types and subplate arrangements. Selective developmental cell death, gene expression and selective vulnerability all argue for superficial vs. deep compartmentalization of the human subplate. Whether these differences represent differential developmental origin, function or have differential involvement in various human pathologies remains to be determined.
Acknowledgments
This work was supported by the MRC and Wellcome Trust. Oeschger FM holds a Berrow Scholarship, Lincoln College Oxford. Ip BK holds an Anatomical Society Studentship. We would like to thank Dr Juan Montiel for his expert advice.
References
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Antonini A, Shatz CJ. Relation between putative transmitter phenotypes and connectivity of subplate neurons during cerebral cortical development. Eur J Neurosci. 1990;2:744–761. doi: 10.1111/j.1460-9568.1990.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Arias MS, Baratta J, Yu J, et al. Absence of selectivity in the loss of neurons from the developing cortical subplate of the rat. Brain Res Dev Brain Res. 2002;139:331–335. doi: 10.1016/s0165-3806(02)00582-5. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y, Ishida M, Kaneko T, et al. Organization and development of corticocortical associative neurons expressing the orphan nuclear receptor Nurr1. J Comp Neurol. 2003;466:180–196. doi: 10.1002/cne.10875. [DOI] [PubMed] [Google Scholar]
- Ayoub AE, Kostović I. New horizons for the subplate zone and its pioneering neurons. Cereb Cortex. 2009;19:1705–1707. doi: 10.1093/cercor/bhp025. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, et al. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 post conceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex. 2008;18:1536–1548. doi: 10.1093/cercor/bhm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Development of layer I and the subplate in the rat neocortex. Exp Neurol. 1990;107:48–62. doi: 10.1016/0014-4886(90)90062-w. [DOI] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Carney RSE, Bystron I, López-Bendito G, et al. Comparative analysis of extra-ventricular mitoses at early stages of cortical development in rat and human. Brain Struct Funct. 2007;212:37–54. doi: 10.1007/s00429-007-0142-4. [DOI] [PubMed] [Google Scholar]
- Catalano S, Shatz CJ. Activity–dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delalle I, Evers P, Kostović I, et al. Laminar distribution of neuropeptide Y-immunoreactive neurons in human prefrontal cortex during development. J Comp Neurol. 1997;379:515–522. doi: 10.1002/(sici)1096-9861(19970324)379:4<515::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, et al. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2006;439:79–83. doi: 10.1038/nature04264. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Bell JE, Matthews N, et al. Zn-binding globulin in human fetal brain and liver: a marker for passive blood/CSF transfer of protein. Neuropathol Appl Neurobiol. 1993a;19:82–90. doi: 10.1111/j.1365-2990.1993.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Dziegielewska KM, Reader M, Matthews N, et al. Synthesis of the foetal protein fetuin by early developing neurons in the immature neocortex. J Neurocytol. 1993b;22:266–272. doi: 10.1007/BF01187125. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry. 2003;8:821–831. doi: 10.1038/sj.mp.4001399. [DOI] [PubMed] [Google Scholar]
- Failor S, Nguyen V, Darcy DP, et al. Neonatal cerebral hypoxia–ischemia impairs plasticity in rat visual cortex. J Neurosci. 2010;30:81–92. doi: 10.1523/JNEUROSCI.5656-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland RJ, Cherry TJ, Preware PO, et al. Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol. 2003;460:266–279. doi: 10.1002/cne.10654. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Hersman MN, Darlington RB. Patterns of vertebrate neurogenesis and the paths of vertebrate evolution. Brain Behav Evol. 1998;52:232–242. doi: 10.1159/000006566. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB, Nicastro N. Developmental structure in brain evolution. Behav Brain Sci. 2001;2:263–278. [PubMed] [Google Scholar]
- Frazier K, Williams S, Kothapalli D, et al. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- Friauf E, Shatz CJ. Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. J Neurophysiol. 1991;66:2059–2071. doi: 10.1152/jn.1991.66.6.2059. [DOI] [PubMed] [Google Scholar]
- Friedlander MJ, Torres-Reveron J. The changing roles of neurons in the cortical subplate. Front Neuroanat. 2009;3:15. doi: 10.3389/neuro.05.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen S, Heuer H, Christ S, et al. CTGF expression during mouse embryonic development. Cell Tissue Res. 2003;312:175–188. doi: 10.1007/s00441-003-0712-6. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, et al. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ. Spontaneous synaptic activity of subplate neurons in neonatal rat somatosensory cortex. Cereb Cortex. 2001;11:400–410. doi: 10.1093/cercor/11.5.400. [DOI] [PubMed] [Google Scholar]
- Hern WM. Correlation of fetal age and measurements between 10 and 26 weeks of gestation. Obstet Gynecol. 1984;63:26–32. [PubMed] [Google Scholar]
- Hevner RF, Zecevic N. Pioneer neurons and interneurons in the developing subplate: molecular markers, cell birthdays, and neurotransmitters. In: Erzurumlu RS, Guido W, Molnár Z, editors. Development and Plasticity in Sensory Thalamus and Cortex. New York: Springer; 2006. pp. 1–17. doi: 10.1007/978-0-387-38607-2_1. [Google Scholar]
- Higashi S, Molnár Z, Kurotani T, et al. Prenatal development of neural excitation in rat thalamocortical projections studied by optical recording. Neuroscience. 2002;115:1231–1246. doi: 10.1016/s0306-4522(02)00418-9. [DOI] [PubMed] [Google Scholar]
- Higashi S, Hioki K, Kurotani T, et al. Functional thalamocortical synapse reorganization from subplate to layer IV during postnatal development in the reeler-like mutant rat (Shaking rat Kawasaki) J Neurosci. 2005;25:1395–1406. doi: 10.1523/JNEUROSCI.4023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerder A. UK: University of Oxford; 2007. Mouse cortical subplate neurones: molecular markers, connectivity and development. DPhil Thesis. [Google Scholar]
- Hoerder-Suabedissen A, Wang WZ, Lee S, et al. Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cereb Cortex. 2009;19:1738–1750. doi: 10.1093/cercor/bhn195. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Berbel P. Connections of visual areas 17 and 18 after neonatal injections of ibotenic acid. Analysis of an experimental cortical network: II. J Neural Transplant Plast. 1991;2:29–54. doi: 10.1155/NP.1991.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judas M, Milosevic NJ, Rasin MR, et al. 2003. Complex patterns and simple architects: molecular guidance cues for developing axonal pathways in the telencephalon; pp. 1–32. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Ann Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Shatz CJ. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 2006;51:627–638. doi: 10.1016/j.neuron.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, et al. Role of subplate neurons in functional maturation of visual cortical columns. Science. 2003;301:521–525. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- König N, Roch G, Marty R. The onset of synaptogenesis in rat temporal cortex. Anat Embryol. 1975;148:73–87. doi: 10.1007/BF00315564. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. PNAS. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I. Prenatal development of nucleus basalis complex and related fiber systems in man: a histochemical study. Neuroscience. 1986;17:1065–1077. doi: 10.1016/0306-4522(86)90077-1. [DOI] [PubMed] [Google Scholar]
- Kostović I, Goldman-Rakic PS. Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol. 1983;219:431–447. doi: 10.1002/cne.902190405. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev. 2007;31:1157–1168. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Kostović I, Molliver ME. A new interpretation of the laminar development of cerebral cortex: synaptogenesis in different layers of neopallium in the human fetus. Anat Rec. 1974;178:395. [Google Scholar]
- Kostović I, Rakic P. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9:219–242. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Kostović I, Rakic P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci. 1984;4:25–42. doi: 10.1523/JNEUROSCI.04-01-00025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kostović I, Vasung L. Insights from in vitro fetal magnetic resonance imaging of cerebral development. Semin Perinatol. 2009;33:220–233. doi: 10.1053/j.semperi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Lindsay S, Copp AJ. MRC-Wellcome Trust Human Developmental Biology Resource: enabling studies of human developmental gene expression. Trends Genet. 2005;21:586–590. doi: 10.1016/j.tig.2005.08.011. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Molnár Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, et al. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund RD, Mustari MJ. Development of the geniculocortical pathway in rat. J Comp Neurol. 1977;173:289–305. doi: 10.1002/cne.901730206. [DOI] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin M, Shatz CJ. Neurogenesis of the cat's primary visual cortex. J Comp Neurol. 1985;242:611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Early prenatal ontogenesis of the cerebral cortex (neocortex) of the cat (Felis domestica). A Golgi study. Anat and Embryol. 1971;134:117–145. doi: 10.1007/BF00519296. [DOI] [PubMed] [Google Scholar]
- McQuillen PS, Ann Sheldon R, Shatz CJ, et al. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinecke DL, Rakic P. Expression of GABA and GABAa receptors by neurons of the subplate zone in developing primate occipital cortex: evidence for transient local circuits. J Comp Neurol. 1992;317:91–101. doi: 10.1002/cne.903170107. [DOI] [PubMed] [Google Scholar]
- Meyer G. Genetic control of neuronal migrations in human cortical development. Adv Anat Embryol Cell Biol. 2007;189:1–111. [PubMed] [Google Scholar]
- Miesenböck G, Kevrekidis IG. Optical imaging and control of genetically designated neurons in functioning circuits. Annu Rev Neurosci. 2005;28:533–563. doi: 10.1146/annurev.neuro.28.051804.101610. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. Directing neuron-specific transgene expression in the mouse CNS. Curr Opin Neurobiol. 2006;16:577–584. doi: 10.1016/j.conb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Møllgård K, Jacobsen M. Immunohistochemical identification of some plasma proteins in human embryonic and fetal forebrain with particular reference to the development of the neocortex. Dev Brain Res. 1984;13:497–502. doi: 10.1016/0165-3806(84)90076-2. [DOI] [PubMed] [Google Scholar]
- Møllgård K, Reynolds ML, Jacobsen M, et al. Differential immunocytochemical staining for fetuin and transferrin in the developing cortical plate. J Neurocytol. 1984;13:497–502. doi: 10.1007/BF01148077. [DOI] [PubMed] [Google Scholar]
- Møllgård K, Dziegielewska KM, Saunders NR, et al. Synthesis and localization of plasma proteins in the developing human brain. Integrity of the fetal blood-brain barrier to endogenous proteins of hepatic origin. Dev Biol. 1988;128:207–221. doi: 10.1016/0012-1606(88)90283-7. [DOI] [PubMed] [Google Scholar]
- Molliver ME, Van der Loos H. The ontogenesis of cortical circuitry: the spatial distribution of synapses in somesthetic cortex of newborn dog. Ergeb Anat Entwicklungsgesch. 1970;42:5–53. [PubMed] [Google Scholar]
- Molnár Z. Development and evolution of thalamocortical interactions. Eur J Morphol. 2000;38:313–320. [PubMed] [Google Scholar]
- Molnár Z, Blakemore C. How do thalamic axons find their way to the cortex? Trends Neurosci. 1995;18:389–397. doi: 10.1016/0166-2236(95)93935-q. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Cheung AFP. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Métin C, Stoykova A, et al. Comparative aspects of cerebral cortical development. Eur J Neurosci. 2006;23:921–934. doi: 10.1111/j.1460-9568.2006.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JRL, et al. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Moore AR, Filipovic R, Mo Z, et al. Electrical excitability of early neurons in the human cerebral cortex during the second trimester of gestation. Cereb Cortex. 2009;19:1795–1805. doi: 10.1093/cercor/bhn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HBM, Kostović I, et al. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 1988;271:355–386. doi: 10.1002/cne.902710306. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Sugino K, Hempel CM. The problem of neuronal cell types: a physiological genomics approach. Trends Neurosci. 2006;29:339–345. doi: 10.1016/j.tins.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Oeschger FM, Wang WZ, Ginet V, et al. Subplate subpopulations in the hypoxic-ischemic neonatal rat brain. 2010. Winter Meeting of the Anatomical Society of Great Britain and Ireland, January 5th–7th, 2010, Oxford, UK.
- Perkins L, Hughes E, Srinivasan L, et al. Exploring cortical subplate evolution using magnetic resonance imaging of the fetal brain. Dev Neurosci. 2008;30:211–220. doi: 10.1159/000109864. [DOI] [PubMed] [Google Scholar]
- Piñon MC, Jethwa A, Jacobs E, et al. Dynamic integration of subplate neurons into the cortical barrel field circuitry during postnatal development in the Golli-tau-eGFP (GTE) mouse. J Physiol. 2009;587:1903–1915. doi: 10.1113/jphysiol.2008.167767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DJ, Aslam S, Tasker L, et al. Fates of the earliest generated cells in the developing murine neocortex. J Comp Neurol. 1997;377:414–422. [PubMed] [Google Scholar]
- Rakic P. Genesis of the dorsal lateral geniculate nucleus in the rhesus monkey: site and time of origin, kinetics of proliferation, routes of migration and pattern of distribution of neurons. J Comp Neurol. 1977;176:23–52. doi: 10.1002/cne.901760103. [DOI] [PubMed] [Google Scholar]
- Reynolds ML, Møllgård K. The distribution of plasma proteins in the neocortex and early allocortex of the developing sheep brain. Anat Embryol. 1985;171:41–60. doi: 10.1007/BF00319053. [DOI] [PubMed] [Google Scholar]
- Rickmann M, Chronwall BM, Wolff JR. On the development of non-pyramidal neurons and axons outside the cortical plate: the early marginal zone as a pallial anlage. Anat Embryol (Berl) 1977;151:285–307. doi: 10.1007/BF00318931. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl− transporter KCC2 and carbonic anhydrase CAVII. J Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford M. MRI of the Neonatal Brain. London: W.B. Saunders, Elsevier Science Ltd; 2002. [Google Scholar]
- Safadi FF, Xu J, Smock SL, et al. Expression of connective tissue growth factor in bone: its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196:51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Ek CJ, Habgood MD, et al. Barriers in the brain: a renaissance? Trends Neurosci. 2008;31:279–286. doi: 10.1016/j.tins.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, et al. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Solá ML, González-Delgado FJ, Pueyo-Morlans M, et al. Neurons in the white matter of the adult human neocortex. Front Neuroanat. 2009;3:7. doi: 10.3389/neuro.05.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cereb Cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Blumenfeld B, Wu C, et al. Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb Cortex. 2004;12:1310–1327. doi: 10.1093/cercor/bhh092. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Palva JM, Andersson S, et al. Slow endogenous activity transients and developmental expression of K+-CI− cotransporter 2 in the immature human cortex. Eur J Neurosci. 2005;22:2799–2804. doi: 10.1111/j.1460-9568.2005.04459.x. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Watakabe A, Ichinohe N, Ohsawa S, et al. Comparative analysis of layer-specific genes in mammalian neocortex. Cereb Cortex. 2007;17:1918–1933. doi: 10.1093/cercor/bhl102. [DOI] [PubMed] [Google Scholar]
- Zecevic N. Cellular composition of the telencephalic wall in human embryos. Early Hum Dev. 1993;32:131–149. doi: 10.1016/0378-3782(93)90007-h. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Verney C. Development of the catecholamine neurons in the human embryos and fetuses, with special emphasis on the innervation of the cerebral cortex. J Comp Neurol. 1995;351:509–535. doi: 10.1002/cne.903510404. [DOI] [PubMed] [Google Scholar]
- Zhao C, Kao JPY, Kanold PO. Functional excitatory microcircuits in neonatal cortex connect thalamus and layer 4. J Neurosci. 2009;29:15479–15488. doi: 10.1523/JNEUROSCI.4471-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


