Abstract
In the adult human telencephalon, subcortical (gyral) white matter contains a special population of interstitial neurons considered to be surviving descendants of fetal subplate neurons [Kostovic & Rakic (1980) Cytology and the time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol9, 219]. We designate this population of cells as superficial (gyral) interstitial neurons and describe their morphology and distribution in the postnatal and adult human cerebrum. Human fetal subplate neurons cannot be regarded as interstitial, because the subplate zone is an essential part of the fetal cortex, the major site of synaptogenesis and the ‘waiting’ compartment for growing cortical afferents, and contains both projection neurons and interneurons with distinct input–output connectivity. However, although the subplate zone is a transient fetal structure, many subplate neurons survive postnatally as superficial (gyral) interstitial neurons. The fetal white matter is represented by the intermediate zone and well-defined deep periventricular tracts of growing axons, such as the corpus callosum, anterior commissure, internal and external capsule, and the fountainhead of the corona radiata. These tracts gradually occupy the territory of transient fetal subventricular and ventricular zones.The human fetal white matter also contains distinct populations of deep fetal interstitial neurons, which, by virtue of their location, morphology, molecular phenotypes and advanced level of dendritic maturation, remain distinct from subplate neurons and neurons in adjacent structures (e.g. basal ganglia, basal forebrain). We describe the morphological, histochemical (nicotinamide-adenine dinucleotide phosphate-diaphorase) and immunocytochemical (neuron-specific nuclear protein, microtubule-associated protein-2, calbindin, calretinin, neuropeptide Y) features of both deep fetal interstitial neurons and deep (periventricular) interstitial neurons in the postnatal and adult deep cerebral white matter (i.e. corpus callosum, anterior commissure, internal and external capsule and the corona radiata/centrum semiovale). Although these deep interstitial neurons are poorly developed or absent in the brains of rodents, they represent a prominent feature of the significantly enlarged white matter of human and non-human primate brains.
Keywords: deep fetal interstitial neurons, deep (periventricular) interstitial neurons, intermediate/subventricular zone, subplate neurons, subplate zone, superficial (gyral) interstitial neurons
Introduction
Neurons situated in the white matter of the adult nervous system are usually described as white matter cells, interstitial cells or interstitial neurons (Kostovic & Rakic, 1980; Okhotin & Kalinichenko, 2003; Suárez-Solá et al. 2009). As demonstrated in the accompanying historical review (Judaš et al. 2010), the use of the adjective ‘interstitial’ is best suited for describing these cells for both terminological reasons and historical precedence. As the terms ‘interstitial cells’ and ‘white matter cells’ describe equally well neurons, astrocytes and oligodendrocytes situated among white matter fibre tracts, one should use the term ‘interstitial neurons’. However, it should be noted that ‘interstitial neurons’ are not just neurons situated between axons of the cerebral white matter, but those located between any axons – including peripheral nerves. Thus, an additional qualification is needed to refer precisely to interstitial neurons of the cerebral white matter. Finally, the findings presented in this study demonstrate that one should distinguish at least two distinct and topographically separated populations of cerebral interstitial neurons in both fetal and adult human brain.
On the basis of direct autoradiographic evidence obtained in both monkeys (Kostovic & Rakic, 1980) and cats (Luskin & Shatz, 1985) it is usually assumed that interstitial neurons located in the gyral and sulcal white matter are surviving descendants of fetal subplate neurons. However, these surviving subplate neurons represent just one subset of cerebral interstitial neurons, because (as we will demonstrate in the present study) there is another population of the so-called deep interstitial neurons, which display no developmental continuity with the supblate zone. These deep fetal interstitial neurons, distinct from subplate neurons, were first described in the human fetal brain on the basis of combined Nissl, Golgi and acetylcholinesterase (AChE) histochemistry critera (Kostović et al. 1981; Kostović, 1984). In this study we use several histochemical and immunocytochemical methods to further characterize deep fetal interstitial neurons as well as to trace their fate in postnatal and adult human brains.
Therefore, we propose the following terms to describe two major populations of interstitial neurons in both fetal and adult human brain (see Fig. 1): (i) subplate neurons (in the fetal subplate zone) and superficial (gyral) interstitial neurons (for subplate neurons that survive in the gyral/sulcal white matter), and (ii) deep fetal interstitial neurons (for neurons located in the fetal white matter, i.e. the intermediate/subventricular zone and periventricular fibre tracts) and deep (periventricular) interstitial neurons (for neurons situated in the centrum semiovale and periventricular fibre tracts in the adult brain).
Fig. 1.
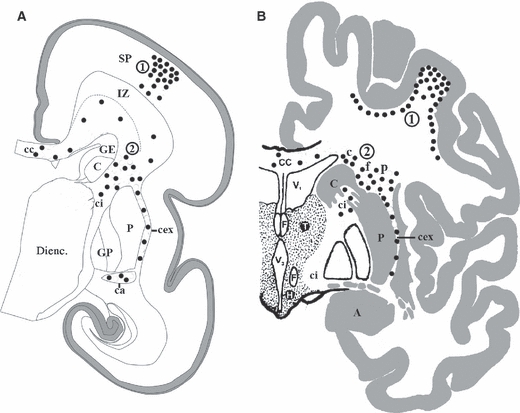
Schematic representation of superficial (encircled number 1) and deep periventricular (encircled number 2) populations of neurons (black dots) analysed in this study, as depicted during midgestation (A) and in the adult brain (B). The first population consists of fetal subplate neurons and their descendants in the adult brain, i.e. the superficial (gyral) interstitial neurons. The second population consists of deep fetal interstitial neurons (located within the fetal white matter, i.e. the intermediate zone and periventricular fibre bundles) as well as deep periventricular interstitial neurons in the adult brain. A, amygdala; C, caudate nucleus; c, interstitial neurons capping the caudate; ca, anterior commissure; cc, corpus callosum; cex, external capsule; ci, internal capsule; DIENC., diencephalon; F, fornix; f, interstitial neurons in the fountainhead of the internal capsule; GE, ganglionic eminence; GP, globus pallidus; H, hypothalamus; IZ, intermediate zone; P, putamen; p, interstitial neurons capping the putamen; SP, subplate; T, thalamus; V1, lateral ventricle; V3, third ventricle.
Our superficial (gyral) interstitial neurons were designated by Kostovic & Rakic (1980) as all cells with their somata clearly situated below the sixth neocortical layer and well within the subjacent white matter of both gyral crowns and sulcal bottoms. The highest concentration of interstitial neurons is encountered in close proximity to the cortex and their number decreases towards the deep white matter (Kostovic & Rakic, 1980). Depending on the size and orientation of the cerebral convolutions, some interstitial neurons may be separated by 2–3 mm from the cortex in monkey and up to 4 mm in humans (Kostovic & Rakic, 1980). Nissl-stained interstitial neurons display typical features of neurons: large size, fusiform or polymorphic shape, voluminous cytoplasm with abundant Nissl bodies, a recognizable proximal dendrite, and a large, pale nucleus containing dispersed heterochromatin and a prominent, darkly stained nucleolus, and finally a well-outlined axon hillock (Kostovic & Rakic, 1980).
Our deep (periventricular) interstitial neurons correspond to neurons located in segments I and II of the cerebral white matter, as defined in a classical study (Von Monakow, 1905) and described in detail in our previous publications (Kostović et al. 2002; Judaš et al. 2005). It is important to note that segment I encompasses the so-called central or periventricular white matter (including corpus callosum, anterior commissure, fornix and centrally placed associative fibre tracts). Thus, deep (periventricular) interstitial neurons do not necessarily reside in the immediate vicinity of cerebral ventricles. Deep interstitial neurons are also located in segment II, which represents the region of the corona radiata, i.e. the fountainhead of the capsula interna, as well as in the adjacent parts of segment III (centrum semiovale). In the fetal human brain, deep (periventricular) interstitial neurons are located in the fetal white matter (intermediate/subventricular zone) and are topographically separated from subplate neurons – the fetal subplate zone is part of the fetal cortex and not the fetal white matter (for review, see Kostović et al. 2002; Kostović & Judaš, 2007; Kostović & Judaš, 2010).
Materials and methods
We analysed postmortem human brain tissue with no macroscopically or microscopically discernible signs of neuropathological alterations or neurodevelopmental disorders. The tissue specimens are part of the extensive Zagreb Neuroembryological Collection (Kostović et al. 1991a) and were obtained during regular autopsies at the Departments of Pathology and Forensic Medicine (University of Zagreb School of Medicine) from fetuses after spontaneous or medically indicated abortions, neonates born prematurely or at term, as well as infants, children and adult subjects with no previous record of neurological or psychiatric disorders and with non-neurological causes of death. The sampling of tissue was approved by the Institutional Review Board (Ethical Committee). The age of fetuses (N = 13) ranged from 15 postconceptional weeks (PCW) to the newborn at term and was estimated on the basis of crown–rump length and/or pregnancy records (analysed ages: 15, 16, 17, 18, 20, 21, 22, 23, 24, 25, 26 and 37 PCW; newborn 2-day-old). In addition, we analysed brain tissue of six infants and children (ages: 15 and 39 postnatal days, 2 and 3 postnatal months, 3 and 12 years) and six adults (age range: 21–57 years). To delineate cytoarchitectonic boundaries and cellular compartments of the fetal telencephalic wall, every 10th section was stained by the Nissl method, and additional age-matched Nissl-stained serial sections were used from the Zagreb Neuroembryological Collection. The AChE histochemistry was performed on frozen 60-μm-thick sections, according to Lewis's modification of Koelle's method, as described previously (Kostovic & Rakic, 1980; Kostović, 1986).
Nicotinamide-adenine dinucleotide phosphate-diaphorase (NADPH-diaphorase) histochemistry
The brains were fixed in 4% paraformaldehyde solution, buffered with 0.1 m phosphate-buffered saline (PBS) (pH 7.4) for a period of 24–48 h, and then cut in several coronal blocks. These blocks of tissue were cryoprotected by immersion in a graded series of sucrose solutions (concentrations 5–30%) at 4 °C and then cut on the cryostat (Leitz, Germany). Cryostat sections (40–50 μm thick) were stained according to the standard direct NADPH-diaphorase (NADPH-d) protocol (Ellison et al. 1987; Judaš et al. 1999). Briefly, the freshly prepared incubation solution consisted of 50 mL of 0.1 m PBS (pH 8.0) with 1 mL of 0.8% Triton X-100 (Sigma, St Louis, MO, USA), 1 mm beta-NADPH-d (Sigma), and 0.8 mm nitro-blue tetrazolium (Sigma). Free-floating or slide-mounted sections were incubated for 3–7 h at 37 °C and the reaction was terminated by transfer of stained sections into the 0.1 m PBS. The sections were then rinsed with distilled water, mounted, dried overnight, dehydrated in a graded series of ethanol, briefly cleared with xylol, and coverslipped by using the Permount medium (Fisher, Pittsburgh, PA, USA). The specificity of the histochemical reaction was confirmed by omitting either NADPH-d or nitro-blue tetrazolium from the incubation solution (sections treated in this way remained completely unstained).
Immunohistochemistry
The brain tissue was fixed as described above, and tissue blocks were embedded in paraffin and cut in 15-μm-thick serial sections in the coronal, horizontal or sagittal plane. To confirm the neuronal nature of interstitial cells, we applied immunohistochemical labelling with the following neuron-specific primary antibodies: (i) anti-neuron-specific nuclear protein (monoclonal mouse IgG1, dilution 1 : 1000; Chemicon, Temecula, CA, USA); (ii) anti-microtubule-associated protein-2 (monoclonal mouse IgG, dilution 1 : 200; Sigma); (iii) anti-calbindin-D-28K (monoclonal mouse IgG1, dilution 1 : 3000; Sigma); (iv) anti-calretinin-22k (monoclonal mouse IgG, dilution 1 : 2000; Swant, Switzerland), and (v) anti-neuropeptide Y (monoclonal rabbit IgG, dilution 1 : 3000; gift from Dr H.B.M. Uylings, Amsterdam). Sections were pretreated for 20 min in 0.3% hydrogen peroxide in the 3 : 1 mixture of methanol and redistilled water, washed for 10 min in PBS, and immersed for 2 h in the blocking solution [PBS containing 3% bovine serum albumin and 0.5% triton X-100; all from Sigma] at room temperature (20°C) to prevent non-specific background staining. Sections were thereafter incubated with primary antibodies for 18 h at 4 °C, washed again, and incubated with secondary biotinylated anti-mouse or anti-rabbit antibodies diluted in blocking solution (1 : 200) for 1 h at room temperature (Vectastain ABC kit; Vector Laboratories, Burlingame, CA, USA). Sections were then incubated in Vectastain ABC reagent (streptavidin–peroxidase complex) for 1 h at room temperature, rinsed in PBS for 10 min, and finally the peroxidase activity was visualized with Ni-3,3-diaminobenzidine (Sigma). Sections were dehydrated in a graded series of alcohol, cleared in xylene and coverslipped with Histamount (National Diagnostic). Negative controls were performed by replacing the primary antibody solution with blocking solution during the incubation procedure. Qualitative analysis of stained sections was performed using an upright microscope (Olympus Provis AX70), and images were captured with a digital camera (Nikon DXM1200).
Results
Prenatal development of subplate neurons and their postnatal persistence as subcortical (gyral) interstitial neurons
Nitrinergic neurons are already present and numerous in the human fetal telencephalon during the first half of gestation and, in a 15 PCW fetus (the youngest specimen available for this study), they were present in the basal forebrain, basal ganglia, and in the developing neocortical anlage (Judaš et al. 1999), where they appear first in the subplate zone (Fig. 2), probably 1 or 2 weeks before 15 PCW (younger specimens were not available, but at 15 PCW subplate nitrinergic neurons are already numerous and display several dendrites with initial branching). In the course of the following few weeks, their number progressively increases, and from about 18 PCW NADPH-d subplate neurons are the most numerous and most conspicuous nitrinergic neurons of the neocortical anlage. However, their morphology significantly changes during the last trimester of gestation (Fig. 2). During midgestation, nitrinergic subplate neurons display an intense NADPH-d reactivity and frequently have unusual morphological features, such as grossly distended proximal dendrites (Fig. 2, 18–21 PCW). The network of beaded NADPH-d fibres within the subplate is only moderately developed. However, in the newborn, nitrinergic subplate neurons remain very numerous, display a variety of morphological types, continue to grow and have very long and extensively branched dendrites, which together with NADPH-d fibres of unidentified origin form a prominent network of NADPH-d-positive processes (Fig. 2).
Fig. 2.
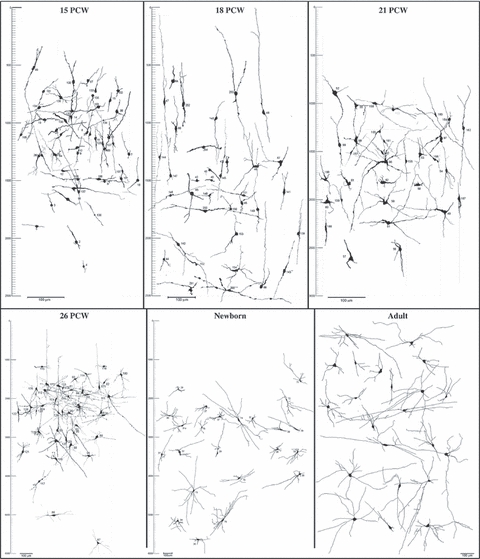
Composite drawings of nitrinergic (NADPH-diaphorase-stained) subplate (15–26 postconceptional weeks – dorsolateral frontal cortex, see Fig. 1A) and superficial (gyral) interstitial neurons (newborn and adult – middle frontal gyrus, see Fig. 1B). Scale bars to the left denote the subpial depth (in μm, short notches; in mm, long notches), and bars represent 100 μm. Note that nitrinergic neurons remain numerous and well developed below the cortical plate (in fetus) and cortex (in newborn and adult) at all analysed ages and continue to display a variety of morphological types. Note also that bars representing the same magnification (100 μm) decrease with increasing age (in order to fit drawings into a single plate), i.e. that there is a significant postnatal increase in neuronal and dendritic arborization size.
At 3 and 12 years (Figs 3, 4, 5) and in the adult brain (Fig. 4B,C) nitrinergic interstitial neurons in the gyral white matter remain numerous, well developed and display a variety of triangular and pyramidal-like (Fig. 4) as well as bipolar and fusiform (Fig. 5) shapes. Some of them are clearly apposed to adjacent blood vessels (Fig. 3C) and at least some send long ascending axons to the overlying cortex (Fig. 3D). They are most numerous within the gyral crowns (where they are predominantly multipolar and triangular) and at the cortical/white matter border and become less numerous towards the deep white matter and at the bottom of cortical sulci where they are predominantly bipolar and fusiform.
Fig. 3.
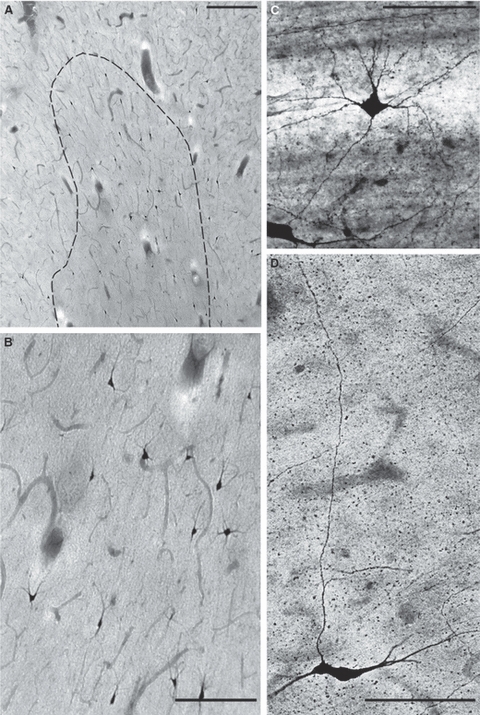
Nitrinergic (NADPH-diaphorase-stained) interstitial neurons in the gyral white matter (right inferior frontal gyrus) at 3 years (A,B; B is enlarged part of A) and 12 years (C,D). Note the multipolar nitrinergic interstitial neuron closely apposed to the blood vessel (C) and fusiform interstitial neuron, which sends a long ascending axon to the overlying cortex (D). Scale bars: 150 μm (A,B) and 100 μm (C,D).
Fig. 4.
Triangular (A,B,F) and pyramidal-like (C,D,E) nitrinergic (NADPH-diaphorase-stained) interstitial neurons in the gyral white matter of the human frontal (premotor) cortex at 3 years (A), 12 years (D,E,F) and in 57-year-old adult brain (B,C). Bars represent 20 μm.
Fig. 5.
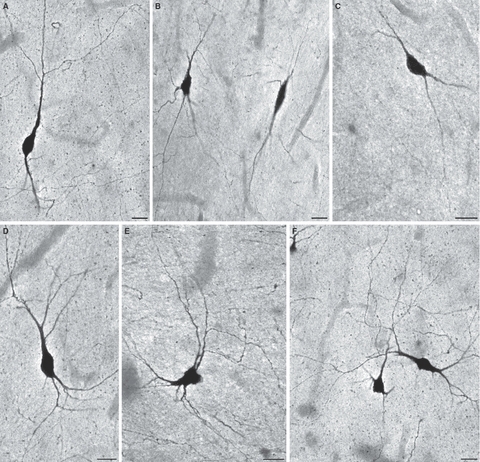
Fusiform bipolar (A,B,C) and bitufted (D,E,F) nitrinergic (NADPH-diaphorase-stained) interstitial neurons in the gyral white matter of the human frontal cortex (middle and inferior frontal gyrus) in a 12-year-old girl. Such neurons predominate at the bottom of cortical sulci, but they are also present deep within gyral crowns and at the cortical/white matter border in sulcal walls. Bars represent 20 μm.
Deep interstitial neurons are present in fetal and adult periventricular white matter
In the human fetal brain, deep interstitial neurons are present in the fetal white matter, i.e. in the intermediate zone (and adjacent part of the subventricular zone) as well as in well-defined periventricular fibre tracts such as the corpus callosum, anterior commissure and internal and external capsule. In the adult brain, deep (periventricular) interstitial neurons are present in the corpus callosum and anterior commissure, internal and external capsule as well as within the deep, central part of the cerebral white matter (the fountainhead of the internal capsule, adjacent white matter representing the dorsal cap of the caudate and putamen, and less frequently in the midst of the centrum semiovale).
Interstitial neurons of the external capsule
Numerous nitrinergic (NADPH-d-stained) interstitial neurons were present in the external capsule during the fetal period as well as in the newborn and adult brains (Fig. 6). Although at 18 PCW (Fig. 6A) they are predominantly bipolar and oriented in parallel with dorso-ventrally running fibres of the external capsule, by 26 PCW (Fig. 6B–G) they develop extensive dendritic arborizations and display a variety of morphological types such as bipolar, bitufted, multipolar, stellate, triangular and pyramidal-like (Fig. 6). Many of these neurons extend their dendrites without respect to the course of external capsule fibres or even perpendicular to it (Fig. 6B,C,E,F,G,I,K). During the second half of gestation and in the newborn (Fig. 6H,I), interstitial neurons of the external capsule continue to grow and display extensive and highly branched dendritic arborizations even in the adult brain (Fig. 6J,K). Although the external capsule in the postnatal and adult brain represents a bundle of tightly packed parallel axons, it should be noted that many interstitial neurons do not assume a ‘squeezed’ elongated fusiform shape but remain multipolar and variably oriented.
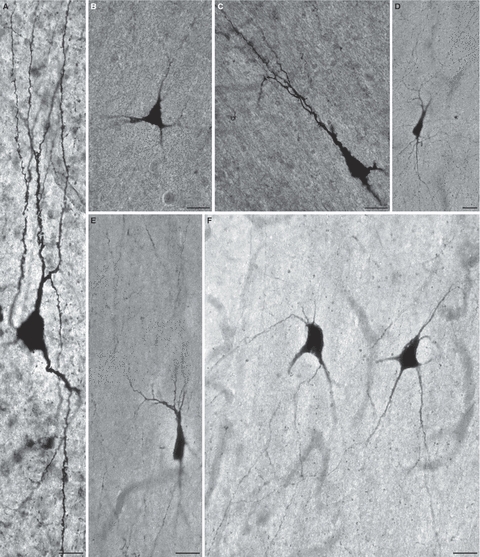
Fig. 6.
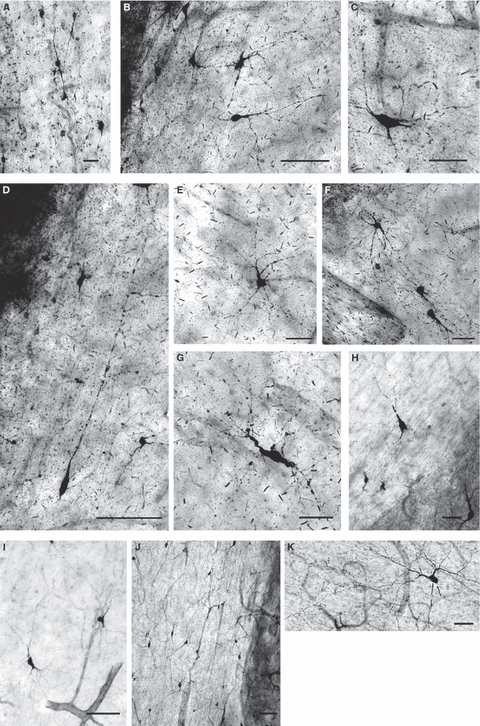
The fetal external capsule of the human fetal [A, 18 postconceptional weeks (PCW); B–G, 26 PCW], newborn (H,I) and adult (J,K) brain contains numerous nitrinergic (NADPH-diaphorase-stained) interstitial neurons that display a variety of sizes and shapes, such as bipolar (A,D), bitufted (C,I), multipolar (B,K), triangular and pyramidal-like (E,F,H) as well as bizarrely shaped types that are not usually encountered in the postnatal brain (G). Note that, with respect to the ventro-dorsal course of external capsule fibres, many cells are oriented perpendicularly or at various angles (B,C,E,F,G,I,K), whereas others remain aligned with fibre bundles (A,D,J). Bars represent 20 μm (A), 50 μm (C,E–H) and 100 μm (B,D,I,J).
Interstitial neurons of the anterior commissure and corpus callosum
Nitrinergic interstitial cells were present in the anterior commissure in both the fetal and newborn brain (Fig. 7) but were observed only occasionally in the adult brain. However, interstitial cells were continuously present in the corpus callosum of prenatal, postnatal and adult brains (Fig. 7I,J,K) as we described in detail in a separate recent study from our laboratory (Jovanov-Milošević et al. 2010). These interstitial neurons were mostly bipolar or fusiform, but multipolar (Fig. 7C,H,K) and triangular (Fig. 7H,I) forms were also present. At least some of these neurons also express neuropeptide Y (Fig. 7) in both fetuses and newborns.
Fig. 7.
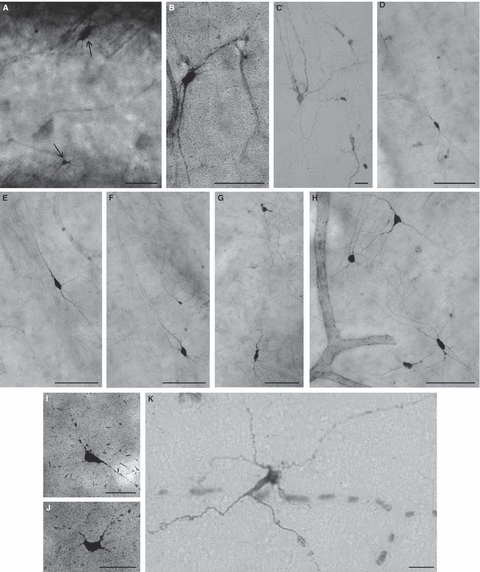
Deep fetal interstitial cells within the anterior commissure (A–H) and corpus callosum (I,J,K) visualized by NADPH-diaphorase histochemistry (A,B,D–J) and neuropeptide Y immunocytochemistry (C,K) at 23 postconceptional weeks (PCW) (C,K), 25 PCW (A,B,I,J) and in 2-day-old newborn (D–H). Note that most of these neurons are fusiform/bipolar, but some are multipolar (C,H,K) or triangular (H,I). Bars represent 100 μm (A–H) and 50 μm (I,J,K).
Interstitial neurons of the fetal intermediate zone, fountainhead of the internal capsule and adjacent parts of the centrum semiovale
Nitrinergic interstitial neurons were present in the fetal intermediate zone during midgestation (18–26 PCW) as well as throughout the entire postnatal period and in the adult brain (Fig. 8). These neurons were not only nitrinergic (Fig. 8G,H), but also expressed neuropeptide Y (Fig. 8A,C–F) and calbindin (Fig. 8B). They also displayed a variety of morphological types and sizes, but they were usually less complex than those situated in the external capsule or corpus callosum.
Fig. 8.
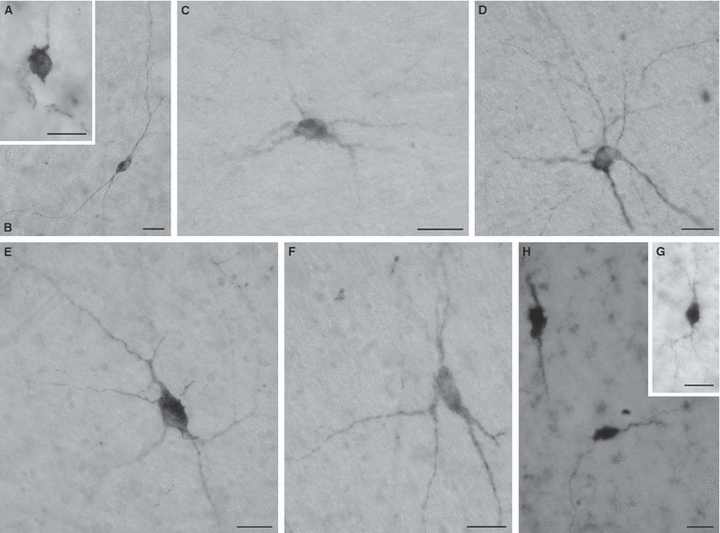
Throughout the entire postnatal period (A, 15 days; B, 39 days; C, 2 months; D,E,F, 3 years; G,H, 57 years), bipolar (B,H) or multipolar (C–F) interstitial neurons can be found in the deep white matter (centrum semiovale and corona radiata) of the human prefrontal cortex. These deep interstitial neurons may contain neuropeptide Y (A,C–F), calbindin (B) and neuronal nitric oxide synthase (G,H). In all figures, bars represent 20 μm.
Although numerous nitrinergic neurons were present within the internal capsule in both the developing and adult brain (not shown – for explanation, see Discussion), the largest and most conspicuous population of deep interstitial neurons was situated not within the internal capsule but at its fountainhead, i.e. its junction with the intermediate zone (in the fetal brain) or centrum semiovale (in postnatal and adult brains). In addition, that population of interstitial neurons spread medially and laterally to occupy a 2–3-mm-wide zone of the deep white matter capping the dorsal tip of the caudate nucleus and putamen (Figs 9, 10). During the midfetal period (Fig. 9E,F,G), many of these interstitial neurons also express neuropeptide Y (Fig. 9B,C,D). In the newborn brain (Figs 9A, 10A–H), nitrinergic interstitial neurons were numerous, well-developed and displayed a variety of morphological types with long dendrites that ramify and course without any particular respect to the orientation of axonal bundles in which they are embedded. Basically the same description can be applied to these neurons in the adult brain (Fig. 10I–M), although their cell-packing density seems to be decreased due to the enormous postnatal growth of the white matter volume.
Fig. 9.

The midfetal internal capsule contains numerous neuropeptide Y-immunoreactive interstitial neurons [B–D, 23 postconceptional weeks (PCW)] and NADPH-diaphorase-stained nitrinergic neurons (E–G, 25 PCW). Nitrinergic neurons are especially numerous and well developed at the fountainhead of the internal capsule in a 2-day-old newborn (A, see also Fig. 10). Bars represent 250 μm (A), 10 μm (B–D), 100 μm (E) and 50 μm (F,G).
Fig. 10.
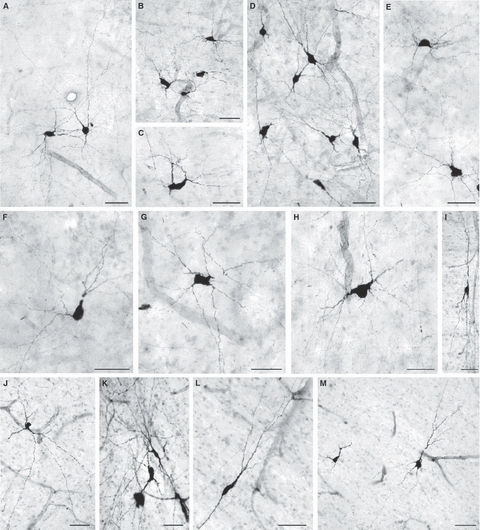
In a 2-day-old newborn (A–H) and in a 22-year-old adult human brain (I–M) numerous well-developed deep interstitial nitrinergic (NADPH-diaphorase-stained) neurons of various morphological types (bipolar, triangular or multipolar) are present in the deep white matter dorsal to the caudate nucleus (A,B) and putamen (C,D) as well as in the fountainhead of the internal capsule (E–M) (for exact location, see c, f and p in Fig. 1B). Many of these neurons are located in the vicinity (or even close apposition to) of blood vessels (A,B,D,G,H,J). Bars represent 50 μm.
Discussion
In this study we used several markers of neuronal chemical phenotypes, but we selected NADPH-d histochemistry (which selectively displays neurons producing nitric oxide) as a method of choice because it enables Golgi-like visualization of nitrinergic neurons and thus provides an opportunity to easily follow their prenatal and postnatal dendritic growth and morphological differentiation. It should be noted that we refer exclusively to the so-called Type I nitrinergic neurons, i.e. neurons that display strong NADPH-d-staining and a Golgi-like appearance (for review on types of nitrinergic neurons, see Yan et al. 1996a,b; Judaš et al. 1999). It should also be noted that nitrinergic neurons represent only a subset of fetal subplate or adult interstitial neurons. Indeed, in one of the early studies (Meyer et al. 1992) it was suggested that nitrinergic neurons represent only about 3% of all interstitial neurons (and about 30% of non-pyramidal interstitial neurons); in other words, they may be a dominant population of cortical nitrinergic neurons, but only a small fraction of the entire population of interstitial neurons. Thus, our findings cannot be generalized to all types of fetal subplate or adult interstitial neurons. Nevertheless, tracing the developmental fate of a defined (nitrinergic) subset of neurons enabled us to recognize two major and distinct populations of cerebral interstitial neurons in the fetal and adult human brain – superficial (gyral) and deep (periventricular).
The major findings of our study can be summarized as follows. (i) Nitrinergic neurons represent a large subset of fetal subplate neurons, which appear early (at 15 PCW) and many of these neurons survive in postnatal and adult brain as superficial (gyral) interstitial neurons. (ii) Numerous nitrinergic neurons are present in defined fibre tracts that surround the pallidum and putamen (internal and external capsule, anterior commissure) in both fetal and adult brain; many of these neurons probably represent displaced basal forebrain cholinergic neurons (see below for explanation). (iii) Deep interstitial neurons are also present in the corpus callosum from the midfetal period into adulthood; as these so-called intracallosal neurons have been described in detail in a separate publication (Jovanov-Milošević et al. 2010), they will not represent a major focus of our discussion. (iv) Finally, a prominent population of deep interstitial neurons inhabits the fountainhead of the internal capsule and neighbouring parts of the centrum semiovale in both fetal and adult human brain; these hitherto undescribed neurons seem to represent a unique population of interstitial neurons. Although these neurons may be most prominently developed in the human brain, we hypothesize that a similar population of interstitial neurons would exist in non-human primates or, more generally, in all large gyrencephalic mammals with an abundant cerebral white matter.
The developmental fate and potential functional roles of subplate neurons in rodents and carnivora have been extensively discussed in a number of excellent reviews (Allendoerfer & Shatz, 1994; Molnár et al. 2006; Clancy et al. 2009; Friedlander & Torres-Reveron, 2009; Luhmann et al. 2009; Suárez-Solá et al. 2009; Kanold & Luhmann, 2010). With respect to the human subplate zone, we have already published a series of articles discussing various aspects of its transient nature, morphologically and neurochemically diverse neurons, input–output connectivity and involvement in transient cortical circuitry (Kostović, 1990; Kostovic & Rakic, 1990; Kostović & Judaš, 2002, 2006, 2007, 2010; Kostović et al. 2002; Judaš et al. 1999, 2005; Kostović & Jovanov-Milošević, 2006; see also Vasung et al. 2010) as well as its potential involvement in the pathogenesis of developmental brain disorders (Kostović & Judaš, 2007, 2010; Kostović et al. 2010; Judaš et al. 2010 this issue). In addition, on the basis of extensive Golgi-stained material, we have already described the development and morphological types of human subplate neurons from 10 PCW to 3 postnatal months (Kostovic & Rakic, 1980, 1990; Mrzljak et al. 1988, 1990, 1992). Therefore, in this Discussion we focus exclusively on comparisons with other studies describing nitrinergic subplate neurons and adult interstitial neurons, as well as those describing possible equivalents of human deep periventricular interstitial neurons in other species.
Fetal subplate and postnatal superficial (gyral) interstitial neurons
Morphological diversity
First of all, it should be noted that human subplate and interstitial neurons continue to grow and morphologically differentiate throughout the fetal and early postnatal period. Although the rapid differentiation of subplate neurons into five neuronal types between 17 and 25 PCW coincided with the ingrowth of thalamocortical (Kostovic & Goldman-Rakic 1983; Kostovic & Rakic 1984; Kostovic & Rakic, 1990), basal forebrain cholinergic (Kostović, 1986), and cortico-cortical afferent fibres (DeAzevedo et al. 1997) into the subplate zone, subplate neurons continued with further dendritic growth even after these afferents had relocated to the cortical plate, up to the seventh postnatal month (Mrzljak et al. 1988, 1990, 1992). Fusiform neurons with very long dendrites were the most frequently impregnated interstitial cell type in the gyral white matter after birth (Mrzljak et al. 1990), but slightly modified pyramidal neurons and multipolar non-pyramidal interstitial neurons were also present (Kostovic & Rakic, 1980; Mrzljak et al. 1990).
Neurochemical diversity
It should also be noted that the neurochemical diversity of subplate and interstitial neurons surpasses the diversity of their morphological types. If we restrict our attention just to studies of fetal and adult human and monkey cerebral cortex, a number of classical neurotransmitters (GABA, glutamate, monoamines), neuropeptides, and transmitter-related enzymes or other signalling molecules have been described. In humans, subplate neurons express GABA (Schiffmann et al. 1988; Yan et al. 1992; Masood et al. 1993; Zecevic & Milosevic, 1997), vesicular GABA transporter, a potassium/chloride co-transporter (KCC2) (Bayatti et al. 2008), various glutamate receptors (Lee & Choi, 1992; Andersen et al. 1995; Ritter et al. 2001; Talos et al. 2006), AChE (Kostovic & Rakic, 1980; Kostović et al. 1981; Kostović, 1984), and nicotinic acetylcholine receptors (Schröder et al. 2001). In the monkey, subplate neurons also express GABA (Huntley et al. 1988; Meinecke & Rakic, 1992; Tomioka & Rockland, 2007) and GABA-A receptors (Huntley et al. 1990; Meinecke & Rakic, 1992). GABA-positive fibres and somata have also been detected in the cortical white matter of adult monkeys (Hendrickson et al. 1981; Schwartz et al. 1988). Finally, choline-acetyltransferase-immunoreactive subplate neurons were observed in fetal monkey cortex (Hendry et al. 1987). Human subplate and interstitial neurons also express various neuropeptides, i.e. neuropeptide Y (Masood et al. 1993; Delalle et al. 1997; Uylings & Delalle, 1997; Colombo & Bentham, 2006; Bayatti et al. 2008), somatostatin (Sorensen, 1982; Kostović et al. 1991b), and substance P (Masood et al. 1993). In the monkey, subplate and interstitial neurons also contain a variety of neuropeptides, i.e. neuropeptide Y (Huntley et al. 1988; Hayashi et al. 1989; Kuljis & Rakic, 1989; Mori, 1996), cholecystokinin (Hayashi et al. 1989), somatostatin (Huntley et al. 1988; Yamashita et al. 1989; Iritani & Satoh, 1991), substance P (Yamashita et al. 1990; Mehra & Hendrickson, 1993) and vasoactive intestinal polypeptide (Benson et al. 1991). Finally, at least some human subplate and interstitial neurons also contain calcium-binding proteins, such as calbindin (Yan et al. 1996b; Ulfig, 2002; Suárez-Solá et al. 2009) and calretinin (Gabbott et al. 1997; Ulfig, 2002; Suárez-Solá et al. 2009). Parvalbumin-stained neurons appear in a sparse band of cells in layer 6 and the upper subplate at 26 weeks, but parvalbumin cells represented only a small fraction of the total human subplate population (Honig et al. 1996). Human subplate and interstitial neurons also express cytoskeletal markers such as microtubule-associated protein-2 (Sims et al.1988; Colombo & Bentham, 2006; Bayatti et al. 2008) and SMI 32, a marker for non-phosphorylated neurofilament high molecular weight (Haynes et al. 2005). They also express important signaling molecules such as reelin (Suárez-Solá et al. 2009), transcription factor Tbr1 (Bayatti et al. 2008; Suárez-Solá et al. 2009), and p75 low-affinity nerve growth factor receptor (Kordower & Mufson, 1992).
Nitrinergic subplate and interstitial neurons
Our own findings (Judaš et al. 1999; this study), as well as the findings of others (Yan et al. 1996a; Yan & Ribak, 1997; Ohyu & Takashima, 1998), clearly demonstrate that nitrinergic human subplate neurons appear at 14–15 PCW and that they represent the most prominent population of nitrinergic cortical neurons during the entire prenatal and postnatal development. The presence of a significant population of nitrinergic neurons within the subcortical white matter has been reported in almost all studies of the adult mammalian neocortex (for review, see Judaš et al. 1999). In the adult monkey brain, NADPH-d interstitial neurons are numerous and predominantly located within the first 100–200 μm of the subcortical white matter (Sandell, 1986; Gabbott & Bacon, 1996a,b; Yan et al. 1996b; Barone & Kennedy, 2000), but also occur in the deep white matter (Aoki et al. 1993; Hashikawa et al. 1994; Dombrowski & Barbas, 1996). There are also regional differences in their number (Barone & Kennedy, 2000). For example, nitrinergic interstitial neurons in the monkey prefrontal cortex are abundant below association areas (where the intracortical distribution of nitrinergic neurons is low) but they are clearly less numerous beneath olfactory and limbic areas (where the intracortical distribution of nitrinergic neurons is high) (Dombrowski & Barbas, 1996). Interstitial nitrinergic neurons have been variably described as aspiny multipolar (Kowall & Beal, 1988; Meyer et al. 1992; Egberongbe et al. 1994), predominantly bipolar (Norris et al. 1996) or sparsely spinous horizontally oriented large cells with oval somata, somatic spines, and filopodia-like appendages (Lüth et al. 1994) or even as densely spiny cells with elongated multipolar somata oriented in parallel to the cortical/white matter border (Fischer & Kuljis, 1994). Their axons and dendrites form a dense network of NADPH-d-positive fibres in the subcortical white matter and some of their processes reach infragranular or even supragranular layers (Meyer et al. 1992; Fischer & Kuljis, 1994). A number of NADPH-d-positive fibres of unidentified origin also cross the cortical/white matter border (Sandell, 1986) and some thick NADPH-d-positive fibres can be followed from the white matter into layer IV, showing a dense plexus and being connected with pericellular NADPH-d baskets in layers IV–VI (Lüth et al. 1994).
What do we know about relative numbers of the different neurotransmitter phenotypes of interstitial neurons?
From the data available for human, non-human primate, carnivore and rodent interstitial cells, it is clear that only a fraction of these neurons is GABAergic and that some of them are glutamatergic. However, we still do not have detailed data on relative numbers of different neurotransmitter phenotypes of either subplate or interstitial neurons in any species. Such analyses should represent one of the priority lines of research in the future. In this section, we review data available on human and monkey interstitial neurons, with some comparisons with rodents. In the monkey, nitrinergic interstitial neurons represent a substantial portion of the total cortical nitrinergic neurons – over 50% in the monkey auditory cortex (Cipolloni & Pandya, 1991) and about 40% in the monkey medial prefrontal cortex (Gabbott & Bacon, 1996b). The proportion of nitrinergic interstitial neurons in a total population of cortical nitrinergic neurons is even more impressive in the adult human cortex (Unger & Lange, 1992; DeFelipe, 1993; Egberongbe et al. 1994; Lüth et al. 1994; Garbossa et al. 2005) where they represent 60–87% of all cortical NADPH-d-positive neurons (Kowall & Beal, 1988; Fischer & Kuljis, 1994; Norris et al. 1996).
The neurochemical nature of NADPH-d- and nitric oxide synthase (NOS)-immunoreactive interstitial neurons has been investigated in a number of co-localization studies in both humans and other mammals (for review, see Judaš et al. 1999). In the human cortex, the majority of nitrinergic interstitial neurons also express somatostatin and neuropeptide Y (Kowall & Beal, 1988; Unger & Lange, 1992). In the monkey cortex, only 58% of nitrinergic interstitial neurons also contain GABA (Yan et al. 1996b), but it seems that these neurons below the monkey auditory cortex do not contain GABA (Cipolloni & Pandya, 1991). About 70% of the nitrinergic interstitial neurons in the subcortical white matter of monkey and human neocortex also contain muscarinic m2 receptors, and approximately 90% of these cells are rich in AChE (Smiley et al. 1998). Finally, nitrinergic neurons in the monkey cerebral cortex are essentially always immunoreactive for somatostatin and neuropeptide Y, but do not express calbindin (Smiley et al. 2000). These neurons comprise about 30% of the somatostatin cells and about 60% of the neuropeptide Y cells (Smiley et al. 2000).
As already mentioned, according to some estimates (Meyer et al. 1992) nitrinergic neurons represent only about 3% of all interstitial neurons (and about 30% of non-pyramidal interstitial neurons); in other words, they may be a dominant population of cortical nitrinergic neurons, but only a small fraction of the entire population of interstitial neurons. If this turns out to be true, than subcortical (gyral) interstitial neurons indeed represent a huge population of neurons in the adult human telencephalon, because our findings as well as the findings of most of the other above-mentioned studies clearly demonstrate that NADPH-d-positive neurons are abundantly present in the subcortical white matter.
Possible functional roles of interstitial neurons
It is fair to say that at present possible functional roles of interstitial neurons in the adult human and monkey brain remain largely unknown. Nitrinergic axons often form perivascular fibre networks (DeFelipe, 1993) and contact blood vessels (Regidor et al. 1993; Schottler et al. 1996; Yan et al. 1996b; Yan & Ribak, 1997). Thus, it has frequently been suggested that nitrinergic subcortical interstitial neurons are involved in local regulation of cortical microvascular circulation (Okhotin & Kupriyanov, 1997; Estrada & DeFelipe, 1998; Okhotin & Kalinichenko, 2003; Suárez-Solá et al. 2009). On the basis of data obtained in rodents, it has been recently proposed that the majority of interstitial neurons are glutamatergic and may function as a sort of cortical gatekeeper involved in modulation of information flow within the overlying cortex (Friedlander & Torres-Reveron, 2009). However, it is difficult to extrapolate such data from rodents to humans without prior knowledge about the exact proportions of glutamatergic vs. GABAergic interstitial neurons in humans and non-human primates.
Deep interstitial neurons surrounding the globus pallidus and putamen may represent displaced basal forebrain cholinergic neurons
The presence of numerous interstitial neurons in fibre tracts surrounding basal ganglia was previously noted in classical Nissl and Golgi studies (Ramón y Cajal, 1911; Foix & Nicolesco, 1925). Das and Kreutzberg (1968) described these neurons as a neuronal shell or globular net virtually enclosing the globus pallidus, extending to the basal forebrain and medullary lamina separating the pallidum from the putamen, and regarded them as typical isodendritic neurons (Das & Kreutzberg, 1968). That concept of a neuronal shell enclosing the globus pallidus was extensively confirmed in subsequent studies of the magnocellular cholinergic basal forebrain system in both human and monkey, by means of various markers of these neurons such as AChE, choline-acetyltransferase, nerve growth factor and nerve growth factor receptors (trk1, p75). Displaced cholinergic neurons (usually interpreted as belonging to the Ch4 group or basal nucleus of Meynert) were documented within the internal and external capsule, anterior commissure and medullary laminae separating two segments of the globus pallidus as well as the globus pallidus from the putamen (Mesulam et al. 1983; Mesulam & Mufson, 1984; Satoh & Fibiger, 1985; Kordower et al. 1988, 1989, 1994; Mesulam & Geula, 1988; Allen et al. 1989; Mufson et al. 1989). Although there is no clear evidence that these cholinergic neurons also synthesize nitric oxide, NADPH-d-positive fibres were noted in the human external capsule (Ellison et al. 1987) and NOS-immunoreactive neurons (Satoh et al. 1995), and neuropeptide Y-immunoreactive neurons were found in the monkey external capsule and medullary laminae surrounding human globus pallidus (Lehéricy et al. 1993). However, no NOS-immunoreactive neurons were found in the monkey anterior commissure (Satoh et al. 1995).
Although direct proof (i.e. co-localization of choline-acetyltransferase and NADPH-d/NOS-1 reactivity in the same neurons) is still lacking in both humans and monkeys, it seems probable that nitrinergic neurons present in the fetal and adult human internal and external capsule and the anterior commissure do really belong to a population of displaced basal forebrain cholinergic neurons, on the basis of their common topographical and morphological features. In fact, this assumption was the reason why the investigation described here was not focused on interstitial neurons within the internal capsule, but exclusively on the population situated in the fountainhead of the internal capsule and neighbouring parts of the centrum semiovale (see below and Fig. 1B, c, f and p).
Is there an equivalent population of deep fetal interstitial neurons in non-human mammals?
The presence of GABAergic tangentially migrating neurons was noted in the intermediate zone/subventricular zone of embryonic rats (Chronwall & Wolff, 1980; Wolff et al. 1984; Lauder et al. 1986; Van Eden et al. 1989; Cobas et al. 1991; DeDiego et al. 1994). These fetal interstitial neurons were generated briefly at embryonic day (E)12 and seemed to migrate from the lateral to medial part of the telencephalic wall from E14 to E18, when they were first observed within the corpus callosum (DeDiego et al. 1994). They did not seem to become incorporated within the cortical plate as some of them postnatally become white matter interstitial cells (DeDiego et al. 1994). Similar GABAergic deep fetal interstitial neurons were also described in mouse (Del Rio et al. 1992, 2000), monkey (Schwartz & Meinecke, 1992) and human (Yan et al. 1992) fetal brain. In fact, such cells were previously described by the Golgi method in the fetal rabbit telencephalon (Stensaas, 1967a,b;). In their study in mouse, Del Rio et al. (2000) concluded that the intermediate zone/subventricular zone neurons constituted a particular GABAergic population (distinct from subplate neurons and unique to rodents), which includes resident cells and tangentially migrating postmitotic neurons spatially related to the development of callosal connections (Del Rio et al. 2000). However, we clearly demonstrated that deep fetal interstitial neurons are also present in the human fetal brain. Although nitrinergic interstitial neurons were indeed noted in the subcortical white matter of adult rodents (Hedlich et al. 1990; Valtschanoff et al. 1993; Yan et al. 1994), and if we accept that the rodent homologue of the subplate is layer VIb or VII (for review, see Reep, 2000; Clancy et al. 2009), some of these rodent neurons may represent a homologue of human deep (periventricular) interstitial neurons. However, interstitial neurons observed in the white matter of adult cats (Mizukawa et al. 1988; Valverde & Facal-Valverde, 1988; Riche et al. 1995) and sheep (Northington et al. 1996) clearly correspond to surviving subplate neurons and thus do not represent an equivalent population for deep fetal interstitial neurons in humans.
However, in both rodents and carnivores there is simply no equivalent for a large population of deep human interstitial neurons located in the fountainhead of the internal capsule and the neighbouring parts of the centrum semiovale dorsal to the dorsal tips of the caudate nucleus and putamen. Therefore, we suggest that this unique population of deep interstitial neurons is characteristic (and probably best developed) for the human brain. However, it is reasonable to assume that it is not human-specific, and that it would probably be found in the corresponding location of apes, rhesus monkeys and – in general – many large mammals with large, slowly developing, gyrencephalic brains with an abundant cerebral white matter.
Acknowledgments
This work was supported by a Croatian Ministry of Science, Education and Sport grant no. 108-1081870-1878 (to M.J.) and Unity through Knowledge Fund (UKF) grant (director Ivica Kostović). The excellent technical assistance of Zdenka Cmuk, Danica Budinšćak and Božica Popović is greatly appreciated.
References
- Allen SJ, Dawbarn D, Spillantini MG, et al. Distribution of beta-nerve growth factor receptors in the human basal forebrain. J Comp Neurol. 1989;289:626–640. doi: 10.1002/cne.902890408. [DOI] [PubMed] [Google Scholar]
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connec-tions between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Andersen DL, Tannenberg AEG, Burke CJ, et al. Develop-mental rearrangements of cortical glutamate-NMDA receptor binding sites in late human gestation. Dev Brain Res. 1995;88:178–185. doi: 10.1016/0165-3806(95)00101-i. [DOI] [PubMed] [Google Scholar]
- Aoki C, Fenstemaker S, Lubin M, et al. Nitric oxide synthase in the visual cortex of monocular monkeys as revealed by light and electron microscopic immunocytochemistry. Brain Res. 1993;620:97–113. doi: 10.1016/0006-8993(93)90275-r. [DOI] [PubMed] [Google Scholar]
- Barone P, Kennedy H. Non-uniformity of neocortex, areal heterogeneity of NADPH-diaphorase reactive neurons in adult macaque monkeys. Cereb Cortex. 2000;10:160–174. doi: 10.1093/cercor/10.2.160. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, et al. A molecular neuro-anatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex. 2008;18:1536–1548. doi: 10.1093/cercor/bhm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DL, Isakson PJ, Jones EG. In situ hybridization reveals VIP precursor mRNA-containing neurons in monkey and rat neocortex. Mol Brain Res. 1991;9:169–174. doi: 10.1016/0169-328x(91)90145-n. [DOI] [PubMed] [Google Scholar]
- Chronwall B, Wolff JR. Prenatal and postnatal development of GABA-accumulating cells in the occipital neocortex of rat. J Comp Neurol. 1980;190:187–208. doi: 10.1002/cne.901900113. [DOI] [PubMed] [Google Scholar]
- Cipolloni PB, Pandya DN. Golgi, histochemical, and immunocytochemical analyses of the neurons of auditory-related cortices of the rhesus monkey. Exp Neurol. 1991;114:104–122. doi: 10.1016/0014-4886(91)90088-t. [DOI] [PubMed] [Google Scholar]
- Clancy B, Teague-Ross TJ, Nagarajan R. Cross-species analyses of the cortical GABAergic and subplate neural populations. Front Neuroanat. 2009;3:20. doi: 10.3389/neuro.05.020.2009. (Vol. 3, article 20, pp. 1–14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobas A, Fairén A, Alvarez-Bolado G, et al. Prenatal development of the intrinsic neurons of the rat neocortex: a comparative study of the distribution of GABA-immunoreactive cells and the GABA-A receptor. Neuroscience. 1991;40:375–397. doi: 10.1016/0306-4522(91)90127-a. [DOI] [PubMed] [Google Scholar]
- Colombo JA, Bentham C. Immunohistochemical analysis of subcortical white matter astroglia of infant and adult primate brains, with a note on resident neurons. Brain Res. 2006;1100:93–103. doi: 10.1016/j.brainres.2006.04.116. [DOI] [PubMed] [Google Scholar]
- Das GD, Kreutzberg GW. Evaluation of interstitial nerve cells in the central nervous system. A correlative study using acetylcholinesterase and Golgi techniques. Ergeb Anat Entwicklungsgesch. 1968;41:1–59. [PubMed] [Google Scholar]
- DeAzevedo LC, Hedin-Pereira C, Lent R. Callosal neurons in the cingulate cortical plate and subplate of human fetuses. J Comp Neurol. 1997;386:60–70. doi: 10.1002/(sici)1096-9861(19970915)386:1<60::aid-cne7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- DeDiego I, Smith-Fernández A, Fairén A. Cortical cells that migrate beyond area boundaries: characterization of an early neuronal population in the lower intermediate zone of prenatal rats. Eur J Neurosci. 1994;6:983–997. doi: 10.1111/j.1460-9568.1994.tb00593.x. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. A study of NADPH diaphorase-positive axonal plexuses in the human temporal cortex. Brain Res. 1993;615:342–346. doi: 10.1016/0006-8993(93)90047-q. [DOI] [PubMed] [Google Scholar]
- Del Rio JA, Soriano E, Ferrer I. The development of GABA immunoreactivity in the neocortex of the mouse. J Comp Neurol. 1992;326:501–526. doi: 10.1002/cne.903260403. [DOI] [PubMed] [Google Scholar]
- Del Rio JA, Martínez A, Auladell C, et al. Developmental history of the subplate and developing white matter in the murine neocortex. Neuronal organization and relationship with the main afferent systems at embryonic and perinatal stages. Cereb Cortex. 2000;10:784–801. doi: 10.1093/cercor/10.8.784. [DOI] [PubMed] [Google Scholar]
- Delalle I, Evers P, Kostovic I, et al. Laminar distribution of neuropeptide Y-immunoreactive neurons in human prefrontal cortex during development. J Comp Neurol. 1997;379:515–522. doi: 10.1002/(sici)1096-9861(19970324)379:4<515::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Dombrowski SM, Barbas H. Differential expression of NADPH diaphorase in functionally distinct prefrontal cortices in the rhesus monkey. Neuroscience. 1996;72:49–62. doi: 10.1016/0306-4522(95)00539-0. [DOI] [PubMed] [Google Scholar]
- Egberongbe YI, Gentleman SM, Falkai P, et al. The distribution of nitric oxide synthase immunoreactivity in the human brain. Neuroscience. 1994;59:561–578. doi: 10.1016/0306-4522(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Ellison DW, Kowall NW, Martin JB. Subset of neurons characterized by the presence of NADPH-diaphorase in human substantia innominata. J Comp Neurol. 1987;260:233–245. doi: 10.1002/cne.902600207. [DOI] [PubMed] [Google Scholar]
- Estrada C, DeFelipe J. Nitric oxide-producing neurons in the neocortex, morphological and functional relationship with intraparenchymal microvasculature. Cereb Cortex. 1998;8:193–203. doi: 10.1093/cercor/8.3.193. [DOI] [PubMed] [Google Scholar]
- Fischer HC, Kuljis RO. Multiple types of nitrogen monoxide synthase-/NADPH diaphorase-containing neurons in the human cerebral neocortex. Brain Res. 1994;654:105–117. doi: 10.1016/0006-8993(94)91576-8. [DOI] [PubMed] [Google Scholar]
- Foix C, Nicolesco J. Les Noyaux Gris Centraux et la Région Mésencéphalo-sousoptique. Paris: Mason & Cie; 1925. [Google Scholar]
- Friedlander MJ, Torres-Reveron J. The changing roles of neurons in the cortical subplate. Front Neuroanat. 2009;3:15. doi: 10.3389/neuro.05.015.2009. (Vol. 3, article 15, pp. 1–8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PLA, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. J Comp Neurol. 1996a;364:567–608. doi: 10.1002/(SICI)1096-9861(19960122)364:4<567::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol. 1996b;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Jays PR, Bacon SJ. Calretinin in human medial prefrontal cortex (areas 24a,b,c, 32 and 25) J Comp Neurol. 1997;38:389–410. doi: 10.1002/(sici)1096-9861(19970519)381:4<389::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Garbossa D, Fontanella M, Tomasi S, et al. Differential distribution of NADPH-diaphorase histochemistry in human cerebral cortex. Brain Res. 2005;1034:1–10. doi: 10.1016/j.brainres.2004.10.049. [DOI] [PubMed] [Google Scholar]
- Hashikawa T, Leggio MG, Hattori R, et al. Nitric oxide synthase immunoreactivity colocalized with NADPH-diaphorase histochemistry in monkey cerebral cortex. Brain Res. 1994;641:341–349. doi: 10.1016/0006-8993(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamashita A, Shimizu K, et al. Ontogeny of cholecystokinin-8 and glutamic acid decarboxylase in cerebral neocortex of macaque monkey. Exp Brain Res. 1989;74:249–255. doi: 10.1007/BF00248857. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Borenstein NS, Desilva TM, et al. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484:156–167. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- Hedlich A, Lüth HJ, Werner L, et al. GABAerge NADPH-diaphorase-positive Martinottizellen im visuellen Cortex der Ratte. J Hirnforsch. 1990;31:681–687. [PubMed] [Google Scholar]
- Hendrickson AE, Hunt SP, Wu JY. Immunocytochemical localization of glutamic acid decarboxylase in monkey striate cortex. Nature. 1981;292:605–607. doi: 10.1038/292605a0. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, Killackey HP, et al. Choline acetyltransferase-immunoreactive neurons in fetal monkey cerebral cortex. Brain Res. 1987;465:311–317. doi: 10.1016/0165-3806(87)90252-5. [DOI] [PubMed] [Google Scholar]
- Honig LS, Herrmann K, Shatz CJ. Developmental changes revealed by immunohistochemical markers in human cerebral cortex. Cereb Cortex. 1996;6:794–806. doi: 10.1093/cercor/6.6.794. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Hendry SHC, Killackey HP, et al. Temporal sequence of neurotransmitter expression by developing neurons of fetal monkey visual cortex. Dev Brain Res. 1988;43:69–96. doi: 10.1016/0165-3806(88)90154-x. [DOI] [PubMed] [Google Scholar]
- Huntley GW, deBlas AL, Jones EG. GABA-A receptor immunoreactivity in adult and developing monkey sensory-motor cortex. Exp Brain Res. 1990;82:519–535. doi: 10.1007/BF00228794. [DOI] [PubMed] [Google Scholar]
- Iritani I, Satoh K. Distribution of somatostatin-immunoreactive cell bodies and fibers in the neocortex of Macaca fuscata. Synapse. 1991;9:50–59. doi: 10.1002/syn.890090108. [DOI] [PubMed] [Google Scholar]
- Jovanov-Milošević N, Petrović D, Petanjek Z, et al. Mor-phology, molecular phenotypes and distribution of neurons in developing human corpus callosum. Eur J Neurosci. 2010 doi: 10.1111/j.1460-9568.2010.07400.x. in press. [DOI] [PubMed] [Google Scholar]
- Judaš M, Šestan N, Kostović I. Nitrinergic neurons in the developing and adult human telencephalon: transient and permanent patterns of expression in comparison to other mammals. Microsc Res Tech. 1999;45:401–419. doi: 10.1002/(SICI)1097-0029(19990615)45:6<401::AID-JEMT7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Judaš M, Radoš M, Jovanov-Milošević N, et al. Structural, immunocytochemical and MR imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. Am J Neuroradiol. 2005;26:2671–2684. [PMC free article] [PubMed] [Google Scholar]
- Judaš M, Sedmak G, Pletikos M. Early history of subplate and interstitial neurons: from Theodor Meynert (1867) to the discovery of the subplate zone (1974) J Anat. 2010;217:344–367. doi: 10.1111/j.1469-7580.2010.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Mufson EJ. Nerve growth factor receptor-immunoreactive neurons within the developing human cortex. J Comp Neurol. 1992;323:25–41. doi: 10.1002/cne.903230104. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bartus RT, Bothwell M, et al. Nerve growth factor receptor immunoreactivity in the nonhuman primate (Cebus apella): distribution, morphology, and colocalization with cholinergic enzymes. J Comp Neurol. 1988;277:465–486. doi: 10.1002/cne.902770402. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bartus RT, Marciano FF, et al. Telencephalic cholinergic system of the New World monkey (Cebus apella): morphological assessment and analysis of the projection to amygdala. J Comp Neurol. 1989;279:528–545. doi: 10.1002/cne.902790403. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chen EY, Sladek JR, Jr, et al. TRK-immunoreactivity in the monkey central nervous system: forebrain. J Comp Neurol. 1994;349:20–35. doi: 10.1002/cne.903490103. [DOI] [PubMed] [Google Scholar]
- Kostović I. Prenatal development of interstitial neurons in the “white” matter of the human telencephalon. Soc Neurosci Abstr. 1984;10:47. [Google Scholar]
- Kostović I. Prenatal development of nucleus basalis complex and related fiber systems in man: a histochemical study. Neuroscience. 1986;17:1047–1077. doi: 10.1016/0306-4522(86)90077-1. [DOI] [PubMed] [Google Scholar]
- Kostović I. Structural and histochemical reorganization of the human prefrontal cortex during perinatal and postnatal life. Prog Brain Res. 1990;85:131–147. doi: 10.1016/s0079-6123(08)62682-5. [DOI] [PubMed] [Google Scholar]
- Kostović I, Goldman-Rakic PS. Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol. 1983;219:431–447. doi: 10.1002/cne.902190405. [DOI] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milošević N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. Correlation between sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002;267:1–6. doi: 10.1002/ar.10069. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. Prolonged coexistence of transient and permanent circuitry elements in the developing cerebral cortex of fetuses and preterm infants. Dev Med Child Neurol. 2006;48:388–393. doi: 10.1017/S0012162206000831. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev. 2007;31:1157–1168. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr. 2010;99:1119–1127. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Cytology and the time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 1980;9:219–242. doi: 10.1007/BF01205159. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci. 1984;4:25–42. doi: 10.1523/JNEUROSCI.04-01-00025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kostović I, Kelović Z, Mrzljak L, et al. Distribution and morphology of interstitial acetylcholinesterase (AChE) reactive neurons in the fiber bundles of the human fetal telencephalon. Neurosci Lett. 1981;(Suppl 7):S288. [Google Scholar]
- Kostović I, Judaš M, Kostović-Knežević Lj, et al. Zagreb Research Collection of human brains for developmental neurobiologists and clinical neuroscientists. Int J Dev Biol. 1991a;35:215–230. [PubMed] [Google Scholar]
- Kostović I, Štefulj-Fučić A, Mrzljak L, et al. Prenatal and perinatal development of the somatostatin-immunoreactive neurons in the human prefrontal cortex. Neurosci Lett. 1991b;124:153–156. doi: 10.1016/0304-3940(91)90082-5. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M, Radoš M, et al. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M, Sedmak G. Developmental history of the subplate zone, subplate neurons and interstitial white matter neurons: relevance for schizophrenia. Int J Dev Neurosci. 2010 doi: 10.1016/j.ijdevneu.2010.09.005. in press. [DOI] [PubMed] [Google Scholar]
- Kowall NW, Beal MF. Cortical somatostatin, neuropeptide Y, and NADPH diaphorase neurons: normal anatomy and alterations in Alzheimer's disease. Ann Neurol. 1988;23:105–114. doi: 10.1002/ana.410230202. [DOI] [PubMed] [Google Scholar]
- Kuljis RO, Rakic P. Distribution of neuropeptide Y-containing perikarya and axons in various neocortical areas in the macaque monkey. J Comp Neurol. 1989;280:383–392. doi: 10.1002/cne.902800305. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Han VKM, Henderson P, et al. Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. Neuroscience. 1986;19:465–493. doi: 10.1016/0306-4522(86)90275-7. [DOI] [PubMed] [Google Scholar]
- Lee H, Choi BH. Density and distribution of excitatory amino acid receptors in the developing human fetal brain: a quantitative autoradiographic study. Exp Neurol. 1992;118:284–290. doi: 10.1016/0014-4886(92)90185-s. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Hirsch EC, Cervera-Piérot P, et al. Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer's disease. J Comp Neurol. 1993;330:15–31. doi: 10.1002/cne.903300103. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Kilb W, Hanganu-Opatz IL. Subplate cells: amplifiers of neuronal activity in the developing cerebral cortex. Front Neuroanat. 2009;3:19. doi: 10.3389/neuro.05.019.2009. (Vol. 3, article 19, pp. 1–11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin MB, Shatz CJ. Studies of the earliest generated cells of the cat's visual cortex: cogeneration of subplate and marginal zones. J Neurosci. 1985;5:1062–1075. doi: 10.1523/JNEUROSCI.05-04-01062.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüth HJ, Hedlich A, Hilbig H, et al. Morphological analyses of NADPH-diaphorase/nitric oxide synthase positive structures in human visual cortex. J Neurocytol. 1994;23:770–782. doi: 10.1007/BF01268089. [DOI] [PubMed] [Google Scholar]
- Masood F, Wadhwa S, Bijlani V. An immunohistochemical study of neurotransmitter profiles in developing human visual cortex. Int J Dev Neurosci. 1993;11:387–397. doi: 10.1016/0736-5748(93)90010-b. [DOI] [PubMed] [Google Scholar]
- Mehra RD, Hendrickson AE. A comparison of the development of neuropeptide and MAP2 immunocyto-chemical labeling in the macaque visual cortex during prenatal and postnatal development. J Neurobiol. 1993;24:101–124. doi: 10.1002/neu.480240109. [DOI] [PubMed] [Google Scholar]
- Meinecke DL, Rakic P. Expression of GABA and GABA-A receptors by neurons of the subplate zone in developing primate occipital cortex: evidence for transient local circuits. J Comp Neurol. 1992;317:91–101. doi: 10.1002/cne.903170107. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol. 1988;275:216–240. doi: 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Neural inputs into the nucleus basalis of the substantia innominata (Ch4) in the rhesus monkey. Brain. 1984;107:253–274. doi: 10.1093/brain/107.1.253. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, et al. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata) and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Meyer G, Wahle P, Castaneyra-Perdomo A, et al. Morphology of neurons in the white matter of the adult human neocortex. Exp Brain Res. 1992;88:204–212. doi: 10.1007/BF02259143. [DOI] [PubMed] [Google Scholar]
- Mizukawa K, Vincent SR, McGeer PL, et al. Reduced nicotinamide adenine dinucleotide phosphate (NADPH)-diaphorase-positive neurons in cat cerebral white matter. Brain Res. 1988;461:274–281. doi: 10.1016/0006-8993(88)90257-0. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Metin C, Stoykova A, et al. Comparative aspects of cerebral cortical development. Eur J Neurosci. 2006;23:921–934. doi: 10.1111/j.1460-9568.2006.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S. Neuropeptide Y-like immunoreactivity in area 17 of chimpanzee. Okajimas Folia Anat Jpn. 1996;73:219–229. doi: 10.2535/ofaj1936.73.5_219. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HBM, Kostovic I, et al. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 1988;271:355–386. doi: 10.1002/cne.902710306. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HBM, Van Eden CG, et al. Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Prog Brain Res. 1990;85:185–222. doi: 10.1016/s0079-6123(08)62681-3. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HBM, Kostović I, et al. Prenatal development of neurons in the human prefrontal cortex. II. A quantitative Golgi study. J Comp Neurol. 1992;316:485–496. doi: 10.1002/cne.903160408. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Bothwell MA, Hersh LB, et al. Nerve growth factor receptor immunoreactive profiles in the normal aged human basal forebrain: colocalization with cholinergic neurons. J Comp Neurol. 1989;285:196–217. doi: 10.1002/cne.902850204. [DOI] [PubMed] [Google Scholar]
- Norris PJ, Faull RLM, Emson PC. Neuronal nitric oxide synthase (nNOS) mRNA expression and NADPH-diaphorase staining in the frontal cortex, visual cortex and hippocampus of control and Alzheimer's disease brains. Mol Brain Res. 1996;41:36–49. doi: 10.1016/0169-328x(96)00064-2. [DOI] [PubMed] [Google Scholar]
- Northington FJ, Koehler RC, Traystman RJ, et al. Nitric oxide synthase 1 and nitric oxide synthase 3 protein expression is regionally and temporally regulated in fetal brain. Dev Brain Res. 1996;95:1–14. doi: 10.1016/0165-3806(96)00051-x. [DOI] [PubMed] [Google Scholar]
- Ohyu J, Takashima S. Developmental characteristics of neuronal nitric oxide synthase (nNOS) immunoreactive neurons in fetal to adolescent human brains. Dev Brain Res. 1998;110:193–202. doi: 10.1016/s0165-3806(98)00107-2. [DOI] [PubMed] [Google Scholar]
- Okhotin VE, Kalinichenko SG. Subcortical white matter interstitial cells: their connections, neurochemical specialization, and role in the histogenesis of the cortex. Neurosci Behav Physiol. 2003;33:177–194. doi: 10.1023/a:1021778015886. [DOI] [PubMed] [Google Scholar]
- Okhotin VE, Kupriyanov VV. Neurovascular relationships in the human neocortex. Neurosci Behav Physiol. 1997;27:482–488. doi: 10.1007/BF02463888. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Histologie du Systeme Nerveux de l’Homme & des Vertébrés. Tome II. Paris: A. Maloine; 1911. [Google Scholar]
- Reep RL. Cortical layer VII and persistent subplate cells in mammalian brains. Brain Behav Evol. 2000;56:212–234. doi: 10.1159/000047206. [DOI] [PubMed] [Google Scholar]
- Regidor J, Edvinsson L, Divac I. NOS neurones lie near branchings of cortical arteriolae. Neuroreport. 1993;4:112–114. doi: 10.1097/00001756-199301000-00030. [DOI] [PubMed] [Google Scholar]
- Riche D, Foutz AS, Denavit-Saubie M. Developmental changes of NADPH-diaphorase neurons in the forebrain of neonatal and adult cat. Dev Brain Res. 1995;89:139–145. doi: 10.1016/0165-3806(95)00114-s. [DOI] [PubMed] [Google Scholar]
- Ritter LM, Unis AS, Meador-Woodruff JH. Ontogeny of ionotropic glutamate receptor expression in human fetal brain. Dev Brain Res. 2001;127:123–133. doi: 10.1016/s0165-3806(01)00126-2. [DOI] [PubMed] [Google Scholar]
- Sandell JH. NADPH diaphorase histochemistry in the macaque striate cortex. J Comp Neurol. 1986;251:388–397. doi: 10.1002/cne.902510309. [DOI] [PubMed] [Google Scholar]
- Satoh J, Fibiger HC. Distribution of central cholinergic neurons in the baboon (Papio papio). I. General morphology. J Comp Neurol. 1985;236:197–214. doi: 10.1002/cne.902360205. [DOI] [PubMed] [Google Scholar]
- Satoh K, Arai R, Ikemoto K, et al. Distribution of nitric oxide synthase in the central nervous system of Macaca fuscata: subcortical regions. Neuroscience. 1995;66:685–696. doi: 10.1016/0306-4522(95)00040-p. [DOI] [PubMed] [Google Scholar]
- Schiffmann S, Campistron G, Tugendhaft P, et al. Immunocytochemical detection of GABAergic nerve cells in the human temporal cortex using a direct gamma-aminobutyric acid antiserum. Brain Res. 1988;442:270–278. doi: 10.1016/0006-8993(88)91512-0. [DOI] [PubMed] [Google Scholar]
- Schottler F, Collins JL, Fergus A, et al. Structural interactions between NOS-positive neurons and blood vessels in the hippocampus. NeuroReport. 1996;7:966–968. doi: 10.1097/00001756-199603220-00028. [DOI] [PubMed] [Google Scholar]
- Schröder H, Schütz U, Burghaus L, et al. Expression of the alpha4 isoform of the nicotinic acetylcholine receptor in the fetal human cerebral cortex. Dev Brain Res. 2001;132:33–45. doi: 10.1016/s0165-3806(01)00293-0. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Meinecke ML. Early expression of GABA-containing neurons in the prefrontal and visual cortices of rhesus monkeys. Cereb Cortex. 1992;2:16–37. doi: 10.1093/cercor/2.1.16. [DOI] [PubMed] [Google Scholar]
- Schwartz ML, Zheng DS, Goldman-Rakic PS. Periodicity of GABA-containing cells in primate prefrontal cortex. J Neurosci. 1988;8:1962–1970. doi: 10.1523/JNEUROSCI.08-06-01962.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims KB, Crandall JE, Kosik KS, et al. Microtubule-associated protein 2 (MAP2) immunoreactivity in human fetal neocortex. Brain Res. 1988;449:192–200. doi: 10.1016/0006-8993(88)91037-2. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Mesulam MM. Infracortical interstitial cells concurrently expressing M2-muscarinic receptors, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate-diaphorase in the human and monkey cerebral cortex. Neuroscience. 1998;84:755–769. doi: 10.1016/s0306-4522(97)00524-1. [DOI] [PubMed] [Google Scholar]
- Smiley JF, McGinnis JP, Javitt DC. Nitric oxide synthase interneurons in the monkey cerebral cortex are subsets of the somatostatin, neuropeptide Y, and calbindin cells. Brain Res. 2000;863:205–212. doi: 10.1016/s0006-8993(00)02136-3. [DOI] [PubMed] [Google Scholar]
- Sorensen KV. Somatostatin localization and distribution in the cortex and the subcortical white matter of human brain. Neuroscience. 1982;7:1227–1232. doi: 10.1016/0306-4522(82)91129-0. [DOI] [PubMed] [Google Scholar]
- Stensaas LJ. The development of hippocampal and dorsolateral pallial region of the cerebral hemisphere in fetal rabbits. II. Twenty millimeter stage, neuroblast morphology. J Comp Neurol. 1967a;129:71–84. doi: 10.1002/cne.901310403. [DOI] [PubMed] [Google Scholar]
- Stensaas LJ. The development of hippocampal and dorsolateral pallial regions of the cerebral hemisphere in fetal rabbits. IV. Forty-one millimeter stage, intermediate lamina. J Comp Neurol. 1967b;131:409–422. doi: 10.1002/cne.901310402. [DOI] [PubMed] [Google Scholar]
- Suárez-Solá ML, González-Delgado FJ, Pueyo-Morlans M, et al. Neurons in the white matter of the adult human neocortex. Front Neuroanat. 2009;3:7. doi: 10.3389/neuro.05.007.2009. (Vol. 3, article 7, pp. 1–6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Follett PL, Folkerth RD, et al. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol. 2006;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka R, Rockland KS. Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. J Comp Neurol. 2007;505:526–538. doi: 10.1002/cne.21504. [DOI] [PubMed] [Google Scholar]
- Ulfig N. Calcium-binding proteins in the human developing brain. Adv Anat Embryol Cell Biol. 2002;165:1–92. [PubMed] [Google Scholar]
- Unger JW, Lange W. NADPH-diaphorase-positive cell populations in the human amygdala and temporal cortex: neuroanatomy, peptidergic characteristics and aspects of aging and Alzheimer's disease. Acta Neuropathol. 1992;83:636–646. doi: 10.1007/BF00299414. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Delalle I. Morphology of neuropeptide Y-immunoreactive neurons and fibres in human prefrontal cortex during prenatal and postnatal development. J Comp Neurol. 1997;379:523–540. doi: 10.1002/(sici)1096-9861(19970324)379:4<523::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ, Kharazia VN, et al. Neurons in rat cerebral cortex that synthesize nitric oxide: NADPH diaphorase histochemistry, NOS immunocytochemistry, and colocalization with GABA. Neurosci Lett. 1993;157:157–161. doi: 10.1016/0304-3940(93)90726-2. [DOI] [PubMed] [Google Scholar]
- Valverde F, Facal-Valverde MV. Postnatal development of interstitial (subplate) cells in the white matter of the temporal cortex of kittens: a correlated Golgi and electron microscopic study. J Comp Neurol. 1988;269:168–192. doi: 10.1002/cne.902690203. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Mrzljak L, Voorn P, et al. Prenatal development of GABA-ergic neurons in the neocortex of the rat. J Comp Neurol. 1989;289:213–227. doi: 10.1002/cne.902890204. [DOI] [PubMed] [Google Scholar]
- Vasung L, Huang H, Jovanov-Milošević N, et al. Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J Anat. 2010;217:400–417. doi: 10.1111/j.1469-7580.2010.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Monakow C. Gehirnpathologie. Vienna: Alfred Hölder; 1905. [Google Scholar]
- Wolff JR, Böttcher H, Zetzsche T, et al. Development of GABAergic neurons in rat visual cortex as identified by glutamate decarboxylase-like immunoreactivity. Neurosci Lett. 1984;47:207–212. doi: 10.1016/0304-3940(84)90515-9. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Hayashi M, Shimizu K, et al. Ontogeny of somatostatin in cerebral cortex of macaque monkey: an immunohistochemical study. Dev Brain Res. 1989;45:103–111. doi: 10.1016/0165-3806(89)90012-6. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Shimizu K, Hayashi M. Ontogeny of substance P-immunoreactive structures in the primate cerebral neocortex. Dev Brain Res. 1990;57:197–207. doi: 10.1016/0165-3806(90)90046-2. [DOI] [PubMed] [Google Scholar]
- Yan XX, Ribak CE. Prenatal development of nicotinamide adenine dinucleotide phosphate-diaphorase activity in the human hippocampal formation. Hippocampus. 1997;7:215–231. doi: 10.1002/(SICI)1098-1063(1997)7:2<215::AID-HIPO8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Yan XX, Zheng DS, Garey LJ. Prenatal development of GABA-immunoreactive neurons in the human striate cortex. Dev Brain Res. 1992;65:191–204. doi: 10.1016/0165-3806(92)90179-z. [DOI] [PubMed] [Google Scholar]
- Yan XX, Garey LJ, Jen LS. Development of NADPH-diaphorase activity in the rat neocortex. Dev Brain Res. 1994;79:29–38. doi: 10.1016/0165-3806(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Yan XX, Garey LJ, Jen LS. Prenatal development of NADPH-diaphorase-reactive neurons in human frontal cortex. Cereb Cortex. 1996a;6:737–745. doi: 10.1093/cercor/6.5.737. [DOI] [PubMed] [Google Scholar]
- Yan XX, Jen LS, Garey LJ. NADPH-diaphorase-positive neurons in primate cerebral cortex colocalize with GABA and calcium-binding proteins. Cereb Cortex. 1996b;6:524–529. doi: 10.1093/cercor/6.3.524. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Milosevic A. Initial development of gamma-aminobutyric acid immunoreactivity in the human cerebral cortex. J Comp Neurol. 1997;380:495–506. doi: 10.1002/(sici)1096-9861(19970421)380:4<495::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]


