Abstract
The development of cortical axonal pathways in the human brain begins during the transition between the embryonic and fetal period, happens in a series of sequential events, and leads to the establishment of major long trajectories by the neonatal period. We have correlated histochemical markers (acetylcholinesterase (AChE) histochemistry, antibody against synaptic protein SNAP-25 (SNAP-25-immunoreactivity) and neurofilament 200) with the diffusion tensor imaging (DTI) database in order to make a reconstruction of the origin, growth pattern and termination of the pathways in the period between 8 and 34 postconceptual weeks (PCW). Histological sections revealed that the initial outgrowth and formation of joined trajectories of subcortico-frontal pathways (external capsule, cerebral stalk–internal capsule) and limbic bundles (fornix, stria terminalis, amygdaloid radiation) occur by 10 PCW. As early as 11 PCW, major afferent fibers invade the corticostriatal junction. At 13–14 PCW, axonal pathways from the thalamus and basal forebrain approach the deep moiety of the cortical plate, causing the first lamination. The period between 15 and 18 PCW is dominated by elaboration of the periventricular crossroads, sagittal strata and spread of fibers in the subplate and marginal zone. Tracing of fibers in the subplate with DTI is unsuccessful due to the isotropy of this zone. Penetration of the cortical plate occurs after 24–26 PCW. In conclusion, frontal axonal pathways form the periventricular crossroads, sagittal strata and ‘waiting’ compartments during the path-finding and penetration of the cortical plate. Histochemistry is advantageous in the demonstration of a growth pattern, whereas DTI is unique for demonstrating axonal trajectories. The complexity of fibers is the biological substrate of selective vulnerability of the fetal white matter.
Keywords: axonal pathways, development, fronto-limbic connectivity, human fetal brain, subplate
Introduction
The establishment of long-range axonal pathways is a crucial neurogenetic event in the development of the expanded cerebral cortex of the large primate brain. The long-range axonal pathways form a basis for expanded connectivity related to the increased number and size of cortical areas which, in turn, have also increased the number and size of cortico–subcortical connections. The process of axonal growth of the long pathways is the most complex in the human cerebrum, where an increased number of areas and projection neurons (Rakic, 1988, 2009) is accompanied by an enormous expansion of axonal pathways (Von Monakow, 1905; Polyak, 1927, 1932; Makris et al. 1997; Schmahmann & Pandya, 2006; Petrides & Pandya, 2007). The expanded system of axonal pathways in the human cerebrum develops over a prolonged period of time. It begins at the end of the embryonic period (His, 1904; Hochstetter, 1909; Bartelmez & Dekaban, 1962, Kostović, 1990a,b;) and lasts until the neonatal period (Kostović, 1990b; Kostović & Judaš, 2002, 2006; Berman et al. 2005; Huang et al. 2006, 2009; Huppi & Dubois, 2006; Kostović & Jovanov-Milošević, 2006; Counsell et al. 2007; Kasprian et al. 2008; Kostović et al. 2008; Ment et al. 2009). During this period of growth, the axonal pathways (i) pass the critical morphogenetic points at the diencephalic–telencephalic border and corticostriatal junction (pallial–subpallial boundary) and (ii) change the direction of the growth trajectory passing through the periventricular crossroads (Judaš et al. 2005). They ‘wait’ in the subplate compartment before penetration into the cortical plate (Rakic, 1976, 1977; Kostović & Goldman-Rakic, 1983; Kostović & Rakic, 1984, 1990; Kostović & Jovanov-Milošević, 2006; Kostović & Judaš, 2007). Some of the fiber systems show transient phenomena, such as overgrowth and retraction of axon branches, which extend into the postnatal period (Innocenti & Price, 2005; Tau & Peterson, 2010). The peak of growth of the long axonal pathways, connecting the cortex with the subcortical centers, corresponds to the period when preterm infants are born [22–34 postconceptual weeks (PCW)] and survive due to the support provided by modern neonatal care (Counsell et al. 2007; Volpe, 2008, 2009; Ment et al. 2009; Miller & Ferriero, 2009). In this period the axonal pathways (the ‘white’ matter) are very vulnerable to hypoxic–ischemic injury, which is the basis of selective vulnerability (Miller & Ferriero, 2009; Sherlock et al. 2009; Volpe, 2009).
The application of modern diffusion tensor imaging (DTI) allows the in-vivo analysis of abnormal changes of the axonal pathways during vulnerable periods (Huang et al. 2006, 2009; Kasprian et al. 2008; Kim et al. 2008; Rutherford et al. 2008; Aeby et al. 2009; Hoon et al. 2009; Ment et al. 2009; Ramenghi et al. 2009). It has been shown that the DTI technique is very useful for the 3D study of the macroscopic fiber bundle architecture and their maturational changes. However, from a microscopic point of view, there are several important limitations of DTI. DTI cannot identify the points of origin, chemical properties and ‘waiting’ periods of axonal pathways. When axonal anatomy is complex in terms of the pixel dimension, such anatomical information (e.g. non-uniform tract orientations within a pixel) could be lost. These issues would lead to unknown validity of DTI results if the results deviate from existing anatomical knowledge. Although sophisticated data acquisition and analysis methods have been postulated to ameliorate these issues, we cannot expect that they will be completely resolved. Careful histological studies based on the histochemical preparations of the sequential histological sections of the brain specimens within different developmental phases (Kostović & Molliver 1974; Kostović & Goldman-Rakic, 1983; Krmpotić-Nemanić et al. 1983; Kostović & Rakic, 1984, 1990; Kostović, 1986, 1990b; Kostović et al. 1988, 1989a; Mrzljak et al. 1988, 1992; Kostović & Judaš, 2002, 2006, 2007; Kostović et al. 2002; Judaš et al. 2005; Kostović & Jovanov-Milošević, 2006; Radoš et al. 2006; Petanjek et al. 2008, Jovanov-Milošević et al. 2009, Kostović & Vasung, 2009) will also be important in future DTI analysis.
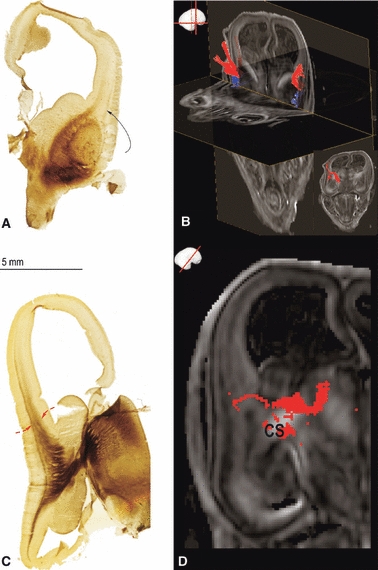
The objective of this review is to compare evidence from the DTI analysis of fetal postmortem brains with the data on histochemically identified pathways. We have correlated the histochemical evidence (acetylcholinesterase (AChE) histochemistry, SNAP-25-immunoreactivity and neurofilament 200 staining) with the DTI reconstruction of pathways [code no. N01-HD-4-3368 and N01-HD-4-3383, University of Maryland Brain and Tissue Bank, for details on DTI data acquisition; for imaging protocol, postprocessing and visualization of DTI, DTI-based fiber tractography and region of interest drawing strategy see Huang et al. (2009)] in order to reconstruct the origin, growth pattern and termination of the axonal pathways. In addition, we have scanned 3-T DTI of the postmortem specimen at 24 PCW [Fig. 9C, similar to the protocol of Huang et al. (2009) but with lower resolution] as well as T2-weighted magnetic resonance imaging (MRI) (Fig. 9D) to elaborate different structural MRI characteristics of the fetal brain. We have examined the period between 8 and 34 PCW.
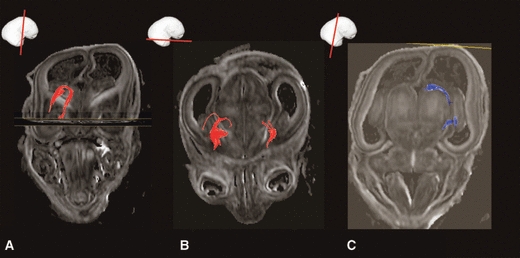
Fig. 9.

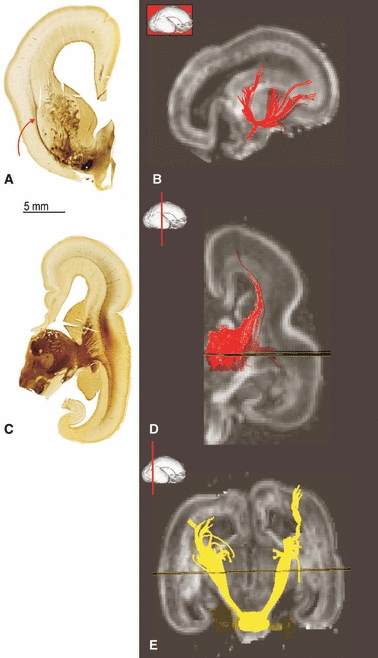
Development of thalamocortical fibres at mid-fetal period. At 19 PCW fibres from different thalamic nuclei/complex (ROI, B) are taking different routs (anterior, superior and posterior thalamic radiation) towards the ‘waiting compartment’-subplate (A) and cortical plate. In C the waiting fibre bundles in subplate, originating from thalamus can be seen by DTI at 24 PCW. For DTI tractography reconstruction in C we have lower the FA threshold level (0.12) due to the highly isotropic subplate in FA images (B). Accumulation of the growing front of thalamocortical fibres in superficial SP is shown by AChE staining (F, X) embedding in ECM rich neuropil shown by fibronectin staining (E, X). In vivo T2 weighted images (D) are also useful in showing subplate (sp) as well as the hiperintense superficial subplate part (D, +) due to the different T2 properties of the fetal laminae. Hippocampus and the enlarged marginal zone are showing similar T2 tissue properties in in vivo T2 weighted images (D, curved arrows). sp, subplate; ci, internal capsule.
Our basic approach in the study of the long axonal pathways of the human fetal cerebrum is illustrated in Fig. 1. This approach uses a combination of structural, histochemical and imaging data of the growing fiber system obtained at the different developmental ages, expressed in the reference to common spatial (cellular, laminar and areal) landmarks. For a detailed timetable of thalamocortical as well as cerebral connection development see Kostović & Jovanov-Milošević (2006) and Kostović & Judaš (2010).
Fig. 1.
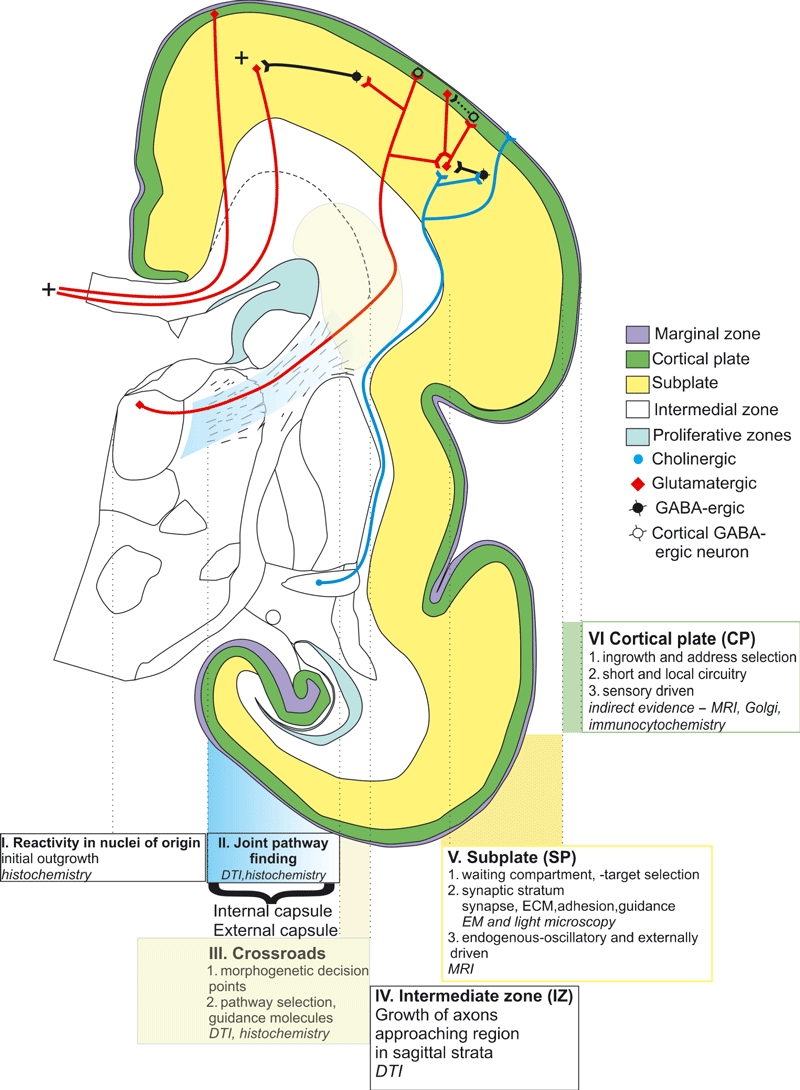
Schematic illustration of our approach to the development of the cortical connectivity in the human brain. DTI, diffusion tensor imaging; ECM, extracellular matrix; EM, electron microscopy; MRI, magnetic resonance imaging.
Using these histological and DTI techniques we have compared the development of axonal pathways of the prefrontal cortex and limbic bundles (Vasung et al. 2009). Our goal was not only to show the differences between the neocortical and allocortical patterns but also to show the possible developmental relationships between these diverse but functionally connected cortical systems (Nauta, 1971; Goldman-Rakic, 1987; Schmahmann & Pandya, 2006; Barbas, 2007; Petrides & Pandya, 2007). We believe that the review of existing data on the development of cerebral connections is a necessary step in studying the consequences of the white matter damage and will facilitate the analysis of structural organization and the possible recovery after perinatal brain damage.
Early initial outgrowth and formation of ‘joint trajectories’ of cerebral pathways (before 10 PCW)
Classical studies on the developing brain have demonstrated that fiber bundles of the major projection pathways appear very early, at the end of the embryonic period, around 8 PCW (His, 1904; Hochstetter, 1909; Bartelmez & Dekaban, 1962; Molliver et al. 1973; Kostović, 1990a; O’Rahilly & Müller, 2006). The standard techniques applied in this study did not stain the fibers directly and the fibers were identified on the basis of topography and pale appearance in the Nissl type of staining (Fig. 2B–D). Applications of histochemical staining techniques showed that the basal forebrain is the early source of afferents in the telencephalon (Fig. 2A). The basal forebrain neurons belong to the magnocellular cholinergic system (Matthews et al. 1974; Mesulam et al. 1983, 1984; Kostović, 1986). These early basal forebrain afferents travel to the pallium via the external capsule, which is well delineated in this early phase (Fig. 2B, curved arrow). Cholinesterase staining also helps in visualizing pathways from the tegmentum, which are probably dopaminergic (Fig. 2A, double curved arrows). Other monoaminergic early afferents were demonstrated with monoamine-fluorescence techniques (Nobin & Bjorklund, 1973; Olson et al. 1973; Zecevic & Verney, 1995). Early afferents from the thalamus form a massive bundle, the so-called cerebral stalk (Fig. 2C, cs) (His, 1904; Hemisferenstiel by Hochstetter, 1909). This massive thalamocortical bundle almost fills the diencephalic–telencephalic (Fig. 2C, red arrows) junction, appearing as a real cerebral stalk (Fig. 2C, cs). Thus, the two major afferent cortical systems from the thalamus and basal telencephalon show early outgrowth and join two major fiber trajectories: (i) the developing cerebral stalk – internal capsule (Fig. 2C, cs) and (ii) the external capsule (Fig. 2B, curved arrow).
Fig. 2.
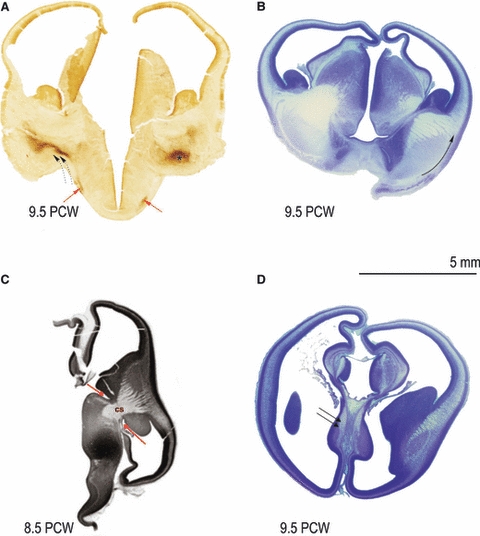
Development of fibre bundles before 10 PCW. Appearance of basal forebrain revealed by AChE histochemistry (A); nucleus basalis complex (asterisk), supraoptic commisure fibres (A, red arrow) and pathways from tegmentum (double dotted arrows) at 9.5 PCW. At 9.5 PCW external capsule (B, along the curved arrow) can be shown by Nissl staining technique. Early formation (at 8.5 PCW) of cerebral stalk (C, cs) and diencephalic-telencephalic sulcus (border- C, shown by two red arrows) as well as the formation of fornix bundle at 9.5 PCW can be shown as Nissl pale staining (D, double arrow). cs, cerebral salk.
The limbic bundles develop along the midline and, at a very early stage, form the compact, well-delineated bundles (Fig. 2D). The most prominent limbic bundle is the fornix (Fig. 2D, double arrow) (His, 1904; Hochstetter, 1909; Macchi, 1951; Bartelmez & Dekaban, 1962; Humphrey, 1967; Rakic & Yakovlev, 1968; Stephan, 1975). The early fornix is a short, slightly curved bundle that connects the hippocampus with the septal area. It is not known whether the early fornix contains more hippocampal-septal or septo-hippocampal fibers. Due to the fact that the pyramidal neurons, at that very early age, are in the process of proliferation of the ventricular zone (Nowakowski & Rakic, 1981), it is very likely that the early fornix contains mostly septo-hippocampal fibers. This is consistent with the early development of the septal area (Kostović et al. 1980).
Afferent pathways invade the corticostriatal junction (around 11 PCW)
A crucial step in axonal pathway development is crossing the border between the pallium and striatum–ganglionic eminence complex, the so-called corticostriatal junction (Molnar & Butler, 2002). An even better term for the corticostriatal junction would be the corticocaudate junction because, in the human brain, only the caudate nucleus is situated in the proximity of the ventricles. From the evolutionary point of view, this corresponds to the pallial-subpallial border (Molnar & Butler, 2002). From the practical, human neuroanatomical point of view, this border is frequently described as the lateral angle of the developing lateral ventricles or thalamocaudate grove. At this border, thalamocortical axons cross with efferent axons and turn towards various pallial regions. The axons for the frontal pallium turn towards the intermediate zone of the frontal portion of the telencephalic vesicles as described for the animal models (Molnar & Blakemore, 1995; Molnar et al. 1998; Sestan et al. 2001; Lopez-Bendito et al. 2002; Molnar & Butler, 2002; Bishop et al. 2003; Lopez-Bendito & Molnar, 2003; Bolz et al. 2004; Price et al. 2006).
This period of development is characterized by the massive invasion of projection fibers into the corticostriatal junction. The fibers arising from the basal telecephalon extend via the external capsule to the outermost portion of the intermediate zone of the developing neopallium (Fig. 3A, curved arrow). For the first time, the external capsule attains a bifurcated appearance where one branch is oriented to the frontal and the other to the parieto-occipital pallium (Fig. 3B). The process of growth of the afferents from the nucleus basalis complex was described in detail by Kostović (1986).
Fig. 3.
Development of afferent fibres at 11PCW revealed by AChE histochemical staining (A, C) and DTI (B, D). The diencephalo-telencephalic junction (C, red arrows) and external capsule (A, curved arrow) can be seen on AChE stained section. The DTI tractography reconstruction shows bifurcating appearance of external capsule (B) and AChE histochemical staining with DTI reveals thalamocortical fibres passing through the cerebral stalk (C, D, cs) originating from the dorsomedial thalamic complex (C, D). cs, cerebral stalk.
The fibers for the temporal and occipital pallium change their trajectory, bending in the ventrocaudal direction (Fig. 3C). The continuity of the thalamocortical projection from the thalamic nuclei, cerebral stalk and internal capsule fanning out into the early thalamic radiation is obvious only in histochemical preparations. DTI imaging (Fig. 3D) shows only the proximal, thick body of the main thalamocortical fiber system. The thickness of the diencephalic–telencephalic junction has increased significantly (Fig. 3C, red arrows) making the diencephalic–telencephalic border less obvious on coronal images through the fetal brain.
The process of growth of the limbic bundles is more advanced than in neopallial regions. The bundle of fornix has a semicircular shape and shows considerable compactness (Fig. 4C). Projection of the amygdala, the neuroendocrine pathway of the stria terminalis, which connects the amygdala with the septal area, is very advanced (Fig. 4A). The fibers connected to the amygdala radiate in all directions (Fig. 4B), providing evidence that the amygdala establishes connections to the different parts of the cortex (Fig. 5C) at a very early stage (13 PCW). The early development of the amygdala fiber system is consistent with the early cytoarchitectonic differentiation of the amygdala (Nikolić & Kostović, 1986) and early origin of the amygdaloid nuclear complex of the human and non-human primate brain (Kordower et al. 1992). It is not possible at this early stage of development to attribute individual fibers from/to the individual nuclei.
Fig. 4.
Development of limbic fibre bundles at 11 PCW revealed by DTI tractography; early formation of subcortical (A, stria terminalis) and cortical (B) amygdala connections as well as the hippocampal pathways (C, fornix).
Fig. 5.

Development of limbic (uncinate fascicle, C), efferent (periventricular fibre system with subcallosal fascicle of Muratoff and cortico-striate fibres, D) and thalamocortical fibres (ThCx A, B) at 13 PCW revealed by Nissl (A) staining and DTI tractography (B, C, D). ThCx, Thalamo-cortical fibers.
Morphogenetic interaction of afferents with the cortical plate (around 13 PCW)
The appearance of thalamocortical (Fig. 5A,B) and basal forebrain afferents in the intermediate zone at an early phase of development raises the question of the developmental and synaptic interactions with the early born and differentiated cortical cells. Before 13 PCW, the cortical plate is composed of densely packed postmigratory neurons, arranged in vertical columns, without significant fibrillar (axonal) content. The fibrillar elements (axons) are distributed in two laminas: the presubplate (below the cortical plate) and marginal zone (above the cortical plate). In these two fibrillar layers the synapses can be found, designating these layers as synaptic strata (Molliver et al. 1973; Kostović & Molliver, 1974; Kostović & Rakic, 1990; Kostović & Judaš, 2007). The prospective postsynaptic elements in the early synaptic strata are the early differentiated presubplate neurons that lie below the cortical plate at the interface with the intermediate zone (Kostović & Rakic, 1990; Meyer, 2007). At around 13 PCW the deep portion of the cortical plate changes dramatically; the cortical cells lose their radial orientation and become constituents of a new layer – the subplate zone. The subplate zone was first described by Kostović & Molliver (1974) (see also Kostović & Jovanov-Milošević 2008). This loose portion of the cortical plate was described as the ‘second’ cortical plate by Poliakov (Poliakov, 1949; Kostović & Rakic, 1990). The neuropil of the ‘second’ cortical plate is plexiform, with a random orientation of the axonal and dendritic processes as well as the fast developing synapses (Kostović & Rakic, 1990; Kostović & Judaš, 2002). This phase of cortical development exists in both human and monkey but was not described in the rodent brain (Bayer & Altman, 1990; De Carlos & O’Leary, 1992; Molnar et al. 1998; Del Rio et al. 2000; Smart et al. 2002). Based on the fact that this ‘second’ cortical plate contains an old neuronal population, randomly oriented neuronal elements and synapses, this event corresponds to the appearance of the thick subplate and was described as the subplate formation stage by Kostović & Rakic (1990). In subsequent phases (15–24 PCW), the cortical plate again becomes condensed (secondary condensation) and contains no synapses. It is not known what causes this first transient lamination of the cortical plate but the presence of the fibrillar elements in the deep portion of the cortical plate indicates the morphogenetic influence of the afferent system. The mostly likely candidate fiber system is the external capsule radiation because this system is the closest to the cortical plate. The fibers from the cholinergic magnocellular nuclei of the basal forebrain run via the external capsule into the external sagittal stratum (Kostović, 1986). The external capsule–external sagittal stratum is an important border between the intermediate zone and subplate (Kostović et al. 2002). The afferents from the external capsule remain permanently at this strategic border between the white matter and cortex. These fibers are closest to the permissive gradients of guidance molecules in the rich extracellular matrix subplate compartment. The beginning of lamination in the cortical plate is the initial step in the establishment of regional differences within the cerebral cortex. Lamination is first observed in the lateral neopallium, external to the angle of the lateral ventricle. The developing subplate is thicker in this midlateral region than in the dorsal neocortical regions. The second cortical plate is thinner in the interhemispheric neopallium and the formation of the ‘second’ plate is absent in the limbic interhemispheric mesocortex (cingular cortex) (Kostović et al. 1989b, 1993). The interpretation of the subplate zone, presented in the present review, differs from the recent revision of the Boulder Committee where the subplate layer is defined immediately after formation of the cortical plate at around 8 PCW (Bayatti et al. 2008b; Bystron et al. 2008).
In the limbic archicortex, the main fibrillar development is in the marginal zone, which is much thicker than the subplate zone of the limbic cortex.
Efferent pathways
At this early phase of fetal development the presence of all efferent pathways cannot be expected, e.g. pyramidal projection neurons from cortical supragranular layers have not yet been born or are in the process of migration (Rakic, 1974). However, cortical cells from the infragranular layer are already in the cortical plate. There is little data about the development of the corticospinal and corticopontine pathways. Eyre et al. (2000) found that the corticospinal pathway reaches the lower spinal cord as early as 24 PCW. It seems that efferent pathways may be present even in the earliest phases, which we present in this study. Indeed, photographs of serial sections through the developing brain show the presence of crura cerebri at early phases (Fig. 5). However, the material presented in this review does not allow the reconstruction of terminal portions of the corticopontine and corticospinal pathways below the brainstem.
In this period there is progress in the growth of the periventricular fiber systems. According to the classification of Von Monakow (1905), the adult brain periventricular white matter, from segments I and II, forms the first periventricular system (Judaš et al. 2005). In segment 1, the corpus callosum is visible after 11 PCW. The periventricular fiber system is situated along the caudate nucleus (Fig. 5D) and, in this position, there are several fiber systems in transient topographical relationships (Judaš et al. 2004). A great proportion of this contingent of fibers is related to the striatum, i.e. the subcallosal fascicle of Muratoff and other corticostriatal fibers (Schmahmann & Pandya, 2006, Vukšić et al. 2008).
Fetal pattern of axonal distribution: sagittal strata and ‘waiting’ subplate compartment (15–18 PCW)
The typical fetal pattern of axonal pathway organization is characterized by the distribution of growing axons within the axonal strata of the intermediate zone and the gradual ingrowth into the deep cortical anlage of the subplate zone. Afferents from the basal forebrain, pons and thalamus may be visualized in both histochemical preparations and DTI tractography (Fig. 6A–D).
Fig. 6.
Demonstration of the basal forebrain fibre bundles running trough the external capsule (A, curved arrow) and external sagittal stratum at 15 PCW (B; tractography reconstruction of basal forebrain fibre system and external capsule). Reconstruction of thalamocortical fibres running trough the internal sagittal stratum at 15 PCW (C) and 17 PCW (D) revealed by AChE staining and DTI tractography. At 15 PCW cortico pontine fibre bundles can be reconstructed using DTI tractography (E).
Thalamocortical axons run predominantly through the internal sagittal stratum (Fig. 6C,D). Basal forebrain axons to the cortex (from the external capsule radiation) run through the most superficial portion of the external sagittal stratum (Fig. 6A,B). The central sagittal stratum contains AChE-negative fibers (Figs 6C and 7C), which are a feature of efferent cortical pathways. All sagittal strata mentioned are part of the intermediate zone fetal ‘white’ matter. For a description of the layers see Kostović et al. (2002), Huang et al. (2006) and Bystron et al. (2008). The intermediate zone with its sagittal strata is situated between the subplate zone and corpus callosum, which runs into the subventricular zone (Fig. 7A–D; CC). This relationship can be appreciated in the section through the rostral portion of the developing telecephalon (Fig. 7B–D).
Fig. 7.
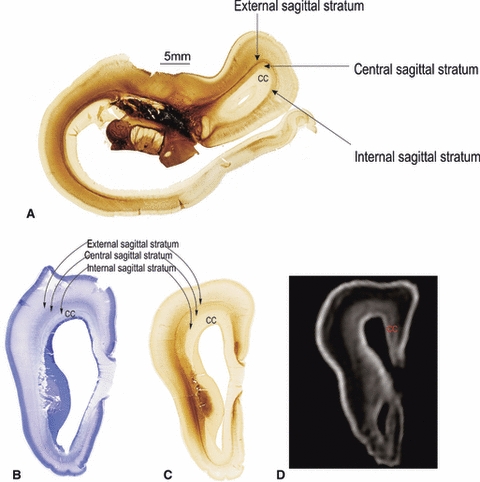
Demonstration of sagittal strata and corpus callosum (CC) revealed by AChE (A, C) and Nissl staining (B) at 18 PCW as well as the MRI T1 weighted image of the same brain (D). CC, corpus callosum.
The most interesting finding is that the massive fiber system from the corpus callosum occupies the subventricular zone and that these fibers join the deep portion of the intermediate zone (Fig. 7B). This laminar relationship shows that there is continuity in fiber systems from the external sagittal stratum (with the external capsule) to the proliferative periventricular zones. Joint fibers of the intermediate zone and corpus callosum form the fiber corridor situated between the differentiating cortical layers (subplate zone and cortical plate) and periventricular proliferative layers (ventricular and subventricular zone). This is the basic architecture of the fetal telecephalon where a central corridor contains afferent and efferent systems. At present it is unknown whether this fiber corridor contains the same axonal guidance molecules as described for the corticostriatal junction or pallial–subpallial boundary (Lopez-Bendito et al. 2006; Maroof & Anderson, 2006).
In our own material findings, we have found high activity of chondroitin sulphate C, which is an essential portion of glycosaminoglycans (Judaš et al. 2005). In addition, we have found intensive staining of SNAP-25 in efferent fibers from the intermedial zone (see also Ulfig et al. 2000). It is also a matter of debate as to what lamina should be considered as a cortical target at this early phase when the cortex is composed of a thick subplate and thinner cortical plate. According to Kostović & Rakic (1990), the subplate is four times thicker than the cortical plate; it is a part of the fetal cortex and separates the cortical plate from the axons in the intermediate zone. We propose that the main source of axonal guidance molecules for entrance into the cortex is the subplate. The essential component of the subplate zone is the extracellular matrix, which builds up to 70% of the neuropil (Kostović et al. 2002). The extracellular matrix of the subplate contains different attractant and repellent molecules, such as fibronectin (Chun & Shatz, 1988; Tuttle et al. 1995; Pearlman & Sheppard, 1996), semaphorins, ephrins and other molecules (Hoerder-Suabedissen et al. 2009; for reviews see Judaš et al. 2003; Uziel et al. 2006). The possible cellular sources of the extracellular matrix and axonal guidance molecules are subplate cells (neurons or glia).
Considering the high activity of extracellular markers and guidance molecules in the subplate, this fetal compartment should be called a ‘guidance compartment’, meaning that afferent axons not only ‘wait’ but also are guided during the growth through the subplate (Rakic, 1977; Kostović & Rakic, 1990; Kostović & Judaš, 2002; Kostović & Jovanov-Milošević, 2006, 2008). In general, the significance of the subplate compartment for the guidance of axons in the cortex is underestimated (Price et al. 2006). A possible reason for this might be that the rodent brain subplate is very thin and exists for a short time, which does not give sufficient resolution in an experimental rodent model.
Different cellular components of the subplate may influence cellular interaction with afferent axons. The subplate is composed of postmigratory neurons, migratory neurons, radial glia, astroglia and microglia, which have a potential developmental role in axonal growth. It should be kept in mind that all efferent axons have to pass through the subplate in order to reach the subcortical trajectories and targets. It is very likely that some of the efferent axons in the subplate interact with the afferent axons in early phases of development. This is consistent with the ‘hand shake’ hypothesis of Molnar & Blakemore (1995). Limbic bundles during the mid-fetal period, fiber pathways for the limbic cortex and limbic subcortical structures are well developed; an angular bundle can be traced from the frontal medial cortex (precingulate, cingulate ventromedial and dorsomedial cortical areas) to the posterior cingulate cortex (Fig. 8). In addition, the cingulum fiber bundle can be well delineated at this phase with the DTI and AChE staining (Fig. 8A). This is the trajectory for most of the cingulate pathways as described in the adult primate cortex (Schmahmann & Pandya, 2006; Petrides & Pandya, 2007). This is also consistent with previous observations in human fetuses (Huang et al. 2006, 2009).
Fig. 8.
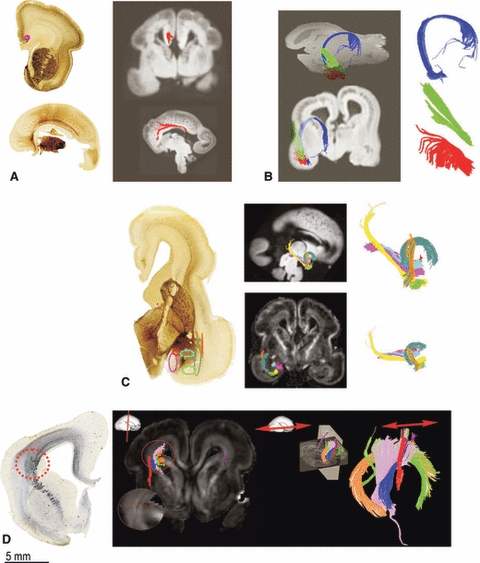
Development of associative pathways and crossroad formation during mid fetal period. Formation of cingulum bundle at 17 PCW is shown by AChE staining and comparative DTI tractography (A, red colour). During 15 PCW (B) and 17 PCW (C) the amygdala connections and crossroad C6 becomes more complex- as revealed by DTI (C). During 19 PCW crossroad C1 formation can be show by SNAP staining and DTI tractography reconstruction (D). The crossroad area C1 is organized by axonal pathways that run in radial (thalamocortical), sagittal (associative) and transverse (callosal) direction (B, C). In D the different ROIs for the crossroad C1 can be seen (I-VI) and thus reconstructed with tractography (different colours represent different ROIs).
The other aspect of limbic pathway organization that deserves special attention is the extremely rich connectivity of the amygdala, which is connected to the orbital cortex via the uncinate bundle (Fig. 8C, sea green). Another voluminous fiber system connects the amygdala with the temporal cortex (Fig. 8B, green and red; Fig. 8C, yellow, pink and blue). This fiber system has the shape of a hand with fingers (Fig. 8B, red). We describe it as the amygdalo-temporal (temporo-amygalar) bundle but tractography does not permit determination of the pathway origin and termination. Other pathways that stream out of and into the amygdala (Fig. 8C) form a part of the periventricular crossroad of pathways (temporal crossroad C6) (Judaš et al. 2005). This periventricular crossroad is situated just in front of the rostral tip of the temporal horn of the lateral ventricle. Thus, our observation strongly indicates early development of the cortico–cortical and cortico–subcortical fronto-limbic connectivity (Kostović & Jovanov-Milošević, 2008; Kostović et al. 2008).
Periventricular crossroads
The analysis of DTI images and histochemical preparations (Figs 6D,E and 8C,D) reveals that periventricular territories adjacent to the internal capsule contain intersections (crossing) of the projection, callosal and associative pathways. These crossroads of pathways were described by von Monakow (1905) and more recently by Judaš et al. (2005). According to Judaš et al. (2005), six crossroad areas can be distinguished in the human telencephalon in fetuses older than 14 PCW. In the crossroad areas, axonal pathways run in radial (thalamocortical), sagittal (associative) and transverse (callosal) directions. The most numerous axonal system in the crossroad areas are projection fibers, which will later form corona radiata. Crossroad areas contain hydrophilic extracellular matrix, which is rich in axonal guidance molecules (Judaš et al. 2005). In addition to thalamocortical fibers, crossroads contain efferent fibers such as the prominent corticopontine fiber system (Fig. 6E). DTI analysis applied in this study allowed visualization of several fiber systems in the periventricular crossroad areas (Figs 6D,E and 8D): thalamocortical, corticopontine, corticocaudate and callosal fiber systems.
Accumulation of the thalamocortical fiber system in the subplate (19–23 PCW)
In a series of studies using AChE histochemistry, Kostović and co-workers (Kostović & Goldman-Rakic, 1983; Krmpotić-Nemanić et al. 1983; Kostović, 1990a,b; Kostović & Rakic, 1990) have shown that thalamocortical fibers accumulate in the superficial subplate after a prolonged ‘waiting’ period. This event occurs almost simultaneously in neocortical regions. The thalamocortical growing front can be traced to the mediodorsal nucleus (Kostović & Goldman-Rakic, 1983). The intense AChE reactivity of the thalamocortical growing front in the superficial subplate (shown in Fig. 9F and marked with ×) is enhanced due to the overlap with AChE staining in ‘cholinergic’ fibers from the magnocellular basal forebrain (Kostović, 1986). The hydrophilic extracellular matrix-rich zone of the subplate can be visualized in conventional T2 in-vivo/in-vitro magnetic resonance images as hyperintense zones beneath the cortical plate (shown in Fig. 9D and marked with +) due to the different hydrophilic organization of the subplate. The event of accumulation of afferent pathways cannot be demonstrated convincingly in DTI preparations (Huppi et al. 2001; Maas et al. 2004; Huang et al. 2006, 2009). In DTI images, thalamocortical pathways can be traced within the anterior, superior and posterior thalamic radiations (Fig. 9A,B). Very few individual fiber bundles reach the subplate (Fig. 9C).
The developmental shifts in histochemical properties and fibrillar organization (Fig. 9E) within the subplate show that this cellular compartment permanently changes in its fibrillar and cellular content. This concept is further supported by the shifts in molecular markers (Bayatti et al. 2008a,b;). The molecular identification of subplate neurons was the research goal for multiple groups (Chun & Shatz, 1988; Meinecke & Rakic, 1992; Delalle et al. 1997; Kwan et al. 2008; Ayoub & Kostović, 2009; Hoerder-Suabedissen et al. 2009; McKellar & Shatz, 2009; Osheroff & Hatten, 2009). Zečević and colleagues have shown that glial cells also show differential distribution within the subplate (Jakovcevski & Zecevic, 2005).
Limbic cortex and limbic bundles
It is not known whether the limbic axonal pathways, related to the cingular, entorhinal and hippocampal cortex, display a waiting period and accumulation phenomena before the penetration of cortical or subcortical targets. In the archipallium of the hippocampus the enlarged marginal zone (Fig. 9D) shows the same histological properties as the subplate zone of the neopallium: enlarged thickness, fibrillar content, extracellular matrix-rich neuropil and intensive synaptogenesis (Kostović et al. 1989b). These basic features were also observed in the marginal zone of limbic (ventral) portions of the cingular cortex (Kostović & Krmpotić-Nemanić, 1976; Kostović & Judaš, 2002) and entorhinal cortex (Kostović et al. 1993).Thus, it is very likely that the marginal zone serves as a ‘waiting’ compartment for limbic axonal pathways. However, in contrast to the neocortical pathways in the subplate, the period of growth of the limbic pathways is shorter and growth trajectories are well delineated. As stated before, the limbic pathways form well-delineated bundles that are different from the neopallium where fibers are arranged in sagittal strata. In addition, limbic pathways grow in the cortical target zones tangentially (Kostović et al. 1989b).
Penetration of the cortical plate (after 24–26 PCW)
The major event in the axonal pathway development is penetration of the thalamocortical and basal forebrain fibers into the cortical plate (Fig. 9C). This event takes place after 24 PCW although some fibers may enter the cortical plate several weeks earlier (Kostović & Goldman-Rakic, 1983) as demonstrated in the AChE preparations. Histochemical activity gradually invades the lower third of the cortical plate (Fig. 10A). At around 28 weeks, the reactivity occupies the deep two-thirds of the neocortical plate (Fig. 10B) with an evident columnar distribution (see also Kostović, 1990a,b;). During later development the AChE reactivity of the cortical plate becomes less dense (Fig. 10C). The ingrowth of thalamocortical fibers into the cortical plate (Counsell et al. 2007) was not directly illustrated in DTI structures. However, the decrease in fractional anisotropy in the frontal cerebrum after 24 PCW (Trivedi et al. 2009) and the decrease of the directionally averaged water apparent diffusion coefficient after 30 PCW (McKinstry et al. 2002) are very likely to be caused by the ingrowth of the afferent fibers (Kostović & Jovanov-Milošević, 2008; Kostović et al. 2008) and reorganization of embryonic columnar organization (McKinstry et al. 2002; Huang et al. 2006). Innervation of the cortical plate with the thalamocortical afferents is a crucial event in the histogenesis and functional organization of the cortex. Thalamocortical afferents cause visible lamination of the cortical plate (Kostović & Judaš, 2002). The ingrowth of thalamocortical fibers is guided with permissive influence or attraction by complex molecular interactions (Molnar & Blakemore, 1995; Molnar et al. 1998; Sestan et al. 2001; Price et al. 2006). The establishment of the first synapses in the human telencephalon after 24 PCW (Molliver et al. 1973) marks possible functional interactions between the thalamic afferents and cortical plate cells. This interaction may induce functional differentiation of cortical layer IV (Wilkemeyer & Angelides 1996; Catalano & Shatz 1998; Anderson & Price 2002) and can be studied by optical recording and current source density analysis (Molnar et al. 2003; Higashi et al. 2005). The most significant event related to the establishment of thalamocortical connectivity is the appearance of somatosensory evoked potentials (Molliver, 1967; Kostović & Jovanov-Milošević, 2006; Vanhatalo & Kaila, 2006; Kostović & Judaš, 2007; Milh et al. 2007). The thalamocortical pathways conducting impulses for pain sensation mature in the same period (Slater et al. 2008; Lagercrantz & Changeux, 2009).
Fig. 10.

Histochemical demonstration of thalamocortical penetration into the cortical plate. At 26 PCW the histochemical activity gradually invades the lower third of the cortical plate (A; X). Around 28 PCW the same reactivity occupies deep two third of the neocortical plate (B; X) and during the later development (36 PCW) the AChE reactivity of the cortical plate becomes less dense (C). After 24 PCW the most massive fibre system is corpus callosum building the massive callosal plate that can be well seen at the coronal sections of the AChE histochemistry (A, B, C; CC). After the 36 PCW the resolution of subplate (SP) can be seen (C) but the external sagittal stratum can be still well delineated (A, B, C; arrows). sp, subplate; CC, corpus callosum.
Long corticocortical pathways (commissural) and development of the corona radiata (26 PCW and older)
The callosal corticocortical pathways develop very soon after the thalamocortical fibers. This sequential callosal growth is several weeks behind the thalamocortical fiber growth. The most massive fiber system in the brain after 24 weeks is the corpus callosum. At the septal (rostral) levels callosal fibers build the massive callosal plate (Fig. 10A,B) (Kostović & Judaš, 2002), which almost fills the periventricular surface of the dorsal telencephalon. This callosal relationship outlines the adult topographical position of the corpus callosum. The massive size of the callosal periventricular plate indicates the initial phase in overgrowth events (Innocenti & Price, 2005). In the early postnatal phases there is a significant decrease of callosal fibers (LaMantia & Rakic, 1994; Innocenti & Price, 2005) and callosal morphogenetic zones (Jovanov-Milošević et al. 2009), which occurs before the period of myelination.
The DTI reconstruction of callosal fibers during late gestation demonstrates an elaborated callosal radiation in all segments of the corpus callosum (Maas et al. 2004; Huang et al. 2006, 2009; Counsell et al. 2007; Kasprian et al. 2008; Ment et al. 2009). These studies did not prove whether/when the callosal fibers actually innervate the cortical plate and did not even show if the callosal fibers penetrate/innervate the subplate. Kostović & Rakic (1990) have proposed that the callosal fibers are the main constituent of the subplate after the thalamocortical fibers have already grown into the cortical plate. Some of the callosal fibers probably never reach the cortical plate. (Schwartz & Goldman-Rakic, 1991).
Classical neuroanatomical studies have encountered serious difficulties in demonstration of the growth of the long associative fibers due to the fact that the glutamatergic axons could not be stained with standard immunocytochemical or histochemical techniques. These fibers are also negative for the cholinesterase. Some of the deep associative pathways, most notably the fronto-occipital fascicle (visualized with SNAP-25), were shown early in midfetal brain by Judaš et al. (2005). According to Huang et al. (2006) the inferior longitudinal fascicle and inferior fronto-occipital fascicle were seen around 17 PCW but do not undergo a significant development during the second trimester. The superior longitudinal fascicle is not prominent even at birth (Huang et al. 2006; Zhang et al. 2007). Our preliminary observations showed different properties of the extracellular matrix at the locations of the associative pathways (Fig. 11).
Fig. 11.

Development of associative fibre bundles revealed by DTI colour coded map (A), GNG staining (B) and PAS staining (C). The prospective trajectories of the associative pathways were indicated by asterisks (A, B) or outlined by colours (C). f. subcal, subcallosal fasciculus; middle long. fasc., middle longitudinal fasciculus; SFL, superior longitudinal fasciculus; inf. long. fasc., inferior longitudinal fasciculus.
We have interpreted the position of the superior crossroad (Judaš et al. 2005) as a prospective trajectory of the superior longitudinal fascicle in our postmortem MRI study.
During the late fetal period there is a major change in the orientation and distribution of the projection fibers. The projection fibers elongate, changing tangential fiber stratification into the corona radiata system. According to Judaš et al. (2005), ‘the transformation of fiber architectonic pattern from predominantly tangential in fetuses to predominantly radial in preterm infants occurs concomitantly with several other events: (i) the development of distal portions of the corona radiata and the centrum semiovale proper, (ii) the dissolution of the transient fetal subplate zone, (iii) the relocation of cortical afferents from the subplate zone into the cortical plate, and (iv) the onset of gyrification (Kostović & Rakic, 1990; Kostović & Judaš, 2002)’ (Fig. 10C).
The individual projection fibers in the corona radiata seem to pass even the border of the subplate (compare with Fig. 8.3.d in Huang et al. 2006). The subplate is still well developed in the cortical gyri, whereas it is reduced in the bottom of the sulci. The presence of the subplate at late fetal age (Kostović, 1990a,b; Kostović & Rakic 1990; Kostović et al. 2002; Judaš et al. 2005) is the main marker of immaturity of the fetal white matter.
In late preterm, the white matter consists of the following segments: segment 1, with the callosum and periventricular fibers; segment 2, the foot of the corona radiata with the periventricular crossroads; segment 3, the centrum semiovale; and segment 4, the gyral white matter. Segment 5 [the (intra)cortical white matter] is not developed (Judaš et al. 2005). The subplate zone interrupts the continuity of the white matter and gyral cortex (Kostović et al. 2002; Judaš et al. 2005; see also classification in Von Monakow, 1905).
Discussion on development of fiber pathways in the cerebral cortex of the fetus
The data about the initial outgrowth, path-finding, trajectories, waiting compartments and target distribution indicate that the fetal part of gestation is the most important for the growth of long projection, associative and commissural pathways in the human cerebrum. It is evident that the neocortical fiber pathways show a different pattern of fiber growth than the limbic fiber systems. The main projection pathway of the limbic nuclei and cortex grows in the form of the compact axonal bundles along the midline (fornix, stria terminalis and cingulum). These bundles elongate and curve parallel to the growth of the hemispheres. However, neopallial fiber pathways change their trajectory at the level of the lateral angles of the ventricles, forming the crossroads, embedded in a rich extracellular matrix with axonal guidance molecules (Molnar & Blakemore, 1995; Molnar et al. 1998; Molnar & Butler, 2002; Judaš et al. 2005; Price et al. 2006). This junction area was described (Molnar & Butler, 2002) as a crucial junction point for the pathway selection of the most commonly demonstrated cortico–subcortical and subcortico–cortical pathways. The involvement of guidance molecules in thalamocortical pathway formation was, among others, most convincingly demonstrated for the Slit/Robo (Bagri et al. 2002), EphRTK/ephrins (Dufour et al. 2003), Nrg1/ErbB4 (Lopez-Bendito et al. 2006) and Semaphorin/Neuropilin1 (Wright et al. 2007) families of proteins. At present it is not known whether the same molecules guide corticostriatal and telencephalo–diencephalic junction fibers in the intermediate zone and sagittal strata. The current evidence is insufficient to form a conclusion about the guidance within the external capsule. The evidence presented in this review clearly shows that the external capsule is a complex system that contains projection fibers from the basal forebrain (Kostović, 1986; Mesulam et al. 1992), associative cortical and corticostriatal pathways. The presence of basal forebrain pathways is not described in the current imaging literature (Makris et al. 1997) and in experimental studies (Schmahmann & Pandya, 2006). The basal forebrain projections through the external capsule were shown to be the earliest modulatory input to the neopallium. The significance of this early afferent modulatory input for the early endogenous (Kostović, 1986; Kostović & Judaš, 2007), oscillatory activity of the cortex was stressed in recent experimental studies (Hanganu & Luhmann, 2004; Hanganu et al. 2009; Luhmann et al. 2009; Yang et al. 2009). The other modulatory inputs arriving at the telencephalon at very early ages are from the monoaminergic nuclei in the brainstem (Nobin & Bjorklund, 1973; Olson et al. 1973; Molliver & Kristt, 1975; Zecevic & Verney, 1995). These earliest afferent pathways, visualized by fluorescent techniques, do not form distinct bundles, and are formed by extremely fine fibers. Therefore, these pathways cannot be demonstrated by a tractography or by the histological techniques presented in this review. The only possible trajectory where these early monoaminergic afferents can be eventually visualized is the medial forebrain bundle in the lateral hypothalamus (Nauta, 1971; Nobin & Bjorklund, 1973; Olson et al. 1973).
The evidence presented in this review supports the concept of the ‘waiting’ period and ‘waiting’ compartments. As presented above, all of the thalamocortical afferents and basal forebrain afferents invade the subplate after 13-14 PCW and ‘wait’ in the subplate compartment for a prolonged period, until after 23 PCW. The concept of the waiting period was introduced by Rakic (1977) and was further corroborated in a series of articles on the development in thalamocortical projection in human and monkey brain (Kostović & Rakic, 1984; Kostović & Goldman-Rakic, 1983; Kostović & Jovanov-Milošević, 2006; Kostović & Judaš, 2002, 2007, 2010). Within this period the tractography can trace long pathways within the trajectories but not the ramification within the subplate. This may be explained by the plexiform isotropic nature of the fibrillar network ‘embedded’ within the fluid rich extracellular matrix of the subplate. It is only after 19 weeks, during the accumulation of the thalamocortical fibers, that some of the fibers can be traced across the subplate. However, the histochemical cholinesterase method was shown to be efficient in the demonstration of the ingrowth of the afferents into the cortical plate.
The indirect evidence for the accumulation of the thalamocortical circuitry within the cortical plate is lamination (Kostović & Judaš, 2002) and changes in isotropy of the cortical plate (Huppi et al. 2001; McKinstry et al. 2002).
Another observed phenomenon of the developmental cerebrum is the axonal distribution and growth in sagittal strata. This stratified pattern of distribution is the predominant pattern in the early development of the cerebral hemisphere (Altman & Bayer, 2002; Bayer & Altman, 2002, 2004, 2005, 2006, 2008; Judaš et al. 2005). The crucial period of the change in stratified development occurs after 28 PCW when the thalamocortical afferents, together with other projection pathways, develop the corona radiata (thalamic radiation, Stabkrantz, Von Monakow, 1905). After this period, the sagittal strata remain well delineated only in the occipital and frontal pole (Judaš et al. 2005).
In the current literature we have found different descriptions and terminology for the classification of the sagittal strata (Makris et al. 1997; Schmahmann & Pandya, 2006). The results presented in this review suggest that the minimum subdivision of the sagittal compartment is into the three sagittal strata (external, central and internal stratum) as suggested by Von Monakow (1905). From the evidence presented in the previous paragraph it is obvious that the data on the development of the sagittal strata are very scanty. Because of this, further studies are needed to describe the content of the individual sagittal strata. This is a very interesting problem for the neuroimaging community because these strata can be easily demonstrated by the tractography method due to the fact that the fibers run in a predominantly anterior–posterior direction.
Significance
The great significance of the prenatal growth of the cerebral pathways is widely accepted in the developmental and prenatal neurological literature. The growing white matter is considered to be the most vulnerable cellular compartment in the preterm brain (Counsell et al. 2007; Leviton & Gressens, 2007; Mathur & Inder, 2009; Ment et al. 2009; Miller & Ferriero, 2009; Volpe, 2009). Lesions of the white matter lead to cognitive impairment in 30–60% of very preterm children, whereas 40% display a mild motor deficit (Mathur & Inder, 2009). A high percentage of very preterm infants have a sensory deficit (Johnston et al. 2001). The most vulnerable locus in the developing white matter of preterm infants is the periventricular crossroads of pathways, which can be damaged by focal leukomalacia (Judaš et al. 2005; Volpe, 2009). As presented above, these loci contain the associative and commissural pathways, which may explain the complex neurodevelopmental and cognitive deficits seen after periventricular lesions in preterm infants (Evrard, 2001; Johnston et al. 2001; Volpe, 2003, 2009; Judaš et al. 2005; Mathur & Inder, 2009; Ment et al. 2009; Miller & Ferriero, 2009).
Acknowledgments
This work was partially supported by UKF fund 07 and Croatian Ministry of Science, Education and Sports grant no. 108-1081870-1876 (to I.K.) and L‘oreals ‘for women in science’ stipend, 2010, L.V.
References
- Aeby A, Liu Y, De Tiege X, et al. Maturation of thalamic radiations between 34 and 41 weeks’ gestation: a combined voxel-based study and probabilistic tractography with diffusion tensor imaging. AJNR Am J Neuroradiol. 2009;30:1780–1786. doi: 10.3174/ajnr.A1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Regional differences in the stratified transitional field and the honeycomb matrix of the developing human cerebral cortex. J Neurocytol. 2002;31:613–632. doi: 10.1023/a:1025787427576. [DOI] [PubMed] [Google Scholar]
- Anderson G, Price DJ. Layer-specific thalamocortical innervation in organotypic cultures is prevented by substances that alter neural activity. Eur J Neurosci. 2002;16:345–349. doi: 10.1046/j.1460-9568.2002.02069.x. [DOI] [PubMed] [Google Scholar]
- Ayoub AE, Kostović I. New horizons for the subplate zone and its pioneering neurons. Cereb Cortex. 2009;19:1705–1707. doi: 10.1093/cercor/bhp025. [DOI] [PubMed] [Google Scholar]
- Bagri A, Marin O, Plump AS, et al. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. J Anat. 2007;211:237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelmez G, Dekaban A. The early development of the human brain. Contr Embryol Carneg Inst. 1962;37:13–32. [Google Scholar]
- Bayatti N, Sarma S, Shaw C, et al. Progressive loss of PAX6, TBR2, NEUROD and TBR1 mRNA gradients correlates with translocation of EMX2 to the cortical plate during human cortical development. Eur J Neurosci. 2008a;28:1449–1456. doi: 10.1111/j.1460-9568.2008.06475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, et al. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex. 2008b;18:1536–1548. doi: 10.1093/cercor/bhm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Development of layer I and the subplate in the rat neocortex. Exp Neurol. 1990;107:48–62. doi: 10.1016/0014-4886(90)90062-w. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Atlas of Human Central Nervous System Development. Boca Raton: CRC Press; 2002. pp. 1–4. [Google Scholar]
- Bayer SA, Altman J. Atlas of Human Central Nervous System Development. Boca Raton: CRC Press; 2004. [Google Scholar]
- Bayer SA, Altman J. Atlas of Human Central Nervous System Development. Boca Raton: CRC Press Taylor & Francis Group; 2005. [Google Scholar]
- Bayer SA, Altman J. Atlas of Human Central Nervous System Development. Boca Raton: CRC Press Taylor & Francis Group; 2006. [Google Scholar]
- Bayer SA, Altman J. Atlas of Human Central Nervous System Development. Boca Raton: CRC Press Taylor & Francis Group; 2008. [Google Scholar]
- Berman JI, Mukherjee P, Partridge SC, et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27:862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Garel S, Nakagawa Y, et al. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol. 2003;457:345–360. doi: 10.1002/cne.10549. [DOI] [PubMed] [Google Scholar]
- Bolz J, Uziel D, Muhlfriedel S, et al. Multiple roles of ephrins during the formation of thalamocortical projections: maps and more. J Neurobiol. 2004;59:82–94. doi: 10.1002/neu.10346. [DOI] [PubMed] [Google Scholar]
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Catalano SM, Shatz CJ. Activity-dependent cortical target selection by thalamic axons. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- Chun JJ, Shatz CJ. A fibronectin-like molecule is present in the developing cat cerebral cortex and is correlated with subplate neurons. J Cell Biol. 1988;106:857–872. doi: 10.1083/jcb.106.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counsell SJ, Dyet LE, Larkman DJ, et al. Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. Neuroimage. 2007;34:896–904. doi: 10.1016/j.neuroimage.2006.09.036. [DOI] [PubMed] [Google Scholar]
- De Carlos JA, O’Leary DD. Growth and targeting of subplate axons and establishment of major cortical pathways. J Neurosci. 1992;12:1194–1211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio JA, Martinez A, Auladell C, et al. Developmental history of the subplate and developing white matter in the murine neocortex. Neuronal organization and relationship with the main afferent systems at embryonic and perinatal stages. Cereb Cortex. 2000;10:784–801. doi: 10.1093/cercor/10.8.784. [DOI] [PubMed] [Google Scholar]
- Delalle I, Evers P, Kostović I, et al. Laminar distribution of neuropeptide Y-immunoreactive neurons in human prefrontal cortex during development. J Comp Neurol. 1997;379:515–522. doi: 10.1002/(sici)1096-9861(19970324)379:4<515::aid-cne4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Dufour A, Seibt J, Passante L, et al. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39:453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- Evrard P. Pathophysiology of perinatal brain damage. Dev Neurosci. 2001;23:171–174. doi: 10.1159/000046138. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Clowry GJ, et al. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain. 2000;123(Pt 1):51–64. doi: 10.1093/brain/123.1.51. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the frontal association cortex and its relevance to dementia. Arch Gerontol Geriatr. 1987;6:299–309. doi: 10.1016/0167-4943(87)90029-x. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Luhmann HJ. Functional nicotinic acetylcholine receptors on subplate neurons in neonatal rat somatosensory cortex. J Neurophysiol. 2004;92:189–198. doi: 10.1152/jn.00010.2004. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Okabe A, Lessmann V, et al. Cellular mechanisms of subplate-driven and cholinergic input-dependent network activity in the neonatal rat somatosensory cortex. Cereb Cortex. 2009;19:89–105. doi: 10.1093/cercor/bhn061. [DOI] [PubMed] [Google Scholar]
- Higashi S, Hioki K, Kurotani T, et al. Functional thalamocortical synapse reorganization from subplate to layer IV during postnatal development in the reeler-like mutant rat (shaking rat Kawasaki) J Neurosci. 2005;25:1395–1406. doi: 10.1523/JNEUROSCI.4023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- His W. Die Entwickelung des menschlichen Gehirns währendder ersten Monate. Leipzig: S. Hirzel; 1904. pp. 91–107. 163–167. [Google Scholar]
- Hochstetter F. Teil. Wien: F. Deuticke; 1909. Beiträge zur Entwicklungsgeschichte des menschlichen Gehirns. 1. [Google Scholar]
- Hoerder-Suabedissen A, Wang WZ, Lee S, et al. Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cereb Cortex. 2009;19:1738–1750. doi: 10.1093/cercor/bhn195. [DOI] [PubMed] [Google Scholar]
- Hoon AH, Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51:697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, et al. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33:27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, et al. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci. 2009;29:4263–4273. doi: 10.1523/JNEUROSCI.2769-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey T. The development of the human hippocampal fissure. J Anat. 1967;101:655–676. [PMC free article] [PubMed] [Google Scholar]
- Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Murphy B, Maier SE, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia. 2005;49:480–491. doi: 10.1002/glia.20134. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Trescher WH, Ishida A, et al. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49:735–741. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Jovanov-Milošević N, Culjat M, Kostović I. Growth of the human corpus callosum: modular and laminar morphogenetic zones. Front Neuroanat. 2009;3:6. doi: 10.3389/neuro.05.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judaš M, Milošević NJ, Rasin MR, et al. Complex patterns and simple architects: molecular guidance cues for developing axonal pathways in the telencephalon. Prog Mol Subcell Biol. 2003;32:1–32. doi: 10.1007/978-3-642-55557-2_1. [DOI] [PubMed] [Google Scholar]
- Judaš M, Radoš M, Jovanov-Milošević N, et al. Structural, immunocytochemical, and mr imaging properties of peri-ventricular crossroads of growing cortical pathways in preterm infants. AJNR Am J Neuroradiol. 2005;26:2671–2684. [PMC free article] [PubMed] [Google Scholar]
- Kasprian G, Brugger PC, Weber M, et al. In utero tractography of fetal white matter development. Neuroimage. 2008;43:213–224. doi: 10.1016/j.neuroimage.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Kim DH, Chung S, Vigneron DB, et al. Diffusion-weighted imaging of the fetal brain in vivo. Magn Reson Med. 2008;59:216–220. doi: 10.1002/mrm.21459. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Piecinski P, Rakic P. Neurogenesis of the amygdaloid nuclear complex in the rhesus monkey. Brain Res Dev Brain Res. 1992;68:9–15. doi: 10.1016/0165-3806(92)90242-o. [DOI] [PubMed] [Google Scholar]
- Kostović I. Prenatal development of nucleus basalis complex and related fiber systems in man: a histochemical study. Neuroscience. 1986;17:1047–1077. doi: 10.1016/0306-4522(86)90077-1. [DOI] [PubMed] [Google Scholar]
- Kostović I. Zentralnervensystem. In: Hinrichsen KV, editor. Humanembryologie. Berlin: Springer-Verlag; 1990a. pp. 381–448. [Google Scholar]
- Kostović I. Structural and histochemical reorganization of the human prefrontal cortex during perinatal and postnatal life. Prog Brain Res. 1990b;85:223–239. doi: 10.1016/s0079-6123(08)62682-5. discussion 239–240. [DOI] [PubMed] [Google Scholar]
- Kostović I, Goldman-Rakic PS. Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol. 1983;219:431–447. doi: 10.1002/cne.902190405. [DOI] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milošević N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milošević N. Subplate zone of the human brain: historical perspective and new concepts. Coll Antropol. 2008;32(Suppl 1):3–8. [PubMed] [Google Scholar]
- Kostović I, Judaš M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002;267:1–6. doi: 10.1002/ar.10069. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. Prolonged coexistence of transient and permanent circuitry elements in the developing cerebral cortex of fetuses and preterm infants. Dev Med Child Neurol. 2006;48:388–393. doi: 10.1017/S0012162206000831. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev. 2007;31:1157–1168. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr. 2010 doi: 10.1111/j.1651-2227.2010.01811.x. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- Kostović I, Krmpotić-Nemanić J. Early prenatal onto-genesis of the neuronal connections in the interhemi-spheric cortex of the human gyrus cinguli. Verh Anat Ges. 1976;70:305–316. [PubMed] [Google Scholar]
- Kostović I, Molliver M. New interpretation of laminar development of cerebral-cortex – synaptogenesis in different layers of neopallium in human fetus. Anat Rec. 1974;178:395. [Google Scholar]
- Kostović I, Rakic P. Development of prestriate visual projections in the monkey and human fetal cerebrum revealed by transient cholinesterase staining. J Neurosci. 1984;4:25–42. doi: 10.1523/JNEUROSCI.04-01-00025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kostović I, Vasung L. Insights from in vitro fetal magnetic resonance imaging of cerebral development. Semin Perinatol. 2009;33:220–233. doi: 10.1053/j.semperi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Kostović I, Kračun I, Kelović Z, et al. The development of “cholinergic” reaction in the septal area of the human fetus. Verh Anat Ges. 1980;74:729–732. [Google Scholar]
- Kostović I, Škavić J, Strinović D. Acetylcholinesterase in the human frontal associative cortex during the period of cognitive development: early laminar shifts and late innervation of pyramidal neurons. Neurosci Lett. 1988;90:107–112. doi: 10.1016/0304-3940(88)90795-1. [DOI] [PubMed] [Google Scholar]
- Kostović I, Lukinović N, Judaš M, et al. Structural basis of the developmental plasticity in the human cerebral cortex: the role of the transient subplate zone. Metab Brain Dis. 1989a;4:17–23. doi: 10.1007/BF00999489. [DOI] [PubMed] [Google Scholar]
- Kostović I, Seress L, Mrzljak L, et al. Early onset of synapse formation in the human hippocampus: a correlation with Nissl-Golgi architectonics in 15- and 16.5-week-old fetuses. Neuroscience. 1989b;30:105–116. doi: 10.1016/0306-4522(89)90357-6. [DOI] [PubMed] [Google Scholar]
- Kostović I, Petanjek Z, Judaš M. Early areal differentiation of the human cerebral cortex: entorhinal area. Hippocampus. 1993;3:447–458. doi: 10.1002/hipo.450030406. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M, Radoš M, et al. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex. 2002;12:536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- Kostović I, Judaš M, Petanjek Z. Structural development of the human prefrontal cortex. In: Nelson C, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2008. pp. 213–237. [Google Scholar]
- Krmpotić-Nemanić J, Kostović I, Kelović Z, et al. Development of the human fetal auditory cortex: growth of afferent fibres. Acta Anat (Basel) 1983;116:69–73. [PubMed] [Google Scholar]
- Kwan KY, Lam MM, Krsnik Z, et al. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci USA. 2008;105:16021–16026. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz H, Changeux JP. The emergence of human consciousness: from fetal to neonatal life. Pediatr Res. 2009;65:255–260. doi: 10.1203/PDR.0b013e3181973b0d. [DOI] [PubMed] [Google Scholar]
- LaMantia AS, Rakic P. Axon overproduction and elimination in the anterior commissure of the developing rhesus monkey. J Comp Neurol. 1994;340:328–336. doi: 10.1002/cne.903400304. [DOI] [PubMed] [Google Scholar]
- Leviton A, Gressens P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 2007;30:473–478. doi: 10.1016/j.tins.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Molnar Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Chan CH, Mallamaci A, et al. Role of Emx2 in the development of the reciprocal connectivity between cortex and thalamus. J Comp Neurol. 2002;451:153–169. doi: 10.1002/cne.10345. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Cautinat A, Sanchez JA, et al. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann HJ, Kilb W, Hanganu-Opatz IL. Subplate cells: amplifiers of neuronal activity in the developing cerebral cortex. Front Neuroanat. 2009;3:19. doi: 10.3389/neuro.05.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas LC, Mukherjee P, Carballido-Gamio J, et al. Early laminar organization of the human cerebrum demonstrated with diffusion tensor imaging in extremely premature infants. Neuroimage. 2004;22:1134–1140. doi: 10.1016/j.neuroimage.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Macchi G. The ontogenetic development of the olfactory telencephalon in man. J Comp Neurol. 1951;95:245–305. doi: 10.1002/cne.900950203. [DOI] [PubMed] [Google Scholar]
- Makris N, Worth AJ, Sorensen AG, et al. Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;42:951–962. doi: 10.1002/ana.410420617. [DOI] [PubMed] [Google Scholar]
- Maroof AM, Anderson SA. Off on a tangent: thalamocortical axons traverse a permissive corridor across the basal telencephalon. Neuron. 2006;50:185–188. doi: 10.1016/j.neuron.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Mathur A, Inder T. Magnetic resonance imaging – insights into brain injury and outcomes in premature infants. J Commun Disord. 2009;42:248–255. doi: 10.1016/j.jcomdis.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DA, Nadler JV, Lynch GS, et al. Development of cholinergic innervation in the hippocampal formation of the rat. I. Histochemical demonstration of acetylcholinesterase activity. Dev Biol. 1974;36:130–141. doi: 10.1016/0012-1606(74)90196-1. [DOI] [PubMed] [Google Scholar]
- McKellar CE, Shatz CJ. Synaptogenesis in purified cortical subplate neurons. Cereb Cortex. 2009;19:1723–1737. doi: 10.1093/cercor/bhn194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinstry RC, Mathur A, Miller JH, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12:1237–1243. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- Meinecke DL, Rakic P. Expression of GABA and GABAA receptors by neurons of the subplate zone in developing primate occipital cortex: evidence for transient local circuits. J Comp Neurol. 1992;317:91–101. doi: 10.1002/cne.903170107. [DOI] [PubMed] [Google Scholar]
- Ment LR, Hirtz D, Huppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, et al. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, et al. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience. 1984;12:669–686. doi: 10.1016/0306-4522(84)90163-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Hersh LB, Mash DC, et al. Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: a choline acetyltransferase study. J Comp Neurol. 1992;318:316–328. doi: 10.1002/cne.903180308. [DOI] [PubMed] [Google Scholar]
- Meyer G. Genetic Control of Neuronal Migrations in Human Cortical Development. Berlin: Springer-Verlag Heilderberg; 2007. [PubMed] [Google Scholar]
- Milh M, Kaminska A, Huon C, et al. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb Cortex. 2007;17:1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- Miller SP, Ferriero DM. From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci. 2009;32:496–505. doi: 10.1016/j.tins.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver ME. An ontogenetic study of evoked somesthetic cortical responses in the sheep. Prog Brain Res. 1967;26:78–91. doi: 10.1016/S0079-6123(08)61420-X. [DOI] [PubMed] [Google Scholar]
- Molliver ME, Kristt DA. The fine structural demonstration of monoaminergic synapses in immature rat neocortex. Neurosci Lett. 1975;1:305–310. doi: 10.1016/0304-3940(75)90017-8. [DOI] [PubMed] [Google Scholar]
- Molliver ME, Kostović I, van der Loos H. The development of synapses in cerebral cortex of the human fetus. Brain Res. 1973;50:403–407. doi: 10.1016/0006-8993(73)90741-5. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Blakemore C. How do thalamic axons find their way to the cortex? Trends Neurosci. 1995;18:389–397. doi: 10.1016/0166-2236(95)93935-q. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Butler AB. The corticostriatal junction: a crucial region for forebrain development and evolution. Bioessays. 2002;24:530–541. doi: 10.1002/bies.10100. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci. 1998;18:5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Kurotani T, Higashi S, et al. Development of functional thalamocortical synapses studied with current source-density analysis in whole forebrain slices in the rat. Brain Res Bull. 2003;60:355–371. doi: 10.1016/s0361-9230(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostović I, et al. Prenatal development of neurons in the human prefrontal cortex: I. A qualitative Golgi study. J Comp Neurol. 1988;271:355–386. doi: 10.1002/cne.902710306. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostović I, et al. Prenatal development of neurons in the human prefrontal cortex. II. A quantitative Golgi study. J Comp Neurol. 1992;316:485–496. doi: 10.1002/cne.903160408. [DOI] [PubMed] [Google Scholar]
- Nauta WJ. The problem of the frontal lobe: a reinterpretation. J Psychiatr Res. 1971;8:167–187. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Nikolić I, Kostović I. Development of the lateral amygdaloid nucleus in the human fetus: transient presence of discrete cytoarchitectonic units. Anat Embryol (Berl) 1986;174:355–360. doi: 10.1007/BF00698785. [DOI] [PubMed] [Google Scholar]
- Nobin A, Bjorklund A. Topography of the monoamine neuron systems in the human brain as revealed in fetuses. Acta Physiol Scand Suppl. 1973;388:1–40. [PubMed] [Google Scholar]
- Nowakowski RS, Rakic P. The site of origin and route and rate of migration of neurons to the hippocampal region of the rhesus monkey. J Comp Neurol. 1981;196:129–154. doi: 10.1002/cne.901960110. [DOI] [PubMed] [Google Scholar]
- Olson L, Boreus LO, Seiger A. Histochemical demonstration and mapping of 5-hydroxytryptamine- and catecholamine-containing neuron systems in the human fetal brain. Z Anat Entwicklungsgesch. 1973;139:259–282. doi: 10.1007/BF00519968. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. Embryonic Human Brain: An Atlas of Developmental Stages. Hoboken: John Wiley & Sons; 2006. [Google Scholar]
- Osheroff H, Hatten ME. Gene expression profiling of preplate neurons destined for the subplate: genes involved in transcription, axon extension, neurotransmitter regulation, steroid hormone signaling, and neuronal survival. Cereb Cortex. 2009;19(Suppl 1):i126–i134. doi: 10.1093/cercor/bhp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman AL, Sheppard AM. Extracellular matrix in early cortical development. Prog Brain Res. 1996;108:117–134. [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Kostović I, et al. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov G. Structural organization of the human cerebral cortex during ontogenetic development. In: Sarkisov A, Filimonof I, Preobratenskaya N, editors. Cytoarchitectonics of the Cerebral Cortex in Man (in Russian) Moscow: Medgiz; 1949. pp. 33–92. [Google Scholar]
- Polyak S. An experimental study of association, callosal, and projection fibres of the cerebral cortex of the cat. J Comp Neurol. 1927;44:197–258. [Google Scholar]
- Polyak S. The main afferent fibre systems of the cerebral cortex in primates. Univ Calif Publ Anat. 1932;2:107–207. [Google Scholar]
- Price DJ, Kennedy H, Dehay C, et al. The development of cortical connections. Eur J Neurosci. 2006;23:910–920. doi: 10.1111/j.1460-9568.2006.04620.x. [DOI] [PubMed] [Google Scholar]
- Radoš M, Judaš M, Kostović I. In vitro MRI of brain development. Eur J Radiol. 2006;57:187–198. doi: 10.1016/j.ejrad.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976;261:467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977;278:245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. J Comp Neurol. 1968;132:45–72. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- Ramenghi LA, Rutherford M, Fumagalli M, et al. Neonatal neuroimaging: going beyond the pictures. Early Hum Dev. 2009;85:S75–S77. doi: 10.1016/j.earlhumdev.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Rutherford M, Jiang S, Allsop J, et al. MR imaging methods for assessing fetal brain development. Dev Neurobiol. 2008;68:700–711. doi: 10.1002/dneu.20614. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Pandya DN. Fibre Pathways of the Brain. Oxford: Oxford University Press; 2006. [Google Scholar]
- Schwartz ML, Goldman-Rakic PS. Prenatal specification of callosal connections in rhesus monkey. J Comp Neurol. 1991;307:144–162. doi: 10.1002/cne.903070113. [DOI] [PubMed] [Google Scholar]
- Sestan N, Rakic P, Donoghue MJ. Independent parcellation of the embryonic visual cortex and thalamus revealed by combinatorial Eph/ephrin gene expression. Curr Biol. 2001;11:39–43. doi: 10.1016/s0960-9822(00)00043-9. [DOI] [PubMed] [Google Scholar]
- Sherlock RL, McQuillen PS, Miller SP. Preventing brain injury in newborns with congenital heart disease: brain imaging and innovative trial designs. Stroke. 2009;40:327–332. doi: 10.1161/STROKEAHA.108.522664. [DOI] [PubMed] [Google Scholar]
- Slater R, Cantarella A, Franck L, et al. How well do clinical pain assessment tools reflect pain in infants? PLoS Med. 2008;5:e129. doi: 10.1371/journal.pmed.0050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, et al. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan H. Allocortex. In: Bargmann W, editor. Handbuch der Mikroskopischen Anatomie des Menschen, Vol. 4. Berlin and New York: Springer-Verlag; 1975. [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi R, Husain N, Rathore RK, et al. Correlation of diffusion tensor imaging with histology in the developing human frontal cerebrum. Dev Neurosci. 2009;31:487–496. doi: 10.1159/000229500. [DOI] [PubMed] [Google Scholar]
- Tuttle R, Schlaggar BL, Braisted JE, et al. Maturation-dependent upregulation of growth-promoting molecules in developing cortical plate controls thalamic and cortical neurite growth. J Neurosci. 1995;15:3039–3052. doi: 10.1523/JNEUROSCI.15-04-03039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Neudorfer F, et al. Distribution of SNAP-25 in transient neuronal circuitries of the developing human forebrain. Neuroreport. 2000;11:1259–1263. doi: 10.1097/00001756-200004270-00023. [DOI] [PubMed] [Google Scholar]
- Uziel D, Garcez P, Lent R, et al. Connecting thalamus and cortex: the role of ephrins. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:135–142. doi: 10.1002/ar.a.20286. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Kaila K. Development of neonatal EEG activity: from phenomenology to physiology. Semin Fetal Neonatal Med. 2006;11:471–478. doi: 10.1016/j.siny.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Vasung L, Huang H, Jovanov-Milošević N, et al. Fronto-subcortical connectivity in the human fetal brain: growth of fiber bundles and axonal strata. In: Neuroscience Sf., editor. SFN Annual Meeting. Chicago, IL: Society for Neuroscience; 2009. pp. 121–122. [Google Scholar]
- Volpe JJ. Cerebral white matter injury of the premature infant – more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the Newborn. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Monakow C. Gehirnpathologie. Wien: Alfred Holder; 1905. [Google Scholar]
- Vukšić M, Rados M, Kostović I. Structural basis of developmental plasticity in the corticostriatal system. Coll Antropol. 2008;32:155–159. [PubMed] [Google Scholar]
- Wilkemeyer MF, Angelides KJ. Addition of tetrodotoxin alters the morphology of thalamocortical axons in organotypic cocultures. J Neurosci Res. 1996;43:707–718. doi: 10.1002/(SICI)1097-4547(19960315)43:6<707::AID-JNR7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wright AG, Demyanenko GP, Powell A, et al. Close homolog of L1 and neuropilin 1 mediate guidance of thalamocortical axons at the ventral telencephalon. J Neurosci. 2007;27:13667–13679. doi: 10.1523/JNEUROSCI.2888-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JW, Hanganu-Opatz IL, Sun JJ, et al. Three patterns of oscillatory activity differentially synchronize developing neocortical networks in vivo. J Neurosci. 2009;29:9011–9025. doi: 10.1523/JNEUROSCI.5646-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Verney C. Development of the catecholamine neurons in human embryos and fetuses, with special emphasis on the innervation of the cerebral cortex. J Comp Neurol. 1995;351:509–535. doi: 10.1002/cne.903510404. [DOI] [PubMed] [Google Scholar]
- Zhang J, Evans A, Hermoye L, et al. Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage. 2007;38:239–247. doi: 10.1016/j.neuroimage.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


