Abstract
Amoeboid microglial subpopulations visualized by antibodies against ionized calcium-binding adapter molecule 1, CD68, and CD45 enter the forebrain starting at 4.5 postovulatory or gestational weeks (gw). They penetrate the telencephalon and diencephalon via the meninges, choroid plexus, and ventricular zone. Early colonization by amoeboid microglia–macrophages is first restricted to the white matter, where these cells migrate and accumulate in patches at the junctions of white-matter pathways, such as the three junctions that the internal capsule makes with the thalamocortical projection, external capsule and cerebral peduncle, respectively. In the cerebral cortex anlage, migration is mainly radial and tangential towards the immature white matter, subplate layer, and cortical plate, whereas pial cells populate the prospective layer I. A second wave of microglial cells penetrates the brain via the vascular route at about 12–13 gw and remains confined to the white matter. Two main findings deserve emphasis. First, microglia accumulate at 10–12 gw at the cortical plate–subplate junction, where the first synapses are detected. Second, microglia accumulate in restricted laminar bands, most notably around 19–30 gw, at the axonal crossroads in the white matter (semiovale centre) rostrally, extending caudally in the immature white matter to the visual radiations. This accumulation of proliferating microglia is located at the site of white-matter injury in premature neonates. The spatiotemporal organization of microglia in the immature white and grey matter suggests that these cells may play active roles in developmental processes such as axonal guidance, synaptogenesis, and neurodevelopmental apoptosis as well as in injuries to the developing brain, in particular in the periventricular white-matter injury of preterm infants.
Keywords: brain macrophages, CD45, CD68, development, double immunolabelling, inductible nitric oxide synthase, ionized calcium-binding adapter molecule 1, N-methyl-D-aspartate receptor 1, periventricular leukomalacia, vessels, white-matter crossroads
Introduction
Microglial cells were first described by del Rio Hortega about 90 years ago (del Rio Hortega, 1919). They represent 5–15% of brain cells and constitute the pool of intracerebral resident macrophages (Ling, 1981; Perry et al. 1985). Microglial cells penetrate the brain during early development (Ling, 1981; Hickey & Kimura, 1988; Hutchins et al. 1990), when they exhibit amoeboid features and share similarities regarding surface antigens, morphology, and functional properties with macrophages recruited in disease states (del Rio Hortega, 1932; Esiri et al. 1991; Rezaie & Male, 1999, 2002). As suggested by del Rio Hortega (1932), these amoeboid cells acquire the ramified morphology characteristic of mature microglia (Ling, 1981; Perry et al. 1985; Rezaie & Male, 1999, 2002). During this process, they down-regulate their antigen expression, acquire an intermediate morphology with a few ramifications and, finally, mature into ramified microglia with small cell bodies and long thin processes (Fig. 1). Thus, the decrease in amoeboid cells seen during development correlates with an increase in ramified microglia (del Rio Hortega, 1932; Ling, 1981; Perry et al. 1985; Wierzba-Bobrowicz et al. 1995, 1997). Moreover, in vitro, amoeboid microglia spontaneously show a ramified phenotype associated with loss of the antigenic and functional properties of macrophages (Giulian & Baker, 1986; Giulian, 1987).
Fig. 1.

The three main morphological aspects of microglial cells. From Rezaie & Male (1999).
In the adult brain, ramified microglia constitute the quiescent resident population of cerebral macrophages, which are distributed evenly throughout the brain. These cells are involved in maintaining extracellular homeostasis (Streit et al. 1988; Graeber & Streit, 1990; Banati & Graeber, 1994) and ensuring cerebral immune protection (Streit et al. 1988; Aloisi et al. 2000). In disease states, resting microglial cells can be activated to functional brain macrophages, which undergo amoeboid transformation and up-regulation of macrophage surface markers (Innocenti et al. 1983a,b; Streit et al. 1988; Graeber & Streit, 1990).
The extracerebral origin of cerebral macrophages remains controversial. One hypothesis involves a neuroepithelial origin from a pluripotent precursor, which may be able to give rise to an astrocyte, an oligodendrocyte, or a phagocyte (Hutchins et al. 1989, 1990; Hao et al. 1991; Fedoroff et al. 1997). However, this hypothesis has not been confirmed. Currently, microglial cells are widely believed to originate in the mesoderm from myeloid precursors produced in the yolk sack and, later on, in haematopoietic organs (liver and bone marrow) (Konigsmark & Sidman, 1963; Ling, 1981; Hickey & Kimura, 1988; Hutchins et al. 1990; Chan et al. 2007). Mononuclear phagocytes penetrate the subpial brain during early embryonic development, before vessel development within the brain parenchyma (Choi, 1981; Cuadros et al. 1993; Ling & Wong, 1993; Andjelkovic et al. 1998). Several studies have focused on the microglia that are present during various periods of human brain development (Kershman, 1939; Choi, 1981; Fujimoto et al. 1989; Esiri et al. 1991; Gould & Howard, 1991; Andjelkovic et al. 1998; Rezaie & Male, 1999, 2002; Rezaie, 2003; Rezaie et al. 2005; Billiards et al. 2006).
This review addresses several questions about when and where microglial cells enter and invade the developing human diencephalon and telencephalon. The data were obtained by examining brain sections from embryos and fetuses aged 5–32 postovulatory/gestational weeks (gw), which were subjected to single and double immunolabelling for various markers for microglia–macrophages. Among these markers, ionized calcium-binding adapter molecule 1 (Iba1), a protein that mediates calcium signals, is expressed in ramified microglia, perivascular microglia, and activated microglia in the brain (Ito et al. 1998). CD68 is specifically expressed in the lysosomal granules of macrophages from various human tissues and in microglial cells found in the developing and adult brain. CD45, also called leukocyte common antigen, is expressed on nucleated haematopoietic cells and myeloid-derived leukocytes. In the human brain, microglia express low levels of CD45 (Dick et al. 1997) (Table 1). Relationships between microglia–macrophages and developing blood vessels, as well as microglial proliferation, were assessed using co-detection by the endothelial marker CD34 and the proliferation marker Ki67 (Monier et al. 2006, 2007). Additional studies investigated the possible involvement of microglia–macrophages in the occurrence of periventricular white-matter injury (PWMI) in preterm infants.
Table 1.
Primary antibodies used in Monier et al. (2006) and additional antibodies used for this study.
| Markers | Host species | Dilution | Manufacturer |
|---|---|---|---|
| RCA-1 lectin | 1 : 200 | Vector (Burlingame, CA, USA) | |
| Iba1 | Rabbit | 1 : 1000 | Wako Chemical (Osaka, Japan) |
| CD68 | Mouse | 1 : 800 | Dako (Trappes, France) |
| CD45 | Mouse | 1 : 50 | Dako (Trappes, France) |
| CD34 | Mouse | 1 : 200 | Serotec (Raleigh, NC, USA) |
| MIB1 | Mouse | 1 : 100 | Dako (Trappes, France) |
| Ki67 | Rabbit | 1 : 100 | Dako (Trappes, France) |
| GFAP | Mouse | 1 : 500 | Sigma-Aldrich (St Quentin, France) |
| NMDAr1 | Mouse | 1 : 200 | Chemicon Millipore (Billerica, MA, USA) |
| iNOS | Mouse | 1 : 50 | BD Transduction Lab (Lexington, KY, USA) |
GFAP, glial fibrillary acidic protein; Iba1, ionized calcium-binding adapter molecule 1; iNOS, inductible nitric oxide synthase; NMDAr1, N-methyl-D-aspartate receptor 1; RCA-1, ricinus communis agglutinin-1.
For this review, we reported data obtained from 33 cases (5–23 gw) with no neuropathological abnormality (Monier et al. 2006, 2007). We studied six additional brains from very preterm and preterm infants aged 20–32 gw, as indicated in Table 2. Four brains were free of neuropathological abnormalities and two displayed PWMI. The procedures were approved by the appropriate ethics committee (Comité Consultatif National d-Ethique pour les Sciences de la Vie (CCNESV), approval no. 90294 and Comité d′évaluation de l′éthique des projets biomédicale (CEERB) du GHU Nord no. 09-062). Immunolabelling was achieved using primary antibodies (Table 1) as described in Monier et al. (2006). Double immunolabelling techniques were used to visualize glial fibrillary acidic protein (GFAP, an astrocyte marker)-Iba1 by bright field microscopy and inducible nitric oxide synthase (iNOS)-Iba1 and N-methyl D-aspartate receptor 1 (NMDAr1)-Iba1 by fluorescent markers (Table 1). For both methods, controls were run without the primary antibody to check for the absence of cross-reactivity.
Table 2.
Additional preterm specimens examined using various double immunolabellings for this review in addition to the cases reported by Monier et al. (2006, 2007).
| Age at death (gw) | Tissue processing | Double immunolabellings | Clinical course | |
|---|---|---|---|---|
| 1 | 20 | Freezing | Iba1-iNOS | Spontaneous abortion |
| 2 | 22 | Freezing | Iba1-iNOS | Respiratory distress syndrome–PWMI |
| 3 | 25 | Paraffin | Iba1-GFAP | Palatal cleft |
| 4 | 25 | Freezing | Iba1-NMDAr1 | Spontaneous abortion |
| 5 | 26 | Freezing | Iba1-NMDAr1 | Spontaneous abortion–PWMI |
| 6 | 32 | Paraffin | Iba1-GFAP | Uropathy |
GFAP, glial fibrillary acidic protein; gw, gestational weeks; Iba1, ionized calcium-binding adapter molecule 1; iNOS, inductible nitric oxide synthase; NMDAr1, N-methyl-D-aspartate receptor 1; PWMI, periventricular white-matter injury.
Early routes of amoeboid microglial cell penetration into the embryonic human forebrain and cerebral cortex
The early penetration of amoeboid microglial cells into the human forebrain at 4.5–5.5 gw seems to match the developmental stages at which this event occurs in animal models including rats [embryonic day (E)11] (Ashwell, 1991) and chickens (E2.5 or Hamburger and Hamilton (HH)15 stage) (Cuadros et al. 1993). We found evidence of three early routes of entry: the meninges, choroid plexus, and ventricles. Specific temporal and spatial patterns were found for each of these routes (Monier et al. 2006, 2007).
Choroid plexus and meninges in the embryonic diencephalon–ventral telencephalon
Amoeboid microglial cells in the extracerebral meningeal tissue penetrate the brain early during development by crossing the pial basal lamina (Choi, 1981; Ling & Wong, 1993). This event was documented in 5.5-gw human embryos, in which Iba1-positive amoeboid and intermediate microglia were detected within the parenchyma all along the meningeal tissue bordering the diencephalic–telencephalic fissure (Fig. 2A–C). At this level, as observed on sagittal embryonic sections, amoeboid Iba1-positive cells travelled from the choroid plexus to the eminentia thalami. Interestingly, Iba1-positive cells located in the choroid plexus chiefly expressed CD45, whereas those located deeper in the eminentia thalami parenchyma chiefly expressed CD68 (Fig. 2D,E). These amoeboid cells accumulated in patches, most of which were in or near the developing white-matter tracts such as the anlagen of the internal capsule, external capsule, and optic tracts (Fig. 2B).
Fig. 2.
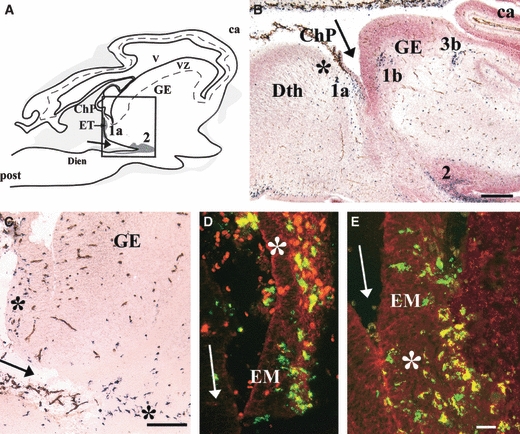
(A) Lateral sagittal section through a 5.5-gestational week (gw) human embryo showing ionized calcium-binding adapter molecule 1 (Iba1)-positive cells issued from the choroid plexus (ChP) and constituting patch 1a in the eminentia thalami (EM/ET). Patch 2 is contiguous to the ditelencephalic fissure (arrows). Grey areas vary in colour depending on the Iba1-positive cell density. The box corresponds to C, where patches of microglial cells are indicated by asterisks. (B,C) Iba1 (black)-CD34 (brown) double labelling and counterstaining with neutral red. (B) Sagittal section through an 8-gw embryo [level indicated in Fig. 2G in Monier et al. (2006)]. Iba1-positive patches 1a, 1b, and 2 are visible near the ditelencephalic fissure (arrow), patch 1a near the presumptive anlage of the internal capsule, and patch 3b at the presumptive anlage of the external capsule. (D,E) High magnification at the level of ChP and ET shown in A. Fluorescent double labelling for Iba1 (green) and CD45 (red) (D) and for Iba1 (green) and CD68 (red) (E) showing co-localization (yellow). Note the preferential CD68 labelling deeper in the EM parenchyma (asterisk in E) compared with the preponderant CD45 labelling in the ChP (asterisk in D). ca, cortical anlagen; Dien, diencephalon; GE, ganglionic eminence; post, posterior; Dth, dorsal thalamus; VZ, ventricular zone; V, ventricle. Scale bars: B, 500 μm; C, 100 μm; D and E, 25 μm. Adapted from Figs 1 and 2 in Monier et al. (2006).
Ventricles and meninges in the developing cerebral cortex
At 4.5 gw, Iba1-positive amoeboid and intermediate extracerebral cells were seen in the lepto-meninges along the cerebral pial surface, contiguous to CD34-positive vessels (Fig. 3A,B). A few amoeboid cells were detected in the marginal zone of the cerebral wall and ventricular zone, and amoeboid cells were visible floating in the ventricular lumen (Fig. 3A). As the cortical plate developed in the lateral cerebral wall at 8 gw, the number of intermediate Iba1-positive cells increased in the ventricular and intermediate zones. These cells were present in the marginal zone but were not seen within the cortical plate (Fig. 3C). The Iba1+/CD45+/CD68+ microglia populating the cortical ventricular zone, subventricular zone, intermediate zone, and subplate in early fetal stages were probably penetrating the ventricular zone border from the ventricles and choroid plexus (Fig. 3C). Amoeboid glial cells of the lepto-meninges populated only the prospective layer 1.
Fig. 3.
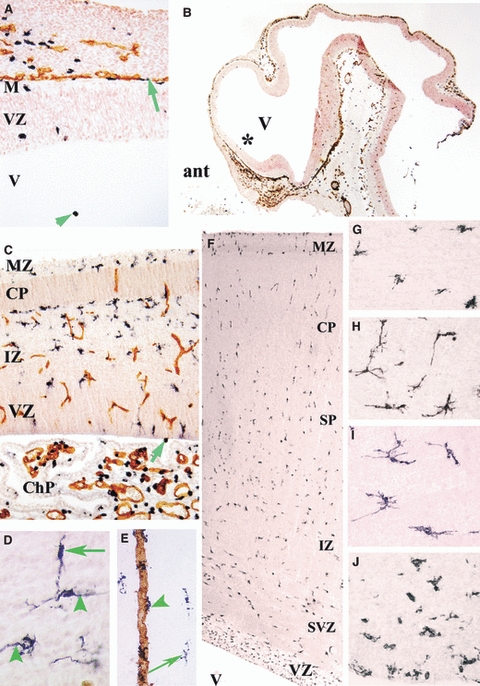
(A–C,E) Ionized calcium-binding adapter molecule 1 (Iba1) (black)-CD34 (brown) double labelling. (A,B) Sagittal section through a 4.5-gestational week (gw) embryo at low (B) and high (A, at the level of the asterisk in B) magnification. In A, Iba1-positive cells are located in the ventricle (V) (green arrowhead) and along the pial surface (green arrow). ant, anterior part of the central nervous system; M, meninges; VZ, ventricular zone. (C) Sagittal cerebral wall and choroid plexus (ChP) at 9 gw; intermediate Iba1-positive cells accumulate under the cortical plate (CP) and in the intermediate zone (IZ), displaying virtually no contiguity with CD34-positive vessel walls. The ChP exhibits numerous amoeboid Iba1-positive microglia. One positive cell close to the ventricular surface (arrow) is visible next to other positive cells in the VZ. MZ, marginal zone. (D) At 12 gw, in the IZ and subplate, Iba1-positive cells exhibit elongated processes that are orientated radially (arrowheads) and tangentially (arrow), consistent with a migratory phenotype. (E) In the subplate at 23.5 gw, Iba1-positive perivascular cells (green arrowhead) are first detected along a CD34-positive vessel wall next to ramified Iba1-positive cells (long arrow). (F–J) At 19 gw, CD45-positive cells are scattered throughout the cingulate cortex (F); they have an intermediate star-like phenotype in the lower CP (G), a tangential and/or radial orientation in the deep subplate (SP) (H), and multiple long multi-directionally orientated processes in the IZ (I). These cells display an intermediate phenotype in the subventricular zone (SVZ) and they are numerous and amoeboid in the VZ (J). Magnification: A, 200×; B, 16×; C, 50×; D,G–J, 400×; E, 100×; F, 20×. Adapted from Figs 1 and 2 in Monier et al. (2007).
Late routes of amoeboid microglial cell penetration: blood vessels and ventricles
The Iba1+/CD45+/CD68+ amoeboid microglial cells were primarily detected in the white-matter tracts of the embryonic forebrain and in the ventricular zone, intermediate zone, and subplate of the cerebral cortex. The first contacts between Iba1+/CD45+/CD68+ amoeboid microglial cells and CD34+ endothelial capillary walls were observed at 10 gw, first within fibre tracts such as the internal capsule (Fig. 4A,C) (Monier et al. 2006). Similar microglial penetration from the vascular compartment was reported in the developing brain of rats at E15 (Ashwell, 1991) and in avian embryos from stage HH21 onward (Cuadros et al. 1993). In the developing cerebral cortex, the first amoeboid microglia–vessel wall contacts were detected at 14 gw but perivascular microglia were visible along radial capillaries in the deep cortical plate and subplate around 22 gw (Fig. 3E). Our results corroborate the hypothesis that microglia may have a dual origin, arising from fetal macrophages in early development and from haematopoietic progenitors at later stages (Rezaie et al. 1997, 2005; Andjelkovic et al. 1998). Chan et al. (2007) have suggested that, late in fetal development, when the bone marrow becomes a haematopoietic organ, cells of monocyte lineage may be recruited to the developing central nervous system, to which they may gain entry via the vasculature (specifically within white-matter areas) (Rezaie, 2007).
Fig. 4.

(A–C) Fetuses aged 9 gestational weeks (gw) (A,B) and 10.5 gw (C). (A) Ionized calcium-binding adapter molecule 1 (Iba1)-CD34 double labelling showing almost no contact between microglial cells (black) and vessels (brown) at 9 gw in the internal capsule (A), whereas one vessel in the white matter contains numerous contiguous microglial cells at 10.5 gw (C, long arrow). Note in C that other blood vessels located in the grey matter on the right in the thalamic anlage medial to the internal capsule, indicated by two arrowheads, do not contain any contiguous positive microglia (arrowheads). (B) Numerous proliferating MIB1 (clone of monoclonal mouse anti-human Ki-67 antigen) (black)-Iba1 (brown) double-labelled microglia in patch 3b at 9 gw (arrows). (D) Coronal sections in a 12-gw embryo; CD45-positive microglia at the border of the third ventricle (IIIrdV) (arrowheads), in the ventricular zone of the IIIrdV, and in the hypothalamic area. (E) Cluster of Iba1-positive cells (4a) in the medial septum at 14.5 gw. AC, anterior commissure; lv, lateral ventricle. (F) Cluster 2 near the optic tract (OT) at 16 gw. (G–J) Double-labelled microglial cells at the same magnification in patch 3b. Cells positive for CD68 (black)-Iba1 (brown) (G,I) and for CD45 (black)-Iba1 (brown) (H,J) in a 13-gw fetus (G,H) and a 23.5-gw fetus (I,J). Note the larger size of amoeboid microglia earlier during development (G,H), especially for CD45-positive cells (H). CD45 labelling is more diffuse in the later-stage specimen (J), whereas CD68 labelling is dot-like (I). Scale bars: A,C, 100 μm; B,G–J, 20 μm; E, 100 μm; F, 300 μm. Adapted from Figs 5–7 in Monier et al. (2006).
Starting at 12 gw, Iba1+/CD45+ amoeboid cells were seen to cross from the third ventricle lumen to the brain (Fig. 4D). However, this route was of lesser importance than the above-mentioned routes and penetration from the lateral ventricles to the cortical anlage (Monier et al. 2007). Such penetration of microglia from the ventricles has been described along the fourth ventricle of the developing avian brain (Cuadros et al. 1993) but not from the third ventricle in other species.
Accumulation of intermediate microglia in clusters in the developing fibre tracts of subcortical areas: diencephalon–ventral telencephalon
As soon as amoeboid microglial cells penetrated the nervous parenchyma, they constituted clusters in or near the developing white-matter tracts (Fig. 2A,B). These clusters exhibited a distinctive distribution in time and space (Tables 3 and 4; Fig. 5). The most prominent early clusters were detected at the junctions of the internal capsule with the thalamus (cluster 3a), internal capsule with the external capsule (cluster 3b), and cerebral peduncle with the internal capsule (cluster 3c) (Tables 3 and 4; Figs 2A,B and 5), as well as in the optic tract (cluster 2) (Fig. 4F). The late clusters were located in the medial septum (Fig. 4E), periventricular hypothalamic area, and corpus callosum (Monier et al. 2006). These clustered microglial cells proliferated actively during early fetal life around 8–12 gw but exhibited no evidence of proliferation from 14 gw onwards (Table 4; Fig. 4B). Such microglial ‘hot spots’ have been reported in white-matter tracts in rodents (Perry et al. 1985; Ashwell, 1991), kittens (Innocenti et al. 1983a), and humans (Kershman, 1939; Andjelkovic et al. 1998).
Table 3.
Location of the microglial cell clusters in the diencephalon–ventral telencephalon as indicated in Figs 4 and 5 and detailed in Monier et al. (2006, 2007).
| Microglial cell clusters | Location |
|---|---|
| 1a | Dorsal to the diencephalic–telencephalic fissure At the level of the eminentia thalami, near the choroid plexus |
| 1b | Edge of the ventricular zone (ganglionic eminence) |
| 2 | Near/in the optic tract, ventral to the diencephalic–telencephalic fissure |
| 3 | In the internal capsule |
| 3a | At the genu of the internal capsule |
| 3b | At the junction between the anterior limb of the internal capsule and the external capsule |
| 3c | At the junction between the posterior limb of the internal capsule and the cerebral peduncle |
| 4a | In the medial septal area |
| 4b | Along the third ventricle in the hypothalamic area |
Table 4.
The temporal sequence and main features of the microglial clusters in the diencephalon and ventral telencephalon.
| Age (gw) | Microglial clusters | Main features |
|---|---|---|
| 5.5–6 | 1a, 2 | No contact with vessel walls, some proliferation |
| 7–11 | 1a,b, 2, 3a,b | Slight contact with vessel walls, increasing proliferation |
| 12 | 1a,b, 2, 3a,b | Contact with vessel walls, extension into the white matter, marked proliferation in microglial clusters |
| 14–17 | 1a,b, 2, 3a,b,c, 4a,b | Contact with vessel walls, extension into the white matter, no proliferation |
| 18–23 | 1a,b, 2, 3a,b,c, 4a,b | Dispersion in the grey matter, no proliferation |
| 19–30 | Axonal crossroads | Proliferation, accumulation in the semi-ovale centre |
Fig. 5.

Coronal anterior (A) and posterior (C) ionized calcium-binding adapter molecule 1 (Iba1)-labelled sections through a 10.5-gestational week (gw) human fetus. Grey areas vary in colour according to the density of Iba1-positive cells. (B,D) Iba1-labelled sections at the level of the corresponding boxes in A and C. Cluster 1a is in the thalamus (Th) extending along the dorsal Th (A,C), cluster 1b in the ventricular zone of the ganglionic eminence (GE) zone, and cluster 3a in the genu of the internal capsule (IC) (B). Patches 3a, 3b, and 3c are located at the junction of the IC with the Th, external capsule (EC), and cerebral peduncle (CP), respectively. Patch 2 surrounds the optic tract (OT). ChP, Choroid plexus; VZ, ventricular zone. Scale bars: B and C, 500 μm. From Fig. 3 in Monier et al. (2006).
These microglia clusters, which are transient structures specific to the developing brain, may be ‘waiting sites’ for glial cells that have entered the brain and will subsequently colonize the parenchyma (del Rio Hortega, 1932; Innocenti et al. 1983a). Their location at meeting points between the main white-matter tracts is highly suggestive of a role in eliminating exuberant axons, promoting neuroaxonal growth, and guiding axonal pathways (Innocenti et al. 1983a,b; Perry et al. 1985; Chamak et al. 1995; Rezaie, 2003). Findings in kittens suggest that the presence of microglia clusters in the white matter may coincide with the elimination of transient trans-callosal projections (Innocenti et al. 1983a). However, the exact significance of these clusters remains unproven, as microglial cells have been reported to accumulate at specific white-matter sites after the corresponding axons reach their final target (Earle & Mitrofanis, 1997).
Antigenic features of microglial–macrophage subpopulations
Antibody directed against the Iba1 (Ito et al. 1998) identifies macrophages/monocytes and labels the largest microglial population, including brain microglia at any stage of activation (Monier et al. 2006, 2007). Iba1 labelling was seen in double-labelling experiments on ricinus communis agglutinin-1 (RCA-1)-positive cells, CD68-positive cells, and most of the CD45-positive cells (Figs 2D,E and 4G–J). However, at about 12 gw, a few CD45-positive cells at the edges of the brain parenchyma did not express Iba1 (Monier et al. 2006). The early amoeboid microglia preferentially expressed CD45 and also expressed Iba1 (Fig. 2D,E), supporting a haematopoietic leukocyte origin (Ling, 1981; Hickey & Kimura, 1988). Close to their primary locations, as microglia accumulated in clusters, the expression of Iba1, RCA-1 and CD68 macrophagic markers was prominent, whereas CD45 immunoreactivity diminished (Fig. 2D,E). In these clusters, labelling was typically Iba1 > RCA-1 > CD68 > CD45 at the early developmental stages. Around 18 gw, the labelling pattern for Iba1/RCA-1/CD45/CD68 became much more uniform, concomitantly with an increasingly homogeneous distribution of microglia, which were seen mainly in their ramified form. We noted a shift in microglial cells from large amoeboid cells with high CD45 and CD68 immunoreactivity early in development to small ramified cells with lower expression levels of these markers (Fig. 4G–J).
Colonization by microglia–macrophages in the cerebral wall
Migration of microglia–macrophages in the developing cerebral cortex
In the cortical layers and white matter, intermediate microglial cells migrated from the ventricular zone to the deep cortical plate, along radial and tangential pathways (Fig. 3D,F–J). Similar radial and tangential migration of microglia has been reported in several regions of the developing central nervous system of vertebrates, such as the retina, optic tectum, and cerebellum (Navascues et al. 2000; Marin-Teva et al. 2004). The inside-out radial progression may be mechanically supported by radial glial fibres, similar to that of migrating neurons (Rakic, 1977). Tangential migration in the intermediate zone (prospective white matter) was similar to the migration observed along the axonal bundles of large white-matter fascicles such as the internal capsule or optic tract (Monier et al. 2006). Guidance cues, such as semaphorins and netrins, have been described for the migration of cells of oligodendrocytic lineage in developing vertebrates (Spassky et al. 2002; Tsai & Miller, 2002). In parallel, expression of the monocycle chemoattractant protein-1 (MCP-1) and chemokine macrophage inflammatory protein-1 (MIP-1α) was detected in the upper layers of the human cerebral cortex between 16 and 22 gw (Rezaie & Male, 1999). Interactions between microglia and adhesion molecules on blood vessels or extracellular matrix components may also be involved in microglial displacement within the developing brain.
Accumulation of amoeboid microglia at the subplate–cortical plate boundary at 9–11 gestational weeks
A remarkable feature in the cerebral wall around 9–11 gw is the concentration of amoeboid microglial cells aligned at the lower edge of the cortical plate at the boundary with the presubplate (Fig. 3B). These cells expressed various markers such as RCA, Iba1, CD68, and CD45 (Fig. 6A,B) (Monier et al. 2007), and many of them were actively proliferating (Fig. 6D). They were still present and proliferating at 12–13 gw (Rezaie et al. 2005). The transient subplate appears early in the human fetal brain and disappears during the last third of gestation and early infancy (Kostovic & Molliver, 1974; Kostovic & Rakic, 1990). It contains mature neurons and receives the first afferent connections entering the cortex, most notably thalamocortical afferents (Shatz et al. 1988; Allendoerfer & Shatz, 1994) and catecholaminergic axons (Verney et al. 1993; Zecevic & Verney, 1995). These cortical afferents were restricted to the subplate-intermediate zone and did not penetrate the cortical plate, as shown for the catecholaminergic axons exhibiting tyrosine hydroxylase immunoreactivity (Fig. 6C). These amoeboid microglia accumulated at the sites of first synapsis development at the cortical plate–subplate junction and in the marginal zone during this period in the human cortex (Kostovic & Molliver, 1974). Microglial cells may promote the death of neuronal cells engaged in synaptogenesis, as observed in the rat cerebellum (Marin-Teva et al. 2004). These various hypotheses regarding the possible role for microglia at this particular location remain to be tested.
Fig. 6.

(A–C) Contiguous sections through the dorsal frontal cortex of a 10.5-gestational week (gw) embryo showing intermediate ionized calcium-binding adapter molecule 1 (Iba1)-positive (A) and CD68-positive (B) cells aligned at the junction between the cortical plate (CP) and subplate (SP) (arrowheads). (C) Tyrosine hydroxylase-immunolabelled contiguous section showing catecholaminergic cortical axonal afferents toward the cerebral cortex, restricted at this developmental stage to the intermediate zone and SP (Zecevic & Verney, 1995). (D) On a contiguous section, numerous double-labelled Iba1 (brown)-MIB1 (clone of monoclonal mouse anti-human Ki-67 antigen) (black) cells (arrowheads), i.e. proliferating microglia–macrophages, in the SP, most notably at the junction with the CP. (E,F) Iba1 labelling of a coronal dorso-frontal section through the foramen of Monro showing a cluster of Iba1-positive cells at axonal crossroads at 22 gw (E, long arrow) and 23.5 gw (F, long arrow). IZ, intermediate zone; SVZ, subventricular zone; VZ, ventricular zone; V, ventricle. (G,H) High magnification of Iba1-positive (G) and CD68-positive (H) intermediate microglial cells in the cluster located in the dorsal occipital cortex of a 19-gw specimen. (I,J) Double labelling for Iba-1 (black, arrows) and glial fibrillary acidic protein (brown) at the crossroads (C2). (I,J) Note, at the same magnification, the high density of Iba1-positive microglia (black) at 25 gw, contrasting with the sparse distribution of these cells at 32 gw. CC, corpus callosum; IZ, intermediate zone; SVZ, subventricular zone; V, ventricle; VZ, ventricular zone. Scale bars: A–C, 50 μm; E, 3 mm; F, 500 μm; D,G–J, 20 μm. Adapted from Figs 2 and 3 in Monier et al. (2007) and additional data.
However, microglial cells may have other functions in the developing cerebral cortex. They are capable of phagocytosis and may be involved in developmental apoptosis (Perry et al. 1985; Ferrer et al. 1990; Ashwell, 1991; Rakic & Zecevic, 2000). In our specimens, microglial cells accumulated in the transient dorsal hippocampus during involution of this structure. Correlations between microglial cells containing intracellular pyknotic debris and pyknotic figures were demonstrated at the same site in the developing rat brain (Ashwell, 1991). Using the terminal deoxynucleotidyl transferase dUTP nick end labelling technique, Rakic & Zecevic (2000) documented apoptotic cell death in the human cortical subplate at the end of the first trimester of gestation, at the time that we and others (Rezaie et al. 2005) observed intermediate microglia in this layer.
Evolution of the distribution pattern of microglia–macrophages in the cerebral cortical wall
After the amoeboid microglia penetrate through the ventricular border and invade the developing cerebral layers in the inside-out direction, intermediate microglia invade the cortical plate between 10 and 13 gw (Table 5). From 14–19 gw onward, the distribution of Iba1+/CD45+ microglial cells was similar throughout the cortical mantle and cell morphology varied across layers (Fig. 3F–J) (Monier et al. 2007). During the second half of gestation, the subventricular and ventricular zones associated with the white matter contained higher densities of microglia/macrophages compared with the subplate layer and cortical plate (Table 5). However, the distribution of microglia/macrophages was heterogeneous.
Table 5.
Overview of ionized calcium-binding adapter molecule 1 labelling of microglia in the various layers of the frontal cerebral cortex and white matter at different ages [in gestational weeks (gw)].
| Age (gw) | Marginal zone | Cortical plate | Intermediate zone (white matter) | Ventricular zone |
|---|---|---|---|---|
| 4–8 | ++ | 0 | ++ | ++ |
| 9–13 | ++ | +/− | +++ | +++ |
| 14–19 | ++ | + | ++++ | ++++ |
| 19–30 | ++ | ++ | ++++ | +++ |
| 31–35 | + | + | ++ | +++ |
Accumulation in the axonal crossroads at 19–30 gestational weeks at sites of potential subsequent white-matter injury
Interestingly, in the 19–30-gw specimens, a tangential band of Iba1-positive cells was seen in the intermediate zone at the junction with the deep subplate of the dorsal cortex corresponding to the prospective semiovale centre (Fig. 6E–I). This cluster of intermediate cells extended medially toward the cingulum bundle and laterally toward the external capsule. It was visible along the entire rostro-caudal extension of the dorsal cerebral cortex, at the level of the basal ganglia, thalamus, and dorsal occipital cortex. These intermediate Iba1-positive cells (Fig. 6G) clearly expressed CD68 (Fig. 6H) and exhibited a more diffuse expression of CD45. In this band, cell density varied across specimens (Fig. 6F). These packed microglial cells were consistently associated with numerous glial fibrillary acidic protein-positive astrocytes (Fig. 6I). Microglia had few points of contact with vessels and some evidence of proliferation was seen. In addition, the germinal zone and ependymal primordium near the microglial cluster also exhibited numerous amoeboid and intermediate Iba1-positive cells (Fig. 6F). The cluster of intermediate microglia (Fig. 6E–I) was located at the intersection of numerous callosal, associative, and thalamocortical axons involved in motor, sensory, and associative systems (Judas et al. 2005). We noticed that, in preterm infants at 30–35 gw, this cluster of intermediate microglial cells decreased drastically in density, as indicated in Fig. 6J. This area is the site where PWMI develops in preterm infants.
Microglial cells may interact with the extracellular matrix and/or axonal guidance molecules that provide cues to axonal projections on their way to their targets (Judas et al. 2005). Also, one cannot exclude phagocytosis of transient axons in these areas. Thus, findings in kittens suggest possible elimination of exuberant trans-callosal projections by microglia during development (Innocenti et al. 1983a,b; Rochefort et al. 2002).
Possible involvement of microglia–macrophages in periventricular white-matter injury in preterm infants
As mentioned in the previous paragraph, the semiovale centre, which contains the microglia–macrophage cluster at the crossroads of axons, is the site that is vulnerable to PWMI in very preterm babies (Judas et al. 2005; Monier et al. 2007). PWMI is the main cause of cerebral palsy in preterm and neonatal infants. Currently, the large increase in preterm births is causing an increase in the number of babies with encephalopathy in neonatal intensive care units (review in Volpe, 2009). Very preterm infants born before 30 gw are at high risk for encephalopathy and about 10% of them develop severe neurodevelopmental sequelae. Moreover, more than 50% of these infants go on to develop cognitive, behavioural, attentional, or socialization deficits of variable severity (Larroque et al. 2008; Volpe, 2009). Although defective myelination has been identified as a major component of white-matter damage (review in Khwaja & Volpe, 2008), a recent study has shown that preoligodendrocytes were still present at PWMI sites in the human brain (Billiards et al. 2008). Pathological studies showed astrogliosis and microglial activation in PWMI (Folkerth, 2005; Verney et al. 2009). The microglia–macrophages may play a role in the lesion via expression of the N-methyl-D-aspartate receptor 1, which we detected on activated macrophages at sites of PWMI (Fig. 7A,B). In parallel, these cells expressed inducible nitric oxide synthase when activated as macrophages (Fig. 7C,D) (Haynes et al. 2009). Expression of interleukin (IL)-1β, IL-2, IL-6, and tumour necrosis factor-α was demonstrated on microglia–macrophages in neonates with PWMI (Yoon et al. 1997; Kadhim et al. 2001, 2002). However, the specific role for microglial–macrophage activation in the pathophysiology of PWMI remains a matter of debate.
Fig. 7.

Cases with no neuropathological lesions [A, 20 gestational weeks (gw); C, 25 gw] and with periventricular white-matter injury (PWMI) (B, 22 gw; D, 26 gw). Fluorescent double labelling for ionized calcium-binding adapter molecule 1 (Iba1) (green) and N-methyl-D-aspartate receptor 1 (NMDAr1) (red) (A,B) and for Iba1 (green) and inductible nitric oxide synthase (iNOS) (red) (C,D) showing co-localization in the amoeboid–intermediate microglia located in the C2 crossroads in the cases with PWMI. In B, note the double-labelled macrophages (yellow, long arrows) and the non-double-labelled (i.e. less activated) microglia (short arrows). Other cells exhibit dot-like red NMDAr1 labelling (asterisk in B) and iNOS labelling (short arrow in D). Scale bars: A–D, 20 μm.
In rodents, activated microglia were detected transiently during early normal postnatal development, within fibre tracts (cingulum and internal and external capsules), in a distribution reminiscent of that found in the normal human brain (Milligan et al. 1991, Baud et al. 2004; Olivier et al. 2005; Monier et al. 2007). In various animal models of PWMI involving excitotoxicity, inflammation, and hypoxia/ischaemia/asphyxia, microglia–macrophage activation was the first cellular event detected in or around the lesion following the insult (Tahraoui et al. 2001; Mallard et al. 2003; Baud et al. 2004; Olivier et al. 2005). In our work, rat pups subjected to experimentally induced prenatal hypoxia were found at birth to have cystic lesions in the cingulum with an early increase in microglia–macrophages. This increase correlated with the increase in the apparent diffusion coefficient detected in the same pups using in-vivo magnetic resonance imaging (Baud et al. 2004).
Taken together, these data suggest that microglia–macrophages may play a role in the pathophysiology of PWMI in preterm infants.
Acknowledgments
This work was supported by grants from the INSERM (French National Institute of Health and Medical Research). We thank F. Narcy, N. Zecevic, Y. Hillion, A.-L. Delezoide, and H. Adle-Biassette for helping us to obtain the postmortem human specimens used for our study.
References
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Ria F, Adorini L. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today. 2000;21:141–147. doi: 10.1016/s0167-5699(99)01512-1. [DOI] [PubMed] [Google Scholar]
- Andjelkovic AV, Nikolic B, Pachter JS, et al. Macrophages/microglial cells in the human central nervous system during development: an immunohistochemical study. Brain Res. 1998;814:13–25. doi: 10.1016/s0006-8993(98)00830-0. [DOI] [PubMed] [Google Scholar]
- Ashwell K. The distribution of microglia and cell death in the fetal rat forebrain. Dev Brain Res. 1991;58:1–12. doi: 10.1016/0165-3806(91)90231-7. [DOI] [PubMed] [Google Scholar]
- Banati RB, Graeber MB. Surveillance, intervention and cytotoxicity: is there a protective role for microglia? Dev Neurosci. 1994;16:114–127. doi: 10.1159/000112098. [DOI] [PubMed] [Google Scholar]
- Baud O, Daire JL, Dalmaz Y, et al. Gestational hypoxia induces white matter damage in neonatal rats: a new model of periventricular leukomalacia. Brain Pathol. 2004;14:1–10. doi: 10.1111/j.1750-3639.2004.tb00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiards SS, Haynes RL, Folkerth RD, et al. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497:199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- Billiards SS, Haynes RL, Folkerth RD, et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 2008;18:153–163. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamak B, Dobbertin A, Mallat M. Immunohistochemical detection of thrombospondin in microglia in the developing rat brain. Neuroscience. 1995;69:177–187. doi: 10.1016/0306-4522(95)00236-c. [DOI] [PubMed] [Google Scholar]
- Chan WY, Kohsaka S, Rezaie P. The origin and cell lineage of microglia: new concepts. Brain Res Rev. 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Choi BH. Hematogenous cells in the central nervous system of developing embryos and fetuses. J Comp Neurol. 1981;196:683–694. doi: 10.1002/cne.901960412. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Martin C, Coltey P, et al. First appearance, distribution and origin of macrophages in the early development of the avian nervous system. J Comp Neurol. 1993;330:113–129. doi: 10.1002/cne.903300110. [DOI] [PubMed] [Google Scholar]
- Dick AD, Pell M, Brew BJ, Foulcher E, Sedgwick JD. Direct ex vivo flow cytometric analysis of human microgial cells CD14 expression: examination of central nervous system biopsy specimens from HIV-scropositive patients with other neurological diseases. AIDS. 1997;11:1699–1708. doi: 10.1097/00002030-199714000-00006. [DOI] [PubMed] [Google Scholar]
- Earle KL, Mitrofanis J. Identification of transient microglial cell colonies in the forebrain white matter of developing rats. J Comp Neurol. 1997;387:371–384. doi: 10.1002/(sici)1096-9861(19971027)387:3<371::aid-cne4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Al Izzi MS, Reading MC. Macrophages, microglial cells, and HLA-DR antigens in fetal and infant brain. J Clin Pathol. 1991;44:102–106. doi: 10.1136/jcp.44.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff S, Zhai R, Novak JP. Microglia and astroglia have a common progenitor cell. J Neurosci Res. 1997;50:477–486. doi: 10.1002/(SICI)1097-4547(19971101)50:3<477::AID-JNR14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Bernet E, Soriano E, et al. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39:451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Folkerth RD. Neuropathologic substrate of cerebral palsy. J Child Neurol. 2005;20:940–948. doi: 10.1177/08830738050200120301. [DOI] [PubMed] [Google Scholar]
- Fujimoto E, Miki A, Mizoguti H. Histochemical study of the differentiation of microglial cells in the developing human cerebral hemispheres. J Anat. 1989;166:253–264. [PMC free article] [PubMed] [Google Scholar]
- Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res. 1987;18:155–171. doi: 10.1002/jnr.490180123. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Howard S. An immunohistological study of macrophages in the human fetal brain. Neuropathol Appl Neurobiol. 1991;17:383–390. doi: 10.1111/j.1365-2990.1991.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: immune network in the CNS. Brain Pathol. 1990;1:2–5. doi: 10.1111/j.1750-3639.1990.tb00630.x. [DOI] [PubMed] [Google Scholar]
- Hao C, Richardson A, Fedoroff S. Macrophage-like cells originate from the neuroepithelium in culture: Characterization and properties of macrophage-like cells. Int J Dev Neurosci. 1991;9:1–14. doi: 10.1016/0736-5748(91)90067-v. [DOI] [PubMed] [Google Scholar]
- Haynes RL, Folkerth RD, Trachtenberg FL, et al. Nitrosative stress and inducible nitric oxide synthase expression in periventricular leukomalacia. Acta Neuropathol. 2009;118:391–399. doi: 10.1007/s00401-009-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hutchins KD, Dickson DW, Kure K, et al. Evidence that microglia in the human brain may be derived from the neurectoderm. J Neuropathol Exp Neurol. 1989;48:342. [Google Scholar]
- Hutchins KD, Dickson DW, Rashbaum WK, et al. Localization of morphologically distinct microglial populations in the developing human fetal brain: implications for ontogeny. Brain Res Dev Brain Res. 1990;55:95–102. doi: 10.1016/0165-3806(90)90109-c. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Koppel H, Clarke S. Transitory macrophages in the white matter of the developing visual cortex: I. Light and electron microscopic characteristics and distribution. Dev Brain Res. 1983a;11:39–53. doi: 10.1016/0165-3806(83)90200-6. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Clarke S, Koppel H. Transitory macrophages in the white matter of the developing visual cortex: II. Development and relations with axonal pathways. Dev Brain Res. 1983b;11:55–66. doi: 10.1016/0165-3806(83)90201-8. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, et al. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Judas M, Rados M, Jovanov-Milosevic N, et al. Structural, immunocytochemical, and mr imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. Am J Neuroradiol. 2005;26:2671–2684. [PMC free article] [PubMed] [Google Scholar]
- Kadhim H, Tabarki B, Verellen G, et al. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- Kadhim H, Tabarki B, De Prez C, et al. Interleukin-2 in the pathogenesis of perinatal white matter damage. Neurology. 2002;58:1125–1128. doi: 10.1212/wnl.58.7.1125. [DOI] [PubMed] [Google Scholar]
- Kershman J. Genesis of microglia in the human brain. Arch Neurol Psychiatry. 1939;41:24–50. [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsmark BW, Sidman RL. Origin of brain macrophages in the mouse. J Neuropathol Exp Neurol. 1963;22:643–676. doi: 10.1097/00005072-196310000-00006. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Molliver ME. A new interpretation of laminar development of cerebral cortex: synaptogenesis in different layers of neopallium in the human fetus. Anat Rec. 1974;178:395. [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Larroque B, Ancel PY, Marret S, EPIPAGE Study Group et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371:813–820. doi: 10.1016/S0140-6736(08)60380-3. [DOI] [PubMed] [Google Scholar]
- Ling EA. The origin and nature of microglia. In: Fedoroff S, Hertz L, editors. Advances in Cellular Neurobiology. New York: Academic; 1981. pp. 33–82. [Google Scholar]
- Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- Mallard C, Welin AK, Peebles D, et al. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res. 2003;28:215–223. doi: 10.1023/a:1022368915400. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, et al. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Milligan CE, Cunningham TJ, Levitt P. Differential immunochemical markers reveal the normal distribution of brain macrophages and microglia in the developing rat brain. J Comp Neurol. 1991;314:125–135. doi: 10.1002/cne.903140112. [DOI] [PubMed] [Google Scholar]
- Monier A, Evrard P, Gressens P, et al. Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J Comp Neurol. 2006;499:565–582. doi: 10.1002/cne.21123. [DOI] [PubMed] [Google Scholar]
- Monier A, Adle-Biassette H, Delezoide A-L, et al. Microglial colonization of developing human cortex. J Neuropathol Exp Neurol. 2007;66:372–382. doi: 10.1097/nen.0b013e3180517b46. [DOI] [PubMed] [Google Scholar]
- Navascues J, Calvente R, Marin-Teva JL, et al. Entry, dispersion and differentiation of microglia in the developing central nervous system. An Acad Bras Cienc. 2000;72:91–102. doi: 10.1590/s0001-37652000000100013. [DOI] [PubMed] [Google Scholar]
- Olivier P, Baud O, Evrard P, et al. Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol. 2005;64:998–1006. doi: 10.1097/01.jnen.0000187052.81889.57. [DOI] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977;278:245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. Eur J Neurosci. 2000;12:2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Rezaie P. Microglia in the human nervous system during development. Neuroembryology. 2003;2:18–31. [Google Scholar]
- Rezaie P. Microglia – from origins to differentiation, distribution and dynamics within the mammalian central nervous system. In: Dheen ST, Ling EA, editors. Trends in Glial Research, Basic and Applied. Kerala: Research Signpost; 2007. pp. 1–41. [Google Scholar]
- Rezaie P, Male D. Colonization of the developing human brain and spinal cord by microglia: A review. Microsc Res Tech. 1999;45:359–382. doi: 10.1002/(SICI)1097-0029(19990615)45:6<359::AID-JEMT4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Male D. Differentiation, ramification and distribution of microglia within the central nervous system examined. Neuroembryology. 2002;1:29–43. [Google Scholar]
- Rezaie P, Cairns NJ, Male DK. Expression of adhesion molecules on human fetal cerebral vessels: relationship to microglial colonisation during development. Brain Res Dev Brain Res. 1997;104:175–189. doi: 10.1016/s0165-3806(97)00153-3. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Dean A, Male D, et al. Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex. 2005;15:938–949. doi: 10.1093/cercor/bhh194. [DOI] [PubMed] [Google Scholar]
- del Rio Hortega P. El “tercer clemento“ de los centros nerviosos. Bol Soc Esp Biol. 1919;9:68–120. [Google Scholar]
- del Rio Hortega P. Microglia. Section X. In: Penfield W, editor. Cytology and Cellular Pathology of the Nervous System. New York: Hoeber; 1932. pp. 481–534. [Google Scholar]
- Rochefort N, Quenech'du N, Watroba L, et al. Microglia and astrocytes may participate in the shaping of visual callosal projections during postnatal development. J Physiol Paris. 2002;96:183–192. doi: 10.1016/s0928-4257(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Chun JJ, Luskin MB. The role of the subplate in the development of the mammalian telencephalon. In: Peters A, Jones EG, editors. Cerebral Cortex. New York: Plenum Press; 1988. pp. 35–58. [Google Scholar]
- Spassky N, de Castro F, Le Bras B, et al. Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J Neurosci. 2002;22:5992–6004. doi: 10.1523/JNEUROSCI.22-14-05992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Tahraoui SL, Marret S, Bodénant C, et al. Central role of microglia in neonatal excitotoxic lesions of the murine periventricular white matter. Brain Pathol. 2001;11:56–71. doi: 10.1111/j.1750-3639.2001.tb00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Miller RH. Glial cell migration directed by axon guidance cues. Trends Neurosci. 2002;25:173–175. doi: 10.1016/s0166-2236(00)02096-8. [DOI] [PubMed] [Google Scholar]
- Verney C, Milosevic A, Alvarez C, et al. Immuno-cytochemical evidence of well-developed dopaminergic and noradrenergic innervations in the frontal cerebral cortex of human fetuses at midgestation. J Comp Neurol. 1993;336:331–344. doi: 10.1002/cne.903360303. [DOI] [PubMed] [Google Scholar]
- Verney C, Fallet-Bianco C, Pogledic I, et al. Characteristics of Glial Cells in Diffuse Versus Focal White Matter Lesions of Preterm Infants. Paris: Euroglia; 2009. [Google Scholar]
- Volpe JJ. The encephalopathy of prematurity – brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 2009;16:167–178. doi: 10.1016/j.spen.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Gwiazda E, Poswinska Z. Morphological study of microglia in human mesencephalon during the development and aging. Folia Neuropathol. 1995;33:77–83. [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lechowicz W, Kosno-Kruszewska E. A morphometric evaluation of morphological types of microglia and astroglia in human fetal mesencephalon. Folia Neuropathol. 1997;35:29–35. [PubMed] [Google Scholar]
- Yoon BH, Romero R, Kim CJ, et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177:406–411. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Verney C. Development of the catecholamine neurons in human embryos and fetuses, with special emphasis on the innervation of the cerebral cortex. J Comp Neurol. 1995;351:509–535. doi: 10.1002/cne.903510404. [DOI] [PubMed] [Google Scholar]


