Abstract
Although renal cell carcinoma (RCC) can metastasize to almost every organ, the most common metastatic sites are the lungs, abdomen, bones and brain. We present a rare case of a 72-year-old male with a large left RCC with simultaneous bilateral adrenal metastasis. In the process of surgical treatment, he underwent left radical nephrectomy with ipsilateral adrenalectomy. Due to the poor general condition of the patient, and also to prevent adrenal insufficiency, the right adrenal mass was preserved, without imposing any hazard to the patient. Systemic immunotherapy was initiated and the patient is still alive 1 year after surgery.
Key Words: Adrenal metastasis, Renal cell carcinoma, Radical nephrectomy, Adrenal insufficiency, Immunotherapy
Introduction
Although renal cell carcinoma (RCC) can metastasize to almost every organ, the most common metastatic sites are the lungs, abdomen, bones and brain. The possibility of bilateral adrenal metastasis (BAM) is very low (<0.5%). The remote but existing risk of developing contralateral adrenal metastasis (CAM) after primary radical nephrectomy supports the idea of sparing the adrenal gland in suitable patients who undergo radical nephrectomy.
Case Presentation
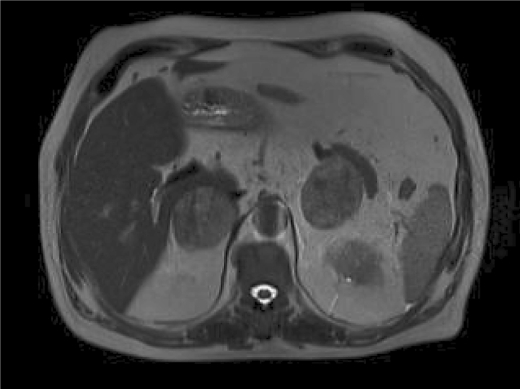
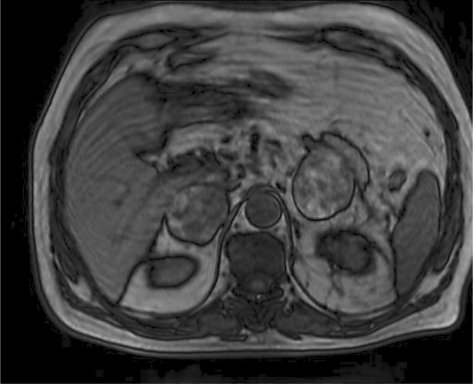
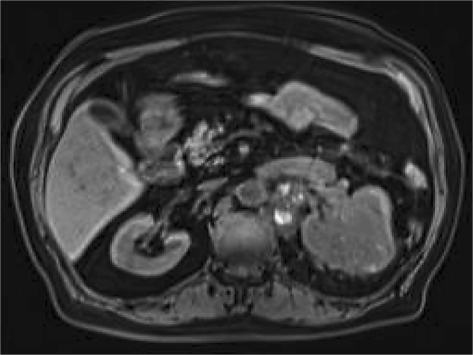
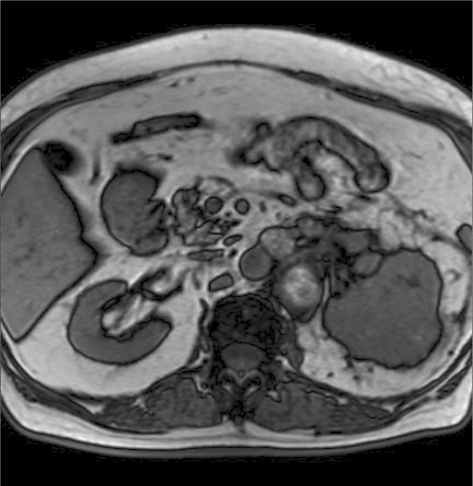
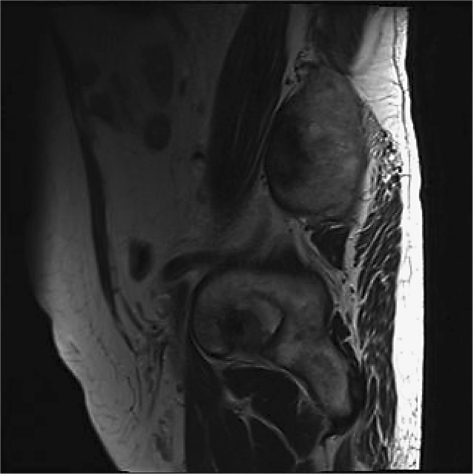
The patient is a 72-year-old Iranian man who was admitted due to a left renal mass. He complained of intermittent gross hematuria in the 6 months before admission. He revealed no history of medical disorders like hypertension or diabetes mellitus. No history of any previous surgery was found. He was an obese man with a body mass index of 28. On physical examination, vital signs were normal and, due to a fatty abdomen, no mass was detectable. No lower extremity edema, varicose veins or peripheral lymphadenopathies were noted. Hematological examination was normal. In the biochemistry evaluation, only a serum creatinine level of 1.7 mg/dl was noted. Liver function test was normal. Chest X-ray was unremarkable. In abdominal ultrasonography, a left renal mass of 14 × 8 × 6.5 cm with bilateral adrenal masses (each 4 × 5 cm) were reported. In abdominal magnetic resonance imaging (MRI), the reported mass with BAM was revealed (fig. 1, fig. 2, fig. 3, fig. 4, fig. 5). The clinical TNM stage of the patient was T3N1M1. The patient was scheduled for left radical nephrectomy. With midline abdominal incision, laparotomy was performed. Standard left radical nephrectomy with resection of the ipsilateral adrenal metastasis with splenectomy (due to adhesions) was performed. Due to the poor general condition of the patient, severe adhesion of the mass, its large size and more than 2,000 ml of bleeding, the resection of the CAM was postponed. The weight of the resected mass was 3,375 g. Postoperative condition of the patient was uneventful. Only a mild elevation of the serum creatinine level to 2.5 mg/dl was seen. He was discharged on the 4th postoperative day in good condition. Pathologic examination of the nephrectomy sample was positive for RCC with adrenal metastasis, clear cell type, Fuhrman nuclear grade 3. The greatest tumor diameter was 9.5 cm. The tumor extends through the renal capsule to the peri-renal adipose tissue. Invasion of the renal vein is seen without any lymph node involvement. Due to adrenal metastasis, tyrosine kinase inhibitors were the recommended first line of treatment, but due to financial constraints, the patient refused their use and we were obligated to prescribe immunotherapy as an every day intramuscular injection of a-interferon (5 million units) for 2 consecutive months, without any major systemic reactions. Due to unknown causes, the patient had proceeded to end stage renal disease 3 months after operation, and he underwent regular hemodialysis after that. Finally, he was alive at the end of the first year after operation.
Fig. 1.
MRI of the abdomen. BAM is evident.
Fig. 2.
MRI of the abdomen. BAM is evident.
Fig. 3.
Abdominal MRI, the left renal mass is evident.
Fig. 4.
Abdominal MRI, the left renal mass is evident.
Fig. 5.
Abdominal MRI, the left renal mass is evident.
Discussion
The behavior of RCC is unpredictable; metastasis may be found synchronously with the primary tumor or in various organs many years after treating the primary RCC [1], following routes of spread and patterns that are not yet fully understood. Adrenal metastasis is a good example, as it can be synchronous or metachronous to the renal neoplasm, ipsilateral, contralateral or bilateral, solitary or part of a diffuse metastatic disease. Direct extension of RCC to the adrenal gland is uncommon. Adrenal metastases may result from either antegrade hematogenous spread or antegrade venous spread, although neither has been proven [2]. Although RCC can metastasize to almost every organ, the most common metastatic sites are the lungs, abdomen, bones and brain [3]. In addition to being able to metastasize to numerous different organs, RCC can recur or metastasize many years after resection of the primary tumor [4].
Whereas metastasis of RCC to different sites is not uncommon, CAM is rare. In one autopsy study of >400 patients who had undergone nephrectomy for RCC, the contralateral adrenal gland was the sole site of metastatic involvement in only 2.5% [3]. In another retrospective study of a large series [5], 610 radical nephrectomy cases were studied. The incidence of CAM was 1.1% (7/610 patients), while the incidence of ipsilateral metastasis was 3.4%. In 3 of 7 cases (0.5%), BAM occurred simultaneously with primary RCC in the kidney. In another study by Antonelli et al. [6], in over 1,179 patients the incidence of adrenal metastasis was 3.7%. In particular, the metastasis was synchronous in 2.7%, metachronous in 1%, ipsilateral in 1.9%, contralateral in 1.5%, and bilateral in 0.2% (2/914) of patients. The histological subtype of the adrenal metastasis was always concordant with that of the renal neoplasm. We postponed contralateral adrenalectomy due to the poor general condition of the patient at the time of surgery and also the fear of adrenal insufficiency, without increasing any risk of bilateral adrenal insufficiency to the patient. There were no differences in survival between patients with synchronous or metachronous appearance of adrenal metastasis, or between those with a uni-, contra- or bilateral metastasis [6]. Plawner [7] showed that the 5-year survival of patients operated on for asynchronous solitary RCC metastases to the contralateral adrenal gland was lower than that for patients with synchronous adrenal metastases, namely 20 and 40%, respectively. Patients in whom the contralateral gland is affected are dependent on hormone replacement following bilateral adrenal removal. The post-adrenalectomy state created is associated with occasional morbidity and, perhaps, even mortality due to acute adrenal insufficiency [8]. Although hormone replacement therapy does not place a burden on young patients, it may become more relevant in later life when compliance becomes relatively poor [9]. The remote but existing risk of developing CAM after primary radical nephrectomy supports the idea of sparing the adrenal gland in suitable patients who undergo radical nephrectomy. In a prospective study, ipsilateral adrenal involvement (IADI) during radical nephrectomy was reported in 2% of cases [10]. Thus, the need for routine adrenalectomy during radical nephrectomy has been questioned, especially because the risk of an ipsilateral tumor developing after adrenal-sparing nephrectomy is low [10].
Some patients benefit from contralateral adrenalectomy for metastatic RCC, and previously published experience also indicates that the surgical treatment of contralateral RCC metastasis is worthwhile in selected patients [11, 12]. The available information on the outcome of the surgical treatment of metastatic RCC to the contralateral adrenal gland is limited and incomplete [13]. Adjuvant treatment may improve the outcome. Patients with IADI had significantly larger primary tumors, higher pT stages and histologic grades, and higher percentages of upper pole involvement, microvascular invasion, spindel-cell-type tumors, lymph node metastasis (LNM), and distant metastasis (DM) outside the ipsilateral adrenal gland (IAd) than control patients [14]. Multivariate analysis revealed that tumor size greater than 5.5 cm, pT stage of 3 or higher, LNM, and DM outside IAd, but not upper pole involvement, were significant predictors of IADI [14]. In the Moudouni et al. study [15] on 15 patients with adrenal involvement in RCC, tumor stage correlated with probability of adrenal spread, with T3-4 and T1-2 accounting for 13.4 and 0.9% of cases, respectively. Upper pole intrarenal RCC was most likely to spread with local extension to the adrenal gland, representing 53.3% of adrenal involvement.
In contrast, multifocal, lower pole and mid region RCC tumors metastasized hematogenously, representing 21.4, 7 and 14% of adrenal metastasis, respectively. The morbidity of adrenalectomy is minimal except in those patients with synchronous CAM in whom the impact of adrenal insufficiency can be devastating. Disease-specific and overall survival of those undergoing radical nephrectomy, with or without adrenalectomy, are similar. The survival of patients with widespread metastatic disease is historically poor regardless of whether adrenalectomy is performed. There is evidence for a survival advantage in patients with isolated adrenal metastasis, although this group comprises no more than 2% of those undergoing surgery for renal tumors [16].
On the basis of the study by Onishi et al. [17], patients who underwent the operation on the contralateral adrenal gland showed an unfavorable survival. Regarding the combination treatment with surgery, patients who received the interferon and interleukin-2 treatment showed a favorable survival. Several studies suggest that metastatic RCC (not by direct extension) in the IAd should be regarded as stage m+ (DM) and that the need and benefit of ipsilateral adrenalectomy during surgery for RCC are extremely limited [18, 19]. On the basis of the Yokoyama and Tanaka study [20], unilateral adrenalectomy might cause an irreversible impairment of the reserve of adrenocortical function, so unconditional ipsilateral adrenalectomy with radical nephrectomy for RCC should be avoidable, and thus preserve the reserve of adrenocortical function, as preoperative imaging, especially thin-slice multidetector helical computed tomography, can detect adrenal involvement with RCC in most cases.
Conclusion
In a prospective study, IADI during radical nephrectomy was reported in 2% of cases [10]. Thus, the need for routine adrenalectomy during radical nephrectomy has been questioned, especially because the risk of an ipsilateral tumor developing after adrenal-sparing nephrectomy is low [10]. Sparing the IAd in radical nephrectomy would avoid the risk of adrenal insufficiency if the development of a tumor necessitates removing the contralateral gland, either at the time of nephrectomy or later [13]. Furthermore, the metastatic organs and the application of cytokines must be considered in the treatment of advanced RCC. In spite of general advise about the removal of adrenal masses (especially metachronous ones) we preserved the right adrenal mass to prevent the need for glucocorticoid and mineralocorticoid substitution therapy and to decrease the surgical risk for the patient. Furthermore, with initiation of post-surgical interferon therapy, the expected prognosis of the patient did not change.
References
- 1.Mesurolle B, Mignon F, Travagli JP, Meingan P, Vanel D. Late presentation of solitary contralateral adrenal metastasis of renal cell carcinoma. Eur Radiol. 1997;7:557–558. doi: 10.1007/s003300050204. [DOI] [PubMed] [Google Scholar]
- 2.Ertl CW, Darras FS. Solitary metachronous contralateral adrenal metastasis from renal cell carcinoma. Urology. 1999;54:162. doi: 10.1016/s0090-4295(98)00565-2. [DOI] [PubMed] [Google Scholar]
- 3.Saitoh H, Nakayama M, Nakamura K, Satoh T. Distant metastasis of renal adenocarcinoma in nephrectomized cases. J Urol. 1982;127:1092–1095. doi: 10.1016/s0022-5347(17)54243-3. [DOI] [PubMed] [Google Scholar]
- 4.O'Dea MJ, Zincke H, Utz DC, Bernatz PE. The treatment of renal cell carcinoma with solitary metastasis. J Urol. 1978;120:540–542. doi: 10.1016/s0022-5347(17)57264-x. [DOI] [PubMed] [Google Scholar]
- 5.von Knoblosh R, Hegele A, Kalble T, Hofmann R. Management of contralateral adrenal metastasis from renal cell carcinoma: possibility of inferior vena cava tumor thrombus. Scand J Urol Nephrol. 2000;34:109–113. doi: 10.1080/003655900750016715. [DOI] [PubMed] [Google Scholar]
- 6.Antonelli A, Cozzoli A, Simoene C, Zani D, Zanotelli T, Portesi E, Cosciani Cunico S. Surgical treatment of adrenal metastasis from renal cell carcinoma: a single-centre experience of 45 patients. BJU Int. 2006;97:505–508. doi: 10.1111/j.1464-410X.2006.05934.x. [DOI] [PubMed] [Google Scholar]
- 7.Plawner J. Results of surgical treatment of kidney cancer with solitary metastasis to contralateral adrenal. Urology. 1991;37:223–236. doi: 10.1016/0090-4295(91)80291-e. [DOI] [PubMed] [Google Scholar]
- 8.Kessler OJ, Mukamel E, Weinstein R, Gayer E, Konichezky M, Servadico C. Metachronous renal cell carcinoma metastasis to the contralateral adrenal gland. Urology. 1998;51:539–543. doi: 10.1016/s0090-4295(97)00698-5. [DOI] [PubMed] [Google Scholar]
- 9.O'Riordain DS, Farley DR, Young WF, Jr, Grant CS, van Heerden JA. Long-term outcome of bilateral adrenalectomy in patients with Cushing's syndrome. Surgery. 1994;116:1088–1094. [PubMed] [Google Scholar]
- 10.Kletscher BA, Qian J, Bostwick DG, Blute ML, Zincke H. Prospective analysis of the incidence of ipsilateral adrenal metastasis in localized renal cell carcinoma. J Urol. 1996;155:1844–1846. [PubMed] [Google Scholar]
- 11.Stein A, Mecz Y, Sova Y, Lurie M, Lurie A. Synchronous and metachronous contralateral adrenal metastases from primary renal cell carcinoma. Urol Int. 1997;58:58–60. doi: 10.1159/000282949. [DOI] [PubMed] [Google Scholar]
- 12.Barnes RD, Abratt RP, Cant PJ, Dent DM. Synchronous contralateral adrenal metastasis from renal cell carcinoma: a 7 year survival following resection. Aust NZ J Surg. 1995;65:540–541. doi: 10.1111/j.1445-2197.1995.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 13.Lau WK, Zincke H, Lohse CM, Cheville JC, Weaver AL, Blute ML. Contralateral adrenal metastasis of renal cell carcinoma: treatment, outcome and a review. BJU Int. 2003;91:775–779. doi: 10.1046/j.1464-410x.2003.04237.x. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Nakazawa H, Marumo K, Ozono S, Igarashi T, Shinohara N, Fukuda M, Tsushima T, Naito S, Hayakawa M. Risk factors for ipsilateral adrenal involvement in renal cell carcinoma. Urology. 2008;72:354–358. doi: 10.1016/j.urology.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 15.Moudouni SM, En-Nia I, Patard JJ, Manunta A, Guille F, Lobel B. Real indications for adrenalectomy in renal cell carcinoma. Scand J Urol Nephrol. 2002;36:273–277. doi: 10.1080/003655902320248236. [DOI] [PubMed] [Google Scholar]
- 16.O'Malley RL, Godoy G, Kanofsky JA, Taneja SS. The necessity of adrenalectomy at the time of radical nephrectomy: a systematic review. J Urol. 2009;181:2009–2017. doi: 10.1016/j.juro.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Onishi T, Ohishi Y, Iizuka N, Shirakawa H, Hatano T, Makino H, Tomita M. Surgical treatment of advanced renal cell carcinoma. Nippon Hinyokika Gakkai Zasshi. 1995;86:1505–1513. doi: 10.5980/jpnjurol1989.86.1505. [DOI] [PubMed] [Google Scholar]
- 18.Shalev M, Cipolia B, Guille F, Staerman F, Lobel B. Is ipsilateral adrenalectomy a necessary component of radical nephrectomy? J Urol. 1995;153:1415–1417. [PubMed] [Google Scholar]
- 19.Sandock DS, Seftel AD, Resnick MI. Adrenal metastases from renal cell carcinoma: role of ipsilateral adrenalectomy and definition of stage. Urology. 1997;49:28–31. doi: 10.1016/S0090-4295(96)00388-3. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama H, Tanaka M. Incidence of adrenal involvement and assessing adrenal function in patients with renal cell carcinoma: is ipsilateral adrenalectomy indispensable during radical nephrectomy? BJU Int. 2005;95:526–529. doi: 10.1111/j.1464-410X.2005.05332.x. [DOI] [PubMed] [Google Scholar]