Abstract
In this observational study, we compared erythrocyte membrane fatty acids in infants consuming formula supplemented with docosahexaenoic acid (DHA) and arachidonic acid (ARA) with those consuming other types of milks. In 110 infants who were participants in a cohort study of otherwise healthy children at risk for developing type 1 diabetes, erythrocytes were collected at approximately 9 months of age, and fatty acid content was measured as a percentage of total lipids. Parents reported the type of milk the infants consumed in the month of and prior to erythrocyte collection: infant formula supplemented with ARA and DHA (supplemented formula), formula with no ARA and DHA supplements (non‐supplemented formula), breast milk, or non‐supplemented formula plus breast milk. Membrane DHA (4.42 versus 1.79, P < 0.001) and omega‐3 fatty acid (5.81 versus 3.43, P < 0.001) levels were higher in infants consuming supplemented versus non‐supplemented formula. Omega‐6 fatty acids were lower in infants consuming supplemented versus non‐supplemented formula (26.32 versus 29.68, P = 0.023); ARA did not differ between groups. Infants given supplemented formula had higher DHA (4.42 versus 2.81, P < 0.001) and omega‐3 fatty acids (5.81 versus 4.45, P = 0.008) than infants drinking breast milk. In infants whose mothers did not receive any dietary advice, use of supplemented formula is associated with higher omega‐3 and lower omega‐6 fatty acid status.
Keywords: arachidonic acid, docosahexaenoic acid, breastfeeding, infant feeding, infant formula, infant feeding behavior
Introduction
Docosahexaenoic acid (DHA; 22:6ω3) and arachidonic acid (ARA; 20:4ω6) are two long‐chain polyunsaturated fatty acids (LCPUFA) that are important in infant development of neural functioning (Innis 1991; Youdim et al. 2000) and visual acuity (Neuringer et al. 1988). Linoleic acid (LA: 18:2ω6) and α‐linolenic acid (ALA; 18:3ω3) are the parent compounds of ARA and DHA, respectively. Both LA and ALA are considered to be essential fatty acids because they cannot be produced endogenously by humans. LA is commonly found in nut, seed, and vegetable oils and is the most abundant omega‐6‐fatty acid in the diet. ALA is the most common omega‐3‐fatty acid in the Western diet and is found in green leafy vegetables, nut, seed, and vegetable oils.
In the past decade, extensive research has been done regarding LCPUFA and infant diets. While human breast milk is a source of DHA and ARA, until recently, traditional infant formulas had not been a good source of DHA and ARA because formulas had only been required to be supplemented with LA, although many were supplemented with both LA and ALA (US Food and Drug Administration 2004). As recent data indicate that 33% of newborns are formula‐fed at birth, and increasing to 69% of infants by 6 months of age (Beck et al. 2002; Li et al. 2002), levels of ARA and DHA in the infant diet are of concern.
In 2001, many companies started supplementing their formulas with varying levels of DHA and ARA, in order to more closely resemble breast milk. Numerous studies have looked at the safety and efficacy of DHA and ARA supplementation, and its impact on growth and development or visual acuity, in randomized controlled trials of term (reviewed in Wright et al. 2006; Rosenfeld et al. 2009; Simmer et al. 2008a), and pre‐term infants (Innis et al. 2002; Clandinin et al. 2005), and reviewed in Simmer et al. (2008b). However, no studies have examined the changing fatty acid status of consumption of formulas supplemented with DHA and ARA in a population that is not selected to be in a controlled intervention and environment, where the participants are free to choose their own method of feeding. The Diabetes and Autoimmunity Study in the Young (DAISY) is an observational longitudinal cohort study that is following otherwise healthy infants who are at increased risk for developing type 1 diabetes and who were recruited between 1993 and 2004. Omega‐3 fatty acid status in this population is particularly important because it is a marker of bioavailability of fatty‐acid supplements (Innis & Hansen 1996; Vidgren et al. 1997; Clandinin et al. 2005) and has been associated with diabetes‐related autoimmunity (Norris et al. 2007) as well as other illnesses (reviewed in Assisi et al. 2006; Breslow 2006; Calder 2006). The results of this study could be used to infer from larger population surveys where it is only possible to obtain self‐reported information on formula use, that those infants reported to be consuming DHA and ARA supplemented formulas actually have a corresponding difference in membrane fatty acid status than those infant consuming non‐supplemented formulas.
Nursing mothers in DAISY were not given dietary advice nor were parents advised to select certain types of formula. Because the supplemented formulas became available for purchase during the time period that DAISY was recruiting newborns, we were in the unique position to closely observe children whose parents chose to give them non‐supplemented or supplemented formulas. In this study, we measured the fatty acid content of erythrocytes in DAISY infants to determine if we could detect differences in fatty acid status between children consuming supplemented formula and those consuming non‐supplemented formula or breast milk as their sole source of milk.
Key messages
-
•
This study compares erythrocyte membrane fatty acids in infants consuming formula supplemented with docosahexaenoic acid (DHA) and arachidonic acid (ARA) with those consuming other types of milks.
-
•
Infants consuming supplemented formula had significantly higher levels of DHA, omega‐3, and lower levels of omega‐6 fatty acids than infants consuming non‐supplemented formula.
-
•
There was no significant difference between levels of ARA in infants consuming supplemented formula versus non‐supplemented formula.
-
•
Infants consuming supplemented formula had significantly higher levels of DHA and omega‐3 fatty acids than infants consuming breast milk.
-
•
In this observational study, infants fed supplemented formula had higher omega‐3 and lower omega‐6 fatty acid levels.
Materials and methods
Study population
The DAISY is a prospective study of two groups of young children at increased risk for developing type 1 diabetes (Rewers et al. 1996). One group consists of unaffected first‐degree relatives of patients with type 1A diabetes, identified and recruited between birth and 8 years of age through the Barbara Davis Center for Childhood Diabetes in Denver, Colorado, other diabetes care clinics and the Colorado IDDM Registry (Kostraba et al. 1992). The second group consists of babies born at St. Josephs Hospital in Denver, who were screened for diabetes‐susceptibility alleles in the human leucocyte antigen (HLA) region. The St. Josephs Hospital newborn population is representative of the general population of the Denver Metropolitan area. An umbilical cord blood sample was sent to Roche Molecular Systems, Inc. (Alameda, CA, USA) for PCR‐based HLA class II typing. The details of the newborn screening (Rewers et al. 1996) and follow‐up (Norris et al. 2003) have been published elsewhere. The Colorado Multiple Institutional Review Board approved all study protocols. Informed consent was obtained from the parents of each study participant.
Infant diet data
Infant diet data were collected during telephone or face‐to‐face interviews at 3, 6, 9, 12, and 15 months of age. At each interview, mothers were asked to report all types of milk, formulas, foods, vitamins, and other dietary supplements that the infant consumed over the previous 3 months. If a particular item was introduced for the first time during that 3‐month interval, the mother was asked to report the date at first introduction. Breastfeeding initiation and termination were also recorded. The frequency of consumption of each food/milk, in terms of number of servings/feedings per day, was also recorded. DAISY did not collect dietary intake data from mothers who were breastfeeding. No dietary advice was given to the families.
Infant milk feeding groups
Of the children in DAISY from whom we had obtained erythrocytes between the ages of 6 months and 1 year of age (mean, 9.4 months; range, 5.5–12.0 months), we selected 110 infants who could be divided among four groups based on the type of milk product consumed in the month of and 1 month just prior to erythrocyte collection. The four infant milk feeding groups, as reported by the parent were: (1) DHA and ARA supplemented formula (n = 21); (2) non‐supplemented formula as the only source of milk (n = 49); (3) breast milk as the only source of milk (n = 20); and (4) both breast milk and non‐supplemented formula (i.e. formula that was not supplemented with DHA and ARA) (n = 20). In order for an infant to be included in the group consuming both breast milk and non‐supplemented formula (group 4), the parent had to report an average of at least one serving each of breast milk and formula per day. We did not distinguish between protein source (cow's milk or soy) of the infant formula. There were three different brands of supplemented formula fed to the 21 infants: 17 infants were fed one brand (with a current formulation of 34 mg of ARA and 17 mg of DHA per 100 calories), while 3 were fed the second brand (with a current formulation of 21.6 mg of ARA and 8.1 mg of DHA per 100 calories), and 1 infant was fed the third brand (with a current formulation of 34 mg of ARA and 17 mg of DHA per 100 calories). We did not distinguish between varying levels of DHA and ARA in the different brands of formula in our analysis. None of the 110 children in our study had received fish oil or any other fatty acid supplement in the month of, or the month prior to, the erythrocyte collection. While we collected frequency data on intake of general food groups such as meat and vegetables, the data were not specific enough to quantify foods containing varying amounts of omega 3 or 6 fatty acids.
Erythrocyte membrane fatty acid composition
On collection at each clinic visit, erythrocytes from the blood sample were separated within 30 min of standard blood draw, flash frozen in liquid nitrogen and stored at –70°C. Samples were shipped to the University of Florida laboratories of M. Clare‐Salzler and N. J. Szabo, where they were extracted for lipids following the method developed by Bligh and Dyer (1959), and stored at –20°C in sealed cryotubes following flushing with nitrogen gas. The fatty acids present in the lipid isolates were subsequently methylated using the base‐catalysed procedures by Maxwell and Marmer (1983) in preparation for analysis by gas chromatography (Hewlett‐Packard 6890) with mass spectrometric detection (Hewlett‐Packard 5973). Sample components, separated across a CP‐WAX column (25 m × 0.25 mm i.d., 0.2 µm film; Varian, Palo Alto, CA, USA), were identified by comparing the retention times and m/z of selected ions from fatty acids in the samples to those of authentic standards (NuCheckPrep, Elysian, MN, USA; Supelco, St. Louis, MO, USA). Quantitation was determined against five‐point standard curves and reported as percent of total lipids (g fatty acid/100 g RBC lipid).
The total erythrocyte membrane omega‐3 fatty acid percentage was calculated by summing the following: ALA, DHA, Eicosapentaenoic acid (EPA, c20:5ω3), Eicosatrienoic acid (ETE, c20:3ω3), and Docosapentaenoic acid (DPA, c22:5ω3). The total erythrocyte membrane omega‐6 fatty acid was calculated by summing the following: LA, ARA, γ‐Linolenic acid (c18:3ω6), Eicosadienoic acid (c20:2ω6), dihomo‐γ‐Linolenic acid (c20:3ω6) and Docosatetraenoic acid (c22:4ω6).
Statistical analysis
All statistical analyses were conducted using SAS 9.1 (SAS, Cary, NC, USA). Analysis of variance and chi‐square tests of heterogeneity were used to test whether maternal age, HLA‐DR3/4 genotype positivity, first‐degree relative diabetes status, and level of maternal education (some college vs. less) varied among the four feeding groups. Student's t‐tests were used to test whether fatty acid level differed by HLA‐DR3/4 genotype positivity, first degree relative diabetes status, and level of maternal education.
To test the potential presence of a difference between mean membrane fatty acid level and feeding group, the data were analysed with both a non‐parametric Wilcoxon rank sum test and a parametric analysis of variance test. Because the two tests gave comparable results (data not shown), all further analyses were carried out using a parametric analysis of variance test, which allowed adjustment for covariates. Because both high‐risk HLA‐DR3/4 genotype and having a first‐degree relative with type 1 diabetes were selection variables in DAISY, and could influence parental decisions about breastfeeding or selection of formula due to the presumed effect of infant diet on diabetes risk, we adjusted for both of these variables when examining the association between fatty acid level and feeding group. To correct for the number of pairwise comparisons, a Bonferroni correction was performed for each membrane fatty acid.
Fatty acid levels are reported as percent of total fatty acids. For ease of reporting in the text, we report these values as numbers, not as percentages.
Results
Characteristics of the study population by feeding group are shown in Table 1. There were no significant differences between any of the feeding groups for age at blood draw, gender, ethnicity, presence of type 1 diabetes in first degree relative of the DAISY child or HLA‐DR3/4 positivity. While maternal age and maternal education differed by feeding group (Table 1), the erythrocyte fatty acid content did not differ by these characteristics (data not shown).
Table 1.
Characteristics of study population by feeding group
| Feeding group | P‐value* | ||||
|---|---|---|---|---|---|
| Supplemented formula | Non‐supplemented formula | Breast milk | Breast milk and non‐supplemented formula | ||
| (n = 21) | (n = 49) | (n = 20) | (n = 20) | ||
| Age at blood draw, in months | 9.45 (0.79) | 9.41 (1.11) | 9.41 (0.60) | 9.19 (0.39) | 0.77 |
| Percent female | 52.4% | 36.7% | 45.0% | 30.0% | 0.46 |
| Percent non‐Hispanic White | 66.7% | 73.5% | 75.0% | 65.0% | 0.84 |
| Maternal age, in years | 30.83 (4.35) | 29.04 (5.62) | 32.06 (4.14) | 32.49 (3.38) | 0.02 |
| Percent of mothers with some college education | 61.9% | 71.4% | 90.0% | 100% | 0.008 |
| Percent of children with first degree relative with type 1 diabetes | 42.9% | 55.1% | 70.0% | 30.0% | 0.06 |
| Percent positive for HLA DR3/4 | 12.0% | 20.4% | 20.0% | 15.0% | 0.71 |
Values are mean (SD) or percent. From analysis of variance or chi square test of heterogeneity.
The unadjusted mean levels of erythrocyte membrane fatty acids and the Bonferroni‐corrected P‐values from the analysis adjusted for HLA‐DR3/4 genotype positivity and first‐degree relative diabetes status, are presented in Fig. 1. Erythrocyte membrane DHA levels were higher in infants fed supplemented formula (mean = 4.42) compared with those fed non‐supplemented formula (mean = 1.79; P < 0.001) (Fig. 1A). Children fed breast milk (mean = 2.81) had higher levels of DHA than children fed non‐supplemented formula (P < 0.001), but lower DHA levels than those fed supplemented formula (P < 0.001). Children in the breast milk and non‐supplemented formula feeding group showed similar relationships (Fig. 1A). Mean total omega‐3 fatty acids in erythrocyte membranes were higher in infants fed supplemented formula (mean = 5.81) compared with those fed non‐supplemented formula (mean = 3.43; P < 0.001) (Fig. 1B). Children in the breast milk feeding group had higher levels of omega‐3 fatty acids (mean = 4.45) than children in the non‐supplemented formula group (P = 0.04), but lower omega‐3 fatty acid levels than those in the supplemented formula group (P = 0.008) (Fig. 1B).
Figure 1.
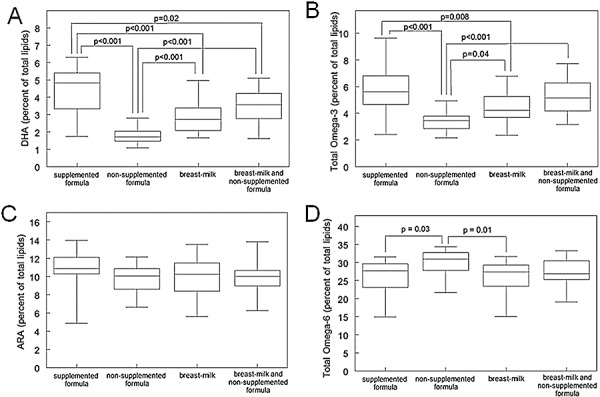
Box plots showing the percent of erythrocyte membrane fatty acid levels in DHA (A), total omega‐3 fatty acids (B), ARA (C), and total omega‐6 fatty acids (D), for the following feeding types: DHA and ARA supplemented formula, (n = 21), non‐supplemented formula as the only source of milk, (n = 49), breast milk as the only source of milk, (n = 20), and both breast milk and non‐supplemented formula, (n = 20). Boxes represent the 25th to 75th percentile of the data. The mean is represented by the line through each box. Lower whiskers represent the 5th to 25th percentile of the data, while upper whiskers represent the 75th to 95th percentile of the data. In each chart, lines connecting a pair of box plots indicate that there is significant difference between them. The P‐value for the pair is above. All P‐values were Bonferroni corrected for 6 tests. Reported P‐values are adjusted for HLA‐DR 3/4 genotype positivity and first‐degree relative diabetes status, while means and standard deviations are unadjusted.
While the mean ARA was higher in the supplemented formula group (mean = 10.74) compared with the non‐supplemented formula (mean = 9.80) and the breast milk (mean = 9.89) feeding groups, these differences were not significant (Fig. 1C). Total omega‐6 fatty acid levels were lower in infants fed supplemented formula (mean = 26.32) than those fed non‐supplemented formula (mean 29.68; P = 0.03) (Fig. 1D). Omega‐6 fatty acid levels were lower in infants fed breast milk (mean = 25.86) compared with infants fed non‐supplemented formulas (P = 0.01) (Fig. 1D).
Discussion
Several studies have examined the relationships between supplemented infant formula, omega‐3 and omega‐6 fatty acid status, and infant development, pre‐term infant growth, and visual acuity. However, most of these studies have been conducted in a clinical trial setting where infants are randomly assigned to receive a certain type of formula. Under these circumstances, the setting is controlled and may not represent what would happen in subjects in a community who are not selected for clinical trials. The DAISY study has the advantage of being an observational study, where mothers are given no advice on their diet or on what to feed their infants. From the time that DHA and ARA supplemented formulas became available on the market, we have been able to observe the choices in feeding patterns that DAISY mother's made. Use of supplemented formulas has increased in DAISY from 3% of infants in 2001 to 36% in 2002 to 70% of infants in 2005 (J. Norris, unpublished data). In the beginning, mothers likely chose to use ARA and DHA supplemented formulas because they believed them to be healthier. Later on, use of supplemented formulas became less of a parental choice and more of an availability issue, as supplemented formulas became a larger and larger proportion of formulas on the market in the United States.
The efficacy of DHA and ARA supplemented formulas has been a controversial issue for many years. While the formulas are considered to be safe and are generally well‐tolerated (Birch et al. 1998; Lucas et al. 1999; 2006, 2008), studies show mixed results as far as increases in development or visual acuity (reviewed in Wright et al. 2006; Rosenfeld et al. 2009; 2008a, 2008b). Our study shows that infants who are fed supplemented formula have higher levels of DHA and omega‐3 fatty acids in erythrocyte membranes than infants fed non‐supplemented formulas. Omega‐3 fatty acid content of erythrocyte membranes is relevant because it is a marker of bioavailability of fatty acid supplements (Innis & Hansen 1996; Vidgren et al. 1997; Clandinin et al. 2005), and has been associated with risk of diabetes‐related autoimmunity (Norris et al. 2007), cardiovascular disease, chronic inflammatory diseases and mental health illnesses (reviewed in Assisi et al. 2006; Breslow 2006; Calder 2006).
We also found that even though supplemented formulas have higher amounts of both DHA and ARA, the erythrocyte membrane ARA content was not increased in children drinking supplemented formulas. In addition, levels of omega‐6 fatty acids were actually lower in infants that were fed supplemented formula compared with those fed non‐supplemented formula. This may be explained by the competitive nature of these two fatty acids, where, when both are available, DHA is preferentially incorporated into the membrane over ARA. Therefore, diets where the omega‐3 to omega‐6 ratio is high result in increased omega‐3 and DHA erythrocyte fatty acid content (Wander & Patton 1991; Romon et al. 1995). In our study, the infants fed supplemented formula were likely consuming higher levels of DHA from the formula, which was preferentially incorporated, leading to the lower levels of omega‐6 fatty acids.
In our population, the levels of DHA and omega‐3 fatty acids in erythrocyte membranes of the infants fed supplemented formula were higher than that of children who received breast milk as their sole source of milk. This is interesting because one of the purposes of supplementing infant formulas with DHA and ARA was to make formulas more similar to breast milk in their fatty acid composition. As this specific comparison was not the main focus of our study, our findings are difficult to interpret, because we did not measure the levels of ARA and DHA in breast milk given to the child, nor did we obtain maternal diet data, which is an important determinant of the levels ARA and DHA in breast milk. Additional work in this area may be important in assessing the need for and benefits of increasing the intake of DHA (either via supplements or dietary change) of lactating women.
As this was an observational study, we did not control or limit the other non‐milk sources of omega‐3 and omega‐6 fatty acids in the diets of our study population. While we confirmed via parental report that none of the children in our study were taking dietary fatty acid supplements, the children may have been eating foods that had varying levels of omega‐3 or omega‐6 fatty acids. We do not believe that this is a serious limitation, as most 9‐month‐old infants would be unlikely to eat large quantities of any of the common foods high in omega‐3 and omega‐6 fatty acids, such as fish, oils and meats. We also did not collect information on the cost of supplemented versus non‐supplemented formula, or the receipt of WIC (Women, Infants, and Children) supplements by mothers. Both of these variables might have influenced which type of formula parents chose to purchase. In addition to the cost of formula, there is the issue of availability of supplemented versus non‐supplemented formula, which may have changed over the course of the study. When DHA/ARA supplemented formulas were first introduced, they were fairly rare (only one or two brands were available, initially). As time passed, these formulas became more common‐place, and non‐supplemented formulas became more rare (e.g. it is currently very difficult to find non‐supplemented formulas in stores). The availability may also have influenced cost, with the supplemented formulas being more expensive when they were initially released, but decreasing in price as they became more commonplace. Due to the observational nature of this study, we also did not measure any functional outcomes, such as development or visual acuity. However, other than an increased risk for development of type 1 diabetes, the infants in this study were generally healthy, and we found it more feasible to measure the intermediate outcome of fatty acid status, rather than less frequent poor outcomes.
In conclusion, in healthy infants whose mothers did not receiving any dietary advice, those who were fed DHA and ARA supplemented formulas had higher erythrocyte membrane levels of DHA and total omega‐3 fatty acids than infants who drank non‐supplemented formula or breast milk alone. The results of this study may provide potentially useful data on a population or community basis; and may support survey studies that are trying to infer the benefit of DHA and ARA supplementation of formula, without having to test a biomarker. Additionally, this study may provide important surrogate for fatty acid status in studies for which only data on infant diet are available. Studies such as these are important, as they are similar to the need to translate excellent randomized clinical trial results to effects that can be observed in the community.
Source of funding
This research was supported by NIH grants R01‐DK49654, R01‐DK32493, and the Diabetes Endocrine Research Center, Clinical Investigation & Bioinformatics Core P30 DK 57516.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors would like to acknowledge the dedicated and talented staff of the DAISY study for their clinical, data and laboratory support. We are indebted to all the children and their families who generously volunteered their time and knowledge.
References
- Assisi A., Banzi R., Buonocore C., Capasso F., Di M., Michelacci V. et al. (2006) Fish oil and mental health: the role of n‐3 long‐chain polyunsaturated fatty acids in cognitive development and neurological disorders. International Clinical Psychopharmacology 21, 319–336. [DOI] [PubMed] [Google Scholar]
- Beck L.F., Morrow B., Lipscomb L.E., Johnson C.H., Gaffield M.E., Rogers M. et al. (2002) Prevalence of selected maternal behaviors and experiences, Pregnancy Risk Assessment Monitoring System (PRAMS), 1999. MMWR Surveillance Summaries 51, 1–27. [PubMed] [Google Scholar]
- Birch E.E., Hoffman D.R., Uauy R., Birch D.G. & Prestidge C. (1998) Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatric Research 44, 201–209. [DOI] [PubMed] [Google Scholar]
- Bligh E.G. & Dyer W.J. (1959) A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Breslow J.L. (2006) n‐3 Fatty acids and cardiovascular disease. American Journal of Clinical Nutrition 83, S1477–S1482. [DOI] [PubMed] [Google Scholar]
- Calder P.C. (2006) n‐3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. American Journal of Clinical Nutrition 83, S1505–S1519. [DOI] [PubMed] [Google Scholar]
- Clandinin M.T., Van Aerde J.E., Merkel K.L., Harris C.L., Springer M.A., Hansen J.W. et al. (2005) Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. Journal of Pediatrics 146, 461–468. [DOI] [PubMed] [Google Scholar]
- Hoffman D.R., Wheaton D.K., James K.J., Tuazon M., Diersen‐Schade D.A., Harris C.L. et al. (2006) Docosahexaenoic acid in red blood cells of term infants receiving two levels of long‐chain polyunsaturated fatty acids. Journal of Pediatric Gastroenterology and Nutrition 42, 287–292. [DOI] [PubMed] [Google Scholar]
- Hoffman D., Ziegler E., Mitmesser S.H., Harris C.L. & Diersen‐Schade D.A. (2008) Soy‐based infant formula supplemented with DHA and ARA supports growth and increases circulating levels of these fatty acids in infants. Lipids 43, 29–35. [DOI] [PubMed] [Google Scholar]
- Innis S.M. (1991) Essential fatty acids in growth and development. Progress in Lipid Research 30, 39–103. [DOI] [PubMed] [Google Scholar]
- Innis S.M. & Hansen J.W. (1996) Plasma fatty acid responses, metabolic effects, and safety of microalgal and fungal oils rich in arachidonic and docosahexaenoic acids in healthy adults. American Journal of Clinical Nutrition 64, 159–167. [DOI] [PubMed] [Google Scholar]
- Innis S.M., Adamkin D.H., Hall R.T., Kalhan S.C., Lair C., Lim M. et al. (2002) Docosahexaenoic acid and arachidonic acid enhance growth with no adverse effects in preterm infants fed formula. Journal of Pediatrics 140, 547–554. [DOI] [PubMed] [Google Scholar]
- Kostraba J.N., Gay E.C., Cai Y., Cruickshanks K.J., Rewers M.J., Klingensmith G.J. et al. (1992) Incidence of insulin‐dependent diabetes mellitus in Colorado. Epidemiology 3, 232–238. [DOI] [PubMed] [Google Scholar]
- Li R., Ogden C., Ballew C., Gillespie C. & Grummer‐Strawn L. (2002) Prevalence of exclusive breastfeeding among US infants: The Third National Health and Nutrition Examination Survey (phase II, 1991–1994). American Journal of Public Health 92, 1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A., Stafford M., Morley R., Abbott R., Stephenson T., MacFadyen U. et al. (1999) Efficacy and safety of long‐chain polyunsaturated fatty acid supplementation of infant‐formula milk: a randomised trial. Lancet 354, 1948–1954. [DOI] [PubMed] [Google Scholar]
- Maxwell R.J. & Marmer W.N. (1983) Systematic protocol for the accumulation of fatty acid data from multiple tissue samples: tissue handling, lipid extraction and class separation, and capillary gas chromatographic analysis. Lipids 18, 453–459. [DOI] [PubMed] [Google Scholar]
- Neuringer M., Anderson G.J. & Connor W.E. (1988) The essentiality of n‐3 fatty acids for the development and function of the retina and brain. Annual Review of Nutrition 8, 517–541. [DOI] [PubMed] [Google Scholar]
- Norris J.M., Barriga K., Klingensmith G., Hoffman M., Eisenbarth G., Erlich H.A. et al. (2003) Timing of initial cereal exposure in infancy and risk of islet autoimmunity. Journal of the American Medical Association 290, 1713–1720. [DOI] [PubMed] [Google Scholar]
- Norris J.M., Yin X., Lamb M.M., Barriga K., Seifert J., Hoffman M. et al. (2007) Omega‐3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. Journal American Medical Association 298, 1420–1428. [DOI] [PubMed] [Google Scholar]
- Rewers M., Bugawan T.L., Norris J.M., Blair A., Beaty B., Hoffman M. et al. (1996) Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY). Diabetologia 39, 807–812. [DOI] [PubMed] [Google Scholar]
- Romon M., Nuttens M.C., Théret N., Delbart C., Lecerf J.M., Fruchart J.C. et al. (1995) Comparison between fat intake assessed by a 3‐day food record and phospholipid fatty acid composition of red blood cells: results from the monitoring of cardiovascular disease‐Lille study. Metabolism 44, 1139–1145. [DOI] [PubMed] [Google Scholar]
- Rosenfeld E., Beyerlein A., Hadders‐Algra M., Kennedy K., Singhal A., Fewtrell M. et al. (2009) IPD meta‐analysis shows no effect of LC‐PUFA supplementation on infant growth at 18 months. Acta Paediatrica 98, 91–97. [DOI] [PubMed] [Google Scholar]
- Simmer K., Patole S.K. & Rao S.C. (2008a) Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database of Systematic Review CD000376. [DOI] [PubMed]
- Simmer K., Schulzke S.M. & Patole S. (2008b) Longchain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database of Systematic Review CD000375. [DOI] [PubMed]
- U.S. Food and Drug Administration (2004) Federal Food, Drug, and Cosmetic Act. Sections 401–416.
- Vidgren H.M., Agren J.J., Schwab U., Rissanen T., Hanninen O. & Uusitupa M.I. (1997) Incorporation of n‐3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid‐rich oil among healthy young men. Lipids 32, 697–705. [DOI] [PubMed] [Google Scholar]
- Wander R.C. & Patton B.D. (1991) Comparison of three species of fish consumed as part of a Western diet: effects on platelet fatty acids and function, hemostasis, and production of thromboxane. American Journal of Clinical Nutrition 54, 326–333. [DOI] [PubMed] [Google Scholar]
- Wright K., Coverston C., Tiedeman M. & Abegglen J.A. (2006) Formula supplemented with docosahexaenoic acid (DHA) and arachidonic acid (ARA): a critical review of the research. Journal for Specialists in Pediatric Nursing 11, 100–112. [DOI] [PubMed] [Google Scholar]
- Youdim K.A., Martin A. & Joseph J.A. (2000) Essential fatty acids and the brain: possible health implications. International Journal of Developmental Neuroscience 18, 383–399. [DOI] [PubMed] [Google Scholar]


