Abstract
For bone tissue engineering, the benefits of incorporating mesenchymal stem cells (MSCs) into porous scaffolds are well established. There is, however, little consensus on the effects of or need for MSC handling ex vivo. Culture and expansion of MSCs adds length and cost, and likely increases risk associated with treatment. We evaluated the effect of using uncultured bone marrow mononuclear cells (bmMNCs) encapsulated within fibrin glue hydrogels and seeded into porous scaffolds to regenerate bone over 12 weeks in an 8-mm-diameter, critical-sized rat cranial defect. A full factorial experimental design was used to evaluate bone formation within model poly(L-lactic acid) and corraline hydroxyapatite scaffolds with or without platelet-rich plasma (PRP) and bmMNCs. Mechanical push-out testing, microcomputed tomographical analyses, and histology were performed. PRP showed no benefit for bone formation. Cell-laden poly(L-lactic acid) scaffolds without PRP required significantly greater force to displace from surrounding tissues than control (cell-free) scaffolds, but no differences were observed during push-out testing of coral scaffolds. For bone volume formation as analyzed by microcomputed tomography, significant positive overall effects were observed with bmMNC incorporation. These data suggest that bmMNCs may provide therapeutic advantages in bone tissue engineering applications without the need for culture, expansion, and purification.
Introduction
Tissue engineering approaches to regenerating lost or damaged tissues often focus on the use of adult stem cells. Among many available sources, mesenchymal stem cells (MSCs) derived from bone marrow or adipose tissue are commonly used as multipotent autologous cell sources and can be collected through relatively noninvasive methods.1 A number of studies have demonstrated the advantages of using MSCs for the repair or regeneration of multiple tissue types,2–9 and multiple clinical trials are underway investigating the use of MSCs for the treatment of both acute and chronic conditions.10
Despite many studies establishing the benefits of MSCs, a number of factors remain unaddressed regarding their widespread use. First, there is little to no standardization of MSC harvest, expansion, and characterization,11–14 and studies suggest that these factors may play a significant role in the success or failure of applications employing these cells.15–21
In addition to raising questions about the standardization and optimization of culture techniques, ex vivo expansion of autologous MSCs requires a delay between harvest and treatment, a likely increased cost of treatment to the patient, and introduces the risk for contamination or adverse cellular changes related to the expansion.22–24 Limited long-term data are available to adequately understand the risks of using culture-expanded MSC in clinical therapy, and, although early reports are promising,25 a number of concerns, including those of genomic instability and resultant tumorigenesis, remain.26–30 Additionally, it is unclear how patient-to-patient variability and changes in cellular function related to age or other demographic factors may impact the use and success of such cell-based therapies.31–37
One potential method to avoid many of the problems associated with harvest and subsequent culture of MSCs is to use uncultured cell sources that may contain MSCs. Such an approach greatly diminishes the number and purity of the available multipotent cell population and, in certain cases of cardiovascular repair and regeneration, has been shown to limit the efficacy of cell-based therapies.38,39 In contrast, Samdani et al. directly compared bone marrow mononuclear cells (bmMNCs) with culture-expanded MSCs in a spinal cord injury model and found that bmMNCs offered a greater protective benefit and reduced scar formation relative to MSC-treated injuries.40
Previous studies in bone and cartilage tissue engineering have shown significant therapeutic benefits associated with the use of uncultured whole bone marrow or bmMNCs.41–44 The ability to perform the necessary cell harvest and any purification steps perioperatively and the relative ease of clinical translation for these one-step processes were cited as advantages to more commonly investigated techniques involving culture-expanded MSCs.40,45 Gan et al. found that marrow MNCs enriched perioperatively using a cell processor performed as well as bone grafts in spinal fusion.45 Muschler et al. found similar results for spinal fusion using a matrix enrichment procedure to increase bmMNC engraftment.46 Further supplementing uncultured whole marrow with growth factors such as transforming growth factor-beta1 has also been shown to have a beneficial effect on healing of critical-sized bone defects.47
Due to the decreased number of multipotent cells available without in vitro expansion and purification and the decreased amount of time available for seeding cell–scaffold constructs during perioperative construct fabrication, cell delivery vehicles have been used to ensure MSC localization to a defect site or within a scaffold. The presence and properties of these delivery vehicles, which are primarily hydrogels such as fibrin glue,48–50 alginate,51 collagen,52 hydroxypropylmethylcellulose,53 and platelet-rich gels,54 can greatly influence cell survival and differentiation. Catelas et al. and Ho et al. previously demonstrated the balance between MSC proliferation and differentiation achieved by modulating the fibrinogen concentration within fibrin gels.55,56 Similar effects have also been seen in collagen gels.57
In the present study, we hypothesized that bone formation within a critical-sized defect would be enhanced using uncultured bmMNCs encapsulated in fibrin gels for delivery of the cells within model scaffolds made of poly(L-lactic acid) (PLLA) or coralline hydroxyapatite (HA). Scaffold properties were also expected to play a significant role on bone formation. Platelet-rich plasma (PRP) was included for potential growth factor delivery to the encapsulated cells and hypothesized to further enhance bone formation. All materials used were U.S. Food and Drug Administration regulated, and an attempt to minimize processing procedures was made to simulate an entire harvest and construct fabrication protocol that could be reasonably completed over the course of a single operation.
Materials and Methods
Experimental design
This study used a full factorial design with three factors tested at two levels each (scaffold material: coral or PLLA; PRP: presence or absence; uncultured marrow MNCs: presence or absence), resulting in a total of eight experimental groups. The groups are described in Table 1. For each group, a total sample size of 14 was used, with 6 samples per group randomly assigned for mechanical (push-out) testing and the remaining 8 used for microcomputed tomography (μCT) and histology.
Table 1.
Experimental Groups Tested
| Designation | Scaffold material | PRP | bmMNC |
|---|---|---|---|
| P– | PLLA | − | − |
| PP- | PLLA | + | − |
| P-M | PLLA | − | + |
| PPM | PLLA | + | + |
| C– | Coral | − | − |
| CP- | Coral | + | − |
| C-M | Coral | − | + |
| CPM | Coral | + | + |
PRP, platelet-rich plasma; bmMNC, bone marrow mononuclear cell; PLLA, poly(L-lactic acid).
Materials
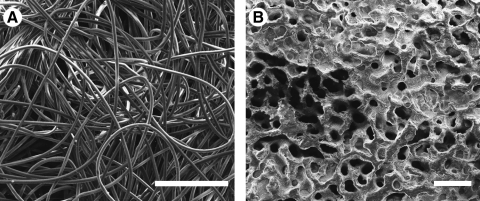
Coralline HA scaffolds (Pro Osteon 200, Interpore Cross International) measuring 8 mm in diameter × 2 mm in height and sterilized by gamma irradiation were used as supplied by the manufacturer. Nonwoven PLLA fiber mesh scaffolds (Concordia Medical) measuring 8 mm in diameter × 1 mm in height with a volumetric porosity of 90%, 300–500 μm pore sizes, and a 40 μm fiber diameter were sterilized using ethylene oxide and placed in a sterile biosafety cabinet to ventilate for a minimum of 24 h before use. Scanning electron micrographs of the surface of the coral and PLLA scaffolds are shown in Figure 1. Fibrin glue (Tisseel™, Baxter Healthcare Corp.) consisting of a human fibrinogen complex (or sealer protein) and human thrombin was used as described below. The fibrinogen complex possibly contains osteogenic growth factors such as transforming growth factor β and basic fibroblast growth factor.55
FIG. 1.
Scanning electron micrographs of the PLLA (A) and coral (B) scaffolds. Scale bars represent 500 μm. PLLA, poly(L-lactic acid).
Animal use
All animal work was performed in accordance with protocols approved by the Rice University Institutional Animal Care and Use Committee. A total of 134 healthy, male syngeneic Fisher 344 rats (Harlon Bioproducts) aged 12 weeks old and weighing 225–249 g were used in this study. Twenty-two rats were used as bone marrow and/or blood donors, and the remaining 112 rats were used in surgery.
PRP harvest and processing
PRP was freshly harvested the day of surgery from donor rats. Rats were euthanized under general anesthesia via CO2 asphyxiation; upon confirmation of death, a cardiac puncture was performed to withdraw ∼5 mL of blood into a sterile syringe containing 500 μL sterile filtered 3.8% sodium citrate (Sigma-Aldrich). Blood from at least two donors was pooled into sterile 15 mL centrifuge tubes (BD Biosciences) and placed on ice. PRP was isolated using a standard laboratory centrifuge (Precision Durafuge 100; Thermo Fisher Scientific) according to a previously established method.58 Briefly, after centrifugation at 400 g for 10 min with no brake applied, the supernatant was sterilely pipetted to a second sterile centrifuge tube, which was then centrifuged at 800 g for 10 min with a brake applied at the end of the cycle. The supernatant of platelet-poor plasma was then removed, and the remaining PRP was returned to ice. Platelet enrichment between peripheral blood and PRP from eight donor rats was confirmed during a preliminary study using a veterinary hematology analyzer (scil Vet abc, scil animal care company). Before use, PRP fibrinogen content was quantified using a spectrophotometric method.59
Bone marrow harvest and construct preparation
On the day of surgery, bone marrow was harvested from the long bones of the hind limbs of euthanized donor rats. Under sterile conditions, the femora and tibiae were isolated, stripped of soft tissue, and placed into sterile minimum essential medium alpha (α-MEM, HyClone Laboratories Inc.). The epiphyses were then removed. Bone marrow was flushed by piercing the diaphyses with a 16-gauge syringe and delivering heparinized phosphate-buffered saline (HyClone Laboratories Inc.). Marrow was disaggregated by serially passaging through 18 followed by 20-gauge syringes. A cell suspension was created by vacuum filtering the marrow through a 100-μm nylon filter (Millipore Corporation). MNCs were then isolated by Ficoll density gradient centrifugation (Accuspin™ System Histopaque®-1077, ρ = 1.077 g/mL; Sigma-Aldrich) performed according to the manufacturer's instructions. Cells were washed three times and resuspended in sterile Tris-buffered saline (TBS; Sigma-Aldrich) or PRP to a final concentration of 6 × 107 cells/mL.
PLLA and coral scaffolds were prewet in gradient ethanol beginning 24 h before seeding and/or implantation. Both the fibrinogen complex and thrombin components of the fibrin glue were freshly prepared each morning according to the manufacturer's instructions, and the fibrinogen concentration was verified using the Ellis method.59 The fibrinogen concentration was diluted with TBS to 102 mg/mL, and the thrombin solution was diluted with 30 mM sterile CaCl2 in TBS to a concentration of 200 IU/mL.
For all experimental groups, 50 μL each of fibrinogen complex and thrombin were delivered to the scaffold, resulting in 100 μL of fibrin glue with final concentrations of 17 mg fibrinogen/mL and 100 IU thrombin/mL. For scaffolds loaded with bmMNCs without PRP, volumes of cells suspended in TBS and fibrinogen complex were added in a 2:1 ratio to a sterile centrifuge tube and vortexed. For scaffolds loaded with PRP, the bmMNC/PRP suspension or PRP alone was added to an equal volume of fibrinogen complex diluted with TBS and vortexed such that the final fibrinogen concentration within the PRP/fibrinogen complex solution was 34 mg/mL. For all experimental groups, after vortexing, 50 μL of the fibrinogen complex solution was then pipetted onto each prewet scaffold within separate wells of an ultra-low attachment 24-well plate (Corning Inc.) that was then placed within a 37°C incubator for 5 min to allow the fibrinogen complex to soak the scaffold. After this, 50 μL of thrombin solution was pipetted onto the scaffolds, which were then returned to the incubator for 15 min. After 15 min, a thin layer of α-MEM was added to the well to cover the scaffold. Scaffolds were returned to the incubator until implantation.
Animal surgery, euthanasia, and implant harvest
Critical-sized, 8-mm-diameter defects within the rat calvarium were created under general inhalational anesthesia, filled with freshly prepared implants, closed, and cared for postoperatively as previously described.60 After 12 postoperative weeks, animals were euthanized according to established and approved methods,60 and the implants and surrounding tissues were harvested with a high-speed surgical drill (TPS®; Stryker) and 701 cutting bur. Before harvest, samples were randomly designated for mechanical testing or μCT/histology. After harvest, samples to be mechanically tested were stripped of any extraneous soft tissues superficial or deep to the implant, immediately placed in ice-cold phosphate-buffered saline, and mechanically tested within 20 min. Samples designated for μCT/histology were individually placed in 10% neutral buffered formalin and stored on a shaker table at 4°C for 72 h.
Mechanical push-out testing
Push-out testing was performed to determine the interfacial shear strength of the implanted constructs after 12 weeks. Test were performed on a mechanical testing bench (858 Mini Bionix II; MTS Systems Corp.) using a custom-made push-out and support jigs allowing 1 mm of clearance between the construct and the support jig around the entire circumference of the construct to minimize the effect of the testing jig on the interfacial stress distribution.61 Tissue samples were oriented with the construct centered over the support jig, and the push-out jig applied a vertical force on the constructs at a constant rate of 0.5 mm/min. Testing was stopped after a peak force was reached.
μCT imaging and analysis
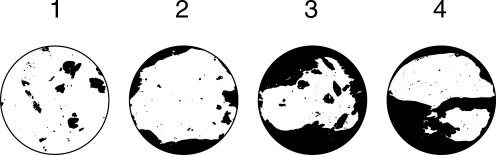
Bone volume and extent of bridging within the explanted samples were analyzed by μCT (SkyScan 1172 high-resolution micro-CT; SkyScan) as previously described.62 For PLLA constructs, lower and upper threshold indices were 70 and 255, respectively. For coral constructs, threshold indices of 90 and 255 were used. For PLLA samples, axial maximum intensity projections (MIPs) were generated, and bony bridging was evaluated by three observers (J.D.K., P.P.S., and F.K.K.) according to the scoring system described in Table 2.
Table 2.
Scoring Guide for Bony Bridging and Union Using Maximum Intensity Projections from Microcomputed Tomography
| Description | Score |
|---|---|
| Bony bridging over entire span of defect at longest point (8 mm) | 4 |
| Bony bridging over partial length of defect | 3 |
| Bony bridging only at defect borders | 2 |
| Few bony spicules dispersed throughout defect | 1 |
| No bone formation within defect | 0 |
 | |
Histological processing
Fixed samples were serially dehydrated in graded ethanol (70%–100%). Samples with PLLA constructs were left undecalcified and embedded in methylmethacrylate. Samples with coral constructs were mildly decalcified with EDTA and embedded in methyl methacrylate. Serial 5-μm coronal sections through the center of the construct were then cut using a rotation microtome (Leica) and stained with hematoxylin and eosin, von Kossa/van Gieson (PLLA constructs only), or Goldner's trichrome according to established methods.63
Light microscopy and histological scoring
Stained sections were analyzed and imaged using a standard light microscope with attached digital camera (Eclipse E600; Nikon Instruments Inc.). Three observers (J.D.K., P.P.S., and F.K.K.) evaluated each of the sections according to a previously used scoring system, shown in Table 3, for assessing the construct–bone interface and the bone growth within the construct pores.62
Table 3.
Quantitative Histological Scoring Parameters
|
Description |
Score |
|---|---|
| Hard tissue response at scaffold–bone interface | |
| Direct bone to implant contact without soft interlayer | 4 |
| Remodeling lacuna with osteoblasts and/or osteoclasts at surface | 3 |
| Majority of implant is surrounded by fibrous tissue capsule | 2 |
| Unorganized fibrous tissue (majority of tissue is not arranged as capsule) | 1 |
| Inflammation marked by an abundance of inflammatory cells and poorly organized tissue | 0 |
| Hard tissue response within the pores of the scaffold | |
|---|---|
| Tissue in pores is mostly bone | 4 |
| Tissue in pores consists of some bone within mature, dense fibrous tissue, and/or a few inflammatory response elements | 3 |
| Tissue in pores is mostly immature fibrous tissue (with or without bone) with blood vessels and young fibroblasts invading the space with few macrophages present | 2 |
| Tissue in pores consists mostly of inflammatory cells and connective tissue components in between (with or without bone) OR the majority of the pores are empty or filled with fluid | 1 |
| Tissue in pores is dense and exclusively of inflammatory type (no bone present) | 0 |
Statistical analyses
Continuous variable data are represented as means ± standard deviation. Ordinal variables are presented as frequencies. All analyses were performed using R version 2.10.0 (R Foundation for Statistical Computing). The a priori defined significance level for all statistical tests was 0.05.
Peak loads obtained from push-out testing and percentage bone volumes were analyzed using two-way analyses of variance (bmMNC × PRP). Post hoc multiple comparisons were made using the step-down Holm-Sidak test.64
MIP and histology scoring were analyzed using a nonparametric method for analyzing two-way ordinal data from factorial experiments.65,66 Statistical significance for main effects and interaction were determined using analyses of variance-type statistics, and multiple comparisons were made based on estimated 95% confidence intervals.
Results
PRP processing
Approximately 250 μL of PRP was obtained from 4.5 mL peripheral blood per donor rat. Preliminary studies showed an 8.6 ± 3.2-fold platelet enrichment in the PRP compared to peripheral blood. Fibrinogen concentrations in the PRP were 14.2 ± 4.5 mg/mL. To keep the fibrinogen concentration constant in the gel fraction of all constructs, the fibrinogen complex concentration in constructs with PRP was slightly lower than in those without.
Construct fabrication and animal surgeries
Constructs were successfully fabricated, with each construct containing 100 μL of gel with, where appropriate, 1.5 × 106 bmMNCs/scaffold, 17 mg/mL fibrinogen and 100 IU/mL thrombin within the gel, and 25 μL PRP. Gel was well formed within the pores of the scaffolds ∼1 min after the addition of thrombin.
One animal died intraoperatively and was replaced before the completion of the study. All other animals survived the surgery and postoperative period without complications. At the time of euthanasia and sample harvest, there were no signs of gross infection or adverse tissue response surrounding the implants.
Mechanical push-out testing
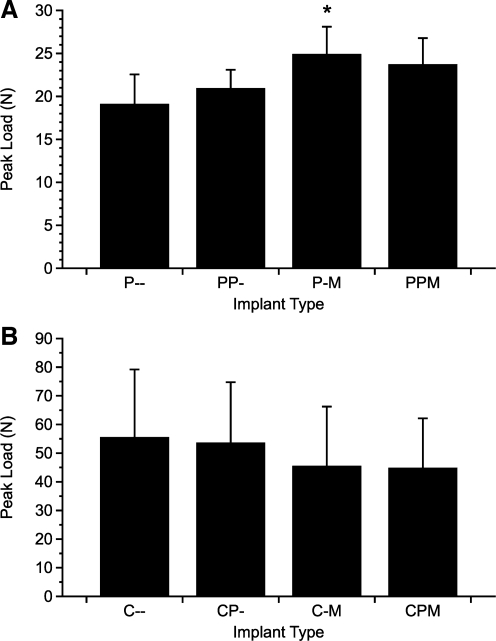
Push-out testing results in the form of peak loads are shown in Figure 2. Due to differences in the Young's moduli of the scaffold materials, direct comparisons between coral and PLLA constructs cannot be made.61 For the PLLA constructs, the group containing only bmMNCs (P-M) required significantly more force to displace the construct than the control (P–) constructs. Among the PLLA constructs, no other significant differences were found. Overall, the presence of bmMNCs had a significant effect on the push-out force (p < 0.005), whereas PRP and the interaction of PRP and bmMNCs were not significant.
FIG. 2.
Results of mechanical push-out testing. Immediately after harvest, constructs were placed on a custom testing jig and a vertical force was applied. The peak load required to displace the implants from the surrounding tissues was recorded for both PLLA constructs (A) and coral constructs (B). Data are reported as mean ± standard deviation (n = 6). Statistical significance between groups and the corresponding control is denoted by *.
No significant differences in push-out strength were found for the coral constructs. The overall effect of bmMNCs seeded onto the scaffolds, the presence of PRP, and any interaction between these factors were also insignificant.
μCT analysis
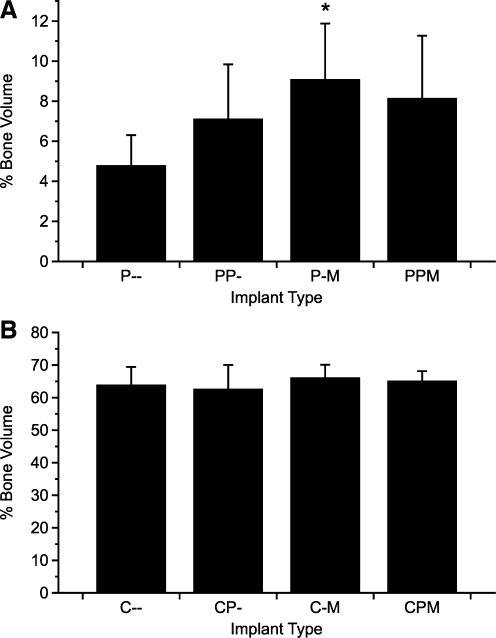
Results from μCT imaging were analyzed to determine the volume percentage of regenerated bone within the constructs (Fig. 3). For PLLA constructs, significant differences in bone formation again existed only between the control constructs and the group containing only bmMNCs (P-M). For coral constructs, no significant differences were present in pairwise comparisons; however, for both PLLA and coral constructs, the addition of bmMNCs had a significant overall effect on bone formation. The effect of PRP and the interaction of PRP and bmMNCs were not significant for both types of constructs.
FIG. 3.
Percent object volume of bone formation within the cranial defect. Explanted constructs and surrounding tissues were scanned by microcomputed tomography and analyzed to determine the percentage of volume within the defect space occupied by mineralized tissue for both PLLA constructs (A) and coral constructs (B). The reported values for the coral constructs include the volume of the scaffold. Data are reported as mean ± standard deviation (n = 8). Statistical significance between groups and the corresponding control is denoted by *.
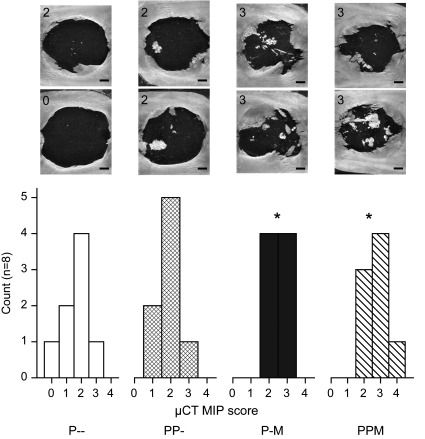
Bony bridging across defects filled with PLLA constructs was evaluated by scoring MIPs as shown in Figure 4. Again, the presence of bmMNCs had a significant effect on bony bridging, but the effect of PRP and the interaction of PRP with bmMNCs were not significant. Pairwise comparisons found the observed bridging between both groups containing bmMNCs (P-M, PPM) to be significantly different from both groups without bmMNCs (P–, PP-). Complete bridging through the midline of the defect was only observed in one sample from group PPM.
FIG. 4.
Scores of bony bridging across the cranial defects. Explanted PLLA constructs were scored by three observers according to the criteria in Table 2. Histograms of the scores are reported for each type of construct, and significant differences between construct scores and the control (P–) score are denoted by *. Images depict representative maximum intensity projections along with the corresponding score assigned to these samples (upper left corner of each image). Scale bars (lower right corners) indicate 1 mm.
Histology
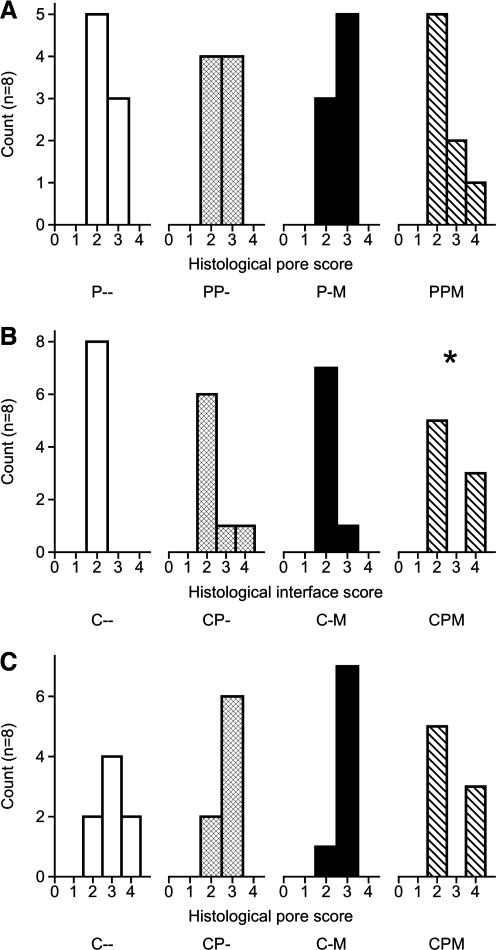
Histological sections were analyzed and scored to evaluate the tissue response at the bone–construct interface and the tissue growth within the construct pores. Representative histological samples are shown in Figure 5. For the defects filled with PLLA constructs, no significant differences between groups or significant effects were observed. A well-organized fibrous capsule surrounded each PLLA construct; thus, each sample received a score of 2 characterizing the interface. The scores reflecting the tissue response within the pores of the PLLA constructs are shown in Figure 6A.
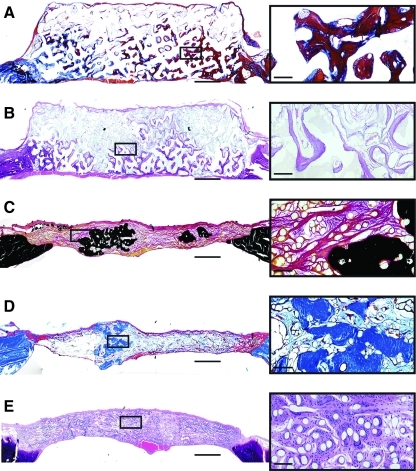
FIG. 5.
Representative histological sections from the experimental groups. Group and stain are as follows: (A) CP-, Goldner's trichrome; (B) CPM, hematoxylin and eosin; (C) P-M, Von Kossa/Van Gieson; (D) PPM, Goldner's trichrome; (E) P–, hematoxylin and eosin. Scale bars in the full size images on the left represent 1 mm, while in the magnified micrographs to the right, the scale bars represent 100 μm. In A, mineralized tissue is stained blue or deep red due to the partial demineralization required during processing. In (D), mineralized tissue is stained bright blue, whereas osteoid is stained deep red.
FIG. 6.
Scores from histological analysis of tissue response within the construct pores (A, C) and at the implant–tissue interface (B). Histology samples were scored by 3 observers according to the criteria in Table 3. Histograms of the scores are reported for each type of construct, and significant differences between construct scores and the control (P– or C–) score are denoted by *. At the interface between the PLLA constructs and the surrounding tissue, all samples received a score of 2.
The results of scoring the tissue response at the interface and within the pores of the coral constructs are shown in Figure 6B and C, respectively. No significant differences in the tissue response within the construct pores were found between groups; however, there was a statistically significant interaction between the bmMNCs and the PRP on the tissue response both at the interface and within the pores of the constructs. Neither factor had a significant effect when considered alone. Additionally, there was a statistically significant difference between the tissue response at the construct interface for the coral constructs with both bmMNCs and PRP (CPM) and the control (C–) constructs.
Discussion
To facilitate the translation of many tissue engineering technologies for clinical use, standardization or simplification of the approaches taken is necessary.67 In the present study, we hypothesized that a process that could be completely performed over the course of a single surgical intervention could be developed incorporating uncultured bmMNCs within U.S. Food and Drug Administration–regulated materials for enhanced bone formation within a critical-sized rat cranial defect. For many of the analyses used, this was indeed the case. Uncultured bmMNCs encapsulated within a fibrin gel inside the pores of PLLA scaffolds regenerated significantly more bone within the defect, achieved greater bony bridging of the defect, and were better incorporated into the cranium as measured by mechanical testing than were cell-free controls.
When comparing cell-laden and cell-free coral constructs, the results were not as clear. The presence of bmMNCs did not significantly increase the bone formation within the scaffolds as detected by μCT, and the peak force needed to display the cellular constructs was less than that needed to displace the cell-free scaffolds, although not significantly so. The presence of PRP had no effect on bone formation as determined by mechanical testing and μCT regardless of scaffold material or whether bmMNCs were also present; however, a significant interaction was observed between PRP and bmMNCs when evaluating the tissue formation within the pores and at the interface of the coral constructs by histology.
Scaffold material played an obvious role in bone formation, mechanical integrity of the implanted constructs, and the effect of bmMNC incorporation. Differences in composition, pore morphology, and mechanical properties between the two scaffolds prevent direct comparison of results obtained using the different scaffolds; however, the studies demonstrate the importance of scaffold physicochemical properties on bone formation. PLLA was chosen as a polymeric scaffold due to its regulatory history, long-term maintenance of mechanical properties, and because MSCs have previously been demonstrated to differentiate well on PLLA fiber meshes.68 Polymeric fiber meshes with similar fiber diameters and densities have previously been used to characterize the effect of MSC culture and differentiation in the rat cranial defect.69
Coralline HA bone grafts have long been used both in research and clinical applications.69 In this study, bone formation in coral scaffolds was not significantly different across groups; however, for μCT analysis of bone formation there was an overall significant effect of bmMNC incorporation. This result was somewhat surprising, as previous work has demonstrated MSC osteogenic differentiation and ingrowth on similar scaffolds.70–72 Although reports showing little to no osteogenesis on coralline HA scaffolds do exist,73 bony ingrowth did occur in all of the tested formulations using coral.
The use of PRP did not significantly affect bone formation in this study. This result is similar to those reported elsewhere in rats,74 although there are multiple reports in humans75 and animals,76 including rats,77,78 of PRP enhancing bone formation. Still, evaluating the efficacy of PRP remains complicated. PRP in combination with uncultured bmMNCs has been found to increase bone regeneration in humans,79 and rat PRP gels have similarly been shown to increase in vitro osteogenic differentiation of rat MSCs.80,81 Similarly, a significant interaction was found between PRP and uncultured bmMNCs by histological scoring of coral constructs, but evaluation by μCT and mechanical testing did not reflect a significant interaction. Other studies have shown that PRP does not increase in vitro or in vivo differentiation of rat MSCs when compared with the effect of growth factors such as bone morphogenetic protein-782 and was found to inhibit MSC differentiation in a study comparing PRP and bone morphogenetic protein-2.83
A number of considerations regarding the variability and evaluation of PRP must be factored in when analyzing the discordant results for PRP use found in the literature. First, processing techniques, donor factors, and overall platelet enrichment play a significant role in the determining the actual composition of any PRP.84–89 Further, there is an established interspecies variation in the growth factors contained in and osteogenic capability of PRP, with human PRP having higher growth factor concentrations and eliciting an increased osteogenic response compared to rat or goat PRP.81,90 Additionally, PRP effectiveness may in part be modulated by scaffold and cell differentiation state for applications involving cell-laden constructs.91,92 Kasten et al. found that PRP increased alkaline phosphatase activity in undifferentiated MSCs but not in those precultured in osteogenic media91; however, in the present study PRP was only found to significantly interact with uncultured bmMNCs when examining histological sections.
The present study had a number of strengths. First, it further establishes the utility of uncultured bmMNCs in bone formation, including demonstrating a mechanical benefit for cell-laden PLLA scaffolds. Additionally, the study uses only commercially available materials that have already been approved for clinical use, meaning other parameters, such as the explicit role of fibrin glue in cell delivery, the relative effect of culture and expansion of bmMNCs on bone formation, and optimal seeding densities can be explored with minimal variability in the substrate materials. This may also allow for more rapid clinical evaluation of the results. The effect of the scaffold on bone formation using PRP and bmMNCs was also demonstrated by the difference in results for PLLA and coral constructs. Finally, mechanical effects of substrate stiffness were carefully controlled by maintaining a constant fibrinogen concentration across samples; thus, any effect seen for PRP addition would have likely been based on bioactive factor delivery.
The primary weaknesses of the present study lie primarily in scope, and thus future studies are necessary to draw more definitive conclusions from the current study. As mentioned in the previous paragraph, future studies are needed to determine whether the benefits of using a simplified process that can be performed over the course of a single operation outweigh any benefits that may exist with MSC expansion and culture. Similarly, future studies should incorporate more time points to determine if there are any temporal effects of PRP addition. Finally, more sophisticated analyses should be done to determine the role of the delivered bmMNCs in bone formation, as the ability of a seeded cell population to recruit host osteoprogenitors may be more important for bone regeneration than the regenerative capability of the seeded cells.93
A number of interesting results were found in this study. First, bmMNCs increased bone formation and construct incorporation for PLLA scaffolds, but the benefit in coral scaffolds was not clear. For mechanical testing of coral constructs, bmMNC incorporation within scaffolds did not provide a significant change in implant mechanical properties, although a significant overall effect was found for μCT analysis. In a similar study by Le Nihouannen et al., fibrin glue and granules of 60/40 HA/β-tri-calcium phosphate (β-TCP) cement were mixed with and without freshly isolated bone marrow and placed within defects in rabbit femora.94 After 12 weeks, there was no difference in bone formation within the defects as detected by μCT. Ueno et al. did, however, demonstrate bone regeneration in rat calvarial defects filled with β-TCP granules and whole marrow.95 Similarly, Becker et al. compared bone marrow and concentrated bmMNC seeding on β-TCP scaffolds and found that constructs seeded with bone marrow induced significantly more bone regeneration than those with bmMNCs, which were not statistically different from controls.96
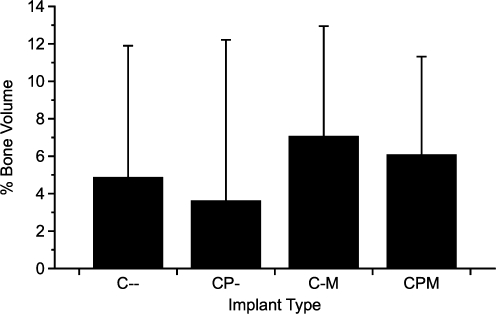
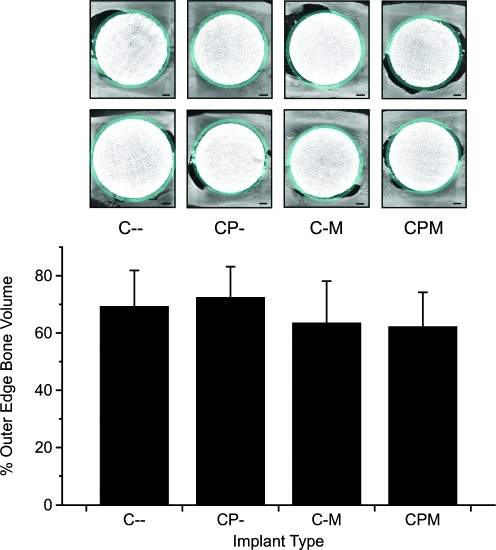
On the basis of these studies, whole marrow may be a better cell source for scaffolds where significant adsorption of potentially osteogenic proteins from the acellular marrow fraction may occur. Additionally, bone formation within the coral scaffolds was similar to that of PLLA scaffolds, but not significant when analyzed by pairs, when the object volume of control (unimplanted) coral scaffolds was not considered (Fig. 7). Since the coral scaffolds cannot be differentiated from newly regenerated bone by μCT, variability in scaffold volume may have masked any effect of differences between the experimental groups. Further, we noticed bone resorption around some of the coral scaffolds during μCT imaging. Although the resorption did not vary between groups as determined by imaging and quantifying the bone around the border of the scaffolds (Fig. 8) or histological scoring of the tissue response at the coral construct interface, this resorption likely affected the relatively high variability of the mechanical testing results. Interestingly, the group of coral constructs with the lowest overall interfacial shear strength (CPM) appeared to be significantly more integrated with the host bone compared to control (C–) groups by histology. These data may indicate that histological evaluation of the host–scaffold interface, because of its two-dimensional nature, does not accurately reflect the mechanical integration of the constructs as the failure of a majority of the constructs occurred at the bone–construct interface.
FIG. 7.
Average volume of regenerated bone within coral scaffolds. The average volume of unimplanted coral scaffolds (n = 10, mean volume 70.89% ± 4.79%) was subtracted from the volume of explanted samples (n = 8), leaving an estimate of the bone regeneration within each coral construct. Data shown are means ± adjusted standard deviations.
FIG. 8.
Bone growth or resorption around the outer volume of the coral constructs was quantified. Representative images (top) denote with false coloring the border where bone was quantified around the defect. Scale bars represent 1 mm. The figure (bottom) shows the average percent bone volume present in the outer ring (n = 8, error bars = standard deviation). No significant differences were found.
The use of fibrin glue may also have confounded the findings with respect to PRP use. Fibrin glue and modifications thereof have been shown to be an effective delivery medium for multipotent cells and growth factors in a number tissue engineering applications.97–100 Platelet-enriched fibrin glue has been shown to be an effective substrate for bone-tissue engineering applications both in the presence and absence of encapsulated MSCs.101,102 Ito et al. showed an increase in bone regeneration in a canine mandibular defect using MSC/PRP/fibrin glue hybrid constructs compared to MSC/fibrin glue constructs alone after 4 and 8 weeks103; however, other studies have reported no benefit to similar hybrid constructs.104 Fibrin glue encapsulation alone likely imparts a benefit in the delivery and retention of cells within the scaffold or defect.48,105,106 In previous studies demonstrating a benefit for PRP and MSCs in combination but not in comparison with other delivery methods or encapsulation substrates,107,108 the benefit may be due in large part to the role of the PRP as a delivery vehicle for the MSCs,109 given the otherwise short-term survival of seeded MSCs.110
The need for a cell delivery vehicle may be dependent on the type of scaffold used; in a study by Haasper et al., no benefit of cellular delivery by fibrin glue to scaffolds to which cells adhere more efficiently, such as bone matrix, has been demonstrated.111 Fibrin glue may still play an important role in applications where bioactive factor delivery is appropriate and controllable and easily tuned release kinetics are desired.112–114
Because previous experiments have explicitly established that the fibrinogen concentration within the fibrin glue affects MSC differentiation and proliferation,55,56 an intermediate fibrinogen concentration (17 mg/mL), close to reported optimal values,115 was selected to allow cells to proliferate while still providing some substrate stiffness. In constructs with PRP, the amount of fibrinogen complex incorporated into the final glue was reduced to keep the fibrinogen concentration constant. Because the fibrinogen complex may also contain growth factors, this reduction in concentration may have offset any benefits of growth factor delivery by PRP.
Conclusions
The many emerging technologies in tissue engineering provide a number of options and important considerations for researchers and clinicians. Efficacy, cost, and risk must be assessed, and researchers focused on quickly translating technologies must weigh regulatory pathways as well. The present study characterized scaffold, uncultured bmMNCs, and PRP combinations to determine their relative effectiveness in regenerating bone within a critical-sized rat cranial defect. PLLA scaffolds laden with bmMNCs delivered within fibrin glue regenerated significantly greater bone than control groups. Cells seeded on coral scaffolds had no significant effect on bone formation. By most methods of evaluation, PRP was not found to have a benefit in any formulation. Taken in the context of previous reports in the literature, these results provide further indication that a complex interplay occurs between scaffolds, cells, growth factors, and the host environment. A translational approach employing freshly harvested bmMNCs is likely beneficial compared to a strictly biomaterials-based approach in some cases; however, further work is needed to better understand when such an approach is warranted.
Acknowledgments
Work in the area of bone tissue engineering is supported by the National Institutes of Health (R01 DE017441, A.G.M.) and a gift from Celthera, Inc. (A.G.M.). The authors would like to thank Dr. Laura Suggs and Dr. Christy Zhang for their technical advice, as well as Baxter Biosciences and Interpore Cross International for generously donating materials for this study. J.D.K. acknowledges support from Baylor College of Medicine Medical Scientist Training Program (NIH T32 GM07330), Rice Institute of Biosciences and Bioengineering's Biotechnology Training Grant (NIH T32 GM008362), and a training fellowship from the Keck Center Nanobiology Training Program of the Gulf Coast Consortia (NIH Grant No. 5 T90 DK070121-04). P.P.S. acknowledges support from the Robert and Janice McNair Foundation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Porada C.D. Zanjani E.D. Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 2.Liu G. Li Y. Sun J. Zhou H. Zhang W. Cui L., et al. In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood derived mesenchymal stem cells on partially demineralized bone matrix. Tissue Eng Part A. 2010;16:971. doi: 10.1089/ten.TEA.2009.0516. [DOI] [PubMed] [Google Scholar]
- 3.Guo X. Park H. Young S. Kretlow J.D. van den Beucken J.J. Baggett L.S., et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6:39. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macchiarini P. Jungebluth P. Go T. Asnaghi M.A. Rees L.E. Cogan T.A., et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 5.Hare J.M. Traverse J.H. Henry T.D. Dib N. Strumpf R.K. Schulman S.P., et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satar B. Karahatay S. Kurt B. Ural A.U. Safali M. Avcu F., et al. Repair of transected facial nerve with mesenchymal stromal cells: histopathologic evidence of superior outcome. Laryngoscope. 2009;119:2221. doi: 10.1002/lary.20610. [DOI] [PubMed] [Google Scholar]
- 7.Oh J.Y. Kim M.K. Shin M.S. Lee H.J. Ko J.H. Wee W.R., et al. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 8.Ringden O. Uzunel M. Sundberg B. Lonnies L. Nava S. Gustafsson J., et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21:2271. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]
- 9.Kuo Y.R. Goto S. Shih H.S. Wang F.S. Lin C.C. Wang C.T., et al. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation. 2009;87:1769. doi: 10.1097/TP.0b013e3181a664f1. [DOI] [PubMed] [Google Scholar]
- 10.Giordano A. Galderisi U. Marino I.R. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 11.Sensebe L. Clinical grade production of mesenchymal stem cells. Biomed Mater Eng. 2008;18:S3. [PubMed] [Google Scholar]
- 12.Frohlich M. Grayson W.L. Wan L.Q. Marolt D. Drobnic M. Vunjak-Novakovic G. Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther. 2008;3:254. doi: 10.2174/157488808786733962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sensebe L. Bourin P. Producing MSC according GMP: process and controls. Biomed Mater Eng. 2008;18:173. [PubMed] [Google Scholar]
- 14.Brinchmann J.E. Expanding autologous multipotent mesenchymal bone marrow stromal cells. J Neurol Sci. 2008;265:127. doi: 10.1016/j.jns.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Pountos I. Corscadden D. Emery P. Giannoudis P.V. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury. 2007;38(Suppl 4):S23. doi: 10.1016/s0020-1383(08)70006-8. [DOI] [PubMed] [Google Scholar]
- 16.Lange C. Cakiroglu F. Spiess A.N. Cappallo-Obermann H. Dierlamm J. Zander A.R. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol. 2007;213:18. doi: 10.1002/jcp.21081. [DOI] [PubMed] [Google Scholar]
- 17.Schuh E.M. Friedman M.S. Carrade D.D. Li J. Heeke D. Oyserman S.M., et al. Identification of variables that optimize isolation and culture of multipotent mesenchymal stem cells from equine umbilical-cord blood. Am J Vet Res. 2009;70:1526. doi: 10.2460/ajvr.70.12.1526. [DOI] [PubMed] [Google Scholar]
- 18.Frith J.E. Thomson B. Genever P. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- 19.Oedayrajsingh-Varma M.J. van Ham S.M. Knippenberg M. Helder M.N. Klein-Nulend J. Schouten T.E., et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 20.Seeger F.H. Tonn T. Krzossok N. Zeiher A.M. Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 21.Agata H. Asahina I. Watanabe N. Ishii Y. Kubo N. Ohshima S., et al. Characteristic change and loss of in vivo osteogenic abilities of human bone marrow stromal cells during passage. Tissue Eng Part A. 2010;16:663. doi: 10.1089/ten.TEA.2009.0500. [DOI] [PubMed] [Google Scholar]
- 22.Lepperdinger G. Brunauer R. Jamnig A. Laschober G. Kassem M. Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies? Exp Gerontol. 2008;43:1018. doi: 10.1016/j.exger.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Banfi A. Muraglia A. Dozin B. Mastrogiacomo M. Cancedda R. Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol. 2000;28:707. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 24.Boquest A.C. Shahdadfar A. Fronsdal K. Sigurjonsson O. Tunheim S.H. Collas P., et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centeno C.J. Schultz J.R. Cheever M. Robinson B. Freeman M. Marasco W. Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther. 2010;5:81. doi: 10.2174/157488810790442796. [DOI] [PubMed] [Google Scholar]
- 26.Foudah D. Redaelli S. Donzelli E. Bentivegna A. Miloso M. Dalpra L., et al. Monitoring the genomic stability of in vitro cultured rat bone-marrow-derived mesenchymal stem cells. Chromosome Res. 2009;17:1025. doi: 10.1007/s10577-009-9090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duggal S. Fronsdal K.B. Szoke K. Shahdadfar A. Melvik J.E. Brinchmann J.E. Phenotype and gene expression of human mesenchymal stem cells in alginate scaffolds. Tissue Eng Part A. 2009;15:1763. doi: 10.1089/ten.tea.2008.0306. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn N.Z. Tuan R.S. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2010;222:268. doi: 10.1002/jcp.21940. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y.F. Bosch-Marce M. Okuyama H. Krishnamachary B. Kimura H. Zhang L., et al. Spontaneous transformation of cultured mouse bone marrow-derived stromal cells. Cancer Res. 2006;66:10849. doi: 10.1158/0008-5472.CAN-06-2146. [DOI] [PubMed] [Google Scholar]
- 30.Dahl J.A. Duggal S. Coulston N. Millar D. Melki J. Shahdadfar A., et al. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol. 2008;52:1033. doi: 10.1387/ijdb.082663jd. [DOI] [PubMed] [Google Scholar]
- 31.Stenderup K. Justesen J. Clausen C. Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Aalami O.O. Nacamuli R.P. Lenton K.A. Cowan C.M. Fang T.D. Fong K.D., et al. Applications of a mouse model of calvarial healing: differences in regenerative abilities of juveniles and adults. Plast Reconstr Surg. 2004;114:713. doi: 10.1097/01.prs.0000131016.12754.30. [DOI] [PubMed] [Google Scholar]
- 33.Shi Y.Y. Nacamuli R.P. Salim A. Longaker M.T. The osteogenic potential of adipose-derived mesenchymal cells is maintained with aging. Plast Reconstr Surg. 2005;116:1686. doi: 10.1097/01.prs.0000185606.03222.a9. [DOI] [PubMed] [Google Scholar]
- 34.Crisostomo P.R. Markel T.A. Wang M. Lahm T. Lillemoe K.D. Meldrum D.R. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery. 2007;142:215. doi: 10.1016/j.surg.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Siddappa R. Licht R. van Blitterswijk C. de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25:1029. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- 36.Kretlow J.D. Jin Y.Q. Liu W. Zhang W.J. Hong T.H. Zhou G., et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCann R.M. Marsh D.R. Horner A. Clarke S. Body mass index is more predictive of progenitor number in bone marrow stromal cell population than age in males—expanding the predictors of the progenitor compartment. Tissue Eng Part A. 2010;16:889. doi: 10.1089/ten.TEA.2009.0346. [DOI] [PubMed] [Google Scholar]
- 38.Iwase T. Nagaya N. Fujii T. Itoh T. Murakami S. Matsumoto T., et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66:543. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Mazo M. Gavira J.J. Abizanda G. Moreno C. Ecay M. Soriano M., et al. Transplantation of mesenchymal stem cells exerts a greater long-term effect than bone marrow mononuclear cells in a chronic myocardial infarction model in rat. Cell Transplant. 2010;19:313. doi: 10.3727/096368909X480323. [DOI] [PubMed] [Google Scholar]
- 40.Samdani A.F. Paul C. Betz R.R. Fischer I. Neuhuber B. Transplantation of human marrow stromal cells and mono-nuclear bone marrow cells into the injured spinal cord: a comparative study. Spine (Phila Pa 1976) 2009;34:2605. doi: 10.1097/BRS.0b013e3181bdca87. [DOI] [PubMed] [Google Scholar]
- 41.Okumura M. Ohgushi H. Tamai S. Bonding osteogenesis in coralline hydroxyapatite combined with bone marrow cells. Biomaterials. 1991;12:411. doi: 10.1016/0142-9612(91)90010-8. [DOI] [PubMed] [Google Scholar]
- 42.Ohgushi H. Okumura M. Tamai S. Shors E.C. Caplan A.I. Marrow cell induced osteogenesis in porous hydroxyapatite and tricalcium phosphate: a comparative histomorphometric study of ectopic bone formation. J Biomed Mater Res. 1990;24:1563. doi: 10.1002/jbm.820241202. [DOI] [PubMed] [Google Scholar]
- 43.Ohgushi H. Goldberg V.M. Caplan A.I. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res. 1989;7:568. doi: 10.1002/jor.1100070415. [DOI] [PubMed] [Google Scholar]
- 44.Chang F. Ishii T. Yanai T. Mishima H. Akaogi H. Ogawa T., et al. Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J Orthop Res. 2008;26:18. doi: 10.1002/jor.20470. [DOI] [PubMed] [Google Scholar]
- 45.Gan Y. Dai K. Zhang P. Tang T. Zhu Z. Lu J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29:3973. doi: 10.1016/j.biomaterials.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Muschler G.F. Matsukura Y. Nitto H. Boehm C.A. Valdevit A.D. Kambic H.E., et al. Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res. 2005;(432):242. doi: 10.1097/01.blo.0000149812.32857.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dean D. Wolfe M.S. Ahmad Y. Totonchi A. Chen J.E. Fisher J.P., et al. Effect of transforming growth factor beta 2 on marrow-infused foam poly(propylene fumarate) tissue-engineered constructs for the repair of critical-size cranial defects in rabbits. Tissue Eng. 2005;11:923. doi: 10.1089/ten.2005.11.923. [DOI] [PubMed] [Google Scholar]
- 48.Ryu J.H. Kim I.K. Cho S.W. Cho M.C. Hwang K.K. Piao H., et al. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2005;26:319. doi: 10.1016/j.biomaterials.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 49.Falanga V. Iwamoto S. Chartier M. Yufit T. Butmarc J. Kouttab N., et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 50.Kalbermatten D.F. Kingham P.J. Mahay D. Mantovani C. Pettersson J. Raffoul W., et al. Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J Plast Reconstr Aesthet Surg. 2008;61:669. doi: 10.1016/j.bjps.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 51.Vacanti C.A. Bonassar L.J. Vacanti M.P. Shufflebarger J. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med. 2001;344:1511. doi: 10.1056/NEJM200105173442004. [DOI] [PubMed] [Google Scholar]
- 52.Behravesh E. Mikos A.G. Three-dimensional culture of differentiating marrow stromal osteoblasts in biomimetic poly(propylene fumarate-co-ethylene glycol)-based macroporous hydrogels. J Biomed Mater Res A. 2003;66:698. doi: 10.1002/jbm.a.10003. [DOI] [PubMed] [Google Scholar]
- 53.Trojani C. Boukhechba F. Scimeca J.C. Vandenbos F. Michiels J.F. Daculsi G., et al. Ectopic bone formation using an injectable biphasic calcium phosphate/Si-HPMC hydrogel composite loaded with undifferentiated bone marrow stromal cells. Biomaterials. 2006;27:3256. doi: 10.1016/j.biomaterials.2006.01.057. [DOI] [PubMed] [Google Scholar]
- 54.Vadala G. Di Martino A. Tirindelli M.C. Denaro L. Denaro V. Use of autologous bone marrow cells concentrate enriched with platelet-rich fibrin on corticocancellous bone allograft for posterolateral multilevel cervical fusion. J Tissue Eng Regen Med. 2008;2:515. doi: 10.1002/term.121. [DOI] [PubMed] [Google Scholar]
- 55.Catelas I. Sese N. Wu B.M. Dunn J.C.Y. Helgerson S. Tawil B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006;12:2385. doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 56.Ho W. Tawil B. Dunn J.C.Y. Wu B.M. The behavior of human mesenchymal stem cells in 3d fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Eng. 2006;12:1587. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 57.Fernandes H. Dechering K. Van Someren E. Steeghs I. Apotheker M. Leusink A., et al. The role of collagen crosslinking in differentiation of human mesenchymal stem cells and MC3T3-E1 cells. Tissue Eng Part A. 2009;15:3857. doi: 10.1089/ten.tea.2009.0011. [DOI] [PubMed] [Google Scholar]
- 58.Elgazzar R.F. Mutabagani M.A. Abdelaal S.E. Sadakah A.A. Platelet rich plasma may enhance peripheral nerve regeneration after cyanoacrylate reanastomosis: a controlled blind study on rats. Int J Oral Maxillofac Surg. 2008;37:748. doi: 10.1016/j.ijom.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Ellis B.C. Stransky A. A quick and accurate method for the determination of fibronogen in plasma. J Lab Clin Med. 1961;58:477. [PubMed] [Google Scholar]
- 60.Patel Z.S. Young S. Tabata Y. Jansen J.A. Wong M. Mikos A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhert W.J. Verheyen C.C. Braak L.H. de Wijn J.R. Klein C.P. de Groot K., et al. A finite element analysis of the push-out test: influence of test conditions. J Biomed Mater Res. 1992;26:119. doi: 10.1002/jbm.820260111. [DOI] [PubMed] [Google Scholar]
- 62.Young S. Patel Z.S. Kretlow J.D. Murphy M.B. Mountziaris P.M. Baggett L.S., et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amling M. Priemel M. Holzmann T. Chapin K. Rueger J.M. Baron R., et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 64.Glantz S.A. 6th. New York: McGraw-Hill Medical; 2005. Primer of Biostatistics. [Google Scholar]
- 65.Shah D.A. Madden L.V. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology. 2004;94:33. doi: 10.1094/PHYTO.2004.94.1.33. [DOI] [PubMed] [Google Scholar]
- 66.Brunner E. Dette H. Munk A. Box-type approximations in nonparametric factorial designs. J Am Stat Assoc. 1997;92:1494. [Google Scholar]
- 67.Klouda L. Kretlow J.D. Mikos A. Tailored biomaterials for tissue engineering needs and their clinical translation. In: Mao J.J., editor; Vunjak-Novakovic G., editor; Mikos A.G., editor; Atala A., editor. Translational Approaches in Tissue Engineering and Regenerative Medicine. 1st. Boston, MA: Artech House; 2008. pp. 325–337. [Google Scholar]
- 68.Li W.J. Cooper J.A., Jr. Mauck R.L. Tuan R.S. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Yoon E. Dhar S. Chun D.E. Gharibjanian N.A. Evans G.R. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- 70.Abramovitch-Gottlib L. Geresh S. Vago R. Biofabricated marine hydrozoan: a bioactive crystalline material promoting ossification of mesenchymal stem cells. Tissue Eng. 2006;12:729. doi: 10.1089/ten.2006.12.729. [DOI] [PubMed] [Google Scholar]
- 71.Mygind T. Stiehler M. Baatrup A. Li H. Zou X. Flyvbjerg A., et al. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 2007;28:1036. doi: 10.1016/j.biomaterials.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Flautre B. Descamps M. Delecourt C. Blary M.C. Hardouin P. Porous HA ceramic for bone replacement: role of the pores and interconnections - experimental study in the rabbit. J Mater Sci Mater Med. 2001;12:679. doi: 10.1023/a:1011256107282. [DOI] [PubMed] [Google Scholar]
- 73.Harris C.T. Cooper L.F. Comparison of bone graft matrices for human mesenchymal stem cell-directed osteogenesis. J Biomed Mater Res A. 2004;68:747. doi: 10.1002/jbm.a.20107. [DOI] [PubMed] [Google Scholar]
- 74.Plachokova A.S. van den Dolder J. Stoelinga P.J. Jansen J.A. The bone regenerative effect of platelet-rich plasma in combination with an osteoconductive material in rat cranial defects. Clin Oral Implants Res. 2006;17:305. doi: 10.1111/j.1600-0501.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- 75.Marx R.E. Carlson E.R. Eichstaedt R.M. Schimmele S.R. Strauss J.E. Georgeff K.R. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 76.Kroese-Deutman H.C. Vehof J.W.M. Spauwen P.H.M. Stoelinga P.J.W. Jansen J.A. Orthotopic bone formation in titanium fiber mesh loaded with platelet-rich plasma and placed in segmental defects. Int J Oral Maxillofac Surg. 2008;37:542. doi: 10.1016/j.ijom.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 77.Messora M.R. Nagata M.J. Mariano R.C. Dornelles R.C. Bomfim S.R. Fucini S.E., et al. Bone healing in critical-size defects treated with platelet-rich plasma: a histologic and histometric study in rat calvaria. J Periodontal Res. 2008;43:217. doi: 10.1111/j.1600-0765.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- 78.Fontana S. Olmedo D.G. Linares J.A. Guglielmotti M.B. Crosa M.E. Effect of platelet-rich plasma on the peri-implant bone response: an experimental study. Implant Dent. 2004;13:73. doi: 10.1097/01.id.0000116455.68968.29. [DOI] [PubMed] [Google Scholar]
- 79.Filho Cerruti H. Kerkis I. Kerkis A. Tatsui N.H. da Costa Neves A. Bueno D.F., et al. Allogenous bone grafts improved by bone marrow stem cells and platelet growth factors: clinical case reports. Artif Organs. 2007;31:268. doi: 10.1111/j.1525-1594.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 80.Hu Z.M. Peel S.A. Ho S.K. Sandor G.K. Clokie C.M. Comparison of platelet-rich plasma, bovine BMP, and rhBMP-4 on bone matrix protein expression in vitro. Growth Factors. 2009;27:280. doi: 10.1080/08977190903137819. [DOI] [PubMed] [Google Scholar]
- 81.van den Dolder J. Mooren R. Vloon A.P. Stoelinga P.J. Jansen J.A. Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006;12:3067. doi: 10.1089/ten.2006.12.3067. [DOI] [PubMed] [Google Scholar]
- 82.Roldan J.C. Jepsen S. Miller J. Freitag S. Rueger D.C. Acil Y., et al. Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone. 2004;34:80. doi: 10.1016/j.bone.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Arpornmaeklong P. Kochel M. Depprich R. Kubler N.R. Wurzler K.K. Influence of platelet-rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells. An in vitro study. Int J Oral Maxillofac Surg. 2004;33:60. doi: 10.1054/ijom.2003.0492. [DOI] [PubMed] [Google Scholar]
- 84.Leitner G.C. Gruber R. Neumuller J. Wagner A. Kloimstein P. Hocker P., et al. Platelet content and growth factor release in platelet-rich plasma: a comparison of four different systems. Vox Sang. 2006;91:135. doi: 10.1111/j.1423-0410.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 85.Kevy S.V. Jacobson M.S. Comparison of methods for point of care preparation of autologous platelet gel. J Extra Corpor Technol. 2004;36:28. [PubMed] [Google Scholar]
- 86.Weibrich G. Kleis W.K. Hitzler W.E. Hafner G. Comparison of the platelet concentrate collection system with the plasma-rich-in-growth-factors kit to produce platelet-rich plasma: a technical report. Int J Oral Maxillofac Implants. 2005;20:118. [PubMed] [Google Scholar]
- 87.Kim E.S. Park E.J. Choung P.H. Platelet concentration and its effect on bone formation in calvarial defects: an experimental study in rabbits. J Prosthet Dent. 2001;86:428. doi: 10.1067/mpr.2001.115874. [DOI] [PubMed] [Google Scholar]
- 88.Weibrich G. Kleis W.K. Kunz-Kostomanolakis M. Loos A.H. Wagner W. Correlation of platelet concentration in platelet-rich plasma to the extraction method, age, sex, and platelet count of the donor. Int J Oral Maxillofac Implants. 2001;16:693. [PubMed] [Google Scholar]
- 89.Weibrich G. Hansen T. Kleis W. Buch R. Hitzler W.E. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34:665. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 90.Plachokova A.S. van den Dolder J. van den Beucken J.J. Jansen J.A. Bone regenerative properties of rat, goat and human platelet-rich plasma. Int J Oral Maxillofac Surg. 2009;38:861. doi: 10.1016/j.ijom.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 91.Kasten P. Vogel J. Luginbuhl R. Niemeyer P. Weiss S. Schneider S., et al. Influence of platelet-rich plasma on osteogenic differentiation of mesenchymal stem cells and ectopic bone formation in calcium phosphate ceramics. Cells Tissues Organs. 2006;183:68. doi: 10.1159/000095511. [DOI] [PubMed] [Google Scholar]
- 92.Kasten P. Vogel J. Beyen I. Weiss S. Niemeyer P. Leo A., et al. Effect of platelet-rich plasma on the in vitro proliferation and osteogenic differentiation of human mesenchymal stem cells on distinct calcium phosphate scaffolds: the specific surface area makes a difference. J Biomater Appl. 2008;23:169. doi: 10.1177/0885328207088269. [DOI] [PubMed] [Google Scholar]
- 93.Tasso R. Augello A. Boccardo S. Salvi S. Carida M. Postiglione F., et al. Recruitment of a host's osteoprogenitor cells using exogenous mesenchymal stem cells seeded on porous ceramic. Tissue Eng Part A. 2009;15:2203. doi: 10.1089/ten.tea.2008.0269. [DOI] [PubMed] [Google Scholar]
- 94.Le Nihouannen D. Goyenvalle E. Aguado E. Pilet P. Bilban M. Daculsi G., et al. Hybrid composites of calcium phosphate granules, fibrin glue, and bone marrow for skeletal repair. J Biomed Mater Res A. 2007;81:399. doi: 10.1002/jbm.a.31058. [DOI] [PubMed] [Google Scholar]
- 95.Ueno T. Honda K. Hirata A. Kagawa T. Kanou M. Shirasu N., et al. Histological comparison of bone induced from autogenously grafted periosteum with bone induced from autogenously grafted bone marrow in the rat calvarial defect model. Acta Histochem. 2008;110:217. doi: 10.1016/j.acthis.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 96.Becker S. Maissen O. Ponomarev I. Stoll T. Rahn B. Wilke I. Osteopromotion by a beta-tricalcium phosphate/bone marrow hybrid implant for use in spine surgery. Spine (Phila Pa 1976) 2006;31:11. doi: 10.1097/01.brs.0000192762.40274.57. [DOI] [PubMed] [Google Scholar]
- 97.Zhang G. Drinnan C.T. Geuss L.R. Suggs L.J. Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix. Acta Biomater. 2010;6:3395. doi: 10.1016/j.actbio.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 98.Zhang G. Hu Q. Braunlin E.A. Suggs L.J. Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A. 2008;14:1025. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- 99.Drinnan C.T. Zhang G. Alexander M.A. Pulido A.S. Suggs L.J. Multimodal release of transforming growth factor-beta1 and the BB isoform of platelet derived growth factor from PEGylated fibrin gels. J Control Release. 2010 doi: 10.1016/j.jconrel.2010.03.026. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 100.Zhang G. Wang X. Wang Z. Zhang J. Suggs L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006;12:9. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- 101.Zhu S.J. Choi B.H. Jung J.H. Lee S.H. Huh J.Y. You T.M., et al. A comparative histologic analysis of tissue-engineered bone using platelet-rich plasma and platelet-enriched fibrin glue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:175. doi: 10.1016/j.tripleo.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 102.Findikcioglu K. Findikcioglu F. Yavuzer R. Elmas C. Atabay K. Effect of platelet-rich plasma and fibrin glue on healing of critical-size calvarial bone defects. J Craniofac Surg. 2009;20:34. doi: 10.1097/SCS.0b013e318190ddb9. [DOI] [PubMed] [Google Scholar]
- 103.Ito K. Yamada Y. Naiki T. Ueda M. Simultaneous implant placement and bone regeneration around dental implants using tissue-engineered bone with fibrin glue, mesenchymal stem cells and platelet-rich plasma. Clin Oral Implants Res. 2006;17:579. doi: 10.1111/j.1600-0501.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 104.Tsai C.H. Hsu H.C. Chen Y.J. Lin M.J. Chen H.T. Using the growth factors-enriched platelet glue in spinal fusion and its efficiency. J Spinal Disord Tech. 2009;22:246. doi: 10.1097/BSD.0b013e3181753ae2. [DOI] [PubMed] [Google Scholar]
- 105.Lee L.T. Kwan P.C. Chen Y.F. Wong Y.K. Comparison of the effectiveness of autologous fibrin glue and macroporous biphasic calcium phosphate as carriers in the osteogenesis process with or without mesenchymal stem cells. J Chin Med Assoc. 2008;71:66. doi: 10.1016/S1726-4901(08)70077-7. [DOI] [PubMed] [Google Scholar]
- 106.Kalia P. Coathup M.J. Oussedik S. Konan S. Dodd M. Haddad F.S., et al. Augmentation of bone growth onto the acetabular cup surface using bone marrow stromal cells in total hip replacement surgery. Tissue Eng Part A. 2009;15:3689. doi: 10.1089/ten.TEA.2008.0676. [DOI] [PubMed] [Google Scholar]
- 107.Lucarelli E. Fini M. Beccheroni A. Giavaresi G. Di Bella C. Aldini N.N., et al. Stromal stem cells and platelet-rich plasma improve bone allograft integration. Clin Orthop Relat Res. 2005;(435):62. doi: 10.1097/01.blo.0000165736.87628.12. [DOI] [PubMed] [Google Scholar]
- 108.Yamada Y. Ueda M. Naiki T. Takahashi M. Hata K. Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 2004;10:955. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 109.Lei H. Xiao R. Tang X.J. Gui L. Evaluation of the efficacy of platelet-rich plasma in delivering BMSCs into 3D porous scaffolds. J Biomed Mater Res B Appl Biomater. 2009;91B:679. doi: 10.1002/jbm.b.31444. [DOI] [PubMed] [Google Scholar]
- 110.Giannoni P. Scaglione S. Daga A. Ilengo C. Cilli M. Quarto R. Short-time survival and engraftment of bone marrow stromal cells in an ectopic model of bone regeneration. Tissue Eng Part A. 2010;16:489. doi: 10.1089/ten.TEA.2009.0041. [DOI] [PubMed] [Google Scholar]
- 111.Haasper C. Breitbart A. Hankemeier S. Wehmeier M. Hesse E. Citak M., et al. Influence of fibrin glue on proliferation and differentiation of human bone marrow stromal cells seeded on a biologic 3-dimensional matrix. Technol Health Care. 2008;16:93. [PubMed] [Google Scholar]
- 112.Patel V.V. Zhao L. Wong P. Pradhan B.B. Bae H.W. Kanim L., et al. An in vitro, in vivo analysis of fibrin glue use to control bone morphogenetic protein diffusion, bone morphogenetic protein-stimulated bone growth. Spine J. 2006;6:397. doi: 10.1016/j.spinee.2005.11.006. discussion 4. [DOI] [PubMed] [Google Scholar]
- 113.Woodruff M.A. Rath S.N. Susanto E. Haupt L.M. Hutmacher D.W. Nurcombe V., et al. Sustained release and osteogenic potential of heparan sulfate-doped fibrin glue scaffolds within a rat cranial model. J Mol Histol. 2007;38:425. doi: 10.1007/s10735-007-9137-y. [DOI] [PubMed] [Google Scholar]
- 114.Hou T. Xu J. Li Q. Feng J. Zen L. In vitro evaluation of a fibrin gel antibiotic delivery system containing mesenchymal stem cells and vancomycin alginate beads for treating bone infections and facilitating bone formation. Tissue Eng Part A. 2008;14:1173. doi: 10.1089/ten.tea.2007.0159. [DOI] [PubMed] [Google Scholar]
- 115.Bensaid W. Triffitt J.T. Blanchat C. Oudina K. Sedel L. Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]