Abstract
Background
Previously, we described a new variant of endemic pemphigus foliaceus (EPF) in Colombia, South America (El Bagre-EPF).
Objective
Continuing our characterization of this variant of EPF, we now focus on one of our previously reported clinical findings: the presence of ocular lesions. These ocular lesions are seen in patients having extensive skin involvement, as measured by the Lund and Browder scale, which is generally used for patients with skin burns.
Methods
We specifically searched for evidence of autoreactivity to various eyelid structures in these patients and correlated our immunologic data with the clinical findings. We performed indirect immunofluorescence studies using normal-appearing human eyelid skin from routine blepharoplasties as substrate tissue. We tested sera from 12 patients with El Bagre-EPF and ocular lesions, 5 patients with sporadic (nonendemic) pemphigus foliaceus, and 20 healthy control subjects (10 from the El Bagre-EPF endemic area and 10 from nonendemic areas). We used fluorescein isothiocyanate conjugated goat antiserum to human total IgG/IgA/IgM as a secondary antibody. In addition, we used fluorescein isothiocyanate conjugated antibodies to human fibrinogen, albumin, IgG, IgE, C1q, and C3, Texas Red (Rockland Immunochemicals, Inc, Gilbertsville, PA), Alexa Fluor 555, or Alexa Fluor 594 (Invitrogen, Carlsbad, CA). Ki-67 (a cell proliferation marker) was used to determine the cell proliferation rate, and nuclear counterstaining was performed with either 4′, 6-diamidino-2-phenylindole or Topro III (Invitrogen, Carlsbad, CA).
Results
We observed autoreactivity to multiple eyelid structures, including meibomian glands and tarsal muscle bundles at different levels, and some areas of the epidermis and the dermis close to the isthmus of the eyelids. Tarsal plate autoreactivity was seen in 10 of 12 of the El Bagre-EPF sera and in one control with pemphigus erythematosus. Furthermore, immunoprecipitation using an eyelid sample as a substrate with 1 mmol/L of sodium orthovanodate showed autoreactivity to several antigens, including some of possible lipid origin.
Limitations
The main limitation of this study is the fact that the antigen or antigens remain unknown.
Conclusion
We identified for the first time to our knowledge autoantibodies to meibomian glands and tarsal muscle in El Bagre-EPF. Our findings suggest that the autoantibodies to the ocular structures cause the clinical and histopathological findings in the ocular lesions in El Bagre-EPF.
Keywords: autoimmunity, endemic pemphigus foliaceus, meibomian glands, tarsal muscle
Endemic pemphigus foliaceus (EPF) is an autoimmune disease occurring in specific geographic foci, mostly in South America. Thus, this disorder represents an interesting model to study how the environment, individual genetics, and immunologic factors can influence autoimmune disease.1-4 We have previously reported a new variant of EPF (El Bagre-EPF) in El Bagre and surrounding rural mining municipalities in Colombia, South America, and compared it with its Brazilian counterpart, fogo selvagem (FS).5,6 The El Bagre-EPF variant resembles Senear-Usher syndrome (pemphigus erythematosus [PE] or seborrheic pemphigus), and differs from other types of pemphigus in many respects.5,6 The primary disease antigens in FS are desmogleins (Dsgs), proteins; recently, E-cadherin was also shown to be an antigen.7 In El Bagre-EPF, in addition to Dsg1, plakin family proteins and other unknown antigens have been detected.5,6 Based on our previous clinical studies, we noted ocular alterations in patients with El Bagre-EPF and significant skin involvement, as measured by the Lund and Browder scale used for patients with burn.8 As a result of the preceding concepts and the predominant seborrheic presentation of this disease, we investigated autoimmune reactivity to eyelid components in these patients.
METHODS
We randomly tested 12 sera from patients with El Bagre-EPF who fulfilled clinical, epidemiologic, and immunologic criteria for this disease, and who showed ocular lesions including conjunctivitis-like changes.5,6,9 Five sera from patients with sporadic pemphigus foliaceus (PF) and 20 sera from healthy control subjects (10 from the El Bagre-EPF endemic area and 10 from nonendemic areas) underwent specific immunologic characterization by indirect immunofluorescence (IF), immunoblotting (IB), and immunoprecipitation (IP) as previously described.9 Indirect IF and IP were conducted as described previously,9 with some modifications to evaluate tissues rich in lipids (ie, the rationale for possible antigens that could be lipid-associated molecules including lipoproteins). For this purpose, we added 1 mmol/L of sodium orthovanodate to the eyelid extracts for IP. All samples were tested anonymously to comply with institutional review board requirements. Table I summarizes the results in all El Bagre-EPF sera.
Table I.
Summary of laboratory findings and common ocular findings in El Bagre-endemic pemphigus foliaceus
| Sex | DX | DIF IgG1 |
DIF IgG total |
IIF IgG4 |
IB-160 | IP 45, 200, 117, 89, 34 |
Red eye | Clinical entropion and/or ectropion, trichiasis, blepharophimosis, thinned eyebrows |
|---|---|---|---|---|---|---|---|---|
| F | El Bagre-EPF 1 | (−) | (+++) | (++) | (++) | (+++) | (+++) | (+++) |
| M | El Bagre-EPF 2 | (−) | (++) | (++) | (1/2 +) | (++) | (++) | (++) |
| F | El Bagre-EPF 3 | (−) | (+++) | (+++) | (++) | (+++) | (++) | (++) |
| M | El Bagre-EPF 4 | (−) | (−) | (+) | (−) | (1/2 +) | (+++) | (+++) |
| M | El Bagre-EPF 5 | (−) | (+) | (++) | (1/2 +) | (+) | (+) | (+) |
| M | El Bagre-EPF 6 | (−) | (++) | (+++) | (−) | (++) | (++) | (++) |
| M | El Bagre-EPF 7 | (−) | (++) | (++) | (+++) | (+++) | (+++) | (+++) |
| M | El Bagre-EPF 8 | (−) | (++) | (+++) | (++) | (++++) | (−) | (−) |
| M | El Bagre-EPF 9 | (−) | (++) | (+++) | (++) | (++++) | (++++) | (++++) |
| M | El Bagre-EPF 10 | (−) | (++) | (++) | (−) | (++) | (++) | (++) |
| M | El Bagre-EPF 11 | (−) | (++) | (++) | (+++) | (+++) | (+++) | (+++) |
| M | El Bagre-EPF 12 | (−) | (++) | (+++) | (−) | (++) | (+++) | (+++) |
| M | PE | (−) | (++) | (+++) | (−) | (++) | (++) | (++) |
| M | PF 1 | (−) | (−) | (+++) | (++) | (45 Only) | (+) | (+) |
| M | PF 2 | (−) | (−) | (+++) | (++) | (200, 45, 34) | (++) | (++) |
| M | NHS 1 | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| M | NHS 2 | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
DIF, Direct immunofluorescence; DX, diagnosis; EPF, endemic pemphigus foliaceus; F, female; IB, immunoblotting; IIF, indirect immunofluorescence; IP, immunoprecipitation; M, male; NHS, normal human serum; PE, pemphigus erythematosus; PF, pemphigus foliaceus.
Indirect IF
We obtained eyelid skin samples from aesthetic reductions in Michel medium (Newcomer Supply, Middleton, WI). We created 4 μm—thick cryostat-cut sections and partially fixed them in paraformaldehyde in phophate-buffered saline (PBS). The slides were then rinsed, partial solubilized in PBS-0.5% Triton X-100 pH 6.8 buffer (Roche Applied Science, Mannheim, Germany), and rinsed again in PBS. Next, the samples were incubated with the sera at 1:20 and 1:40 dilutions in PBS-0.05% Triton. Then, the samples were rinsed twice in PBS and incubated again with secondary antibody cocktails diluted in PBS (goat antihuman total IgG/IgA/IgM— fluorescein isothiocyanate [FITC] conjugated) (Zymed, Invitrogen, Carlsbad, CA). In addition, other slides were incubated with other secondary antibodies: FITC-conjugated rabbit antisera to human fibrinogen, albumin, IgA, IgG, and C3 (DakoCytomation, Carpinteria, CA).
Based on the rationale that the eyelid and the tear film barrier are constantly reformed and replenished, we also used rabbit antihuman IgG conjugated with Alexa 488 (Invitrogen) to see whether the continued cell-renewal process could be affected in this autoimmune disease. After these incubations, the slides were rinsed twice in PBS again, and were incubated with an anti-Ki-67 antigen mouse monoclonal antibody at 1:50 dilution (IgG1, immunogen: recombinant protein representing a 1086—base pair Ki-67 motif-containing complementary DNA fragment) (Vector Laboratories, Inc, Burlingame, CA). Ki-67 is a cell proliferation marker, and our Ki-67 antibody cross-reacts with human, rat, and mouse Ki-67. The slides were washed, and then incubated with a secondary antibody for Ki-67, donkey antimouse IgG (heavy and light), at a 1:100 dilution (Jackson Immuno Research Labs, Jackson, ME). Finally, the slides were counterstained either with DAPI (Pierce, Rockford, IL) or Topro III (Invitrogen), washed, coverslipped, and dried overnight at 4°C.
A second set of experiments was performed using conventional, paraffin-embedded skin samples fixed in 10% buffered formalin, initially processed in the L.F. Montgomery Eye Pathology Laboratory of the Department of Ophthalmology at Emory University Medical Center, Atlanta, GA. For this experiment, we developed our own protocol using paraffin-embedded skin slides cut at 4 μm. For antigen retrieval, the sections were incubated at 60°C for 40 minutes to remove excess paraffin. Then, with cotton-tipped swabs, paraffin was manually removed. This procedure was repeated twice at exactly the same oven temperature. The tissues were rinsed twice with extraction buffer (PBS with 0.5% Tween [Sigma-Aldrich, St Louis MO]) at 37°C for a few minutes. The first antibodies (patient and control sera) were added at the same dilution using the extraction buffer, but in this case the incubation was set overnight at 4°C. For secondary antibodies, in addition to those outlined above, we used rabbit antihuman IgG antiserum conjugated with either Alexa Fluor 555 or Alexa Fluor 594 (Invitrogen). Finally, all sections were observed with a microscope (Eclipse 50i, Nikon, Tokyo, Japan) using a Xenon arc light (XBO 75 W) as the light source and a PL APO ×40/0.80 dry objective. The slides were visualized using both a FITC filter and a triple filter (Nikon) (DAPI [Pierce]/FITC/Texas red triple EX 395-410/490-505/560-585, EM 450-490/515-545/600-652). Simultaneously to the IF evaluations, a quick hematoxylin-eosin stain was performed for each case to evaluate the quality of the tissue used as the antigen source.
IP and IB procedures
The sera were evaluated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis using iodine-125-labeled 48-kd tryptic fragments of Dsg1 purified by Concanavalin A affinity chromatography as described previously.5,6,9 We also performed IB using human whole eyelid skin samples, which were extracted in 62.5 mmol/L Tris-HCl buffer (pH 6.0) containing 0.8 mol/L of orthovanodate (Mallinckrodt Baker, Inc, Phillpsburg, NJ).10 To detect possible lipid antigens we used a lysis buffer containing a protease inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany) and 1 mmol/L of sodium orthovanodate. In brief, SDS extractions were performed using eyelids rinsed with ice-cold Tris buffered saline (TBS), pH 8.0. The meibomian glands and adjacent tissues were scraped and lysed in 0.1% SDS in TBS with 2 mmol/L of phenylmethylsulphonyl fluoride, 20 mmol/L of sodium fluoride, 10 mmol/L of sodium pyrophosphate, a protease inhibitor cocktail (Roche Molecular Biochemicals), and 0.8 mol/L of sodium orthovana-date. An equal volume from each sample was centrifuged at 1000g for approximately 30 minutes at 48C. After adding 1% SDS in TBS with phosphatase inhibitors to the low-speed pellets, the mixture was boiled with intermittent vortexing.10 Protein concentrations were determined using a DC protein assay kit (Bio-Rad DC Protein Assay Kit I, Hercules, CA) and the protein content of homogenates was adjusted to equal concentrations. Equal amounts of protein were resolved using NuPage 4% to 12% Bis-Tris gels (Invitrogen) and identified by IB on PVDF membranes (Invitrogen). Molecular weight standards were used in each gel (Invitrogen). We used horseradish peroxidase-conjugated antitotal human IgG/IgA/IgM antiserum (Southern Biotechnology, Birmingham, AL). The blots were examined using chemiluminescence (Western Lightning Chemiluminescence, PerkinElmer Life Sciences Inc) and exposed to radiographic film (Kodak, Rochester, NY). To show the results of the IB, preincubation was performed for 1 hour at room temperature in 5% nonfat dry milk, 0.05% Tween-20 in 10 mmol/L of Tris-HCl, 154 mmol/L NaCl, pH 7.5.10 Then, the membranes were washed twice for 10 minutes with PBS-Tween-20, and then incubated with the first antibody (either patient or control sera), diluted at 1:75 dilution in 1% bovine serum albumin/PBS-Tween-20, and incubated for 2 hours. The membranes were then washed several times with PBS-Tween-20 for 15 minutes. Next, the membranes were incubated with horseradish peroxidase-labeled secondary antibodies, diluted in 1% bovine serum albumin/PBS-Tween-20, and then incubated with the membranes for 90 minutes. Finally, we washed the membranes several times with PBS-Tween-20. After this, we prepared the chemiluminescence reagent (0.125 mL of chemiluminescence reagent/cm2 of membrane) by mixing equal volumes of the enhanced luminol reagent and the oxidizing reagent, and incubated the membrane in the chemiluminescence reagent for 1 to 3 minutes with shaking. The reaction was stopped when the proteins were visualized.10
Ophthalmic clinical evaluation
Ophthalmic clinical evaluations were performed in an El Bagre local hospital by two residents of the Department of Ophthalmology from the Institute of Health Sciences (Instituto de Ciencias de la Salud) in Medellin, Colombia.
RESULTS
Clinical findings
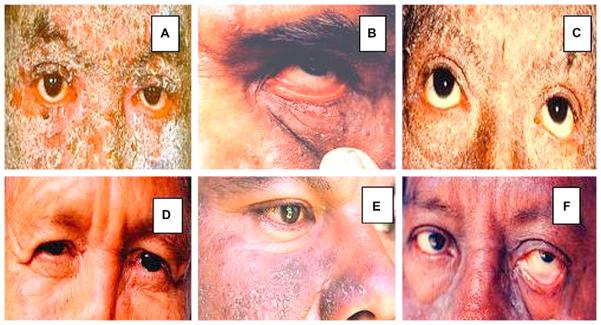
In Table I, we compare major ocular involvement and laboratory findings for the most significant subjects of the study. In Fig 1, we show several pictures revealing ocular area lesions in patients affected by El Bagre-EPF. Our most common clinical findings included: (1) 10 of 12 patients showed meibomianitis and partial occlusion of the meibomian ducts, pingueculae, amblyopia, refractory defects, chalazia, superior tarsal muscle edema or hypertrophy (in the acute stages, likely a consequence of the inflammatory process), red eye, nasal ptyrigia, and melanosis; (2) all 12 patients experienced a sensation of a foreign body in the eye; (3) 3 of 12 patients displayed open angle glaucoma; and (4) 11 of 12 patients displayed cortical-nuclear cataracts. In several cases, bilateral severe swelling, induration, and thickening of eyelids, as well as some conjunctival and lid margin erosions, were appreciated. Fig 1, A, C, and D, show lesions of the palpebral skin, which could indirectly affect eyelid motility (Ameondola).11 The palpebral skin is one of the most delicate structures of the human body. Small undetected blisters or inflammation, especially in exfoliative form, may result in fibrosis; this process could alter the palpebral border, and lead to an oblique direction and contraction of the orbicularis muscle, which would in turn drag the free margin of the eyelid backward. As a consequence, some clinical entropion of the upper lids, tylosis, trichiasis, and blepharophimosis could result, and such conditions are indeed shown. The resulting deviation of the eyelashes of the upper eyelid could cause continuous trauma of the anterior corneal surface, and superficial inflammation in this region, with formation of pannus lesions, vascularized loops, and superficial keratitis, all of which cause severe visual disturbances. The illustrations show rarefaction of the eyebrows, blepharoptosis, blepharophimosis, decrease of the muscular tonus in the lower lid, entropion of the upper lid, slight ectropion of the lower lid, perilimbic vascular invasions, and corneal pannus lesions. Descemetocele causes dryness of the epithelium, infiltration of intima, blisters, and possible fusion of the resulting ulcers. In agreement with the description of Ameondola,11 we found other alterations that included thinned eyebrows, entropion in severe cases, mild blepharophimosis, pseudoptosis, lagophthalmos, muscular atrophy (especially in chronic cases), decreased muscular tone, and inflammation of the tarsal plate (Fig 1). In some cases, descemetocele, perilimbic vascular invasion, corneal pannus lesions, and infiltration of the intima were seen, and in the iris, some diffuse brownish pigmentation with red color was observed as well. Several forms of cataracts were seen, including central opacity in the anterior cortex of the lens, and cortical anterior cataracts, with opaque dots in the entire cortical layer. Intriguingly, the presence of subcapsular cataracts did not differ significantly between the patients and the control subjects. In the control groups of similar age and sex, the main finding was racial melanosis (7/10).
Fig 1.
Clinical images of El Bagre-endemic pemphigus foliaceus (EPF). A, Edema, erythema, and scaling of eyelids with severe ectropion. B, Hyperemia, edema, erythema, and conjunctival injection without mucoid discharge. C, Dry eye and atrophy of superior and inferior tarsal muscles. D, Catarrhal conjunctivitis-like alterations characterized by significant edema of superior and inferior eyelids. Small internal synechiae (as rare finding) resembling nasal ptyrigia in internal cantus of left eye. E, Most common clinical findings include meibomianitis of lower eyelid and partial occlusion of meibomian ducts. F (as in B), Hyperemia, edema, erythema, and conjunctival injection, in this case with mucoid discharge. A, D, C, and F, Thinning of eyebrows.
Immunologic findings
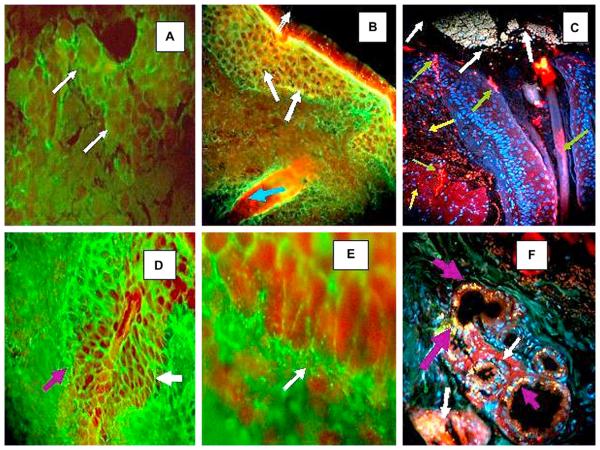
In Fig 2, A, 5 of 12 patients with El Bagre-EPF and one of 5 patients with PF and higher antibody titers to antihuman IgG4 and/or total IgG showed 9 positive intercellular (IC) staining findings in the conjuctiva. Fig 2, A, shows a typical staining seen in a serum from one patient with El Bagre-EPF displaying moderate to bright IC fluorescence among the keratinocytes, and basement membrane zone (BMZ) stain. Control sera consistently revealed negative findings for both of these autoantibody patterns, with the exception of the serum from a seborrheic pemphigus that showed a similar pattern. Fig 2, C and F, present images for the skin samples that were processed with our antigen retrieval technique. In these cases, we were able to see some reactivity to possible cell junctionlike dots when using antibodies to antihuman fibrinogen and antihuman albumin conjugated with FITC. This reactivity was seen around the meibomian glands and their ductus, around the tarsal muscle bundles, and around the isthmus of the eyelid. This dot immunoreactivity seemed to display two different patterns. The first pattern was fine, dotlike puncta, and was noted along the BMZ and ducts of the meibomian glands, as well as around the epidermis and dermis close to the isthmus of the eyelashes. These dot patterns were also consistently seen around the muscle bundles of the tarsal plates (8 of 12 patients with El Bagre-EPF). No control subjects displayed positive results with this pattern with the exception of one patient with seborrheic pemphigus. The second cell junctionlike dot pattern manifested as larger, irregular, and in some cases clumped dots of reactivity, occurring in patterns suggesting polarity within underlying cells. This pattern was observed in some diagonal distributions along the meibomian glands, their BMZ (Figs 2, D and E), ducts, and the tarsal muscle bundles.
Fig 2.
A, B, D, and E, Results of indirect IF (IF) performed after partial fixation with paraformaldehyde. C and F, Results of indirect immunofluorescence performed using paraffin-fixed samples followed by antigen retrieval techniques. A, Using conjuctiva as the antigen source, antihuman conjugated IgG-Alexa 488 (Invitrogen) showed positive ICS and basement membrane zone (BMZ) staining (white arrows). B, Positive intercellular (IC), BMZ, and hair follicle staining using antihuman total IgG antiserum as secondary antibody (green) (fluorescein isothiocyanate [FITC]) (white arrows). Nuclei of cells were stained with Topro III (Invitrogen) (red ). In addition, note strong reactivity of hair follicle bulb to Ki-67 proliferating antigen (red) (Texas Red) (blue arrow). C, Structure (top) that resembles secretion near tear duct, likely of mixed material including mucins, lipids, and other tear components. These components contribute to high, non-Newtonian viscosity of tear film and its low surface tension, features essential for tear film stability (white arrows). In same figure, Ki-67 antigen demonstrated clumped elongated pattern around eyelid base, within isthmus, and in some parts of epidermal layer (red) (green arrows). Positive autoreactivity as small and large dots (red) using Alexa Fluor 555 (Invitrogen) against human IgG (yellow arrows). Nuclei were counterstained with DAPI (Pierce) (blue). D and F, IC and BMZ staining were seen in El Bagre-endemic pemphigus foliaceus using antihuman conjugated IgG FITC (white arrows)(×20). D, BMZ staining of meibomian glands (×100). E, Secretory portion of meibomian gland, in yellow dots, as part of intrinsic fluorescence of these structures (purple arrows). Ki-67 antigen showed positive clumped pattern surrounding involving base of gland ducts on eyelid (white arrows).
In contrast, when we studied the skin samples using the partial fixation of paraformaldehyde (Fig 2, B, D, and F), we were able to see some pattern that reminded us of the IC staining, as seen in pemphigus and along the BMZ of the meibomian glands (×40 and ×20) (Fig 2, D to F). We were thus able to observe that the prefixation of the samples with paraformaldehyde unmasked a true reactivity, which usually in nonfixed skin samples and nonnucleicounterstained samples is routinely considered to be background. In Fig 2, B, C, and F, we were also able to see 3 types of staining patterns using the antiserum to Ki-67 antigen. One pattern of reactivity was seen in single basal cells inside the meibomian glands in close proximity to the detected patient's autoanti-bodies (Fig 2, C) (large blue arrow). The other pattern was seen as an elongated linear staining around some spots of the mostly eyelid isthmus and in the meibomian ductus (Fig 2, C). The final pattern of distribution of Ki-67 was basically exclusive to the secretory part of the ductus of the meibomian glands. This may be related to high levels of cell proliferation.
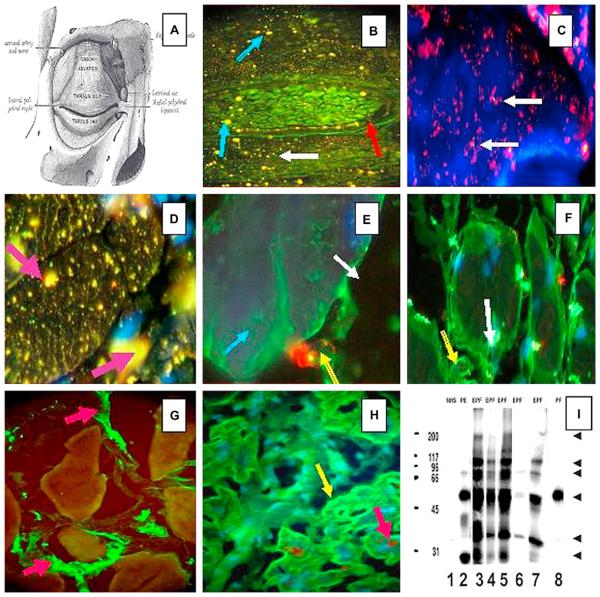
Fig 3 summarizes how we were able to demonstrate, with laboratory findings, those alterations reported by Ameondola11 60 years ago. We were able to demonstrate autoreactivity of the tarsal bundle muscle using eyelid skin as a substrate partially fixed on paraformaldehyde. Consistent with the strong reactivity of tarsal muscle, general muscle atrophy was seen in El Bagre-EPF as described in FS by Ameondola.11 During the past few years, we have noticed possible autoreactivity in some skeletal muscles, and in light of the positive findings of autoreactivity to tarsal muscle, in addition to the fact that some patients with El Bagre-EPF experience significant muscular weakness and sudden death syndrome, we decided to perform some preliminary studies using skeletal and heart muscle. To test this, we used histoarray tissue microarray slides (frozen sections of various normal human organs; 20 organs in duplicate) (Imgenex, San Diego, CA). In this immunoassay, 10 of 12 patients with El Bagre-EPF had positive findings for antitarsal antibodies, and 7 of 10 displayed antibodies to either skeletal or heart muscle (unpublished data). In testing the heart muscle, we used monoclonal anti-connexin-43 antibody (Clone CXN-6; ascites fluid) (Sigma-Aldrich Co, St Louis, MO) as the first antibody, to search for possible colocalization with patients with El Bagre-EPF. As a secondary antibody for the connexin antibody, we used CY3-conjugated, affinity purified donkey antimouse IgG (heavy and light chain) antiserum (red stain). No control subjects had positive findings for indirect IF. Fig 3 shows a series of experiments demonstrating our results. Of particular interest is the finding that one patient's serum with sporadic PE (or seborrheic pemphigus) from Colombia (not from the endemic area), who displayed 75% skin involvement by the Lund and Browder scale, also showed autoantibodies to meibonian glands and to the tarsal and skeletal muscle, but not to the heart. Fig 3, I, shows the results of IP, revealing several antigens that are specifically recognized by the sera from patients with El Bagre-EPF. This image was taken after exposing the membranes to autoradiography film (X-OMAT Blue, Kodak) for 30 seconds. We developed the films by determining an optimum exposure (background vs real bands). Protein bands were quantified by visual examination only, and not with software assistance.
Fig 3.
A, A diagram of the eye superior tarsal muscle (musculus tarsalis superior) showing a panoramic view of a tarsal plaque. (Reprinted from Gray H. Gray's Anatomy of the Human Body. 20th ed. Philadelphia: Lea & Febiger;1918.) B, Direct IF shows a panoramic view of a tarsal plaque with small continuously yellow-greenish dots around the plaque (red arrow). In addition to this, reactivity to larger dots (blue arrows) and smaller ones (white arrow) was observed. These findings were seen using a secondary goat antihuman IgG/IgA/IgM antiserum conjugated with FITC. C, Positive red dots, seen in bundles of tarsal muscle, using antihuman IgG antiserum conjugated with Alexa Fluor 594 (white arrows). D, Using conjugated antihuman IgG Alexa 488 (yellow stains dots), larger bundles of tarsal muscle show positive reactivity of different shapes inside the myocytes (purple arrows). In this case, the reactivity was seen with antihuman fibrinogen FITC conjugate. In addition, thin zig-zag yellowish positivity was seen inside the muscle bundles resembling the staining of sarcoplasmic structures. E, When repeating the experiments using antihuman fibrinogen FITC conjugate alone, the staining shows a positive reactivity outside the myocytes, as seen at higher magnification (green staining) (blue arrow). In addition, there is a structure that resembles a “large-cell junction” (red staining)(yellow arrow) using antihuman IgM antiserum conjugated with rhodamine. The white arrow indicates the extracellular space. F, The white arrow shows autoreactivity to a large structure inside the myocytes using antihuman IgG FITC conjugated. The yellow arrow shows autoreactivity to the plasma membrane. G, We performed IIF testing on heart muscle tissue from sheep, beef, rat, and human, to determine if the reactivity could be observed in multiple species. Positive staining was noted among the muscle bundles (green staining) utilizing FITC conjugated antihuman fibrinogen (red arrows). This staining was noted when rabbit antihuman fibrinogen and albumin antisera were used. H, We used tissue microarray slides as the antigen source with Alexa 488 conjugated and antihuman IgG heavy and light chain (H&L) antiserum. We again showed positivity within muscle bundles (green staining)(yellow arrow). To identify colocalization with the autoreactivity detected by antihuman IgG for gap junctions, we used an antibody to connexin-43 as a control (brown staining)(red arrow). However, the autoreactivity did not superimpose with connexin-43, although confocal microscopy was not performed. (Note that in D, E, F, and H, the cell nuclei were counterstained with DAPI [blue]). I, The sera were tested by IP, and an approximately 45 kDa protein band was strongly recognized by sera with numbers 2, 3, 4, 5, and 7 (and very weakly by number 8). In addition to this band, other antigenic bands were immunoprecipitated, mostly by the El Bagre-EPF patient sera (numbers 2, 3, 4, 5, 7). As a control, we also used normal human serum (NHS) (Lane 1). Lanes 3 to 7 correspond to sera from El Bagre-EPF patients, and lane 8 corresponds to serum from a sporadic PF patient. The molecular weight marker standards are shown in the first lane on the left (200, 117, 96, 66, 45, and 31 kDa, respectively). The arrows on the right point to protein bands of approximately 200, 117, 89, 67 and 34 kDa that were consistently recognized by the El Bagre-EPF sera, and to lesser extent, by the sera from patients with pemphigus erythematosus. Although we show the results of 7 sera, all 12 sera showed similar results.
DISCUSSION
Since the initial report by Francis Senear and Barney Usher regarding PE (ie, Senear-Usher syndrome or seborrheic pemphigus), the anatomic predisposition of this disease for seborrheic areas has been well established.12,13 We have described a new variant of EPF that resembles Senear-Usher syndrome in many aspects.12,13 In our current study, we compared immunologic alterations in the eyelid with the clinical alterations previously observed in patients with El Bagre-EPF. We previously showed that El Bagre-EPF clinically affected predominately seborrheic areas, and that some patients also displayed a predisposition for axillary lesions. The seborrheic areas include the eyelids with modified sebaceous glands (also known as meibomian glands).14,15 Recently, a proteomic analysis of human meibomian gland secretions was reported.16 The analysis demonstrated a complexly large number of heterogeneous molecules, including alpha2-macro-globulin receptor, IgA alpha chain, farnesoid X activated receptor, interferon regulatory factor 3, lacritin precursor, lactotransferrin, lipocalin 1, lysozyme C precursor, potential phospholipid transporting ATPase IK, a transmembrane helix receptor (also termed somatostatin receptor type), and TrkC tyro-sine kinase, development-related NYD-SP21 among others.16 The authors indicated that the meibomian gland secretes a number of proteins into the tear film. It is quite possible that these proteins contribute to the dynamics of the tear film in both healthy and disease conditions. On the other hand, the entire eyelid including the meibomian glands, the tarsal muscle, and the conjunctiva are packed with small blood vessels and are innervated by a fine network of nerves. These nerves are positive to neuron-specific enolase, abundant in smooth and varicose nerve fibers closely apposed to the basement membranes of acini of the meibomian glands. Other neural markers highly expressed in these areas includes the neuropeptide Y and the vasoactive intestinal polypeptide. Nerve fibers and small vessels are also visualized in other eyelid structures, including conjunctiva, epidermis, hair follicles, and subconjunctival and in the lymphoid follicles. We have detected autoantibodies to several nerves and vessels in El Bagre-EPF (Abreu et al, unpublished data).
This study showed for the first time to our knowledge that meibomian gland BMZ and IC junctions seem to be recognized by specific antibodies in patients with El Bagre-EPF and in patients affected by Senear-Usher syndrome. However, the identification of potential antigens is beyond the scope of this article and requires examination. As previously noted, no blisters, scarring, crusts, or pustules have been found clinically on the palms, soles, or oral mucosa of patients with either EPF or sporadic forms of PF (Cazenave pemphigus foliaceus).17-19 Previous studies have shown expression of pemphigus antigens in the epidermis, and that lack of mucosal involvement in PF may be a result of the low expression of Dsg1.18,19 This finding may be inconsistent with ours, because the previous authors did not perform prefixation by paraformaldehyde in the direct and indirect IF or IP. Indeed, we clearly showed a specific antigenic response in those patients with El Bagre-EPF that was different from the results of our own previous studies, performed without these prefixation procedures.8-10
Anatomically, human palms, soles, and oral mucosa do not contain hair follicles or sebaceous glands. The largest numbers of sebaceous glands, and the largest glands, are located on the face and scalp.14,20 Sebaceous glands secrete sebum via a very active holocrine mechanism and could be the explanation for the increased expression of Ki-67.14,20 Significantly, we detected high levels of staining in the BMZ of these glands in patients with El Bagre-EPF and in one sporadic PE control with extensive skin involvement. We also observed different patterns of staining with Ki-67 (a cell proliferation marker), for which the significance and relationship to the pemphigus autoantibodies remain unknown. Thus, we suggest that meibomian gland antigens, including lipids or lipid-associated protein, could be part of this new variant of EPF. Furthermore, these antigens may be present in other types of superficial pemphigus with extensive skin involvement; however, a larger number of samples needs to be tested.
Classically, in pemphigus and bullous pemphigoid, most described antigens are proteins; however, in other diseases, several lipid antigens have been associated with multiple disease processes.21,22 Indeed, lipid rafts are plasma membrane microdomains that have been implicated in the maintenance of diverse cell signaling pathways, such as those mediated by growth factors, morphogens, integrins, and antigen receptors on immune system cells.21,22 Even Dsgs are embedded in this large lipid cell membrane-associated structure. It has been also demonstrated that IgA is secreted by normal human sebaceous and sweat glands. Because it is well known that IgA plays an important role in the inactivation of invading viruses, bacteria, and other antigenic structures on mucous membranes, it appears that IgA in sebum and sweat fulfills a similar function on the outer body surface.23 In fact, some lipid rafts containing a given set of proteins can change their size and composition in response to intra- or extracellular stimuli,24 perhaps inducing abnormal activation of signalling cascades. Eyelid skin involvement has also been reported in sporadic PF. Thus, ocular pemphigus is probably under-diagnosed and its frequency appears to be underestimated.25,26
Our second finding was the autoreactivity detected by indirect IF to several tarsal plate structures, correlating with the tarsal atrophy and other clinical findings described by Ameondola,11 who described tarsal muscle ocular findings not only in PF, but also in FS. Several areas of the tarsal muscle were specifically recognized by the autoantibodies in the sera of patients with El Bagre-EPF. Of note, we did not observe tarsal plate reactivity in sporadic PF sera that did recognize meibomian glands. In addition, some preliminary experiments showed different patterns of autoreactivity to human heart muscle and leg skeletal muscle microarray slides (Imgenex) that did not seem to colocalize with connexin-43, a gap junction protein. In this regard, we need to perform more extensive experiments. We conclude that, in our experiments, the partial prefixation of the skin substrate with paraformaldehyde greatly assisted the process of unmasking target antigens within the meibomian glands and the tarsal muscle. The identity of the antigens in this polyclonal immune response remains to be elucidated. The autoimmune bullous skin diseases, pemphigus (with major subsets pemphigus vulgaris, PF, and paraneoplastic pemphigus) and the more common bullous pemphigoid (with variant disease phenotypes of cicatricial pemphigoid and gestational pemphigoid) may have ocular manifestations. As such, a comparison of all of these bullous diseases needs to be performed.
CAPSULE SUMMARY.
We demonstrate that patients with extensive El Bagre-EPF may have involvement of several ocular structures.
We describe autoantibodies to meibomian glands and tarsal muscle in this form of endemic pemphigus.
Ocular examination should be performed routinely in all patients with pemphigus.
Acknowledgments
We thank Drs Hector Dario Escobar and Liliana Zuluaga, ophthalmologists, from the Institute of Health Science (Instituto de Ciencias de la Salud), Medellin, Colombia, South America, for their clinical evaluation of patients. We also want to thank Dr Weiqing Gao at the Montgomery Eye Pathology Laboratory of the Department of Ophthalmology at Emory University Medical Center for her excellent technical assistance.
Supported by Georgia Dermatopathology Associates (Dr Howard). The El Bagre-endemic pemphigus foliaceus samples were collected with the support of previous grants from the Embassy of Japan in Bogota, Colombia, (Dirección Seccional de Salud de Antioquia), U. de A, Mineros SA (anonymous society) (SA) (Dr Abreu-Velez), Medellin, Colombia, South America, and Emory University Medical Center (Dr Grossniklaus). Our studies were also funded by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by a grant from the Ministry of Health, Labor and Welfare (Research on Intractable Diseases [Dr Hashimoto]).
Abbreviations used
- BMZ
basement membrane zone
- Dsg
desmoglein
- EPF
endemic pemphigus foliaceus
- FITC
fluorescein isothiocyanate
- FS
fogo selvagem
- IB
immunoblotting
- IC
intercellular
- IF
immunofluorescence
- IP
immunoprecipitation
- PBS
phophate-buffered saline
- PE
pemphigus erythematosus
- PF
pemphigus foliaceus
- SDS
sodium dodecyl sulfate
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.Viera J. Pemphigus foliaceus (fogo selvagem): endemic disease of Sao Paolo (Brazil) Arch Dermatol Syph. 1940;211:858. [Google Scholar]
- 2.Proenca N, Ribeira A. Aspectus epidemiologicos do penfigo foliaceo no Brazil [Epidemiologic features of pemphigus foliaceus in Brazil] Rev Assoc Med Bras. 1976;22:281–4. [PubMed] [Google Scholar]
- 3.Castro R, Proenca N. Semelhancas e diferencas entre o fogo selvagem e o penfigo foliaceo de Cazenave [Similarities and differences between South American pemphigus foliaceus and Cazanave's pemphigus foliaceus] An Bras Dermatol. 1983;53:137–9. [Google Scholar]
- 4.Diaz L, Sampaio S, Rivitti EA, Martins CR, Cunha PR, Lombardi C, et al. Endemic pemphigus foliaceus (fogo selvagem): clinical features and immunopathology. J Am Acad Dermatol. 1989;20:657–9. doi: 10.1016/s0190-9622(89)70079-7. [DOI] [PubMed] [Google Scholar]
- 5.Abréu-Vélez AM, Beutner EH, Montoya F, Bollag WB, Hashimoto T. Analyses of autoantigens in a new form of endemic pemphigus foliaceus in Colombia. J Am Acad Dermatol. 2003;49:609–14. doi: 10.1067/s0190-9622(03)00852-1. [DOI] [PubMed] [Google Scholar]
- 6.Abréu-Vélez AM, Hashimoto T, Bollag WB, Tobón-Arroyave S, Abreu-Velez CE, Londoño ML, et al. A unique form of endemic pemphigus in Northern Colombia. J Am Acad Dermatol. 2003;4:599–80. doi: 10.1067/s0190-9622(03)00851-x. [DOI] [PubMed] [Google Scholar]
- 7.Evangelista F, Dasher DA, Diaz LA, Prisayanh PS, Li N. E-cadherin is an additional immunological target for pemphigus autoantibodies. J Invest Dermatol. 2008;128:1710–8. doi: 10.1038/sj.jid.5701260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lund C, Browder N. The estimate of area of burns. Surg Gynecol Obstet. 1944;79:352–8. [Google Scholar]
- 9.Labib RS, Rock B, Martins CR, Diaz LA. Pemphigus foliaceus antigen: characterization of an immunoreactive tryptic fragment from BALB/c mouse epidermis recognized by all patients' sera and major autoantibody subclasses. Clin Immunol Immunopathol. 1990;57:317–29. doi: 10.1016/0090-1229(90)90045-r. [DOI] [PubMed] [Google Scholar]
- 10.Lynch RD, Francis SA, McCarthy KM, Casas E, Thiele C, Schneeberger EE. Cholesterol depletion alters detergent-specific solubility profiles of selected tight junction proteins and the phosphorylation of occluding. Exp Cell Res. 2007;313:2597–610. doi: 10.1016/j.yexcr.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ameondola F. Ocular manifestations of pemphigus foliaceus. Am J Opthalmol. 1949;32:35–44. [PubMed] [Google Scholar]
- 12.Steffen C, Thomas D. The men behind the eponym: Francis E. Senear, Barney Usher, and the Senear-Usher syndrome. Am J Dermatopathol. 2003;5:432–6. doi: 10.1097/00000372-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Castro RM, Augusto DAF, Rivitti EA. Sindrome de Senear-Usher e Fogo selvagem (penfigo foliaceo endemico) An Bras Dermatol. 1988;63(Suppl):264–5. [Google Scholar]
- 14.Serri F, Huber WM. The development of sebaceous glands in man. In: Montagna W, Ellis RA, Silvers AF, editors. Advances in biology of skin. Vol 4. The sebaceous glands. Pergamon Press; New York: 1963. [Google Scholar]
- 15.Den S, Shimizu K, Ikeda T, Tsubota K, Shimmura S, Shimazaki J. Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006;25:651–5. doi: 10.1097/01.ico.0000227889.11500.6f. [DOI] [PubMed] [Google Scholar]
- 16.Tsai PS, Evans JE, Green KM, Sullivan RM, Schaumberg DA, Richards SM, et al. Proteomic analysis of human meibomian gland secretions. Br J Ophthalmol. 2006;90:372–7. doi: 10.1136/bjo.2005.080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro RM, Proença NG. Similarities and differences between Brazilian wild fire and pemphigus foliaceus Cazenave. Hautarzt. 1982;11:574–7. [PubMed] [Google Scholar]
- 18.Stanley JR, Koulu L, Thivolet C. Distinction between epidermal antigens binding pemphigus vulgaris and pemphigus foliaceus autoantibodies. J Clin Invest. 1984;74:313–20. doi: 10.1172/JCI111426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirakata Y, Amagai M, Hanakawa Y, et al. Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol. 1998;110:76–8. doi: 10.1046/j.1523-1747.1998.00085.x. [DOI] [PubMed] [Google Scholar]
- 20.Montagna W, Parakkal PF. The structure and function of skin. 3rd ed. Academic Press; New York: 1974. [Google Scholar]
- 21.Gupta N, DeFranco AL. Visualizing lipid raft dynamics and early signaling events during antigen receptor-mediated B-lymphocyte activation. Mol Biol Cell. 2003;14:432–44. doi: 10.1091/mbc.02-05-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–36. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 23.Metze D, Jurecka W, Gebhart W, Schmidt J, Mainitz M, Niebauer G. Immunohistochemical demonstration of immunoglobulin A in human sebaceous and sweat glands. J Invest Dermatol. 1989;1:13–7. doi: 10.1111/1523-1747.ep13070402. [DOI] [PubMed] [Google Scholar]
- 24.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–9. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 25.Daoud YJ, Foster CS, Ahmed R. Eyelid skin involvement in pemphigus foliaceus. Ocul Immunol Inflamm. 2005;13:389–94. doi: 10.1080/09273940590951025. [DOI] [PubMed] [Google Scholar]
- 26.Palleschi GM, Giomi B, Fabbri P. Ocular involvement in pemphigus. Am J Ophthalmol. 2007;144:149–52. doi: 10.1016/j.ajo.2007.02.046. [DOI] [PubMed] [Google Scholar]