Abstract
High-altitude environments provide ideal testing grounds for investigations of mechanism and process in physiological adaptation. In vertebrates, much of our understanding of the acclimatization response to high-altitude hypoxia derives from studies of animal species that are native to lowland environments. Such studies can indicate whether phenotypic plasticity will generally facilitate or impede adaptation to high altitude. Here, we review general mechanisms of physiological acclimatization and genetic adaptation to high-altitude hypoxia in birds and mammals. We evaluate whether the acclimatization response to environmental hypoxia can be regarded generally as a mechanism of adaptive phenotypic plasticity, or whether it might sometimes represent a misdirected response that acts as a hindrance to genetic adaptation. In cases in which the acclimatization response to hypoxia is maladaptive, selection will favor an attenuation of the induced phenotypic change. This can result in a form of cryptic adaptive evolution in which phenotypic similarity between high- and low-altitude populations is attributable to directional selection on genetically based trait variation that offsets environmentally induced changes. The blunted erythropoietic and pulmonary vasoconstriction responses to hypoxia in Tibetan humans and numerous high-altitude birds and mammals provide possible examples of this phenomenon. When lowland animals colonize high-altitude environments, adaptive phenotypic plasticity can mitigate the costs of selection, thereby enhancing prospects for population establishment and persistence. By contrast, maladaptive plasticity has the opposite effect. Thus, insights into the acclimatization response of lowland animals to high-altitude hypoxia can provide a basis for predicting how altitudinal range limits might shift in response to climate change.
Keywords: acclimatization, countergradient variation, hypoxia tolerance, oxygen transport, physiological genomics
Introduction
High-altitude environments provide fertile ground for investigating mechanisms of physiological adaptation. For air-breathing vertebrates that live at high altitude, hypobaric hypoxia is an unremitting environmental stressor that cannot be mitigated by behavioral avoidance. High-altitude environments are also characterized by low ambient temperatures relative to lowland environments at similar latitudes, and therefore present endothermic animals with the additional physiological challenge of sustaining metabolic heat production in spite of the reduced availability of O2 for aerobic power generation.
In animal species that inhabit a broad range of elevational zones, physiological challenges associated with reduced O2 availability and reduced ambient temperature will tend to increase in severity as a function of altitude. Altitudinal gradients – like latitudinal gradients – are also characterized by relatively uniform and consistent patterns of change in a number of other environmental variables such as net primary productivity. One key difference is that ecotonal transitions across altitudinal gradients occur more rapidly over a given distance than those associated with latitudinal gradients. The spatially fine-grained environmental variation across altitudinal gradients has important implications for the relative roles of genotypic specialization and phenotypic plasticity in physiological adaptation.
When environmental heterogeneity is fine grained, individuals are forced to tolerate a broad range of environmental conditions over the course of their lifetimes. Conversely, when environmental heterogeneity is coarse grained, individuals will experience a more limited range of conditions. An organism's perceived grain of environmental heterogeneity is determined by its life history and dispersal capability. For a set of taxa that are co-distributed across the same altitudinal gradient, spatial variation in environmental conditions would be perceived as fine grained by highly mobile organisms such as birds or bats, and would be perceived as coarse grained by rodents and other organisms that have more restricted dispersal capabilities (Levins, 1968; van Tienderen, 1991; van Tienderen, 1997). Spatially fine-grained environmental heterogeneity is especially conducive to the evolution of phenotypic plasticity, as an increased acclimatization capacity enables organisms to track changes in habitat-specific trait optima. If no single phenotype confers highest fitness across the full range of environments, theory predicts that dispersal among spatially separated subpopulations will generally promote the evolution of phenotypic plasticity over genotypic specialization (Scheiner, 1993; Scheiner, 1998; de Jong and Behera, 2002; Sultan and Spencer, 2002). This theoretical prediction is relevant to animal species that are continuously distributed across steep altitudinal gradients. With regard to the temporal component of environmental heterogeneity, theory predicts that fluctuations that occur on a within-generation timescale will be especially conducive to the evolution of reversible plasticity (Moran, 1992). This would be relevant, for example, to birds that migrate over high mountain passes and therefore have to cope with marked changes in ambient temperature and O2 availability on a daily or seasonal basis.
Environment-dependent variation in the phenotypic expression of a given genotype can be described as a reaction norm, and genotypic variation in reaction norms is referred to as a genotype × environment interaction (Fry, 1992; Via et al., 1995). A given reaction norm can be considered adaptive if the phenotype expressed in each environmental state is in the same direction as the environment-specific optimum favored by selection (Conover and Schultz, 1995; van Tienderen, 1991; van Tienderen, 1997; Ghalambor et al., 2007). During the colonization of a new environment, an adaptive reaction norm would move the mean phenotype closer to the new trait optimum (Price et al., 2003; Ghalambor et al., 2007). By contrast, a maladaptive reaction norm would move the mean phenotype further away from the new optimum, and would therefore require selection on genetically based trait variation to counteract the environmentally induced changes. In cases in which the trait optimum is unchanged in the newly colonized environment, a maladaptive reaction norm would require compensatory genetic changes to restore the ancestral phenotype (Grether, 2005). Thus, maladaptive plasticity can result in a form of cryptic adaptive evolution in which phenotypic similarity between ancestral and derived populations is attributable to the effects of divergent selection that offset environmentally induced trait differences.
Much of our understanding of the acclimatization response to hypoxia derives from studies of animal species that are native to lowland environments. These studies can reveal whether the hypoxia-induced physiological response tends to move the population mean phenotype closer to the fitness optimum of the nonnative high-altitude environment. The nature of the ancestral acclimatization response can therefore indicate whether phenotypic plasticity can be expected to facilitate or impede adaptation to high altitude. Here, we review general mechanisms of physiological acclimatization and genetic adaptation to high-altitude hypoxia in birds and mammals. Rather than reviewing these mechanisms in detail, we evaluate whether the acclimatization response to environmental hypoxia can be regarded generally as a mechanism of adaptive phenotypic plasticity, or whether it represents a misdirected response that works at cross purposes with genetically based physiological adaptation.
The nature of physiological adaptation to high-altitude hypoxia
Under hypoxic conditions, many animals are able to compensate for a reduced O2 supply by suppressing total metabolism, thereby reducing O2 demand (Ramirez et al., 2007). In many endotherms that are native to high-altitude environments, metabolic suppression is not a viable option for coping with reduced O2 availability owing to the exigencies of aerobic thermogenesis and/or aerobic exercise. In birds, for example, O2 consumption increases 15- to 20-fold during flight (Ward et al., 2002). In such cases, matching O2 supply with an undiminished or even increased O2 demand requires an enhanced flux capacity of the O2 transport pathway.
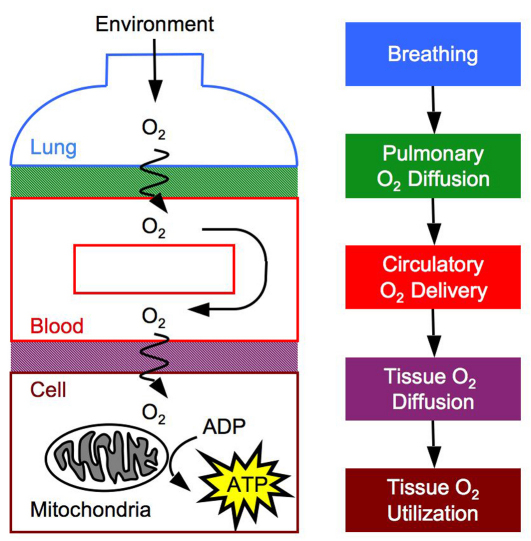
There are several convective and diffusive steps in the O2 cascade from inspired air to the tissue mitochondria where physiological adjustments can preserve the rate of O2 flux in spite of hypoxia, thereby ensuring an uninterrupted supply of O2 to the energy-producing machinery of the cells (Fig. 1). These steps include ventilatory convection, diffusion across the blood–gas interface, circulatory convection, diffusion across the blood–tissue interface (including facilitated diffusion by myoglobin), and O2 utilization by the tissue mitochondria.
Fig. 1.
O2 is transported from atmospheric air to the mitochondria of tissue cells along a pathway with several diffusive and convective steps. The steps in this O2 cascade are breathing, O2 diffusion across the blood–gas interface, circulation of O2 throughout the body, O2 diffusion across the blood–tissue interface to the mitochondria, and O2 utilization to generate ATP by oxidative phosphorylation.
Mechanisms of phenotypic plasticity and genetic adaptation in the O2 cascade
Pulmonary O2 transport
The effectiveness of O2 loading during high-altitude hypoxia is strongly influenced by plasticity in the pulmonary system, which comprises the first two steps in the O2 transport pathway (Fig. 1). The rate of O2 transport in the air compartment of the lungs can be described as the product of ventilation (V) and the inspired–alveolar difference in O2 partial pressure (PO2) (i.e. PIO2–PAO2). High rates of ventilation therefore help maintain a high alveolar PO2, the pressure head for diffusion across the blood–gas interface. The rate of O2 transport across the blood–gas interface can be described as the product of pulmonary O2 diffusion capacity (DL) and the alveolar–blood PO2 difference (PAO2–PbO2). The decrement in PbO2 is therefore mitigated by an enhanced pulmonary O2 diffusion capacity, which is determined by lung morphology and pulmonary perfusion.
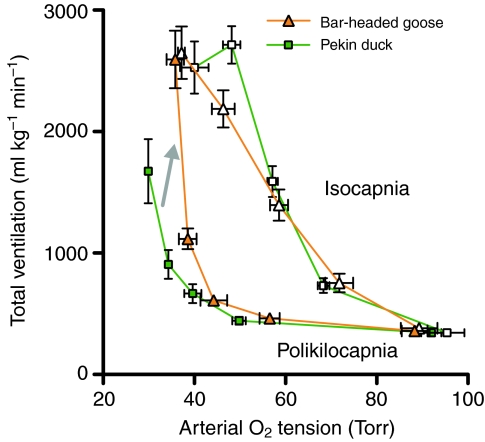
The most important factors affecting ventilation at high altitude are the partial pressures of O2 and CO2 and the pH of arterial blood (Scott and Milsom, 2009). Breathing is stimulated when a decline in arterial PO2 (PaO2; hypoxemia) is sensed by chemoreceptors in the carotid bodies. However, this hypoxic ventilatory response increases respiratory CO2 loss, causing a secondary hypocapnia (low CO2) and alkalosis (high pH) in the blood. Because CO2 and H+ normally stimulate breathing via central and peripheral chemoreceptors, secondary hypocapnia and alkalosis restrain the ventilatory response. The net effect is an increase in ventilation that is dependent on the severity of hypoxia, but the magnitude of this increase is less than if blood CO2 levels had remained constant (Fig. 2) (Scott and Milsom, 2009). The magnitude of the hypoxic ventilatory response is also dependent on the duration of hypoxia (Powell et al., 1998). Compared with the rapid response that occurs within minutes of the onset of hypoxia, several days of acclimatization can further increase ventilation owing to plasticity in the O2 sensing function of the carotid body chemoreceptors and in central integration sites within the neural feedback loop that regulates breathing (Black and Tenney, 1980; Powell, 2007). Over many months at high altitude, this hypoxic ventilatory response can be gradually attenuated by hypoxic desensitization (Powell et al., 1998; Brutsaert, 2007). It is difficult to ascribe adaptive significance to these various responses owing to the multiple functions of breathing. Increases in breathing during the acute response and early phases of acclimatization clearly improve O2 uptake during hypoxia. Conversely, hypoxic desensitization might help to maintain blood CO2/pH homeostasis, reduce respiratory water loss, or reduce the metabolic cost of breathing at the expense of diminished O2 uptake (Powell, 2007).
Fig. 2.
The ventilatory response to environmental hypoxia causes a secondary loss of CO2, and the resulting hypocapnia (low arterial CO2 tension) and alkalosis (high arterial pH) inhibit breathing. As a result, the hypoxic ventilatory response (HVR) when CO2 is experimentally maintained (isocapnia, white symbols) is higher than in the environmentally realistic situation when CO2 is uncontrolled and allowed to fall (poikilocapnia, filled symbols). However, bar-headed geese (orange triangles) are less sensitive to hypocapnia than are low-altitude birds, and thus exhibit an enhanced ventilatory response to acute environmental hypoxia (gray arrow). This is reflected by a higher poikilocapnic HVR in bar-headed geese than in pekin ducks (green squares) but a similar isocapnic HVR. Data are means ± s.e.m., modified from Scott and Milsom (Scott and Milsom, 2007). 1 Torr≈133 Pa.
The morphological capacity for O2 diffusion across the lungs is generally increased in lowlanders after acclimatization to high-altitude hypoxia. Development or prolonged residence at high altitude can increase pulmonary diffusion capacity by increasing the surface area available for diffusion and by reducing the thickness of the pulmonary blood–gas interface (Hsia et al., 2005; Hsia et al., 2007; Ravikumar et al., 2009). Although relatively little is known about how O2 levels control lung morphogenesis during development or during adult life stages (Metzger et al., 2008), these induced changes in lung morphology clearly help improve O2 transport during hypoxia.
In lowland species, the acclimatization response to hypoxia generally involves a maladaptive change in pulmonary perfusion, the physiological component of pulmonary O2 diffusion. Hypoxia directly stimulates the endothelial and smooth muscle cells in pulmonary blood vessels, causing vasoconstriction throughout the lungs and an increase in pulmonary blood pressure that can persist for prolonged durations at high altitude (Reeves and Grover, 1975; Gurney, 2002). This global vasoconstriction impairs O2 diffusion because it can divert blood flow away from the gas exchange surface to pulmonary shunt vessels (Lovering et al., 2008), and the resultant pulmonary hypertension can cause fluid leakage into the air spaces, which in turn causes a thickening of the O2 diffusion barrier (Maggiorini et al., 2001; Eldridge et al., 2006). Hypoxic pulmonary hypertension can also overburden the right ventricle of the heart and can contribute to pathophysiological conditions, such as chronic mountain sickness (Monge and León-Velarde, 1991; León-Velarde et al., 2010).
Some features of the acclimatization response to hypoxia can improve pulmonary O2 transport at high altitude, whereas other features can be counterproductive. Populations at high altitude could therefore benefit from enhancing the facilitatory mechanisms or suppressing the inhibitory ones. Some highland species or populations are characterized by a heightened acute hypoxic ventilatory response, a more effective breathing pattern, a maintained acclimatization response, and/or a prevention of hypoxic desensitization (Black and Tenney, 1980; Beall et al., 1997; Wu and Kayser, 2006; Brutsaert, 2007; Scott and Milsom, 2007; Pichon et al., 2009). The underlying chemoreceptive mechanisms are not necessarily convergent at the cellular level. For example, the enhanced ventilatory response to acute hypoxia in the high-altitude bar-headed goose (Anser indicus) is partly due to an insensitivity to hypocapnia (Fig. 2) (Scott and Milsom, 2007; Scott and Milsom, 2009), whereas responses of high-altitude humans generally involve changes in O2 sensitivity (Brutsaert, 2007). Other highland species or populations exhibit a blunted ventilatory response to acute hypoxia (Blake and Banchero, 1985; Brutsaert et al., 2005), which would be maladaptive at high altitudes without compensatory changes at other steps in the O2 transport pathway that maintain peripheral O2 supply. As might also be the case for hypoxic desensitization, this blunted response might help maintain blood CO2/pH homeostasis or it might reduce the costs of breathing (Hochachka, 1998; Powell, 2007).
The ancestral acclimatization response for the morphological component of pulmonary O2 diffusion will facilitate pulmonary O2 transport at high altitude. However, pulmonary O2 diffusion capacity can be enhanced further in some high-altitude adapted groups that have larger lungs than lowlanders (Brutsaert et al., 1999; Wu and Kayser, 2006; Brutsaert, 2007; Scott et al., 2010). This is particularly true in birds because their lungs are rigid and are ventilated by air sacs. The larger lungs of the high-altitude bar-headed goose (Scott et al., 2010), for example, should afford a greater surface area for diffusion and thus reduce the PO2 difference between parabronchial gas and arterial blood. By contrast, the ancestral acclimatization response for the physiological component of pulmonary O2 diffusion will impede pulmonary O2 transport at high altitudes. Not surprisingly, the pulmonary vasoconstriction response is blunted or lost altogether in species that are adapted to high altitudes (Faraci et al., 1984a; Durmowicz et al., 1993; Groves et al., 1993; Ge et al., 1998; Sakai et al., 2003), which could represent an example of genetic compensation.
Blood O2 transport
Under conditions of hypoxic hypoxemia, when the PO2 of arterial blood (PaO2) is reduced, transport of O2 by blood must meet a dual challenge: (1) it must maintain an adequate delivery of O2 to the peripheral tissues; and (2) it must maintain an adequate PO2 at the vascular supply source to permit O2 diffusion to the tissue mitochondria.
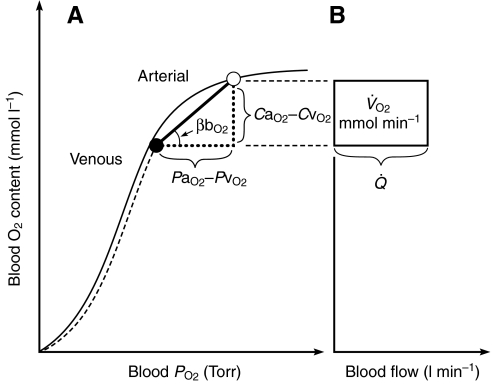
At a given PaO2, O2 delivery can be enhanced by increasing the total cardiac output, 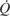 , and by increasing the blood O2 capacitance coefficient, βbO2 (Fig. 3). The latter parameter is defined as the ratio (CaO2–CvO2)/(PaO2–PvO2), where CaO2–CvO2 is the arterial–venous difference in O2 concentration and PaO2–PvO2 is the arterial–venous difference in PO2. With regard to the second challenge mentioned above, the lower critical PO2 at the vascular supply source for tissue oxygenation can be expressed as
, and by increasing the blood O2 capacitance coefficient, βbO2 (Fig. 3). The latter parameter is defined as the ratio (CaO2–CvO2)/(PaO2–PvO2), where CaO2–CvO2 is the arterial–venous difference in O2 concentration and PaO2–PvO2 is the arterial–venous difference in PO2. With regard to the second challenge mentioned above, the lower critical PO2 at the vascular supply source for tissue oxygenation can be expressed as 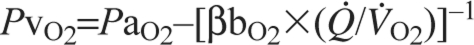 , where
, where 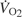 is the rate of O2 consumption by the tissues and the product
is the rate of O2 consumption by the tissues and the product 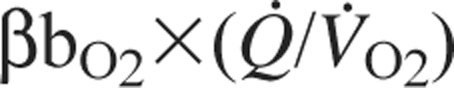 is the specific blood O2 conductance (Dejours et al., 1970; Bouverot, 1985). Because PaO2 is determined by ventilation and O2 equilibration at the blood–gas interface, this equation shows that an increase in the specific blood O2 conductance minimizes the decline in PvO2 under hypoxia, thereby maintaining an adequate pressure head for O2 diffusion to the tissue mitochondria.
is the specific blood O2 conductance (Dejours et al., 1970; Bouverot, 1985). Because PaO2 is determined by ventilation and O2 equilibration at the blood–gas interface, this equation shows that an increase in the specific blood O2 conductance minimizes the decline in PvO2 under hypoxia, thereby maintaining an adequate pressure head for O2 diffusion to the tissue mitochondria.
Fig. 3.
Schematic illustration of blood O2 transport. (A) O2-equilibrium curve under physiochemical conditions prevailing in arterial blood (a, solid curve, open symbol) and venous blood (v, dashed curve, closed symbol). The x-axis measures blood PO2 and the y-axis measures blood O2 content. CaO2–CvO2 denotes the arterial–venous difference in O2 content, PaO2–PvO2, denotes the corresponding difference in PO2, βbO2 denotes the blood O2 capacitance coefficient (see text for details),  denotes cardiac output, and
denotes cardiac output, and 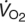 denotes the rate of O2 consumption. (B) The area of the rectangle is proportional to total O2 consumption, which can be enhanced by increasing
denotes the rate of O2 consumption. (B) The area of the rectangle is proportional to total O2 consumption, which can be enhanced by increasing  and/or by increasing the βbO2. Increases in the βbO2 produce a corresponding increase in CaO2–CvO2 through shifts in the shape or position of the O2-equilibrium curve.
and/or by increasing the βbO2. Increases in the βbO2 produce a corresponding increase in CaO2–CvO2 through shifts in the shape or position of the O2-equilibrium curve.
Under severe hypoxia, an increased blood–O2 affinity will tend to maximize βbO2 because the arterial–venous PO2 difference spans the steepest portion of the sigmoidal O2-equilibrium curve (Fig. 3). This can be visualized as an increase in the slope of the line connecting the arterial and venous points on the curve. The resultant increase in the specific blood O2 conductance helps meet the dual challenge mentioned above: it minimizes the inevitable PO2 decrement in the tissue capillaries while preserving a constant CaO2–CvO2 difference and, hence, a constant net O2 flux. Likewise, an increased hemoglobin (Hb) concentration increases CaO2, thereby increasing blood O2 conductance if PaO2, 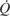 and
and 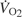 all remain constant. With excessive polycythemia, however, potential advantages of an increased Hb concentration for O2 carrying capacity might be more than offset by a corresponding reduction in
all remain constant. With excessive polycythemia, however, potential advantages of an increased Hb concentration for O2 carrying capacity might be more than offset by a corresponding reduction in 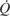 .
.
Erythropoeitic activity
When lowland natives ascend to high altitude, the acclimatization response to hypoxia generally entails correlated increases in hematocrit (Hct) and Hb concentration, but the adaptive significance of this erythropoeitic response is questionable (Bullard, 1972; Monge and Whittenbury, 1976; Winslow and Monge, 1987; Monge and Leon-Velarde, 1991). Under normoxia, a moderately increased Hct augments arterial O2 content and therefore enhances aerobic capacity (Ekblom and Hermansen, 1968; Kanstrup and Ekblom, 1984; Ekblom and Berglund, 1991). However, the highest attainable Hct is not necessarily associated with the highest attainable aerobic power output (Crowell et al., 1959; Crowell and Smith, 1967; Villafuerte et al., 2004; Schuler et al., 2010). This is because the associated increase in blood viscosity produces a higher peripheral vascular resistance that might compromise 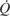 , thereby reducing VO2,max (Guyton and Richardson, 1961; Connes et al., 2006). It is reasonable to expect that a moderately increased Hct could confer a physiological advantage under hypoxia because a greater quantity of O2 can be carried at partial saturations, but experimental results do not support this (McGrath and Weil, 1978; Winslow et al., 1985). In humans at high altitude, evidence suggests that the optimal Hb concentration might actually be quite close to the typical sea level value (Villafuerte et al., 2004).
, thereby reducing VO2,max (Guyton and Richardson, 1961; Connes et al., 2006). It is reasonable to expect that a moderately increased Hct could confer a physiological advantage under hypoxia because a greater quantity of O2 can be carried at partial saturations, but experimental results do not support this (McGrath and Weil, 1978; Winslow et al., 1985). In humans at high altitude, evidence suggests that the optimal Hb concentration might actually be quite close to the typical sea level value (Villafuerte et al., 2004).
In humans living at high altitude, hypoxia-induced polycythemia often vastly exceeds the optimal Hct. For example, in Andean natives living at altitudes of >3000 m, Hct and Hb concentrations can reach values of up to 0.83 and 270 g l–1, respectively (Bouverot, 1985). This excessive polycythemia limits exercise performance, 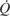 and pulmonary function, and appears to play a key role in the pathogenesis of chronic mountain sickness (Winslow et al., 1985; Monge and León-Velarde, 1991; Rivera-Ch et al., 2007). Richards (Richards, 1960) provided a vivid description of the effects of hypoxia-induced polycythemia in patients suffering from chronic pulmonary disease: “The increased red cell mass produces increased blood volume, which overfills the heart; and this organ, already under strain working against a restricted pulmonary vascular bed, goes into congestive heart failure, which still further increases blood volume. At the same time the added pulmonary vascular engorgement further aggravates the [hypoxia], thus setting up a second vicious cycle.”
and pulmonary function, and appears to play a key role in the pathogenesis of chronic mountain sickness (Winslow et al., 1985; Monge and León-Velarde, 1991; Rivera-Ch et al., 2007). Richards (Richards, 1960) provided a vivid description of the effects of hypoxia-induced polycythemia in patients suffering from chronic pulmonary disease: “The increased red cell mass produces increased blood volume, which overfills the heart; and this organ, already under strain working against a restricted pulmonary vascular bed, goes into congestive heart failure, which still further increases blood volume. At the same time the added pulmonary vascular engorgement further aggravates the [hypoxia], thus setting up a second vicious cycle.”
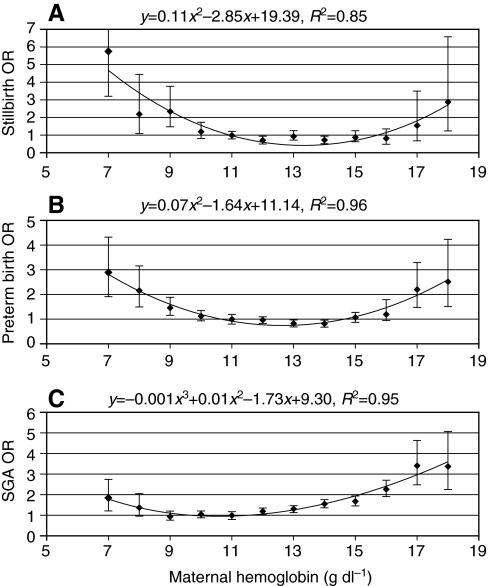
In humans, the potential fitness consequences of excessive polycythemia seem especially clear cut when considering the effects on pregnancy outcomes at high altitude (Moore et al., 2004; Gonzales et al., 2009; Julian et al., 2009a; Julian et al., 2009b). Hospital-based studies have documented that the risk of adverse perinatal outcomes is increased with extreme maternal Hcts (Moore et al., 2001a; Gonzales et al., 2009) (Fig. 4). During pregnancy at high altitude, elevated Hct and the associated increase in blood viscosity contribute to an increased risk of stillbirth, preterm birth, and reduced birth weight, all of which appear to stem from a reduced uterine artery blood flow and, consequently, a reduced rate of O2 delivery to the uteroplacental circulation (Moore et al., 1982; Moore et al., 2001a; Moore et al., 2001b; Zamudio et al., 1995; Wilson et al., 2007; Julian et al., 2008; Julian et al., 2009b). For this reason, acclimatized lowlanders at high altitude generally experience especially high rates of adverse pregnancy outcomes. In humans living at high altitude, the available evidence suggests that hypoxia-induced polycythemia represents an example of maladaptive plasticity.
Fig. 4.
Odds ratios (OR) for stillbirths (A), preterm births (B) and small-for-gestational-age (SGA) births (C) as a function of maternal Hb concentration. Data are compiled from 37,377 women residing at low (150 m) and high (>3000 m) altitudes (2003–2006) (modified from Gonzales et al., 2009).
Blood–O2 affinity
Fine-tuned adjustments in blood–O2 affinity can play a key role in matching O2 supply and O2 demand by shifting the steepest portion of the O2-equilibrium curve within the range of in vivo PO2s (Bouverot, 1985; Samaja et al., 2003). These adjustments could be mediated by changes in the intrinsic Hb–O2 affinity, changes in the sensitivity of Hb to allosteric cofactors that modulate Hb–O2 affinity, and/or changes in the concentration of allosteric cofactors within the erythrocyte (Nikinmaa, 2001; Weber and Fago, 2004; Weber, 2007; Storz and Moriyama, 2008). The latter mechanism involves regulatory changes in the chemical milieu within the erythrocyte and does not require structural modifications of the Hb protein itself. In the enucleated erythrocytes of most mammals, the most potent allosteric cofactor is 2,3-diphosphoglycerate (DPG), a metabolite of glycolysis [for exceptions, see Bunn (Bunn, 1980)]. In the nucleated erythrocytes of birds, the most potent allosteric cofactor is typically inositol pentaphosphate (InsP5), a metabolite of oxidative phosphorylation. Ectothermic vertebrates typically make use of other nucleotide triphosphates (Nikinmaa, 2001). These various allosteric cofactors generally reduce Hb–O2 affinity by preferentially binding and stabilizing the deoxygenated, ‘tense-state’ conformation of the Hb tetramer. Other cofactors such as H+ and Cl– ions exert similar allosteric effects, thereby facilitating O2 unloading in the tissue capillaries (Riggs, 1988; Nikinmaa, 2001; Mäirbaurl, 1994; Jensen, 2004; Weber and Fago, 2004).
In mammals that are native to lowland environments, the acclimatization response to hypoxia typically involves a DPG-induced reduction in Hb–O2 affinity (a rightward shift in the O2-equilibrium curve), which might be mediated primarily by an increase in the erythrocytic concentration of DPG (Lenfant et al., 1968; Lenfant et al., 1971; Mäirbaurl et al., 1993). In humans, for example, a rapid increase in the intracellular DPG concentration occurs within 24 h of ascent to high altitude (>4500 m), and the resultant decrease in Hb–O2 affinity is partially offset by the respiratory alkalosis induced by increased ventilation (Lenfant et al., 1968; Lenfant et al., 1971; Weiskopf and Severinghaus, 1972).
Modulating the erythrocytic concentration of allosteric cofactors can be considered to be a mechanism of phenotypic plasticity because the same genotype (manifest as Hb structure) can express a range of different phenotypes (blood–O2 affinities) depending on the prevailing circumstances. The adaptive significance of the DPG-induced decrease in Hb–O2 affinity at high altitude has been the subject of much debate. Under conditions of moderate hypoxia, a reduction in Hb–O2 affinity can enhance levels of tissue oxygenation by maximizing the capillary–tissue PO2 difference. However, under conditions of severe hypoxia when PaO2 is substantially reduced, a leftward shift of the O2-equilibrium curve is required to allow O2 loading and unloading over the steepest portion of the curve (Turek et al., 1973; Turek et al., 1978a; Turek et al., 1978b; Samaja et al., 1986; Samaja et al., 2003). Even at low altitude, a left-shifted curve might be advantageous when the rate of O2 transfer across the blood–gas interface is diffusion limited, as is the case during intense exercise (Bencowitz et al., 1982). In rats, a pharmacologically increased Hb–O2 affinity was shown to greatly enhance metabolic performance and survival under conditions of extreme hypoxia (Eaton et al., 1974; Turek et al., 1978a; Turek et al., 1978b), and human subjects with mutant Hbs that exhibit increased O2 affinity (Hb Andrews-Minneapolis) maintain normal arterial O2 saturation and undiminished aerobic capacity at high altitude (3100 m) relative to subjects with wild-type Hb (Hebbel et al., 1978). It therefore appears that the DPG response of lowlanders might be maladaptive at high altitudes.
In contrast to the hematological changes that are typically associated with the acclimatization response to hypoxia in lowland mammals, genetically based changes in Hb structure that increase intrinsic O2 affinity or that suppress sensitivity to allosteric cofactors are generally thought to make more important contributions to hypoxia tolerance in species that are high-altitude natives (Bunn, 1980; Monge and León-Velarde, 1991; Storz, 2007; Weber, 2007; Storz and Moriyama, 2008). This is because an increased Hb–O2 affinity promotes pulmonary O2 loading at low PO2, and can thus amplify the effects of pulmonary adaptations to increase PaO2. However, an increased Hb–O2 affinity also hinders O2 unloading in the tissue capillaries. Other modulators of Hb–O2 binding might be important in this regard, such as the Bohr effect (reduction in Hb–O2 affinity at low pH). This trade-off in Hb function between normoxic and hypoxic environments can give rise to trade-offs in whole-organism aerobic performance (Hebbel et al., 1978; Chappell and Snyder, 1984; Chappell et al., 1988).
Tissue O2 transport
Similar to the situation for pulmonary O2 diffusion, the rate of O2 transport from blood to mitochondria in tissues can be described as the product of tissue O2 diffusion capacity (DT) and the blood–mitochondria PO2 difference (PbO2–PmitO2). Under hypoxia, the decrement in PO2 at mitochondria is mitigated by an enhanced O2 diffusion capacity. O2 diffusion at peripheral tissues has both morphological and physiological components. The morphological capacity for O2 diffusion is determined by the density and distribution of capillaries in peripheral tissues, as well as by the cellular arrangement of mitochondria (Fig. 5). Regional tissue perfusion (capillary recruitment) represents the physiological component, which determines the extent to which the morphological capacity for O2 diffusion is realized. Tissue O2 transport will be optimized at high altitudes by full perfusion of abundant and homogeneously spaced capillaries, and by mitochondria that are positioned close to capillaries.
Fig. 5.
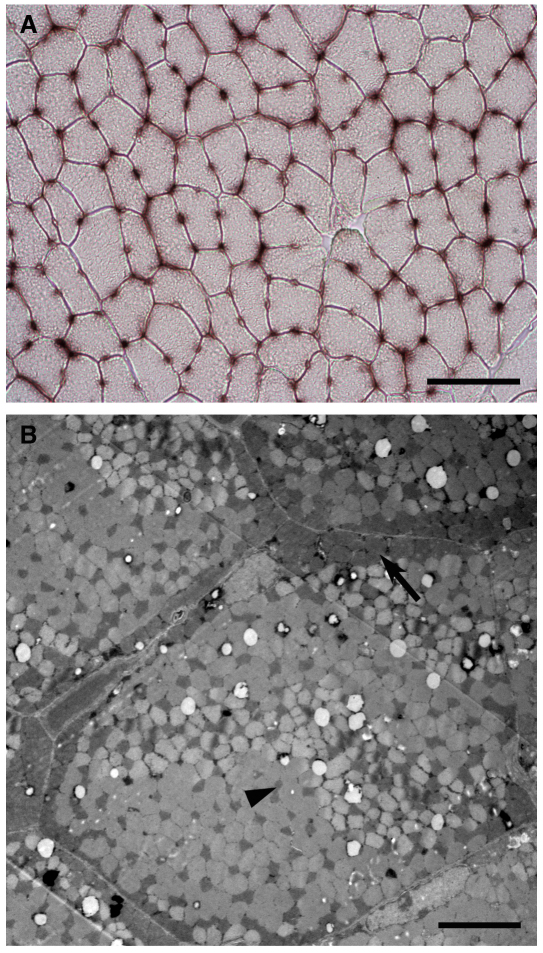
The diffusion capacity for O2 is enhanced in the flight muscle of the bar-headed goose, a species that lives at elevation and migrates over the Himalayas at extremely high altitudes. This is accomplished by an increase in capillarity and a redistribution of mitochondria towards the cell membrane. (A) Histological staining for alkaline phosphatase activity identifies abundant capillaries in the pectoralis muscle of a bar-headed goose (scale bar: 50 μm). (B) Transmission electron micrograph of a fast-oxidative muscle fiber in a bar-headed goose showing the abundant subsarcolemmal mitochondria (arrow) and the less common intermyofibrillar mitochondria (arrowhead) (scale bar: 5 μm). See Scott et al. (Scott et al., 2009a) for details.
It is unclear whether the morphological capacity for O2 diffusion is altered by high-altitude acclimatization in low-altitude species. In general, there is a strong relationship between capillarity and metabolic demand [e.g. mitochondrial abundance (Hepple, 2000)], but capillarity must increase relative to demand for tissue O2 transport to be improved in hypoxia. The issue of whether high-altitude acclimatization increases capillarity has been contentiously debated, based on methodological and conceptual arguments related to both O2 supply and metabolic demand (Mathieu-Costello, 2001). There are examples where capillarity in the muscle has increased during high-altitude acclimatization without any change in mitochondrial abundance (Mathieu-Costello and Agey, 1997), but it is generally thought that any changes in capillarity occur as a secondary consequence of alterations in metabolic demand or cell size (Lewis et al., 1999; Hepple, 2000; Hoppeler and Vogt, 2001). The arrangement of mitochondria within cells must trade-off between reducing the intracellular diffusion distance for O2 and reducing that for intracellular metabolites, which will be optimized when mitochondria are situated in predominantly subsarcolemmal (i.e. clustered near the cell membrane) or intermyfibrillar (i.e. distributed throughout the cell) locations, respectively (Fig. 5B) (Kinsey et al., 2007). Acclimatization to high-altitude hypoxia does not appear to alter mitochondrial distribution in muscle, based on a study of chronic hypoxia exposure in rats (van Ekeren et al., 1992). This contrasts with the proliferation of subsarcolemmal mitochondria that is induced when hypoxia is experienced only during short daily bouts of exercise (Schmutz et al., 2010). Although comprehensive support remains to be collected, low-altitude species appear to exhibit negligible plasticity in the morphological capacity for O2 diffusion during high-altitude acclimatization.
Lowlanders at high altitude might sacrifice perfusion of muscle and some other peripheral tissues. Regional tissue perfusion during hypoxia is regulated by local paracrine signals that promote vasodilation (e.g. nitric oxide) and central signals that regulate vasoactivity in a tissue-specific manner (e.g. sympathetic neural activity). The former mechanism tends to increase blood flow and capillary recruitment, particularly to metabolically active tissues (Heinonen et al., 2010). By contrast, the latter mechanism can promote a redistribution of blood flow away from peripheral tissues during hypoxia in favor of hypoxia-sensitive tissues, such as the heart and brain (Hochachka, 1985). This reduction in blood flow to peripheral tissues might help animals tolerate short periods of O2 lack, but it is not overcome after prolonged acclimatization to high altitude (Kamitomo et al., 1993; Hansen and Sander, 2003). Peripheral O2 supply can remain impaired even when pulmonary and hematological adjustments restore arterial oxygenation to normoxic levels (Calbet et al., 2003), possibly because of the complex integration of feedforward and feedback influences from the central nervous system, the heart and the peripheral tissues (Noakes et al., 2004). The redistribution of blood flow might therefore represent a maladaptive response to high-altitude hypoxia.
In principle, tissue O2 transport could be improved at high altitude by enhanced morphological capacities for O2 diffusion. This has occurred in some high-altitude species, such as the bar-headed goose, which has expanded capillarity in the flight muscle and heart, as well as mitochondria that are redistributed closer to the more abundant capillaries (Fig. 5) (Scott et al., 2009a; Scott et al., 2010). These unique traits are canalized, occurring without any prior exposure to high altitude. The benefit of these specializations is probably restricted to tissues that are active during flight, because running exercise is not well sustained in bar-headed geese during hypoxia (Fedde et al., 1989). However, this phenotype does not appear to be a universal evolutionary response to high altitude, because muscle capillarity is not altered in some high-altitude mammals (Mathieu-Costello, 1989; Brutsaert, 2008). Tissue O2 transport could also be improved by restricting the hypoxia-induced redistribution of blood flow. This hypothesis has not been thoroughly investigated, and the minimal support for this possibility is confounded by differences in arterial O2 loading (Faraci et al., 1984b; Faraci et al., 1985).
Tissue O2 utilization
Tissue O2 utilization is altered in various ways by high-altitude acclimatization in low-altitude species. A reduction in whole-tissue metabolic capacity (i.e. maximal O2 demand) occurs in muscle and other tissues during chronic hypoxia through reductions in cell size and mitochondrial abundance (Hoppeler and Vogt, 2001; Mathieu-Costello, 2001). Reductions in mitochondrial capacity lead to a shift in fuel preference in favour of carbohydrate oxidation at a given rate of ATP turnover (McClelland et al., 1998), which reduces the requirement for O2 by 12–14% (Essop, 2007; Calbet et al., 2009). Chronic hypoxia might in some cases improve the efficiency of mitochondrial O2 utilization (i.e. ADP/O2 consumed) (Zungu et al., 2008) but, in general, mitochondrial function is sustained or even impaired under such conditions (Essop, 2007). The use of anaerobic metabolism in the muscle to synthesize ATP during exercise generally declines after high-altitude acclimatization, possibly because of an improved coordination between O2 supply, glycolytic flux and cellular ATP demand (Hochachka et al., 2002). By contrast, chronic hypoxia has no effect on some traits that could have a profound influence on mitochondrial metabolism during O2 lack, such as the sensitivity of mitochondrial respiration to low O2 (mitochondrial O2 kinetics) (Costa et al., 1997) and the activity of the creatine kinase shuttle (Daneshrad et al., 2001). The general result is that some metabolic traits respond to hypoxia to help match cellular O2 supply and demand, whereas others exhibit negligible plasticity.
Depending on the species in question, ancestral acclimatization responses in tissue O2 utilization could either facilitate or impede high-altitude adaptation. The reduction in metabolic capacity might be beneficial in species that usually sustain sub-maximal rates of aerobic metabolism. Mitochondrial abundance is constitutively reduced in the muscle of Tibetan humans compared with that of native lowlanders, and is not affected by environmental O2 availability (Kayser et al., 1991; Kayser et al., 1996). This might represent an example of genetic assimilation (Pigliucci et al., 2006), because the phenotypic plasticity exhibited by lowlanders has become constitutively expressed in Tibetans. Conversely, reductions in metabolic capacity might be maladaptive in high-altitude birds, which need to sustain near-maximal rates of aerobic metabolism during flight. In the bar-headed goose, for example, aerobic capacity of the flight muscle is expanded by an increased proportion of oxidative fibers, whereas the inherent respiratory capacities, O2 kinetics, and phosphorylation efficiencies of their mitochondria are unaltered (Scott et al., 2009a; Scott et al., 2009b). Human populations at high altitude have also evolved an enhanced preference for carbohydrate oxidation in the muscle and heart, along with a decreased propensity for anaerobic lactate production during exercise (Hochachka et al., 1991; Hochachka, 1998). Unlike the situation in lowlanders, these phenotypes exhibit very little plasticity in highland natives during acclimatization to hypoxia and might therefore represent another case of genetic assimilation. In addition, some traits could be enhanced in high-altitude species that are not plastic in lowlanders, such the creatine kinase shuttle (Scott et al., 2009b), whereas other traits remain unaltered, such as mitochondrial phosphorylation efficiency and O2 kinetics (Scott et al., 2009a).
Another important aspect of O2 utilization at high altitudes that has important consequences for cellular function, but which is not specifically related to matching O2 supply and demand, is the production of reactive O2 species (ROS). ROS production can increase at high altitudes if O2 limitation to cytochrome c oxidase (COX), the enzyme that catalyzes the terminal reduction of O2 in oxidative phosphorylation, causes a shift in mitochondrial redox towards a more reduced state (Aon et al., 2010). Although endogenous antioxidant systems exist to help prevent ROS from causing oxidative damage, the acclimatization response to hypoxia in lowland natives often involves a downregulation of antioxidant capacity (Dosek et al., 2007). With diminished protection, oxidative damage to various cellular components increases at high altitudes, as indicated by increased rates of lipid peroxidation and DNA strand breaks (Currie, 1999; Dosek et al., 2007).
Mechanisms to reduce ROS production or to protect against oxidative damage would be beneficial at high altitude. One possible means of reducing ROS production is to maintain a more oxidized electron transport chain (Brown et al., 2009). This could be the general strategy employed by bar-headed geese, as reflected by an alteration in COX enzyme kinetics (higher substrate affinity) that might allow the electron transport chain to operate at a lower redox state with less reduced-cytochrome c (Scott et al., 2010). This might be caused by a single amino acid substitution in subunit 3 of the protein that alters inter-subunit interactions (Scott et al., 2010). The importance of reducing ROS production in preventing oxidative damage is emphasized by studies comparing high- and low-altitude human populations: oxidative damage at high altitudes is generally less in highlanders even though most (but not all) antioxidant enzyme levels are reduced (Gelfi et al., 2004; Sinha et al., 2009a; Sinha et al., 2009b; Sinha et al., 2010). It is presently unclear how these evolutionary changes relate to the ancestral acclimatization response, but they should nonetheless be important for improving cell function at high altitudes.
Maladaptive plasticity, countergradient variation and high-altitude adaptation
Bullard (Bullard, 1972) and Monge and León-Velarde (Monge and León-Velarde, 1991) described a physiological syndrome characteristic of birds and mammals that are genetically adapted to high-altitude hypoxia. These animals are typically characterized by an elevated Hb–O2 affinity, a normal or slightly increased Hct, thin-walled pulmonary vessels that respond to hypoxemia with moderate hypertension, and increased muscle capillary density. Some of these features are shared with burrowing mammals that are adapted to the chronic hypoxia of subterranean burrow systems (Hall, 1966; Jelkmann et al., 1981; Campbell et al., 2010). By contrast, in lowland-adapted animals, acclimatization to hypoxia typically involves increased erythropoeitic activity, decreased blood–O2 affinity, pulmonary arterial hypertension, and numerous other responses that can potentially impair O2 transport. These physiological responses can overburden the right side of the arterial circulation and can contribute to pathophysiological conditions such as chronic mountain sickness, suggesting that at least some features of the typical acclimatization response to hypoxia are maladaptive. Moreover, evidence suggests that many responses to hypoxia, such as polycythemia and reduced Hb–O2 affinity, do not simply represent pathological overshoots of the phenotypic optimum; instead, the ancestral norm of reaction is actually pointed in the wrong direction.
In humans, the acclimatization response to severe hypoxia includes an increase in Hct (mediated by increased production of erythropoeitin) and a decrease in Hb–O2 affinity (mediated by increased red cell concentrations of DPG and a reduced intracellular pH). For reasons explained above, both of these hematological adjustments are counterproductive under conditions of severe hypoxia. What accounts for this off-target response? One possible explanation is that the increased Hct and decreased Hb–O2 affinity are part of a misdirected response to environmental hypoxia that originally evolved as a response to anemia (Hebbel et al., 1978; Storz, 2010). Environmental hypoxia and anemia both result in reduced levels of tissue oxygenation, but they have different root causes. In the case of anemia, reduced tissue oxygenation is caused by a curtailed oxygen transport capacity of the blood, and can therefore be rectified by increasing the Hct (which increases CaO2) and by decreasing Hb–O2 affinity [which, under normoxia, enhances O2 unloading without compromising O2 uptake in the lungs (Brauner and Wang, 1997)]. In the case of environmental hypoxia, by contrast, reduced tissue oxygenation has an external cause: the reduced PO2 of inspired air, which in turn leads to a reduced O2 saturation of arterial blood. Under these circumstances, O2 delivery might be further compromised by an elevated Hb concentration (due to the associated increase in blood viscosity) and a decreased Hb–O2 affinity (due to reduced pulmonary O2 loading that leads to a further diminution of arterial O2 saturation). In humans and other lowland mammals that experience hypoxic stress at high altitude, the joint increase in Hb concentration and DPG/Hb ratio provide examples of maladaptive phenotypic plasticity, as the induced physiological changes are in the opposite direction of the phenotypic optimum under severe hypoxia.
Pulmonary vasoconstriction is another example of a misdirected response to environmental hypoxia, which probably evolved in terrestrial vertebrates to help match perfusion to ventilation. At low altitude, heterogeneity occurs throughout the lungs and can lead to some regions being slightly hypoxic relative to others. Pulmonary vasoconstriction in these isolated regions is beneficial because it causes preferential shunting of blood to the most oxygenated areas of the lungs. However, as described above, this is maladaptive when hypoxia occurs globally throughout the lungs, causing pulmonary hypertension, a diversion of blood flow away from the gas exchange surface to pulmonary shunt vessels (Lovering et al., 2008), and a thickening of the O2 diffusion barrier due to fluid leakage into the air spaces (Maggiorini et al., 2001; Eldridge et al., 2006). A blunted pulmonary pressor response should therefore be advantageous for O2 uptake in hypoxia and this benefit should outweigh the potential disruption to ventilation–perfusion matching.
In cases in which the acclimatization response to hypoxia is maladaptive, selection will favor an attenuation of the induced phenotypic change. This can give rise to a pattern of countergradient variation (Conover and Schultz, 1995; Grether, 2005) caused by selection on genetically based trait variation that offsets the environmentally induced changes. The blunted erythropoietic and pulmonary vasoconstriction responses to hypoxia in numerous high-altitude species and populations provide possible examples of this phenomenon.
Recent genomic surveys of nucleotide polymorphism in Tibetan highlanders revealed strong evidence for a history of positive selection at EPAS1 and other genes in the hypoxia-inducible factor (HIF) oxygen-signaling pathway (Beall et al., 2010; Bigham et al., 2009; Bigham et al., 2010; Simonson et al., 2010; Yi et al., 2010). The HIF family of transcription factors regulates systemic and cellular oxygen homeostasis by coordinating the transcriptional response to hypoxia. EPAS1 (also known as HIF2α) encodes the oxygen-sensitive α subunit of the heterodimeric HIF2 transcription factor and plays an especially important role in regulating erythropoeisis (Rankin et al., 2007). The studies by Beall et al. (Beall et al., 2010) and Yi et al. (Yi et al., 2010) revealed a striking signal of positive selection at EPAS1, and documented that noncoding nucleotide variants in and around the EPAS1 gene are strongly associated with a reduced Hb concentration in Tibetan highlanders. Previous studies demonstrated that Tibetan highlanders are characterized by low Hb concentrations relative to acclimatized lowlanders, as well as to Andean highlanders that are resident at comparable altitudes (Beall and Reichsman, 1984; Winslow et al., 1989; Beall et al., 1990; Beall et al., 1998; Garruto et al., 2003; Wu et al., 2005; Beall, 2007). Remarkably, Tibetans living at elevations of up to 4000 m present a hematological profile similar to what would be expected at sea level. Evidence suggests that this same blunted erythropoeitic response would be beneficial in other high-altitude human populations as well. According to Villafuerte et al. (Villafuerte et al., 2004): “...the overall symptomatic and physical improvement that comes from reducing Hct to sea level values while at high altitude suggest that Andean humans would be better suited for life at high altitude if they could maintain a [Hb] within the sea level range”. It is possible that Andean highlanders have not evolved a similar mechanism for attenuating the erythropoietic response to hypoxia because of their shorter history of residence at high altitude (Beall et al., 1998; Moore, 2001; Beall, 2006; Beall, 2007).
The Hb concentration of Tibetan highlanders does not fall outside the ancestral range of variation characteristic of closely related lowlanders, such as the Han Chinese. Instead, selection appears to have favored a blunted erythropoietic response such that Hb concentration at high altitude is maintained at the sea-level status quo. This represents a potential example of genetic compensation (Grether, 2005) in which a maladaptive acclimatization response requires selection on genetically based trait variation to restore the ancestral phenotype. Although the mechanism has yet to be uncovered, it is possible that noncoding changes that alter the expression of EPAS1 and other HIF-related genes have recalibrated the homeostatic set point for hypoxia-induced erythropoeisis in Tibetans. It remains to be seen whether Hb concentration represents the direct phenotypic target of selection in Tibetans, or whether changes in Hb concentration simply represent an ancillary effect of selection on some other physiological trait that is altered by regulatory changes in the HIF cascade (Zhuang et al., 1993; Moore et al., 2006).
The cellular regulation of Hb–O2 affinity in high-altitude deer mice provides another potential example of how the acclimatization response to hypoxia might work at cross purposes with genetically based adaptation. Comparisons between highland and lowland deer mice have revealed genetically based differences in baseline DPG/Hb ratios (Snyder, 1982) and Hb–O2 affinity (Snyder et al., 1982; Snyder, 1985; Chappell and Snyder, 1984; Chappell et al., 1988; Storz et al., 2009; Storz et al., 2010). Adult deer mice captured at high altitude showed a 14% reduction in DPG/Hb ratios after 65 days acclimation to sea level, and a 23% reduction after ∼230 days. Common garden experiments under normoxic conditions revealed that the highland mice were characterized by lower baseline DPG/Hb ratios than lowland mice, and experimental crosses revealed that these differences have a genetic basis (Snyder, 1982). Moreover, comparisons of Hb function between highland and lowland mice revealed that genetically based differences in Hb–O2 affinity are largely attributable to a reduced sensitivity to DPG and Cl– ions in the Hbs of highland mice (Storz et al., 2009; Storz et al., 2010). The reduced baseline DPG/Hb ratios and the suppressed anion sensitivity both appear to be adaptive mechanisms for maintaining an elevated blood–O2 affinity and, yet, high-altitude deer mice still exhibit a hypoxia-induced increase in DPG levels. Why hasn't selection abolished the seemingly maladaptive DPG response in high-altitude deer mice? Perhaps for the same reason that Andean humans have not yet evolved a blunted erythropoeitic response to hypoxia. According to Snyder (Snyder, 1982): “...[it might be] that the physiologically mediated DPG response evolved not in the context of high-altitude hypoxia, but in response to other sorts of hypoxic stress encountered by animals living at low altitudes. By that line of thought, the DPG response in high-altitude deer mice is an inextricable feature of the animal's physiological architecture that cannot be modified without pleiotropic repercussions”.
Cases in which induced physiological responses require compensation by genetically based changes suggest that the ancestral acclimatization response might act as a hindrance to high-altitude adaptation. Such cases also suggest the possibility that many phenotypic similarities between highland and lowland populations of the same species could be attributable to the effects of countergradient selection.
Genomic approaches to the study of phenotypic plasticity and genetic adaptation to high altitude
Some of the best prospects for identifying adaptive mechanisms of hypoxia tolerance, and for teasing apart the relative roles of genotypic specialization and phenotypic plasticity, are afforded by genetic and genomic studies of population-level variation in species that are continuously distributed across steep altitudinal gradients. Although much previous work on high-altitude adaptation has focused on comparisons between separate species that have different altitudinal range limits, comparisons between highland and lowland populations of the same species offer several important advantages for identifying genetic mechanisms of adaptation. First, the ability to conduct crosses is crucial for disentangling genetic and environmental sources of phenotypic variation. Second, mechanistic studies of population-level variation provide the greatest experimental power for assessing genotypic differences in fitness-related physiological performance (Garland and Adolph, 1991; Storz and Wheat, 2010). Many mechanisms of physiological adaptation involve modifications of regulatory networks, and newly developed genomic technologies provide a means of investigating the nature of these modifications. In particular, the simultaneous analysis of sequence variation and expression profiles for a set of genes in the same pathway provides a means of identifying both structural and regulatory mechanisms of adaptation.
At the molecular level, phenotypic plasticity is associated with changes in transcriptional regulation (Gracey et al., 2008). Recent common garden experiments that were designed to quantify the proportion of transcriptomic variation that is due to environmental and genetic effects have demonstrated tremendous plasticity of gene expression in species with broad altitudinal distributions (Appenzeller et al., 2006; Cheviron et al., 2008). In rufous-collared sparrows (Zonotrichia capensis), hundreds of genes that are involved in aerobic metabolism and oxidative stress responses are differentially expressed in the skeletal muscle of birds native to high (>4000 m) and low (<2000 m) altitude (Cheviron et al., 2008). However, none of these transcriptional differences persists after one week of sea-level acclimation. These results demonstrate considerable plasticity in the transcriptomic profiles of rufous-collared sparrows, and suggest that many genome-wide expression differences between highland and lowland individuals are environmentally induced.
One especially promising genomic approach to evaluate the relative contributions of phenotypic plasticity and genotypic specialization in high-altitude adaptation is whole transcriptome shotgun sequencing (WTSS) or RNA-seq (Wang et al., 2009). RNA-seq uses next-generation sequencing to simultaneously characterize transcript abundance and sequence polymorphism in thousands of expressed mRNAs. This type of population genomic approach therefore holds much promise for identifying novel molecular mechanisms of phenotypic plasticity and genetic adaptation, particularly for loci whose function and adaptive significance were previously unanticipated. After candidate loci for hypoxia adaptation have been identified, it is then possible to conduct follow-up experiments to measure the physiological effects of altered protein function (in the case of amino acid mutations) or protein expression (in the case of regulatory mutations) (Feder and Walser, 2005; Storz and Wheat, 2010).
Implications for altitudinal range shifts in a warming world
Global climate change has already been shown to impact the physiological tolerances and range limits of many organisms, and both altitudinal and latitudinal range shifts can be expected to continue as warming becomes more severe (Deutsch et al., 2008; Moritz et al., 2008; Huey et al., 2009; Sinervo et al., 2010; Somero, 2010). Populations that are restricted to high altitudes could be at risk of increased competition as lowland species shift their altitudinal ranges towards cooler environments, but this will depend on the capacity of lowlanders to acclimatize and/or adapt to high-altitude hypoxia. In birds and mammals that are native to lowland environments, certain features of the acclimatization response to hypoxia might constrain adaptation to high-altitude environments. This is because some of the induced physiological changes (e.g. polycythemia, increased erythrocytic phosphate/Hb ratios, and pulmonary vasoconstriction) are in the opposite direction of the apparent phenotypic optimum under chronic hypoxia. Insights into the acclimatization response of lowland animals to high-altitude hypoxia can provide a basis for predicting how altitudinal range limits might shift in response to climate change. When lowland animals colonize high-altitude environments, adaptive phenotypic plasticity can mitigate the costs of selection, thereby enhancing prospects for population establishment and persistence (Ghalambor et al., 2007). By contrast, maladaptive plasticity has the opposite effect by increasing the cost of selection, thereby diminishing the prospects of successful colonization.
- βbO2
- blood O2 capacitance coefficient
- CaO2
- arterial O2 concentration
- COX
- cytochrome c oxidase
- CvO2
- venous O2 concentration
- DL
- pulmonary O2 diffusion capacity
- DT
- tissue O2 diffusion capacity
- DPG
- 2,3-diphosphoglycerate
- Hb
- hemoglobin
- Hct
- hematocrit
- HVR
- hypoxic ventilatory response
- OR
- odds ratio
- PO2
- partial pressure of O2
- PaO2
- arterial O2 pressure
- PAO2
- alveolar O2 pressure
- PbO2
- blood O2 pressure
- PIO2
- inspired O2 pressure
- PmitO2
- mitochondrial O2 pressure
- PvO2
- venous O2 pressure
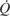
- cardiac output
- ROS
- reactive O2 species
- SGA
- small for gestational age
- V
- ventilation
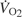
- rate of O2 consumption by tissues
We thank W. Milson and two anonymous reviewers for helpful comments. J.F.S. gratefully acknowledges grant support from the National Science Foundation and the National Institutes of Health/NHLBI. G.R.S. is supported by the Natural Sciences and Engineering Research Council of Canada. Deposited in PMC for release after 12 months.
References
- Aon M. A., Cortassa S., O’Rourke B. (2010). Redox-optimized ROS balance: a unifying hypothesis. Biochim. Biophys. Acta (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller O., Minko T., Qualis C., Pozharov V., Gamboa J., Wang Y. (2006). Gene expression, autonomic function and chronic hypoxia: lessons from the Andes. Clin. Auton. Res. 16, 217-222 [DOI] [PubMed] [Google Scholar]
- Beall C. M. (2006). Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr. Comp. Biol. 46, 18-24 [DOI] [PubMed] [Google Scholar]
- Beall C. M. (2007). Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc. Natl. Acad. Sci. USA 104, 8655-8660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall C. M., Reichsman A. B. (1984). Hemoglobin levels in a Himalayan high altitude population. Am. J. Phys. Anthropol. 63, 301-306 [DOI] [PubMed] [Google Scholar]
- Beall C. M., Brittenham G. M., Macuaga F., Barragan M. (1990). Variation in hemoglobin concentration among samples of high-altitude natives in the Andes and the Himalayas. Am. J. Hum. Biol. 2, 639-651 [DOI] [PubMed] [Google Scholar]
- Beall C. M., Strohl K. P., Blangero J., Williams-Blangero S., Almasy L. A., Decker M. J., Worthman C. M., Goldstein M. C., Vargas E., Villena M., et al. (1997). Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am. J. Phys. Anthropol. 104, 427-447 [DOI] [PubMed] [Google Scholar]
- Beall C. M., Brittenham G. M., Strohl K. P., Blangero J., Williams-Blangero S., Goldstein M. C., Decker M. J., Vargas E., Villena M., Soria R., et al. (1998). Hemoglobin concentration of high altitude Tibetans and Bolivian Aymara. Am. J. Phys. Anthropol. 106, 385-400 [DOI] [PubMed] [Google Scholar]
- Beall C. M., Cavalleri G. L., Deng L., Elston R. C., Gao Y., Knight J., Li C., Li J. C., Liang Y., McCormack M., et al. (2010). Natural selection on EPAS1 (HIF2α) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl. Acad. Sci. USA 107, 11459-11464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencowitz H. Z., Wagner P. D., West J. B. (1982). Effect of change in P50 on exercise tolerance at high altitude: a theoretical study. J. Appl. Physiol. 53, 1487-1495 [DOI] [PubMed] [Google Scholar]
- Bigham A., Mao X., Mei R., Brutsaert T., Wilson M. J., Julian C. G., Parra E. J., Akey J. M., Moore L. G., Shriver M. D. (2009). Identifying positive selection candidate loci for high-altitude adaptation in Andean populations. Hum. Genomics 4, 79-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham A., Bauchet M., Pinto D., Mao X., Akey J. M., Mei R., Scherer S., Julian C. G., Wilson M. J., López-Herráez D., et al. (2010). Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. P., Tenney S. M. (1980). Oxygen transport during progressive hypoxia in high altitude and sea level waterfowl. Respir. Physiol. 39, 217-239 [DOI] [PubMed] [Google Scholar]
- Blake C. I., Banchero N. (1985). Effects of cold and hypoxia on ventilation and oxygen consumption in awake guinea pigs. Respir. Physiol. 61, 357-368 [DOI] [PubMed] [Google Scholar]
- Bouverot P. (1985). Adaptation to Altitude-Hypoxia in Vertebrates. Berlin: Springer-Verlag; [Google Scholar]
- Brauner C. J., Wang T. (1997). The optimal oxygen equilibrium curve: a comparison between environmental hypoxia and anemia. Am. Zool. 37, 101-108 [Google Scholar]
- Brown J. C., McClelland G. B., Faure P. A., Klaiman J. M., Staples J. F. (2009). Examining the mechanisms responsible for lower ROS release rates in liver mitochondria from the long-lived house sparrow (Passer domesticus) and big brown bat (Eptesicus fuscus) compared to the short-lived mouse (Mus musculus). Mech. Ageing Dev. 130, 467-476 [DOI] [PubMed] [Google Scholar]
- Brutsaert T. D. (2007). Population genetic aspects and phenotypic plasticity of ventilatory responses in high altitude natives. Respir. Physiol. Neurobiol. 158, 151-160 [DOI] [PubMed] [Google Scholar]
- Brutsaert T. D. (2008). Do high-altitude natives have enhanced exercise performance at altitude? Appl. Physiol. Nutr. Metab. 33, 582-592 [DOI] [PubMed] [Google Scholar]
- Brutsaert T. D., Soria R., Caceres E., Spielvogel H., Haas J. D. (1999). Effect of developmental and ancestral high altitude exposure on chest morphology and pulmonary function in Andean and European North American natives. Am. J. Hum. Biol. 11, 383-395 [DOI] [PubMed] [Google Scholar]
- Brutsaert T. D., Parra E. J., Shriver M. D., Gamboa A., León-Velarde F. (2005). Ancestry explains the blunted ventilatory response to sustained hypoxia and lower exercise ventilation of Quechua altitude natives. Am. J. Physiol. Reg. Integr. Comp. Physiol. 289, R225-R234 [DOI] [PubMed] [Google Scholar]
- Bullard R. W. (1972). Vertebrates at altitude. In Physiological Adaptations (ed. Yousef M. K., Horvath S. M., Bullard R. W.), pp. 209-225 New York: Academic Press; [Google Scholar]
- Bunn H. F. (1980). Regulation of hemoglobin function in mammals. Am. Zool. 20, 199-211 [Google Scholar]
- Calbet J. A., Robach P., Lundby C. (2009). The exercising heart at altitude. Cell. Mol. Life Sci. 66, 3601-3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet J. A. L., Boushel R., Rådegran G., Søndergaard H., Wagner P. D., Saltin B. (2003). Why is Vo2 max after altitude acclimatization still reduced despite normalization of arterial O2 content? Am. J. Physiol. Reg. Integr. Comp. Physiol. 284, R304-R316 [DOI] [PubMed] [Google Scholar]
- Cambell K. L., Storz J. F., Signore A. V., Moriyama H., Catania K. C., Payson A. P., Bonaventura J., Stetefeld J., Weber R. E. (2010). Molecular basis of a novel adaptation to hypoxic-hypercapnia in a strictly fossorial mole. BMC Evol. Biol. 10, 214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M. A., Snyder L. R. G. (1984). Biochemical and physiological correlates of deer mouse α-chain hemoglobin polymorphisms. Proc. Natl. Acad. Sci. USA 81, 5484-5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M. A., Hayes J. P., Snyder L. R. G. (1988). Hemoglobin polymorphisms in deer mice (Peromyscus maniculatus): physiology of β-globin variants and α-globin recombinants. Evolution 42, 681-688 [DOI] [PubMed] [Google Scholar]
- Cheviron Z. A., Whitehead A., Brumfield R. T. (2008). Transcriptomic variation and plasticity in rufous-collared sparrows (Zonotrichia capensis) along an elevational gradient. Mol. Ecol. 17, 4556-4569 [DOI] [PubMed] [Google Scholar]
- Connes P., Yalcin O., Baskert O., Brun J. F., Hardeman M. (2006). In heath and in a normoxic environment, VO2 max is/is not limited primarily by cardiac output and locomotor muscle blood flow. J. Appl. Physiol. 100, 2099 [DOI] [PubMed] [Google Scholar]
- Conover D. O., Schultz E. T. (1995). Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol. Evol. 10, 248-252 [DOI] [PubMed] [Google Scholar]
- Costa L. E., Mendez G., Boveris A. (1997). Oxygen dependence of mitochondrial function measured by high-resolution respirometry in long-term hypoxic rats. Am. J. Physiol. Cell Physiol. 273, C852-C858 [DOI] [PubMed] [Google Scholar]
- Crowell J. W., Smith E. E. (1967). Determinants of the optimal hematocrit. J. Appl. Physiol. 22, 501-504 [DOI] [PubMed] [Google Scholar]
- Crowell J. W., Ford R. G., Lewis V. M. (1959). Oxygen transport in hemorrhagic shock as a function of hematocrit ratio. Am. J. Physiol. 196, 1033-1038 [DOI] [PubMed] [Google Scholar]
- Currie R. J. (1999). Ascites in poultry: recent investigations. Avian Pathol. 28, 313-326 [DOI] [PubMed] [Google Scholar]
- Daneshrad Z., Novel-Chaté V., Birot O., Serrurier B., Sanchez H., Bigard A. X., Rossi A. (2001). Diet restriction plays an important role in the alterations of heart mitochondrial function following exposure of young rats to chronic hypoxia. Pflügers Arch. 442, 12-18 [DOI] [PubMed] [Google Scholar]
- de Jong G., Behera N. (2002). The influence of life-history differences on the evolution of reaction norms. Evol. Ecol. Res. 4, 1-25 [Google Scholar]
- Dejours P., Garey W. F., Rahn H. (1970). Comparison of ventilatory and circulatory flow rates between animals in various physiological conditions. Respir. Physiol. 9, 108-117 [DOI] [PubMed] [Google Scholar]
- Deutsch C. A., Tewksbury J. J., Huey R. B., Sheldon K. S., Ghalambor C. K., Haak D. C., Martin P. R. (2008). Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 105, 6668-6672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosek A., Ohno H., Acs Z., Taylor A. W., Radak Z. (2007). High altitude and oxidative stress. Respir. Physiol. Neurobiol. 158, 128-131 [DOI] [PubMed] [Google Scholar]
- Durmowicz A. G., Hofmeister S., Kadyraliev T. K., Aldashev A. A., Stenmark K. R. (1993). Functional and structural adaptation of the yak pulmonary circulation to residence at high altitude. J. Appl. Physiol. 74, 2276-2285 [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Skelton T. D., Berger E. (1974). Survival at extreme altitude: protective effect of increased hemoglobin affinity. Science 183, 743-744 [DOI] [PubMed] [Google Scholar]
- Ekblom B., Bergland B. (1991). Effect of erythopoietin administration on mammal aerobic power. Scand. J. Med. Sci. Sports 1, 88-93 [Google Scholar]
- Ekblom B., Hermansen L. (1968). Cardiac output in athletes. J. Appl. Physiol. 25, 619-625 [DOI] [PubMed] [Google Scholar]
- Eldridge M. W., Braun R. K., Yoneda K. Y., Walby W. F. (2006). Effects of altitude and exercise on pulmonary capillary integrity: evidence for subclinical high-altitude pulmonary edema. J. Appl. Physiol. 100, 972-980 [DOI] [PubMed] [Google Scholar]
- Essop M. F. (2007). Cardiac metabolic adaptations in response to chronic hypoxia. J. Physiol. 584, 715-726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci F. M., Kilgore D. L., Fedde M. R. (1984a). Attenuated pulmonary pressor response to hypoxia in bar-headed geese. Am. J. Physiol. 247, R402-R403 [DOI] [PubMed] [Google Scholar]
- Faraci F. M., Kilgore D. L., Fedde M. R. (1984b). Oxygen delivery to the heart and brain during hypoxia: pekin duck vs. bar-headed goose. Am. J. Physiol. 247, R69-R75 [DOI] [PubMed] [Google Scholar]
- Faraci F. M., Kilgore D. L., Fedde M. R. (1985). Blood flow distribution during hypocapnic hypoxia in pekin ducks and bar-headed geese. Respir. Physiol. 61, 21-30 [DOI] [PubMed] [Google Scholar]
- Fedde M. R., Orr J. A., Shams H., Scheid P. (1989). Cardiopulmonary function in exercising bar-headed geese during normoxia and hypoxia. Respir. Physiol. 77, 239-262 [DOI] [PubMed] [Google Scholar]
- Feder M., Walser J. (2005). The biological limitations of transcriptomics in elucidating stress and stress response. J. Evol. Biol. 18, 901-910 [DOI] [PubMed] [Google Scholar]
- Fry J. (1992). The mixed-model analysis of variance applied to quantitative genetics: biological meaning of the parameters. Evolution 46, 540-550 [DOI] [PubMed] [Google Scholar]
- Garland T., Adolph S. C. (1991). Physiological differentiation of vertebrate populations. Annu. Rev. Ecol. Syst. 22, 193-229 [Google Scholar]
- Garruto R. M., Chin C., -T., Weitz C. A., Liu J., -C., Liu R., -L., He X. (2003). Hematological differences during growth among Tibetans and Han Chinese born and raised at high altitude in Qinghai, China. Am. J. Phys. Anthropol. 122, 171-183 [DOI] [PubMed] [Google Scholar]
- Ge R. L., Kubo K., Kobayashi T., Sekiguchi M., Honda T. (1998). Blunted hypoxic pulmonary vasoconstrictive response in the rodent Ochotona curzoniae (pika) at high altitude. Am. J. Physiol. Heart Circ. Physiol. 274, H1792-H1799 [DOI] [PubMed] [Google Scholar]
- Gelfi C., De Palma S., Ripamonti M., Eberini I., Wait R., Bajracharya A., Marconi C., Schneider A., Hoppeler H., Cerretelli P. (2004). New aspects of altitude adaptation in Tibetans: a proteomic approach. FASEB J. 18, 612-614 [DOI] [PubMed] [Google Scholar]
- Ghalambor C. K., McKay J., Carroll S. P., Reznick D. (2007). Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394-407 [Google Scholar]
- Gonzales G. F., Steenland K., Tapia V. (2009). Maternal hemoglobin level and fetal outcome at low and high altitudes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1477-R1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey A. Y., Chaney M. L., Boomhower J. P., Tyburczy W. R., Connor K., Somero G. N. (2008). Rhythms of gene expression in a fluctuating intertidal environment. Curr. Biol. 18, 1-7 [DOI] [PubMed] [Google Scholar]
- Grether G. (2005). Environmental change, phenotypic plasticity and genetic compensation. Am. Nat. 166, E115-E123 [DOI] [PubMed] [Google Scholar]
- Groves B. M., Droma T., Sutton J. R., McCullough R. G., McCullough R. E., Zhuang J., Rapmund G., Sun S., Janes C., Moore L. G. (1993). Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. J. Appl. Physiol. 74, 312-318 [DOI] [PubMed] [Google Scholar]
- Gurney A. M. (2002). Multiple sites of oxygen sensing and their contributions to hypoxic pulmonary vasoconstriction. Respir. Physiol. Neurobiol. 132, 43-53 [DOI] [PubMed] [Google Scholar]
- Guyton A. C., Richardson T. Q. (1961). Effect of hematocrit on venous return. Circ. Res. 9, 157-164 [DOI] [PubMed] [Google Scholar]
- Hall F. G. (1966). Minimal utilized oxygen and the oxygen dissociation curve of blood of rodents. J. Appl. Physiol. 21, 375-378 [DOI] [PubMed] [Google Scholar]
- Hansen J., Sander M. (2003). Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J. Physiol. 546, 921-929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbel R. P., Eaton J. W., Kronenberg R. S., Zanjani E. D., Moore L. G., Berger E. M. (1978). Human llamas: adaptation to altitude in subjects with high hemoglobin oxygen affinity. J. Clin. Invest. 62, 593-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen I. H., Kemppainen J., Kaskinoro K., Peltonen J. E., Borra R., Lindroos M., Oikonen V., Nuutila P., Knuuti J., Boushel R., et al. (2010). Regulation of human skeletal muscle perfusion and its heterogeneity during exercise in moderate hypoxia. Am. J. Physiol. Reg. Integr. Comp. Physiol. (in press). [DOI] [PubMed] [Google Scholar]
- Hepple R. T. (2000). Skeletal muscle: microcirculatory adaptation to metabolic demand. Med. Sci. Sports Exerc. 32, 117-123 [DOI] [PubMed] [Google Scholar]
- Hochachka P. W. (1985). Exercise limitations at high altitude: the metabolic problem and search for its solution. In Circulation, Respiration and Metabolism (ed. Gilles R.), pp. 240-249 Berlin: Springer-Verlag; [Google Scholar]
- Hochachka P. W. (1998). Mechanism and evolution of hypoxia-tolerance in humans. J. Exp. Biol. 201, 1243-1254 [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Stanley C., Matheson G. O., McKenzie D. C., Allen P. S., Parkhouse W. S. (1991). Metabolic and work efficiencies during exercise in Andean natives. J. Appl. Physiol. 70, 1720-1730 [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Beatty C. L., Burelle Y., Trump M. E., McKenzie D. C., Matheson G. O. (2002). The lactate paradox in human high-altitude physiological performance. News Physiol. Sci. 17, 122-126 [DOI] [PubMed] [Google Scholar]
- Hoppeler H., Vogt M. (2001). Muscle tissue adaptations to hypoxia. J. Exp. Biol. 204, 3133-3139 [DOI] [PubMed] [Google Scholar]
- Hsia C. C., Carbayo J. J., Yan X., Bellotto D. J. (2005). Enhanced alveolar growth and remodeling in Guinea pigs raised at high altitude. Respir. Physiol. Neurobiol. 147, 105-115 [DOI] [PubMed] [Google Scholar]
- Hsia C. C. W., Johnson R. L., McDonough P., Dane D. M., Hurst M. D., Fehmel J. L., Wagner H. E., Wagner P. D. (2007). Residence at 3,800-m altitude for 5 mo in growing dogs enhances lung diffusing capacity for oxygen that persists at least 2.5 years. J. Appl. Physiol. 102, 1448-1455 [DOI] [PubMed] [Google Scholar]
- Huey R. B., Deutsch C. A., Tewksbury J. J., Vitt L., Hertz P., Perez A., Garland T. (2009). Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. Lond. B. Biol. Sci. 276, 1939-1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelkmann W., Oberthur W., Kleinschmidt T., Braunitzer G. (1981). Adaptation of hemoglobin function to subterranean life in the mole, Talpa europaea. Respir. Physiol. 46, 7-16 [DOI] [PubMed] [Google Scholar]
- Jensen F. B. (2004). Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol. Scand. 182, 215-227 [DOI] [PubMed] [Google Scholar]
- Julian C. G., Galan H., Wilson M. J., Desilva W., Cioffi-Ragan D., Schwartz J., Moore L. G. (2008). Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R906-R915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian C. G., Wilson M. J., Moore L. G. (2009a). Evolutionary adaptation to high altitude: a view from in utero. Am. J. Hum. Biol. 21, 614-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian C. G., Wilson M. J., Lopez M., Yamashiro H., Tellez W., Rodriguez A., Bigham A. W., Shriver M. D., Rodiruguez C., Vargas E., et al. (2009b). Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1564-R1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitomo M., Alonso J. G., Okai T., Longo L. D., Gilbert R. D. (1993). Effects of long-term, high-altitude hypoxemia on ovine fetal cardiac output and blood flow distribution. Am. J. Obstet. Gynecol. 169, 701-707 [DOI] [PubMed] [Google Scholar]
- Kanstrup I. L., Ekblom B. (1984). Blood volume and hemoglobin concentration as determinants of maximal aerobic power. Med. Sci. Sports. Exerc. 16, 256-262 [PubMed] [Google Scholar]
- Kayser B., Hoppeler H., Claassen H., Cerretelli P. (1991). Muscle structure and performance capacity of Himalayan Sherpas. J. Appl. Physiol. 70, 1938-1942 [DOI] [PubMed] [Google Scholar]
- Kayser B., Hoppeler H., Desplanches D., Marconi C., Broers B., Cerretelli P. (1996). Muscle ultrastructure and biochemistry of lowland Tibetans. J. Appl. Physiol. 81, 419-425 [DOI] [PubMed] [Google Scholar]
- Kinsey S. T., Hardy K. M., Locke B. R. (2007). The long and winding road: influences of intracellular metabolite diffusion on cellular organization and metabolism in skeletal muscle. J. Exp. Biol. 210, 3505-3512 [DOI] [PubMed] [Google Scholar]
- Lenfant C., Torrance J., English E., Finch C. A., Reynafarje C., Ramos J., Faura J. (1968). Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J. Clin. Invest. 47, 2652-2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant C., Torrance J. D., Reynafarje C. (1971). Shift of the O2-Hb dissociation curve at altitude: mechanism and effect. J. Appl. Physiol. 30, 625-631 [DOI] [PubMed] [Google Scholar]
- León-Velarde F., Villafuerte F. C., Richalet J. P. (2010). Chronic mountain sickness and the heart. Prog. Cardiovasc. Dis. 52, 540-549 [DOI] [PubMed] [Google Scholar]
- Levins R. (1968). Evolution in Changing Environments: Some Theoretical Explorations. Princeton, NJ: Princeton University Press; [Google Scholar]
- Lewis A. M., Mathieu-Costello O., McMillan P. J., Gilbert R. D. (1999). Effects of long-term, high-altitude hypoxia on the capillarity of the ovine fetal heart. Am. J. Physiol. Heart Circ. Physiol. 277, H756-H762 [DOI] [PubMed] [Google Scholar]
- Lovering A. T., Romer L. M., Haverkamp H. C., Pegelow D. F., Hokanson J. S., Eldridge M. W. (2008). Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J. Appl. Physiol. 104, 1418-1425 [DOI] [PubMed] [Google Scholar]
- Maggiorini M., Melot C., Pierre S., Pfeiffer F., Greve I., Sartori C., Lepori M., Hauser M., Scherrer U., Naeije R. (2001). High-altitude pulmonary edema is initially caused by an increase in capillary pressure. Circulation 103, 2078-2083 [DOI] [PubMed] [Google Scholar]
- Mairbäurl H. (1994). Red blood cell function in hypoxia at altitude and exercise. Int. J. Sports Med. 15, 51-63 [DOI] [PubMed] [Google Scholar]
- Mairbäurl H., Oelz O., Bärtsch P. (1993). Interactions between Hb, Mg, DPG, ATP, and Cl determine the change in Hb-O2 affinity at high altitude. J. Appl. Physiol. 74, 40-48 [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O. (1989). Muscle capillary tortuosity in high altitude mice depends on sarcomere length. Respir. Physiol. 76, 289-302 [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O. (2001). Muscle adaptation to altitude: tissue capillarity and capacity for aerobic metabolism. High Alt. Med. Biol. 2, 413-425 [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O., Agey P. J. (1997). Chronic hypoxia affects capillary density and geometry in pigeon pectoralis muscle. Respir. Physiol. 109, 39-52 [DOI] [PubMed] [Google Scholar]
- McClelland G. B., Hochachka P. W., Weber J. M. (1998). Carbohydrate utilization during exercise after high-altitude acclimation: a new perspective. Proc. Natl. Acad. Sci. USA 95, 10288-10293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath R. L., Weil J. V. (1978). Adverse effects of normovolemic polycythemia and hypoxia on hemodynamics in the dog. Circ. Res. 43, 793-798 [DOI] [PubMed] [Google Scholar]
- Metzger R. J., Klein O. D., Martin G. R., Krasnow M. A. (2008). The branching programme of mouse lung development. Nature 453, 745-750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge C., León-Velarde F. (1991). Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol. Rev. 71, 1135-1172 [DOI] [PubMed] [Google Scholar]
- Monge C., Whittembury J. (1976). High altitude adaptation in the whole animal. In Environmental Physiology of Animals (ed. Blight J., Cloudsey-Thompson J., MacDonald A.). New York: Wiley; [Google Scholar]
- Moore L. G. (2001). Human genetic adaptation to high altitude. High Alt. Med. Biol. 2, 257-279 [DOI] [PubMed] [Google Scholar]
- Moore L. G., Hershey D. W., Jahnigen D., Bowes W. (1982). The incidence of pregnancy-induced hypertension is increased among Colorado residents at high altitude. Am. J. Obstet. Gynecol. 144, 423-429 [DOI] [PubMed] [Google Scholar]
- Moore L. G., Young D., McCullough R. E., Droma T., Zamudio S. (2001a). Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am. J. Hum. Biol. 13, 635-644 [DOI] [PubMed] [Google Scholar]
- Moore L. G., Zamudio S., Zhuang J., Sun S., Droma T. (2001b). Oxygen transport in Tibetan women during pregnancy at 3,658 m. Am. J. Phys. Anthropol. 114, 42-53 [DOI] [PubMed] [Google Scholar]
- Moore L. G., Shriver M., Bemis L., Hickler B., Wilson M., Brutsaert T., Parra E., Vargas E. (2004). Maternal adaptation to high-altitude pregnancy: an experiment of nature – a review. Placenta 25, S60-S71 [DOI] [PubMed] [Google Scholar]
- Moore L. G., Shriver M., Bemis L., Vargas E. (2006). An evolutionary model for identifying genetic adaptation to high altitude. In Hypoxia and Exercise (ed. Roach R. C., Wagner P. D., Hackett P. H.), pp. 101-118 New York: Springer-Verlag; [DOI] [PubMed] [Google Scholar]
- Moran N. (1992). The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971-989 [Google Scholar]
- Moritz C., Patton J., Conroy C., Parra J., White G., Beissinger S. (2008). Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261-264 [DOI] [PubMed] [Google Scholar]
- Nikinmaa M. (2001). Haemoglobin function in vertebrates: evolutionary changes in cellular regulation in hypoxia. Respir. Physiol. 128, 317-329 [DOI] [PubMed] [Google Scholar]
- Noakes T. D., Calbet J. A. L., Boushel R., Sondergaard H., Radegran G., Wagner P. D., Saltin B. (2004). Central regulation of skeletal muscle recruitment explains the reduced maximal cardiac output during exercise in hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R996-R1002 [DOI] [PubMed] [Google Scholar]
- Pichon A., Zhenzhong B., Favret F., Jin G., Shufeng H., Marchant D., Richalet J. P., Ge R. L. (2009). Long term ventilatory adaptation and ventilatory response to hypoxia in Plateau pika (Ochotona curzoniae): role of nNOS and dopamine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R978-R987 [DOI] [PubMed] [Google Scholar]
- Pigliucci M., Murren C. J., Schlichting C. D. (2006). Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 209, 2362-2367 [DOI] [PubMed] [Google Scholar]
- Powell F. L. (2007). The influence of chronic hypoxia upon chemoreception. Respir. Physiol. Neurobiol. 157, 154-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell F. L., Milsom W. K., Mitchell G. S. (1998). Time domains of the hypoxic ventilatory response. Respir. Physiol. 112, 123-134 [DOI] [PubMed] [Google Scholar]
- Price T., Qvarnstrom A., Irwin D. (2003). The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B. Biol. Sci. 270, 1433-1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J.-M., Folkhow L. P., Blix A. S. (2007). Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu. Rev. Physiol. 69, 113-143 [DOI] [PubMed] [Google Scholar]
- Rankin E. B., Biju M. P., Liu Q., Unger T. L., Rha J., Johnson R. S., Simon M. C., Keith B., Haase V. H. (2007). Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J. Clin. Invest. 117, 1068-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar P., Bellotto D. J., Johnson R. L., Hsia C. C. (2009). Permanent alveolar remodeling in canine lung induced by high-altitude residence during maturation. J. Appl. Physiol. 107, 1911-1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. T., Grover R. F. (1975). High-altitude pulmonary hypertension and pulmonary edema. Prog. Cardiol. 4, 99-118 [Google Scholar]
- Richards D. W. (1960). Homeostasis: its dislocations and perturbations. Perspect. Biol. Med. 3, 238-251 [Google Scholar]
- Riggs A. F. (1988). The Bohr effect. Annu. Rev. Physiol. 50, 181-204 [DOI] [PubMed] [Google Scholar]
- Rivera-Ch M., León-Velarde F., Huicho L. (2007). Treatment of chronic mountain sickness: Critical reappraisal of an old problem. Respir. Physiol. Neurobiol. 158, 251-265 [DOI] [PubMed] [Google Scholar]
- Sakai A., Matsumoto T., Saitoh M., Matsuzaki T., Koizumi T., Ishizaki T., Ruan Z. H., Wang Z. G., Chen Q. H., Wang X. Q. (2003). Cardiopulmonary hemodynamics of blue-sheep, Pseudois nayaur, as high-altitude adapted mammals. Jpn. J. Physiol. 53, 377-384 [DOI] [PubMed] [Google Scholar]
- Samaja M., di Prampero P. E., Cerretelli P. (1986). The role of 2,3-DPG in the oxygen transport at altitude. Respir. Physiol. 64, 191-202 [DOI] [PubMed] [Google Scholar]
- Samaja M., Crespi T., Guazzi M., Vandegriff K. D. (2003). Oxygen transport in blood at high altitude: role of the hemoglobin-oxygen affinity and impact of the phenomena related to hemoglobin allosterism and red cell function. Eur. J. Biochem. 90, 351-359 [DOI] [PubMed] [Google Scholar]
- Scheiner S. (1993). Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 24, 35-68 [Google Scholar]
- Scheiner S. (1998). The genetics of phenotypic plasticity. VII. Evolution in a spatially structured environment. J. Evol. Biol. 11, 303-320 [Google Scholar]
- Schmutz S., Däpp C., Wittwer M., Durieux A. C., Mueller M., Weinstein F., Vogt M., Hoppeler H., Flück M. (2010). A hypoxia complement differentiates the muscle response to endurance exercise. Exp. Physiol. 95, 723-735 [DOI] [PubMed] [Google Scholar]
- Schuler B., Arras M., Keller S., Rettich A., Lundby C., Vogel J., Gassmann M. (2010). Optimal hematocrit for maximal excercise performance in acute and chronic erythropoietin-treated mice. Proc. Natl. Acad. Sci. USA 107, 419-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. R., Milsom W. K. (2007). Control of breathing and adaptation to high altitude in the bar-headed goose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R379-R391 [DOI] [PubMed] [Google Scholar]
- Scott G. R., Milsom W. K. (2009). Control of breathing in birds: implications for high altitude flight. In Cardio-Respiratory Control in Vertebrates: Comparative and Evolutionary Aspects (ed. Glass M. L., Wood S. C.), pp. 429-448 Berlin: Springer-Verlag; [Google Scholar]
- Scott G. R., Egginton S., Richards J. G., Milsom W. K. (2009a). Evolution of muscle phenotype for extreme high altitude flight in the bar-headed goose. Proc. R. Soc. Lond. B. Biol. Sci. 276, 3645-3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. R., Richards J. G., Milsom W. K. (2009b). Control of respiration in flight muscle from the high-altitude bar-headed goose and low-altitude birds. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1066-R1074 [DOI] [PubMed] [Google Scholar]
- Scott G. R., Schulte P. M., Egginton S., Scott A. L. M., Richards J. G., Milsom W. K. (2010). Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol. Biol. Evol. epub ahead of print [DOI] [PubMed] [Google Scholar]
- Simonson T. S., Yang Y., Huff C. D., Yun H., Qin G., Witherspoon D. J., Bai Z., Lorenzo F. R., Xing J., Jorde L. B., et al. (2010). Genetic evidence for high-altitude adaptation in Tibet. Science 329, 72-75 [DOI] [PubMed] [Google Scholar]
- Sinervo B., Mendez-de-la-Cruz F., Miles D. B., Heulin B., Bastiaans E., Villagran-Santa Cruz M., Lara-Resendiz R., Martinez-Mendez N., Calderon-Espinosa M. L., Meza-Lazaro R. N., et al. (2010). Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894-899 [DOI] [PubMed] [Google Scholar]
- Sinha S., Ray U., Saha M., Singh S., Tomar O. (2009a). Antioxidant and redox status after maximal aerobic exercise at high altitude in acclimatized lowlanders and native highlanders. Eur. J. Appl. Physiol. 106, 807-814 [DOI] [PubMed] [Google Scholar]
- Sinha S., Ray U. S., Tomar O. S., Singh S. N. (2009b). Different adaptation patterns of antioxidant system in natives and sojourners at high altitude. Respir. Physiol. Neurobiol. 167, 255-260 [DOI] [PubMed] [Google Scholar]
- Sinha S., Dutta A., Singh S. N., Ray U. S. (2010). Protein nitration, lipid peroxidation and DNA damage at high altitude in acclimatized lowlanders and native highlanders: relation with oxygen consumption. Respir. Physiol. Neurobiol. 171, 115-121 [DOI] [PubMed] [Google Scholar]
- Snyder L. R. G. (1982). 2,3-diphosphoglycerate in high- and low-altitude populations of the deer mouse. Respir. Physiol. 48, 107-123 [DOI] [PubMed] [Google Scholar]
- Snyder L. R. G. (1985). Low P50 in deer mice native to high altitude. J. Appl. Physiol. 58, 193-199 [DOI] [PubMed] [Google Scholar]
- Snyder L. R. G., Born S., Lechner A. J. (1982). Blood oxygen affinity in high- and low-altitude populations of the deer mouse. Respir. Physiol. 48, 89-105 [DOI] [PubMed] [Google Scholar]
- Somero G. (2010). The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine “winners” and “losers”. J. Exp. Biol. 213, 912-920 [DOI] [PubMed] [Google Scholar]
- Storz J. F. (2007). Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. J. Mammal. 88, 24-31 [Google Scholar]
- Storz J. F. (2010). Genes for high altitudes. Science 329, 40-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Moriyama H. (2008). Mechanisms of hemoglobin adaptation to high-altitude hypoxia. High Alt. Med. Biol. 9, 148-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Wheat C. W. (2010). Integrating evolutionary and functional approaches to infer adaptation at specific loci. Evolution 64, 2489-2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Sabatino S. J., Kelly J. K., Ferrand N., Moriyama H., Weber R. E., Fago A. (2009). Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc. Natl. Acad. Sci. USA 106, 14450-14455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Moriyama H., Weber R. E., Fago A. (2010). Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 213, 2565-2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan S., Spencer H. (2002). Metapopulation structure favors plasticity over local adaptation. Am. Nat. 160, 271-283 [DOI] [PubMed] [Google Scholar]
- Turek Z., Kreuzer F., Hoofd L. (1973). Advantage or disadvantage of a decrease of blood oxygen affinity for tissue oxygen supply at hypoxia. A theoretical study comparing man and rat. Pflügers Arch. 342, 185-187 [DOI] [PubMed] [Google Scholar]
- Turek Z., Kreuzer F., Ringnalda B. E. M. (1978a). Blood gases at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflügers Arch. 376, 7-13 [DOI] [PubMed] [Google Scholar]
- Turek Z., Kreuzer F., Turek-Maischeider M., Ringnalda B. E. M. (1978b). Blood O2 content, cardiac output, and flow to organs at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflügers Arch. 376, 201-207 [DOI] [PubMed] [Google Scholar]
- van Ekeren G. J., Sengers R. C., Stadhouders A. M. (1992). Changes in volume densities and distribution of mitochondria in rat skeletal muscle after chronic hypoxia. Int. J. Exp. Pathol. 73, 51-60 [PMC free article] [PubMed] [Google Scholar]
- van Tienderen P. (1991). Evolution of generalists and specialists in spatially heterogenous environments. Evolution 45, 1317-1331 [DOI] [PubMed] [Google Scholar]
- van Tienderen P. (1997). Generalists, specialists, and the evolution of phenotypic plasticity in sympatric populations of distinct species. Evolution 51, 1372-1380 [DOI] [PubMed] [Google Scholar]
- Via S., Gomulkiewicz R., de Jong G., Scheiner S., Schlichting C., van Tienderen P. (1995). Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212-217 [DOI] [PubMed] [Google Scholar]
- Villafuerte F. C., Cárdenas R., Monge C. C. (2004). Optimal hemoglobin concentration and high altitude: a theoretical approach for Andean men at rest. J. Appl. Physiol. 96, 1581-1588 [DOI] [PubMed] [Google Scholar]
- Wang Z., Gerstein M., Snyder M. (2009). RNA-Seq: a revolutionary tool for transcriptomics. Nat. Rev. Genet. 10, 57-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Bishop C. M., Woakes A. J., Butler P. J. (2002). Heart rate and the rate of oxygen consumption of flying and walking barnacle geese (Branta leucopsis) and bar-headed geese (Anser indicus). J. Exp. Biol. 205, 3347-3356 [DOI] [PubMed] [Google Scholar]
- Weber R. E. (2007). High-altitude adaptations in vertebrate hemoglobins. Respir. Physiol. Neurobiol. 158, 132-142 [DOI] [PubMed] [Google Scholar]
- Weber R. E., Fago A. (2004). Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir. Physiol. Neurobiol. 144, 141-159 [DOI] [PubMed] [Google Scholar]
- Weiskopf R. B., Severinghaus J. W. (1972). Lack of effect of high altitude on hemoglobin oxygen affinity. J. Appl. Physiol. 33, 276-277 [DOI] [PubMed] [Google Scholar]
- Wilson M. J., Lopez M., Vargas M., Julian C., Tellez W., Rodriguez A., Bigham A., Armaza J. F., Niermeyer S., Shriver M., et al. (2007). Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1313-R1324 [DOI] [PubMed] [Google Scholar]
- Winslow R., Monge C. (1987). Hypoxia, Polycythemia, and Chronic Mountain Sickness. Baltimore, MD: Johns Hopkins University Press; [Google Scholar]
- Winslow R., Monge C., Brown E., Klein H., Sarnquist F., Winslow N., McNeally S. (1985). Effects of hemodilution on O2 transport in high-altitdue polycythemia. J. Appl. Physiol. 59, 1495-1502 [DOI] [PubMed] [Google Scholar]
- Winslow R. M., Chapman K. W., Gibson C. C., Samaja M., Monge C. C., Goldwasser E., Sherpa M., Blume D. (1989). Different hematologic responses to hypoxia in Sherpas and Quechua Indians. J. Appl. Physiol. 66, 1561-1569 [DOI] [PubMed] [Google Scholar]
- Wu T., Kayser B. (2006). High altitude adaptation in Tibetans. High Alt. Med. Biol. 7, 193-208 [DOI] [PubMed] [Google Scholar]
- Wu T., Wang X., Wei C., Cheng H., Wang X., Li Y., Ge-Dong Zhao H., Young P., Li G., Wang Z. (2005). Hemoglobin levels in Qinghai-Tibet: different effects of gender for Tibetans vs. Han. J. Appl. Physiol. 98, 598-604 [DOI] [PubMed] [Google Scholar]
- Yi X., Liang Y., Huerta-Sanchez E., Jin X., Cuo Z. X. P., Pool J. E., Xu X., Jiang H., Vinckenbosch N., Korneliussen T. S., et al. (2010). Sequencing of fifty human exomes reveals adaptation to high altitude. Science 329, 75-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S., Palmer S. K., Droma T., Stamm E., Coffin C., Moore L. G. (1995). Effect of altitude on uterine artery blood flow during normal pregnancy. J. Appl. Physiol. 79, 7-14 [DOI] [PubMed] [Google Scholar]
- Zhuang J., Droma T., Sun S., Janes C., McCullough R. E., McCullough R. G., Cymerman A., Huang S. Y., Reeves J. T., Moore L. G. (1993). Hypoxic ventilatory responsiveness in Tibetan compared with Han residents of 3,658 m. J. Appl. Physiol. 74, 303-311 [DOI] [PubMed] [Google Scholar]
- Zungu M., Young M. E., Stanley W. C., Essop M. F. (2008). Expression of mitochondrial regulatory genes parallels respiratory capacity and contractile function in a rat model of hypoxia-induced right ventricular hypertrophy. Mol. Cell. Biochem. 318, 175-181 [DOI] [PMC free article] [PubMed] [Google Scholar]