Abstract
Coordination of different motor systems for sound production involves the use of feedback mechanisms. Song production in oscines is a well-established animal model for studying learned vocal behavior. Whereas the online use of auditory feedback has been studied in the songbird model, very little is known about the role of other feedback mechanisms. Auditory feedback is required for the maintenance of stereotyped adult song. In addition, the use of somatosensory feedback to maintain pressure during song has been demonstrated with experimentally induced fluctuations in air sac pressure. Feedback information mediating this response is thought to be routed to the central nervous system via afferent fibers of the vagus nerve. Here, we tested the effects of unilateral vagotomy on the peripheral motor patterns of song production and the acoustic features. Unilateral vagotomy caused a variety of disruptions and alterations to the respiratory pattern of song, some of which affected the acoustic structure of vocalizations. These changes were most pronounced a few days after nerve resection and varied between individuals. In the most extreme cases, the motor gestures of respiration were so severely disrupted that individual song syllables or the song motif were atypically terminated. Acoustic changes also suggest altered use of the two sound generators and upper vocal tract filtering, indicating that the disruption of vagal feedback caused changes to the motor program of all motor systems involved in song production and modification. This evidence for the use of vagal feedback by the song system with disruption of song during the first days after nerve cut provides a contrast to the longer-term effects of auditory feedback disruption. It suggests a significant role for somatosensory feedback that differs from that of auditory feedback.
Keywords: birdsong, vagus nerve, somatosensory, sensorimotor integration, zebra finch
INTRODUCTION
A common feature of motor systems is their use of feedback mechanisms for the maintenance of appropriate output (e.g. Pearson et al., 1998; Diedrichsen et al., 2010). Vocal coordination represents a particularly complex motor control task that requires the simultaneous integration of different feedback modalities, such as acoustic and somatosensory information (Suthers and Zollinger, 2004; Smotherman, 2007). In human speech, real-time acoustic and somatosensory feedback is important for proper motor control of vocalizations (Nasir and Ostry, 2006; Tremblay et al., 2003).
Birdsong is a well-established animal model for studying learned vocal behavior (e.g. Zeigler and Marler, 2008). In addition to the parallels in motor aspects of vocal production, birdsong shares with human speech many aspects of a sensorimotor learning process (Doupe and Kuhl, 2008). During this phase of vocal ontogeny, online use of auditory feedback facilitates development of a highly stereotyped song. It is not clear which, if any, role other feedback mechanisms (somatosensory) play during song ontogeny.
Once vocal learning is complete, song becomes highly stereotyped and there is growing evidence that auditory feedback plays a role in its maintenance. Altered auditory feedback or deafening results in changes of the stereotyped song sequence, but the time course of song deterioration is age dependent and differs between species (Leonardo and Konishi, 1999; Woolley and Rubel, 1997; Okanoya and Yamaguchi, 1997). The on-line use of auditory feedback to correct errors has more recently been demonstrated (Sakata and Brainard, 2008; Tumer and Brainard, 2007; Andalman and Fee, 2009; Sober and Brainard, 2009; Lei and Mooney, 2010), and the adjustments are subtle and specific to frequency control. It is unclear whether the long delay between more global alteration of feedback (deafening, nerve transaction, muting procedures) and song changes indicates a categorically different mechanism of feedback use.
In comparison to this evidence for the use of acoustic feedback, our knowledge about the use of somatosensory feedback in birdsong is very limited. The case of syringeal feedback is puzzling. Although sensory receptors in syringeal muscles or other syringeal tissues have not been identified, afferent fibers in the tracheosyringeal nerve are present. However, deafferentation of the syrinx did not result in a clear change in the song motor pattern (Bottjer and Arnold, 1982; Bottjer and Arnold, 1984).
Evidence for an on-line use of somatosensory feedback during song was found in the respiratory system. In northern cardinals (Cardinalis cardinalis) a short perturbation of respiratory pressure through injection of air during song was found to elicit a compensatory adjustment in expiratory effort (Suthers et al., 2002). Another experimental manipulation of respiratory control during song, however, did not elicit a compensatory response. Chronic reduction in the air volume of the posterior thoracic air sacs did not cause a change in expiratory effort despite a drastic decrease in song amplitude (Plummer and Goller, 2008). Consistent with physiological evidence from receptors in the respiratory system (e.g. Gleeson and Molony, 1989), these findings suggest that the respiratory somatosensory system responds to dynamic changes of air sac volume rather than to a static condition. However, it is not clear which sensory feedback mechanism is involved in the on-line adjustment. The two possible feedback routes are vagal feedback and feedback from respiratory muscles routed to the spinal cord.
Structures similar to neuroepithelial bodies have been described in the air sac membranes (Kubke et al., 2004), and recordings from vagal fibers indicate a response of these putative receptors during the inspiratory phase of respiration. This activity is consistent with the air injection experiment, because the injection would generate a brief inspiration-like expansion of the air sac. In mammals, similar structures exist but, although their vagal innnervation has been confirmed, their function is not yet completely understood (Widdicombe, 2001; Van Lommel et al., 1998). Alternatively, the feedback information mediating the compensatory response could have arisen from muscle spindles in the abdominal expiratory muscle sheet. Spindle-like receptors have been located in the expiratory abdominal muscles of birds (DeWet et al., 1971; Iscoe, 1998).
The vagal nerve carries afferent information about several organs to the central nervous system (Undem and Weinreich, 2005). For example, information from all the broncho-pulmonary sensors is routed through the vagus. This information is integrated and processed in a major center for homeostatic control in the brainstem, the nucleus of the solitary tract (nTS). Our knowledge about the role of broncho-pulmonary feedback processing in birds is very rudimentary and does not parallel what is known about this system in mammals.
Possible routes for the use of feedback information for song control have been described in birds. nTS is connected to the respiratory network (nucleus parambigualis, PAm) (Wild et al., 2009). PAm has connections with the nucleus uvaeformis (Uva), which in turn is connected to HVC (neostriatal song motor control nucleus; acronym used as a proper name). Recently, a functional connection between PAm and HVC was documented, and this connection most likely is important for transmitting respiratory information to telencephalic song motor control areas (Ashmore et al., 2008). Although the specific role of Uva in song generation is not known, this function as a relay station together with its known role as a sensory integration center (Wild, 1994) suggests strongly that it is involved in informing song control centers about respiratory activity. Consistent with this interpretation, bilateral lesioning of Uva in zebra finches disrupts song permanently, whereas unilateral lesion causes transient disruption (Coleman and Vu, 2005).
The effects of vagotomy have been analyzed in different species of mammals and birds (Fedde et al., 1964; Richards, 1968). In general, all previous studies addressed the function of vagal feedback during normal, silent respiration and found that vagal feedback is actively used to control respiratory activity. Although songbirds have not been studied, during normal breathing we expected no major differences from what is known in other avian species. The functional role of vagal feedback during vocal behavior has not been explored in any species. If respiratory vagal feedback is integrated into the somatosensory control of song production, it is plausible that vagotomy could affect the song motor program. The nature of such effects is of interest, particularly whether disrupted vagal feedback might cause changes to song similar to those occurring after disruption of auditory feedback (e.g. decrystallization).
We investigated whether vagal feedback plays an active role in song maintenance by comparing song and accompanying physiological correlates of motor activity before and after unilateral vagotomy. The observed changes to respiratory and syringeal motor gestures suggest that the perturbation of vagal feedback by unilateral vagotomy destabilizes the song motor program rapidly.
MATERIALS AND METHODS
Animals and recording procedure
Seven male zebra finches, Taeniopygia guttata Vieillot 1817, from our colony with various backgrounds in regard to raising conditions and of different ages were studied in this experiment. Each bird was transferred to a recording box and tethered. As soon as the bird resumed singing, a pressure cannula was implanted into a thoracic air sac. After this, the bird was recorded for at least 2 days. Typically, birds sang reliably and frequently enough to allow recording of at least 10 motifs of pre-operative song. Because successful recording of air sac pressure is only possible for a limited time (1–2 weeks), we attempted to maximize recording time after the vagotomy. Therefore, as soon as the bird had sung at least 10 motifs, we performed a unilateral vagotomy and recorded song and air sac pressure for as many days as we could obtain reliable pressure data.
Surgery
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Utah. Before surgery, an elastic belt with a Velcro connector was fitted around the bird's thorax to which a wire was attached for tethering the bird to a counterbalanced tether arm. When the birds resumed singing, preoperative song was recorded and then the first surgical procedure was carried out. The bird was deprived of food and water for 1 h before the surgeries. Isoflurane anesthesia was used for both surgeries.
The surgical procedures and recording methods for air sac pressure and airflow recording have been described in detail elsewhere (Goller and Suthers, 1996; Goller and Daley, 2001). A small hole was made in the abdominal wall, and a flexible cannula (Silastic tubing) was inserted into an anterior thoracic air sac. The cannula was sutured to the ribs, and the free end of the cannula was connected to a pressure transducer (Fujikura FPM-02PG; Tokyo, Japan) mounted on the backpack. Airflow was recorded with custom-built flow sensors (made with GE Thermometrics BB05JA202 thermistors; Billerica, MA, USA) that were inserted into the trachea through the connective tissue between two cartilage rings (right above the location where the interclavicular air sac attaches) and secured in place with a suture and tissue adhesive. The wires were routed subcutaneously to the backpack. Airflow was determined as the voltage required in a feedback circuit (Hector Engineering; Ellettsville, IN, USA) to heat the thermistor bead to a constant temperature (∼60°C).
For vagus transection, the right trunk of the vagus nerve (X) was approached by an incision in the skin in the neck region approximately 2–3 cm below its anastomosis with the hypoglossal nerve (XII) (Bottjer and Arnold, 1984). The connective tissue was removed and the dissected nerve was cut with surgical scissors. The skin incision was closed with surgical suture. We chose to perform the vagotomy on the right branch to keep the effects on the heart minimal (e.g. Estavillo and Burger, 1973).
Data analysis
All the analysis methods described below were programmed in Matlab (version 7.7.0, MathWorks, Natick, MA, USA).
Sound analysis
Songs of each bird before and after the vagotomy were visually inspected for differences using spectrograms. When a difference was identified, at least five representative motifs for each experimental time point were selected and the normalized average power spectral density was calculated. To account for possible variations in the sound amplitude caused by a different radiation direction of the sound, data were normalized. We divided the signal by the integrated signal in the selected period. The power spectral density was calculated using Thomson's multitaper method (Thomson, 1982).
Air-sac pressure analysis for stereotypy
To analyze the level of stereotypy of the motif before and after the vagotomy, 10 motifs were randomly selected at different time points during the experiment corresponding to a given day. Then, to compare the pressure patterns on a syllable-by-syllable basis, the different renditions of the expiratory pulse of the syllable were aligned using the peak (maximum value of the pressure during the syllable), and the time of peak air sac pressure for each syllable was defined as tref. Because of the variable duration between different renditions of an individual air sac pressure pulse corresponding to each song syllable, we used peak pressure for alignment of the expiratory pulses. Peaks in pressure were very distinct and therefore allowed more precise alignment than was possible with a threshold pressure at syllable onset. To do so, we identified the pressure level corresponding to the atmospheric pressure using quiet respiration at the beginning of the song motif, and then for each expiratory pressure pulse of the 10 selected motifs we calculated the intersections of the pressure patterns with the atmospheric level. This gave 10 starting and 10 ending time points for each syllable of the motif. We used the average of these time points to establish a time interval around tref for each syllable (δtl to the left, and δtr to the right). Thus, the syllable was between ti=tref–δtl and tf=tref+δtr, and each syllable was contained in a time series S as:
| (1) |
where x represents the pressure pattern time series. With this procedure, for each syllable 10 time series with the same duration were extracted [Si(α), i=1,..., 10]. Then, for each presentation of the syllable the quadratic difference from the mean (to account for positive or negative deviations from the mean) in each time was calculated as:
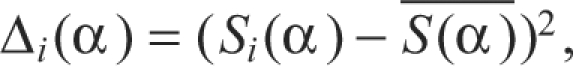 |
(2) |
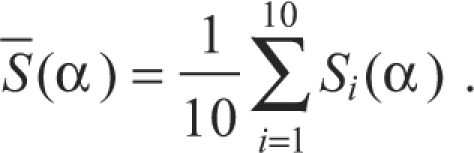 |
(3) |
This method quantifies the variability around the mean for each set of pressure patterns. To assess whether the variability within each expiratory pressure pulse of the motif was higher after than before the vagotomy, a Welch's t-test was performed to compare the quadratic differences at each value of α. This test resulted in a value of P (output of the Welch's t-test) for each value of α. We then added the duration of all time intervals in which the post-vagotomy variation was significantly (0.05 level) higher than the pre-vagotomy variation (DSD: duration of significant difference, the total duration of significantly lower stereotypy of pressure patterns after the vagotomy).
Analysis of the pressure pattern shape
A comparison between the pressure patterns before and after the vagotomy using the same 10 representative song motifs per time point was carried out. This analysis tested whether or not the vagotomy caused a change in the mean air sac pressure pattern (N=10) of the individual expiratory pressure pulses of the motif. Expiratory pressure pulses were identified as continuous supra-atmospheric pressure events during song. These events were interrupted by minibreaths (i.e. events of sub-atmospheric pressure, which typically correspond to silent periods during the song motif). Again, for each syllable at each time point of the pulse a Welch's t-test was performed to test for significant differences in the air sac pressure patterns before and after the vagotomy.
Analysis of temporal variability
The temporal features of each motif of every bird were quantified by measuring the duration of the motif from either the air sac pressure pattern or the sound recording. Coefficients of variation were calculated to assess the variability of motif duration on each day of recording and allow comparison between the different motif durations of different individuals.
Quantification of the pressure pattern complexity
To quantify the modulation characteristics of air sac pressure for individual pulses of the song motif, we fitted a fourth order polynomial (using the least square method) to the expiratory pulse. With this procedure the main shape of the pressure pulse can be reproduced but without the superimposed fine modulations. We then calculated the sum of the absolute value differences between the actual pressure signal and the fitted curve at each time point and calculated it per unit time (ms) to standardize for different syllable durations. We call this metric the pressure modulation index (PMI) and use it to quantitatively assess the modulation complexity of expiratory pressure pulses during the motif independently of their duration.
RESULTS
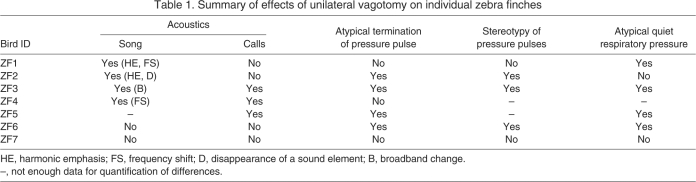
The effects of the unilateral vagotomy on the song motor pattern were analyzed at different levels as detailed below. With the exception of one individual, birds sang readily after the vagotomy. The exceptional bird (ZF5) only attempted one song on the first day and then did not sing again until after the pressure cannula failed on day 4 after the vagotomy. Although this one song contained a syllable interruption, data from this bird were not included in the detailed analysis below. In general, the nature of changes to acoustic behavior and the underlying respiratory motor patterns were highly variable between individuals. Nevertheless, six of the seven birds showed some effect of the vagotomy, as summarized in Table 1.
Table 1.
Summary of effects of unilateral vagotomy on individual zebra finches
Atypical respiratory patterns occur after vagotomy
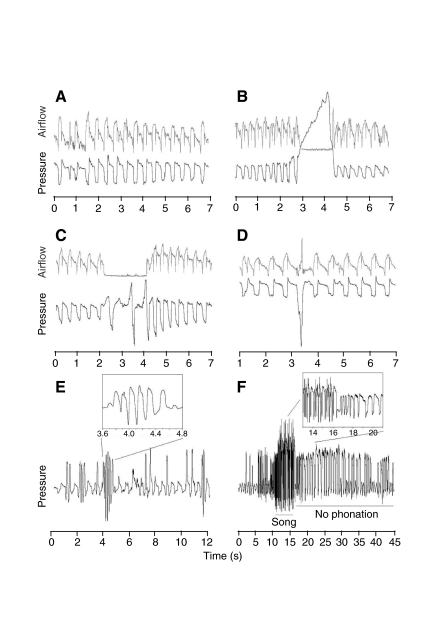
After unilateral vagotomy some notable changes to the silent respiratory pattern were observed in five of the seven birds (see Fig. 1A for an example of silent respiration before the unilateral vagotomy). Atypical respiratory patterns occurred during both inspiration and expiration (Fig. 1C,D), but atypically deep inspirations were most noticeable (Fig. 1D). The atypical nature of the pressure pattern was also accompanied by unusual airflow patterns. For example, despite increased pressurization for three respiratory cycles in Fig. 1C, the airflow is near zero during the expiratory and inspiratory phases, indicating that the syringeal valves were closed during these respiratory events. These pressure signatures were not observed before the vagotomy and do not correspond to signatures typical of other behavior such as defecation (Fig. 1B), swallowing, yawning, preening, etc. (not illustrated). Such atypical respiratory patterns during quiet breathing were only observed after the unilateral vagotomy was performed. For example, in the recordings of ZF1 deep inspirations were present 4 times during the first day after nerve cut and 47 times during the second day. In ZF5, during the first day 103 events were observed and 23 events on the second day. In both birds the atypical respirations were observed throughout the day with no clear accumulation at any specific time.
Fig. 1.
Unilateral vagotomy caused atypical patterns of quiet and vocal respiration. (A) Normal breathing pattern before the vagal transaction in one zebra finch (ZF1), showing fairly regular air sac pressure (bottom trace in black, relative voltage) and tracheal airflow (top trace in gray, relative voltage) patterns. (B) Example of a typical defecation event embedded in normal quiet breathing. Air sac pressure is ramped up and maintained in the expiratory position while the syringeal valves are closed as indicated by zero airflow during this pressurization event. (C,D) Examples of atypical respiratory events after the vagotomy during quiet, non-vocal respiration in two birds (C: ZF1, D: ZF5) show atypical respiratory (air sac pressure, black trace, horizontal line indicates ambient pressure) and airflow patterns (tracheal airflow, gray trace, both inspiratory and expiratory flow are upward deflections; the direction of airflow can be determined from air sac pressure). (C) The change in rhythm of breathing is characterized by expiratory pressure pulses that are longer in duration and modulated, and are separated by short and deep inspirations. However, during these respiratory movements the syringeal valves are closed as indicated by zero airflow. Abnormally deep inspirations (D) were the most commonly observed alteration after the vagotomy. (E,F) Atypical respiratory events occurring after the vagotomy in the context of vocal behavior included sequences of expiratory pulses that do not correspond to the bird's (ZF3) song or calls and did not produce audible phonation. The insets show selected segments with the most pronounced atypical respiratory patterns on a larger scale. A sequence of expiratory pulses separated by short inspirations (minibreaths) was silent (E), and a song bout with audible song was followed by a sequence of pressure pulses and minibreaths that were silent (F).
Atypical respiratory patterns also occurred in the context of vocal behavior (Table 1). Sequences of variable expiratory pulses associated with deep inspirations were generated before or after call or song production. Although the expiratory pulses associated with this activity were of high enough amplitude to cross the phonatory threshold (as judged from the pressure amplitude of calls), no sound was produced during most of these atypical sequences (Fig. 1E,F). The inset in Fig. 1F also shows that the expiratory pulses are variable in the duration and modulation of amplitude, but they do not resemble any of the stereotyped pressure patterns that give rise to calls or song syllables.
These atypical song-like pressure patterns (Fig. 1E,F) were never observed before the vagotomy. ZF3, for example, generated the pressure patterns displayed in Fig. 1E (only pressure patterns with at least three consecutive expiratory modulations of a period less than 0.2 s were counted as atypical) frequently after the nerve transaction: 14 times on the second day, 256 times on the third day, 112 times on the fourth day and 11 times on the fifth day.
In addition, clear and, in some individuals, dramatic changes to the respiratory motor sequence of song were found. These changes included interruption of song in the middle of a syllable, i.e. before the completion of an expiratory pulse and atypical pressure patterns at the end of the syllable (Figs 2 and 3; red and green lines). The modulation pattern of air sac pressure and airflow at the end of the song indicates that a modified version of the expiratory pulse was produced, which is in contrast to terminating song prematurely after the pulse by returning to silent respiration. The air sac pressure patterns after song interruption were not those of regular, silent respiration. Although their amplitude was low and variable, they retained some aspects of the modulation patterns of the expiratory pulse that normally gave rise to the omitted song segment or syllable.
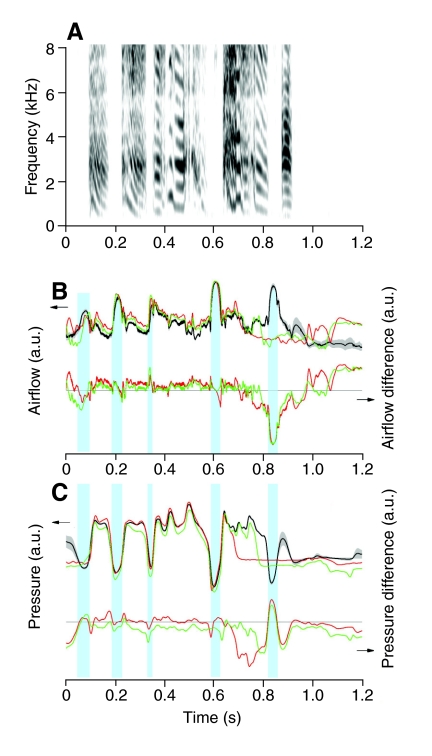
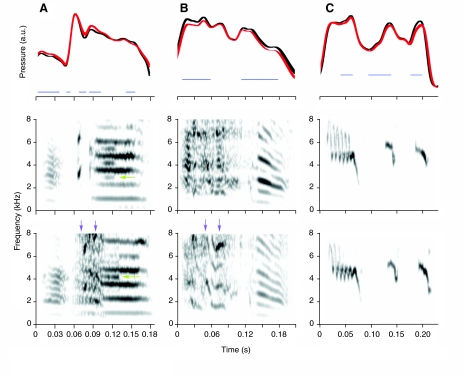
Fig. 2.
Interruptions occurred at various points during a syllable. The intact acoustic structure of the song motif is illustrated spectrographically (A) along with the mean tracheal airflow (top trace in B) and air sac pressure (top trace in C) patterns (black lines with gray area indicate the mean ± 1 s.e.m. of 10 motifs). The two illustrated interruptions of the fourth syllable occurred late (green) and early (red) during the ongoing expiratory pressure pulse. For each interruption the difference in airflow and air sac pressure from the mean value of the complete motif are plotted (bottom traces in B and C; horizontal line indicates zero difference). The largest difference in airflow occurred after early termination of the expiratory pulse when the short deep inspiration (minibreath) of the intact motif was replaced by nearly atmospheric air sac pressure and, consequently, low airflow. Blue shading (B,C) highlights the minibreaths. Note that the last minibreath is only present before the vagotomy (black) and no longer occurs after interruption of the preceding syllable (red and green). a.u., arbitrary units.
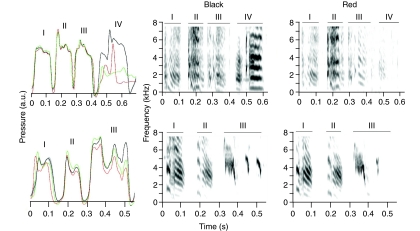
Fig. 3.
Additional examples of interrupted syllables by two other birds (A: ZF3, B: ZF6), from which only air sac pressure was recorded. Mean air sac pressure (black, N=10) ± s.e.m. (gray area) of the motifs before the vagotomy illustrates the high degree of stereotypy. After the nerve cut interruptions of syllables (two examples shown in red and in green) occurred in one syllable. These atypical termination events caused the omission of acoustic elements. In each example, the spectrogram labeled ‘Black’ illustrates the complete motif recorded before the vagotomy and the one labeled ‘Red’ shows the interruption after the vagotomy corresponding to the red pressure trace (on the left). The expiratory pulses and the generated syllables are indicated by Roman numerals for easy identification. Syllables are not simply terminated, but retain some of the modulations at lower overall air sac pressure. Also note the difference in deviation from the pressure modulation pattern of the other syllables between the two individuals.
The alterations of the expiratory pulse also led to striking differences in airflow (Fig. 2) and to acoustic differences (Fig. 3). For example, the drastically reduced level of air sac pressure was not sufficient to cross the threshold value for phonation, thus resulting in an atypically terminated song syllable (Fig. 3B). In addition, with this alteration of the third syllable the song motif was also prematurely terminated.
We explored the respiratory patterns of song in the two individuals with the longest record to assess the time course of the occurrence of atypical interruptions (errors). Interruptions were never observed in the songs of these birds prior to the nerve cut. On the first day after the nerve cut, the percentage of these major errors was 16 and 25%, respectively, and this decreased on subsequent days (Fig. 4). The decrease in the occurrence of errors did not appear to be directly related to the number of song bouts a bird produced on a given day. Interestingly, errors did not occur at a consistent rate during the course of a day, but were concentrated during certain time periods and were not present at others. For example, within the first 24 h of vagotomy, both birds sang throughout the day, but one bird had only two 1–2 h time periods during which errors occurred (Fig. 4A) and the other only one 1 h period (Fig. 4B).
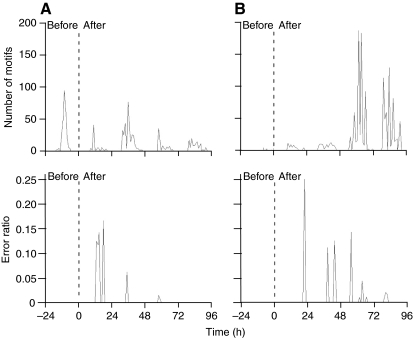
Fig. 4.
Interruptions of a syllable occurred predominantly during the first few days after the vagotomy. Graphs illustrate the number of motifs sung per hour (top) and the fraction of song motifs with atypical termination of a syllable (bottom) for two individuals (A: ZF6, B: ZF3). The error rate does not appear to be related to the absolute amount of singing, and is concentrated during short time periods within the period of singing on each day. ZF3 (B) did not sing frequently after the implantation of the pressure cannula. We proceeded with the vagotomy after 1 day despite the low song output to maximize the time with intact air sac pressure recordings after the nerve cut.
The interruptions of syllables (error) usually occurred during a period of high singing activity. The distribution of time intervals between a bout with an error and its preceding and following bouts was significantly different (the time intervals were significantly shorter) from the distribution of intervals between bouts without errors [Kolmogorov–Smirnov (K–S) test, D=0.014 for ZF3 and D=0.02 for ZF6; the long interval between last bout of the evening and first bout of the subsequent morning was not included in this analysis]. A bout during which an error occurred did not cause an immediate increase in singing because the inter-bout interval to the following bout was not statistically shorter than that to the preceding bout (K–S test, D=0.43 for ZF3 and D=0.42 for ZF6). Furthermore, the production of a motor error during one bout did not increase vocal production in a 2 h period after the occurrence of the error when compared with the preceding 2 h period (two-sided sign test of the difference in the number of motifs produced two hours before and after, P=0.73 for ZF3 and P=0.37 for ZF6).
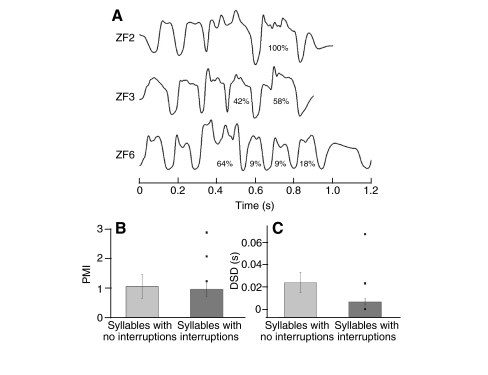
The occurrence of an error typically caused an interruption of the bout (61% of the times for ZF3 and 91% of the times for ZF6). The errors occurred during production of specific syllables of the motif (Fig. 5A). For example, in ZF3 interruptions were observed only in syllables 4 and 5, whereas most errors occurred in syllable 3 of the motif (64%) in ZF6 (Fig. 5A).
Fig. 5.
Atypical interruptions of the pressure patterns during song did not affect all syllables, but occurred towards the end of the motif. (A) For the three birds (ID on the left) with sufficient data to quantify the occurrence of atypical termination of a syllable, the pressure patterns corresponding to their motifs are displayed, and syllables with interrupted pressure patterns are identified by the percentage value of interruptions. (B,C) There is no simple explanation of why certain pressure pulses were interrupted and others were not. A comparison of the degree of modulation of air sac pressure during the expiratory pulses of the song motifs (B, quantified as PMI: pressure modulation index, the degree of modulation per unit time – see Materials and methods), shows that the syllables that were not interrupted had a similar degree of modulation to those that were interrupted (mean ± 1 s.e.m.). The most frequently interrupted pressure pulse (as indicated by the highest percentage in A) for each of the three birds had a high PMI value (black dots above dark gray bar). (C) Interrupted syllables were also not characterized by reduced stereotypy (calculated as duration of significant difference in variation around the mean before and after vagotomy, DSD) of the air sac pressure pulse. Driven by the short syllables of the motif of ZF6 the mean DSD is lower for the interrupted syllables. The set of syllables with the greatest number of interruptions for the three birds is highly variable and therefore does not indicate a correlation between the stereotypy of the pressure pulse and the occurrence of interruptions.
In order to explore whether syllables that were interrupted showed an unusually high degree of modulation in the expiratory pressure, we quantified the degree of modulation for all expiratory pulses using the pressure modulation index (PMI). The mean PMI values corresponding to the set of pressure pulses that were not affected by the vagotomy were not significantly different from those of the set that was interrupted (Welch's t-test, P>0.05) (Fig. 5B). If for each bird only the pressure pulse that was most frequently interrupted is considered, their PMI values tended to be higher than the mean for all pressure pulses. This indicates that most interruptions occurred in pressure pulses with a high degree of modulation.
Another possibility is that the occurrence of interruptions in some pressure pulses is correlated with changes in the stereotypy of the pattern as a consequence of the vagotomy. We assessed stereotypy by an index that quantifies the time of significant differences between pre- and post-vagotomy variation in air sac pressure for each expiratory pulse of the motif (DSD values, see Materials and methods). A comparison of DSD values for the pressure pulses with interruptions to those without interruptions showed no correlation between the stereotypy of the pressure patterns and the interruptions (Fig. 5C).
Modulation of pressure patterns changes after unilateral vagotomy
In addition to these drastic changes and early termination, smaller differences in pressure and flow modulation occurred in the song syllables of some individuals (Figs 2 and 3). For example, the individual whose pressure pattern of song is depicted in Fig. 3A (black line) showed only small variation in pressure modulation during the first three syllables, whereas another bird produced pressure patterns with larger differences in the first two syllables (Fig. 3B). To quantify this variability, we evaluated the stereotypy of respiratory patterns during song at the level of individual expiratory pulses, which correspond to song syllables (Franz and Goller, 2002).
We calculated the mean air sac pressure of a given syllable before and after the vagotomy and statistically compared the means at each point of the pressure pulse. Three examples from three different birds are shown in Fig. 6. Significant differences were found around the areas where pressure is modulated. Some of these changes in the modulation of air sac pressure are likely to drive the observed changes in frequency modulation characteristics and harmonic emphasis of the corresponding sounds.
Fig. 6.
(A–C) Three examples (A: ZF3, B: ZF2, C: ZF6) of air sac pressure pulses (top panels) whose fine modulation of pressure showed significant differences before (black) and after the vagotomy (red). A spectrogram of the pre-vagotomy (top) syllable and post-vagotomy (bottom) syllable illustrates a range of acoustic changes, some of which are likely related to the pressure difference (e.g. range of frequency modulation in C). Other acoustic differences more likely reflect changes in the respective contributions from the two syringeal sound generators (blue and green arrows in A and B).
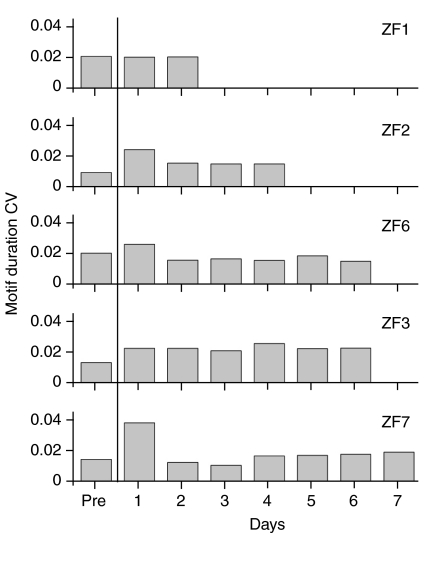
We also assessed temporal stereotypy of the respiratory motor gestures by measuring variation in motif duration before and each day after the vagotomy. There was a modest increase in the coefficient of variation on the first day after the vagotomy (Fig. 7). However, these increases fall within the temporal variation that has been ascribed to changes in motivation for song [coefficient of variation was 3–4% for the duration of expiratory pulses between directed and undirected song, table 1 in Cooper and Goller (Cooper and Goller, 2006); similar to temporal variation reported in Sossinka and Böhner (Sossinka and Böhner, 1980) and Glaze and Troyer (Glaze and Troyer, 2006)] and can therefore not be unambiguously attributed to the disruption of vagal feedback information.
Fig. 7.
Temporal variability of the motif changed moderately over the course of the experiment. Bars show coefficients of variation on each day for completed song motifs in five birds. Pre, pre-vagotomy.
Acoustic changes
We found some consistent acoustic changes in five out of the seven birds, but the effects varied between individuals (Table 1). Acoustic changes occurred in songs and calls, and here we discuss mainly alterations that were not included in the above analysis of air sac pressure patterns. In some cases, new acoustic elements were added to song syllables. For example, in one syllable the high-frequency note was accompanied by broad-band sound after the vagotomy (Fig. 6A, blue arrows), suggesting that the left sound generator is not silenced [as is normally the case during high-frequency syllables (Goller and Cooper, 2004)]. In the next acoustic segment, the longer duration of the frequency component inserted between the main harmonics of the sound also suggests a change in the syringeal gating of sound production (Fig. 6A, green arrows). A syllable of another bird showed remarkable changes in acoustic structure (Fig. 6B, blue arrows), which also indicates changes in the syringeal control of sound production beyond the modest change in air sac pressure for this acoustic segment.
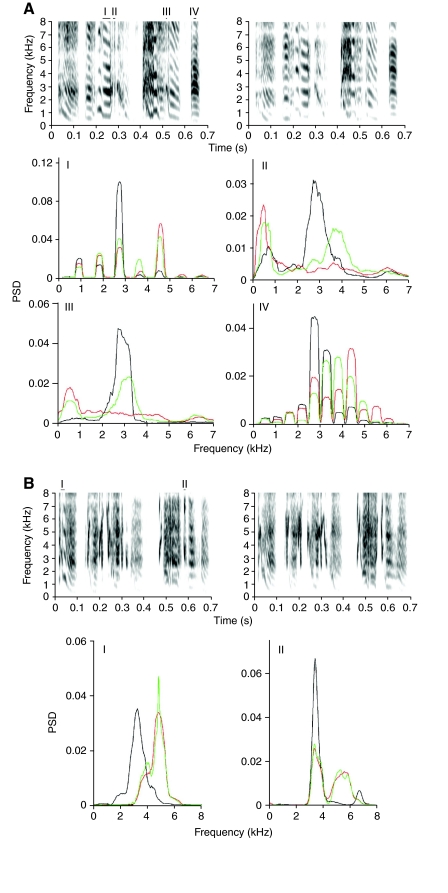
Two additional main effects on song after the vagotomy were a change in harmonic emphasis in some song syllables (Fig. 8A, I and IV) and the disappearance of certain acoustic features of the song (Fig. 8A, II and III). These changes typically occurred on the first few days after the vagotomy, and, in some cases, a partial recovery of acoustic features was observed on subsequent days (Fig. 8A, II and III). In some syllables, frequency modulation patterns and the fundamental frequency of high-frequency syllables showed small changes after the vagotomy (Fig. 8).
Fig. 8.
Representative examples (A: ZF2, B: ZF4) of the effects of unilateral vagotomy on the acoustic structure of song syllables. In the motifs several different segments (I, II, III and IV for A and I and II for B) were identified where differences in the acoustics exist. For each segment power spectral density was compared. (A) Power spectral density curves (mean; N=5) before (black), and 2 days (red) and 4 days (green) after the vagotomy. (B) Power spectral density curves before (black), and 5 days (red), and 12 days (green) after the vagotomy.
Although calls tend to be more variable and were not the main focus of this investigation, we visually inspected spectrographic representations before and after the vagotomy for noticeable changes. In three of the seven birds some changes occurred after the vagotomy (Table 1). The range of observed changes included altered harmonic emphasis, reshaping of the acoustic structure of some segments of calls and altered frequency modulation. It is likely that at least some of these changes can be attributed to the interruption of vagal information processing as respiratory and syringeal origins of the altered acoustic structure can be inferred.
DISCUSSION
In this study, we investigated the effects of disrupted vagal feedback on the song motor program. The effects of vagotomy on normal and stress-enhanced respiration have been studied in a few non-oscine species (Fedde et al., 1964; Richards, 1968), but no information is available in songbirds or for vocal behavior. This study is a first step towards exploring the role of vagal feedback in the performance of a learned vocal behavior, whose generation involves significant changes to the normal respiratory pattern. It is thought that for song production the vocal motor centers exert control over respiratory centers (e.g. Suthers and Margoliash, 2002) to establish the respiratory pattern of song. The resulting respiratory activity of phonation must activate the somatosensory systems and thus provide feedback information. Here we show that disruption of somatosensory feedback by a unilateral vagotomy causes changes in the motor patterns of song.
Afferent fibers in the vagal nerve carry information from CO2 receptors in the lungs (Glesson and Molony, 1989) and from receptors that are most likely located in the air sac membrane and are sensitive to changes in the inflation of air sacs (Kubke et al., 2004). The latter receptors have been implicated in mediating a compensatory change in respiratory effort during song (Suthers et al., 2002). Unilateral vagotomy disrupts the flow of feedback information from both receptor types on one side but information flow on the contralateral side is left intact. In addition, the vagus carries feedback from and motor information to other peripheral organ systems. However, the most parsimonious explanation for the observed effects on song behavior is that they were caused by interrupted information transfer from relevant respiratory receptors in the lungs and the air sacs. Because bilateral vagotomy leads to death within a short time period, only unilateral disruption of feedback is possible. This alteration of feedback is therefore not a complete interruption but constitutes a possible mismatch between the left and right feedback routes. We do not expect the unilateral disruption of vagal feedback to result in a lateralized effect on respiratory control of song because the respiratory motor program for song is symmetrical. In a species with a high degree of syringeal lateralization during song (brown thrasher, Toxostoma rufum) the activation patterns of the left and right expiratory muscles are highly similar (Goller and Suthers, 1999). Thus, even for sounds produced by only one side of the syrinx the activation of the expiratory muscles is symmetrical. This suggests that the respiratory motor program is symmetrical. Vagal feedback from the two sides of the body is most likely integrated so that a unilateral disruption affects the left and right sides symmetrically.
Respiratory changes resemble results of disruption of central control
A striking change in song motor control as a consequence of the vagotomy was the occurrence of interruptions of the respiratory motor patterns, including interruption of the motif and even expiratory pulses of individual syllables. This is noteworthy because it implies that the song motor program of individual syllables is not executed correctly. Experiments where song was interrupted by the presentation of stroboscopic flashes also caused interruptions of the song motif, but these occurred during the silent intervals between syllables, i.e. after completion of individual syllables (Cynx, 1990; Franz and Goller, 2002). This interpretation of acoustic recordings was confirmed by air sac pressure recordings which showed that song interruption coincided with silent inspirations of the motif (Franz and Goller, 2002).
The disruptions of individual syllables observed in this study are similar to song terminations caused by HVC stimulation (Ashmore et al., 2005). Stimulation trains delivered to one HVC caused changes in the pressure modulation pattern of syllables or atypical truncation of an expiratory pressure pulse of song [see figure 5 in Ashmore et al. (Ashmore et al., 2005)]. This similarity in observed effects after HVC stimulation and vagotomy indicates that the feedback disruption in this study may have caused a change in HVC activity, thus causing aberrant motor control of respiration. However, there are also differences between the illustrated example in Ashmore et al. (Ashmore et al., 2005) and the song interruption following vagotomy. Whereas the former shows that the pressure pattern returns to a typical silent respiratory pattern after song termination, we observed a pressure pattern that still retained some modulation characteristics of the intact expiratory pulse of song, but at a much lower amplitude and with significant variation in the modulation patterns. It is unclear from the limited examples whether this discrepancy represents a consistent difference between the effects of HVC stimulation and vagotomy and therefore might hold significance in regard to neural processing.
Several of the effects observed in this study after the vagotomy parallel those observed after a unilateral Uva lesion (Coleman and Vu, 2005). In particular, after the two manipulations the time course of the appearance of disrupted song and of recovery of normal song after some days was similar (see below). Furthermore, the effects on the acoustics of the motif and of the calls also seem remarkably similar. Finally, the occurrence of effects after Uva lesion is also highly variable within and between individuals. This high degree of similarity between the two studies suggests that interrupted information flow to Uva caused by the vagotomy might have changed activity in Uva and, therefore, indirectly altered information flow to the motor control areas of the song circuit.
All motor systems of vocal production are affected
The vagotomy caused changes not just in motor instructions to the respiratory system but also in syringeal and, possibly, upper vocal tract motor control. Although we did not record airflow from many individuals to study syringeal gating of airflow directly, the combination of acoustic recordings with air sac pressure enables an analysis of which motor systems contributed to the observed acoustic effects. Changes in the respiratory motor pattern, such as the termination of expiratory pulses and subsequent modulation of the decreased pressure, could indicate a decoupling of motor control between respiratory and syringeal control. If an adequate air sac pressure is not present, sound production will be terminated irrespective of whether syringeal control still follows the stereotyped motor pattern. However, the airflow recordings indicate that syringeal gating of airflow is most likely also changed simultaneously with the change in pressurization (e.g. green lines in Fig. 2B,C).
In addition, the acoustic output sometimes allows inference about how the two sound generators contributed to the sound (Goller and Cooper, 2004; Suthers and Zollinger, 2004). Some changes in the respective use of the two voices are visible in the spectrograms (Fig. 6) without simultaneous significant changes in the air sac pressure. This evidence, therefore, indicates that the vagotomy also caused a modification of bilateral syringeal control.
The origin of the changes in harmonic emphasis of syllables cannot be determined with certainty. It is possible that a change in the pressure–flow conditions resulting from atypical respiratory and syringeal control caused a change in the harmonic structure of syllables. However, a change in the filter characteristics of the upper vocal tract structures such as oropharyngeal cavity and beak opening dynamics (Goller and Cooper, 2004; Riede et al., 2006; Riede and Suthers, 2009) is more likely to have contributed to the observed acoustic changes.
Together, these observations indicate that the disruption of feedback from the respiratory system caused instabilities in the song motor program that affected all motor systems involved in sound generation and not just the respiratory system. It is therefore highly likely that vagal feedback information is processed by the song production network and does not simply affect processing of motor commands in the respiratory pre-motor areas of the medulla (Wild, 2004). This tentative case for the use of vagal feedback in cortical areas of the songbird song control network parallels similar suggested processing of feedback information in the sensorimotor areas of the mammalian cortex (Ito, 2002).
Different time course of effects between vagotomy and auditory feedback disruption
The evidence described here suggests that the altered feedback exerts a direct or indirect short-term effect on the song control circuit. The largest disruption of song occurred within 1–3 days of the vagotomy, followed by a partial or full recovery of the quantified parameters of song and the associated respiratory patterns. Unilateral Uva lesions cause a similar transient disruption (Coleman and Vu, 2005). This short time scale is in marked contrast to the longer-term effects of changing auditory feedback. In the zebra finch, various methods of chronically disrupting auditory feedback have been employed (e.g. deafening, muting) (Nordeen and Nordeen, 1992; Cooper and Goller, 2004; Hough and Volman, 2002). Despite the variable involvement of other potential feedback mechanisms for some of these methods, all cause a slow degradation of song over the course of weeks or months. The rate of deterioration is age specific, but is in all cases much slower than the almost instantaneous effects observed after the vagotomy. These long-term changes involve major changes to the song motor program and lead to omission of syllables (expiratory pulses) as well as incorporation of new syllables or atypical repetition of syllables (stuttering) (e.g. Leonardo and Konishi, 1999). These long-term effects represent a marked alteration of the song motor pattern and differ from documented on-line use of auditory feedback for small compensations of sound frequency (Sober and Brainard, 2009; Lei and Mooney, 2010; Andalman and Fee, 2009).
It is not clear whether the short time period during which the disruption of the song pattern occurred after vagotomy is the result of a central adjustment to the unilateral disruption of feedback or whether somatosensory feedback use differs in general from the role of auditory feedback in the maintenance of the song motor program. In either case, it is clear that the onset of changes in the motor patterns occurs much earlier than after disruption of auditory feedback.
The time scale of the effects observed with the vagotomy and the rapid recovery process are inconsistent with a re-innervation or a re-anastomosis of the nerve (e.g. Abdalla and King, 1979; Bregeon et al., 2007). The recovery within a few days of the vagotomy could be related to a compensatory mechanism involving a shift to the intact contralateral vagus and could occur at the level of nTS (Undem and Weinreich, 2005), Uva (Coleman and Vu, 2005) or perhaps at the cortical level (HVC). Examples of a compensatory mechanism at the cortical level with a similar rapid time course are found in the vibrissa system of rats and in the somatosensory map of the hand in monkeys (Feldman and Brecht, 2005; Fox and Wong, 2005).
The effects of unilateral vagotomy on song varied between individuals, ranging from no noticable changes (ZF7) to occasional, significant modifications at the level of the respiratory, syringeal and, most likely, upper vocal tract motor patterns (Table 1). These modifications resulted in lower stereotypy of motor gestures and therefore caused changes in the acoustic features of song syllables. It is possible that the altered acoustic feedback resulting from the changes to motor patterns after the vagotomy may have played a role in the rapid recovery.
It is unclear at present why unilateral vagotomy caused such a wide range of effects on song in different individuals. The respective developmental background of each male may have played a role in the extent to which song was affected by vagotomy. A more extensive study comparing different age and tutoring groups is necessary to systematically explore differences in the effects of feedback disruption. Alternatively, the use of feedback information may be greatly individualized and therefore may result in different perturbations of the motor program.
Footnotes
This research was supported by the National Institutes of Health grant DC 04390. We thank the referees for their constructive comments. Deposited in PMC for release after 12 months.
REFERENCES
- Abdalla A. B., King A. S. (1979). The afferent and efferent myelinated fibres of the avian cervical vagus. J. Anat. 128, 135-142 [PMC free article] [PubMed] [Google Scholar]
- Andalman A. S., Fee M. S. (2009). A basal ganglia-forebrain circuit in the songbird biases motor output to avoid mistakes. Proc. Natl. Acad. Sci. USA 106, 12518-12523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore R. C., Wild J. M., Schmidt M. F. (2005). Brainstem and forebrain contributions to the generation of learned motor behavior for song. J. Neurosci. 25, 8543-8554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore R. C., Renk J. A., Schmidt M. F. (2008). Bottom-up activation of the vocal motor forebrain by the respiratory brainstem. J. Neurosci. 28, 2613-2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer S. W., Arnold A. P. (1982). Afferent neurons in the hypoglossal nerve of the zebra finch (Poephila guttata): localization with horseradish peroxidase. J. Comp. Neurol. 210, 190-197 [DOI] [PubMed] [Google Scholar]
- Bottjer S. W., Arnold A. P. (1984). The role of feedback from the vocal organ. I. Maintenance of stereotypical vocalizations by adult zebra finches. J. Neurosci. 4, 2387-2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregeon F., Alliez J. R., Héry G., Marqueste T., Ravailhe S., Jammes Y. (2007). Motor and sensory re-innervation of the lung and heart after re-anastomosis of the cervical vagus nerve in rats. J. Physiol. 581, 1333-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. J., Vu E. T. (2005). Recovery of impared songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. J. Neurobiol. 63, 70-89 [DOI] [PubMed] [Google Scholar]
- Copper B. G., Goller F. (2004). Partial muting leads to age-dependent modification of motor patterns underlying crystallized zebra finch song. J. Neurobiol. 61, 317-332 [DOI] [PubMed] [Google Scholar]
- Cooper B. G., Goller F. (2006). Physiological insights into social-context-dependent changes in the rhythm of the song motor program. J. Neurophysiol. 95, 3798-3809 [DOI] [PubMed] [Google Scholar]
- Cynx J. (1990). Experimental determination of a unit of song production in the zebra finch (Taenopygia guttata). J. Comp. Psychol. 104, 3-10 [DOI] [PubMed] [Google Scholar]
- DeWet P. D., Farrell P. R., Fedde M. R. (1971). Number and morphology of muscles spindles in the transversus abdominis muscle of the chicken. Poult. Sci. 50, 1349-1357 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J., Shadmehr R., Ivry R. B. (2010). The coordination of movement: optimal feedback control and beyond. Trends Cogn. Sci. 14, 31-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe A. J., Kuhl P. K. (2008). Birdsong and human speech: common themes and mechanisms. In Neuroscience of Birdsong (ed. Zeigler H. P., Marler P.), pp. 5-31 Cambridge: Cambridge University Press; [DOI] [PubMed] [Google Scholar]
- Estavillo J., Burger R. E. (1973). Cardiac afferent activity in depressor nerve of the chicken. Am. J. Physiol. 225, 1063-1066 [DOI] [PubMed] [Google Scholar]
- Fedde M. R., Burger R. E., Kitchell R. L. (1964). Electromyographic studies of the effects of bilateral cervical vagotomy on the action of the respiratory muscles of the domestic cock. Poult. Sci. 43, 1119: 1125 [Google Scholar]
- Feldman D. E., Brecht M. (2005). Map plasticity in somatosensory cortex. Science 310, 810-815 [DOI] [PubMed] [Google Scholar]
- Fox K., Wong R. O. L. (2005). A comparison between experience-dependent plasticity in the visual and somatosensory systems. Neuron 48, 465-477 [DOI] [PubMed] [Google Scholar]
- Franz M., Goller F. (2002). Respiratory units of motor production and song imitation in the zebra finch. J. Neurobiol. 51, 129-141 [DOI] [PubMed] [Google Scholar]
- Glaze C. M., Troyer T. W. (2006). Temporal structure in zebra finch song: implication for motor coding. J. Neurosci. 26, 991-1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M., Molony V. (1989). Control of breathing. In Form and Function in Birds, Vol. 4 (ed. King A. S., McLelland J.), pp. 439-484 London: Academic Press [Google Scholar]
- Goller F., Cooper B. G. (2004). Peripheral motor dynamics of song production in the zebra finch. Ann. NY Acad. Sci. 1016, 130-152 [DOI] [PubMed] [Google Scholar]
- Goller F., Daley M. A. (2001). Novel motor gestures for phonation during inspiration enhance the acoustic complexity of birdsong. Proc. R. Soc. Lond. B. Biol. Sci. 268, 2301-2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller F., Suthers R. A. (1996). Role of syringeal muscles in gating airflow and sound production in singing brown thrashers. J. Neurophysiol. 75, 867-876 [DOI] [PubMed] [Google Scholar]
- Goller F., Suthers R. A. (1999). Bilaterally symmetrical respiratory activity during lateralized birdsong. J. Neurobiol. 41, 513-523 [DOI] [PubMed] [Google Scholar]
- Hough G. E., II, Volman F. (2002). Short-term and long-term effects of vocal distortion on song maintenance in zebra finches. J. Neurosci. 22, 1177-1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscoe S. (1998). Control of abdominal muscles. Prog. Neurobiol. 56, 433-506 [DOI] [PubMed] [Google Scholar]
- Ito S. (2002). Visceral region in the rat primary somatosensory cortex identified by vagal evoked potential. J. Comp. Neurol. 444, 10-24 [DOI] [PubMed] [Google Scholar]
- Kubke M. F., Ross J. M., Wild J. M. (2004). Vagal innervation of the air sacs in a songbird, Taenopygia guttata. J. Anat. 204, 283-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H., Mooney R. (2010). Manipulation of a central auditory representation shapes learned vocal output. Neuron 65, 122-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A., Konishi M. (1999). Decrystallization of adult birdsong by perturbation of auditory feedback. Nature 399, 466-470 [DOI] [PubMed] [Google Scholar]
- Nasir S. M., Ostry D. J. (2006). Somatosensory precision in speech production. Curr. Biol. 16, 1918-1923 [DOI] [PubMed] [Google Scholar]
- Nordeen K. W., Nordeen E. J. (1992). Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav. Neural Biol. 57, 58-66 [DOI] [PubMed] [Google Scholar]
- Okanoya K., Yamaguchi A. (1997). Adult Bengalese finches (Lonchura striata var. domestica) require real-time auditory feedback to produce normal song syntax. J. Neurobiol. 33, 343-356 [PubMed] [Google Scholar]
- Pearson K. G., Misiaszek J. E., Fouad K. (1998). Enhancement and resetting of locomotor activity by muscle afferent. Ann. NY Acad. Sci. 860, 203-215 [DOI] [PubMed] [Google Scholar]
- Plummer E. M., Goller F. (2008). Singing with reduced air sac volume causes uniform decrease in airflow and sound amplitude in the zebra finch. J. Exp. Biol. 211, 66-78 [DOI] [PubMed] [Google Scholar]
- Richards S. A. (1968). Vagal control of thermal panting in mammals and birds. J. Physiol. 199, 89-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T., Suthers R. A. (2009). Vocal tract motor patterns and resonances during constant frequency song: the white-throated sparrow. J. Comp. Physiol. A 195, 183-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T., Suthers R. A., Fletcher N. H., Blevins W. E. (2006). Songbirds tune their vocal tract to the fundamental frequency of their song, Proc. Natl. Acad. Sci. USA 103, 5543-5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata J. T., Brainard M. S. (2008). Online contributions of auditory feedback to neural activity in avian song control circuitry. J. Neurosci. 28, 11378-11390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman M. S. (2007). Sensory feedback control of mammalian vocalizations. Behav. Brain Res. 182, 315-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober S. J., Brainard M. S. (2009). Adult birdsong is actively maintained by error correction. Nat. Neurosci. 12, 927-931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossinka R., Böhner J. (1980). Song types in the zebra finch Poephila guttata castanotis. Z. Tierpsychol. 53, 123-132 [Google Scholar]
- Suthers R. A., Margoliash D. (2002). Motor control of birdsong. Curr. Opin. Neurobiol. 12, 684-690 [DOI] [PubMed] [Google Scholar]
- Suthers R. A., Zollinger S. A. (2004). Producing song: the vocal apparatus. Ann. NY Acad. Sci. 1016, 109-129 [DOI] [PubMed] [Google Scholar]
- Suthers R. A., Goller F., Wild J. M. (2002). Somatosensory feedback modulates the respiratory motor program of crystallized birdsong. Proc. Natl. Acad. Sci. USA 99, 5680-5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. (1982). Spectrum estimation and harmonic analysis. Proc. IEEE 70, 1055-1096 [Google Scholar]
- Tremblay S., Shiller D. M., Ostry D. J. (2003). Somatosensory basis of speech production. Nature 423, 866-869 [DOI] [PubMed] [Google Scholar]
- Tumer E. C., Brainard M. S. (2007). Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature 450, 1240-1244 [DOI] [PubMed] [Google Scholar]
- Undem B. J., Weinreich D. (2005). Advances in Vagal Afferent Neurobiology. Boca Raton: Taylor and Francis; [Google Scholar]
- Van Lommel A., Lauwerryns J. M., Berthoud H. R. (1998). Pulmonary neuroepithelial bodies are innervated by vagal afferent nerves: and investigation with in vivo anterograde DiI tracing and confocal microscopy. Anat. Embryol. 197, 325-330 [DOI] [PubMed] [Google Scholar]
- Widdicombe J. (2001). Airway receptors. Respir. Physiol. 125, 3-15 [DOI] [PubMed] [Google Scholar]
- Wild J. M. (1994). Visual and somatosensory inputs to the avian song system via nucleus uvaeformis (Uva) and a comparison with the projections of a similar thalamic nucleus in a nonsongbird, Columba livia. J. Comp. Neurol. 349, 512-535 [DOI] [PubMed] [Google Scholar]
- Wild J. M. (2004). Functional neuroanatomy of the sensorimotor control of singing. Ann. NY Acad. Sci. 1016, 438-462 [DOI] [PubMed] [Google Scholar]
- Wild J. M., Kubke M. F., Mooney R. (2009). Avian nucleus retroambigualis: cell types and projections to other respiratory-vocal nuclei in the brain of the zebra finch (Taeniopygia guttata). J. Comp. Neurol. 512, 768-783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley S. M. N., Rubel E. (1997). Bengalese finches Lonchura striata domestica depend upon auditory feedback for maintenance of adult song. J. Neurosci. 17, 6380-6390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler H. P., Marler P. (2008). Neuroscience of Birdsong, pp. 5-31 Cambridge: Cambridge University Press; [Google Scholar]