Abstract
Protein reinforced supported bilayer membranes (rSBMs) composed of phosphatidyl choline (PC), biotin-PE and Neutravidin were used to coat hybrid microchips composed of polydimethylsiloxane (PDMS) and glass. Since the coatings required a freshly oxidized, hydrophilic substrate, a novel method to rapidly connect reservoirs using plasma oxidation was first developed and found to support up to 5.2 N/cm2 (1.5 N) pull-off force. rSBMs were then assembled in the oxidized hydrophilic channels. The electroosmotic mobility (μeo) of rSBM-coated channels was measured over a 3 h time to evaluate the stability of the coatings for microchip electrophoresis. rSBM-coated microchips with a simple cross design had excellent properties for microchip separations, yielding efficiencies of up to 700,000 plates/m for fluorescent dyes and peptides. The separation performance of rSBM and PC-coated channels was evaluated after repeatedly drying and rehydrating the channels. The separation efficiency of fluorescein on PC-coated devices decreased by 40% after one dehydration cycle and nearly 75% after 3 cycles. In contrast for rSBM-coated devices there was no significant change in the fluorescein efficiency until the third cycle (10% decreased efficiency). rSBM-coated channels were also markedly more stable when placed in a dehydrated state during long-term storage compared to PC-coated channels, and showed reduced chip failure and no reduction in performance for up to one month of dehydrated storage. Finally, rSBM-coated devices were used to perform single-cell cytometry. Microchips that had been dehydrated, stored two weeks, and rehydrated prior to use demonstrated similar performance to newly coated devices for the separation of fluorescein and carboxyfluorescein from single cells. Thus rSBM-coated devices were rugged- withstanding electric fields, prolonged storage under dehydrated conditions, and biofouling by cellular constituents while maintaining excellent separation performance.
Introduction
Biomimetic supported membranes are an attractive surface coating for microchip electrophoresis due to a combination of simplicity, performance, and reliability. They have been used in capillaries1 and on microchips2 to separate a range of proteins with high efficiency. Recently there has been much interest in making supported bilayer membranes (SBMs) air-stable 3–8 to prevent dehydration-induced delamination. Air-stable SBMs would be more practical for real-world applications and commercial development because the coated devices do not have to be freshly prepared for each experiment. Microchips with air-stable SBM coatings could be commercially fabricated and a simple “rehydrate and use” product delivered to end users in biology and medicine.
Protein reinforced SBMs (rSBMs)9,10 are a versatile air-stable coating and also permit a variety of different proteins including antibodies to be tethered from plastic microchip surfaces. rSBMs are fabricated in a similar manner to regular SBMs, and thus share the same robust self-assembly and reproducible formation. Unlike regular SBMs, rSBMs include a small percentage of lipids with a biotin headgroup mixed with standard phospholipids in the membrane. Biotin-binding proteins are then introduced to reinforce the membrane structure. rSBMs also take advantage of the very high-affinity and reliable biotin-avidin interaction to provide a stable surface to which antibodies and other molecules can be tethered without the complexity of chemical functionalization. The ability to precisely control the mole fraction of biotinylated lipids in starting vesicle solutions used to prepare membranes allows for precise control over the density of capture molecules. Previous reports have shown the use of rSBM-coatings to reduce protein adsorption and tether antibodies for highly sensitive immunoassays10, although rSBMs have not been utilized for microchip electrophoresis. While non-specifically adsorbed proteins have been demonstrated for use in microchip CE11,12, rSBMs might further improve the stability and uniformity of protein coatings while providing superior blocking properties. In particular, the use of the neutral protein coating, Neutravidin, on rSBMs might provide two benefits13: 1.) reduced loss of analyte due to decreased electrostatic interactions with charged analytes and 2.) improved separation performance.
In this work, a novel method developed to fabricate poly(dimethylsiloxane) (PDMS) microchip devices coated with rSBMs is described. rSBMs were also tested for use in electrophoretic separations and single cell cytometry. Supported membranes in PDMS microfluidics are normally formed by vesicle fusion in oxidized channels. Because of the enclosed nature of the channels, the channel-oxidation step generally occurs prior to assembly of the device and construction of buffer reservoirs. Since the PDMS surface rapidly returns to a hydrophobic state, a very limited time is available for both reservoir assembly and SBM coating. The assembly of reservoirs on these oxidized PDMS microchips within a short time window is a challenge since existing adhesion methods require either long adhesive curing times or elevated temperatures14–17 which leads to a more rapid recovery of hydrophobic surface properties18. The hydrophobic or native channel surfaces do not support formation of the SBMs. In this report a rapid plasma oxidation-based adhesion method for reservoirs was demonstrated to overcome this challenge, and the reservoir bonding strength was compared with conventional adhesion methods. Next, Neutravidin rSBMs formed in microchannels were characterized for their stability in an electric field by measuring electroosmotic mobility (μeo) over a period of three hours. rSBM-coated simple cross microchips were then assessed for their separation performance using fluorescein and Oregon Green and two fluorescent peptides which were substrates for kinases. To determine whether rSBMs could be assembled within channels and stored for later use, a series of durability tests were carried out comparing the separation performance of dehydrated/rehydrated rSBM-coated channels with regular SBM-coated channels over storage periods of up to two months. Finally, rSBM-coated microfluidic devices were used for separation of single-cell contents.
Experimental
Materials
Egg phosphatidylcholine (PC) and 1,2-dipalmitoyl-snglycero-3-phosphoethanolamine-N-cap biotinyl) (sodium salt) (biotin-PE) were obtained from Avanti (Alabaster, AL). Fluorescein (FL) and Oregon Green carboxylate (OG) were purchased from Invitrogen (Carlsbad, CA). Avidin was acquired from Sigma-Aldrich and Neutravidin was obtained from Pierce. Phosphate buffer (PB) for μeo measurements was prepared with 10 mM or 9 mM potassium phosphate and titrated to pH 7.4. Tris-ves buffer was composed of 10 mM tris and 150 mM NaCl and adjusted to pH 7.4. Tris-sep buffer was comprised of 25 mM tris at pH 8.4 and was used for all separations. ECB-Glu buffer was defined as 135 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM MgCl2,1 mM CaCl2, and 10 mM glucose at pH 7.4. F-ABL was the peptide fluorescein-Glu-Ala-Ile-Tyr-Ala-Ala-Pro-Phe-Ala-Lys-Lys-Lys. F-ABL was synthesized by Anaspec, Inc. (San Jose, CA) with an amidated C terminus. The peptide was also obtained in its phosphorylated form with the phosphate added to the side chain of the Tyr (pF-ABL). The two component PDMS was Sylgard 184 PDMS elastomer (Dow-Corning). One-part PDMS was RTV108 adhesive sealant (General Electric, Fairfield, CT). Tubing (3.2 mm i.d./6.4 mm o.d. Dow Corning Silastic) was obtained from Cole-Parmer, Vernon Hills, IL. Tubing cutter A-329 was purchased from Upchurch, Oak Harbor, WA. A PDC-001 plasma cleaner (Harrick, Ithaca, NY) was used for oxidation of tubing and PDMS. For pull-off testing, a custom-made device was fabricated to suspend weights from the tubing attached to the glass surface. All optical filters were purchased from Semrock, Rochester, NY.
Chip fabrication
For separation experiments, PDMS chips with 30 µm × 30 µm cross channels were formed on silicon molds as described in a previous report.2 The channels for single cell cytometry experiments were constructed from PDMS using soft lithography and bonded to a glass coverslip. The design was similar to the cross channels used for separations, except that the loading channel was 60 µm in width and the separation channel was 20 µm in width. An extra reservoir was added for a cell focusing channel that was 120 µm wide and 200 µm away from the separation channel (on the side of the cell loading reservoir). The master mold used to imprint the channels into PDMS was a silicon wafer fabricated in the UNC CHANL cleanroom facility using conventional photolithographic techniques with SU-8 photoresist. The reservoirs were cut from tubing (3.2 mm I.D.) with a tubing cutter. The reservoirs along with the microchip surfaces to be bonded (glass substrate and PDMS top piece) were cleaned with ethanol and oxidized in a plasma cleaner. The flat side of the tubing was gently pressed against the oxidized substrate and held in place for the amount of time specified. This time was termed the “press time” since 0.5 N was applied during this time. The device was then incubated for a varying “bonding time” during which time the device remained undisturbed. For reservoirs attached with the 2-part PDMS, elastomer and curing agent were mixed in a 10:1 ratio for 10 min and degassed for 15 min under high vacuum. The mixture was then applied with a Q-tip or pipette tip to the tubing, which was subsequently joined to the substrate and baked at 85°C for at least 1 h. For connections with the 1-part PDMS, RTV108 adhesive was applied with a pipette tip to the bottom of tubing and it was pressed into place on the substrate and allowed to cure at least 24 h.
Pull-off strength test
The tubing to PDMS connections were prepared as described above and a custom-made pull-off strength gauge was attached to the tubing (Figure S1). The tubing was held so that the weight added to the pull-off gauge exerted a force vertically downward. Weights were gradually placed inside the gauge until the tubing was pulled-off from the PDMS surface. The total weight and the measured adhesion area were recorded.
Vesicle preparation
Chloroform stocks of lipids were dried in a nitrogen stream and rehydrated with a probe tip sonicator in Tris-ves buffer for 30 min. Vesicles were stored at 4° C for up to 2 weeks before disposal. To make rSBM-coated channels, 5 mol % biotin-PE was dehydrated with 95 mol % PC during preparation. The PC/biotin-PE vesicles were incubated for 1 h in freshly oxidized PDMS chips and the channels were rinsed for 10 min. Then 1 mg/mL avidin or neutravidin in water was incubated in the channels for 1 h and rinsed for 10 min. The biotin-PE/PC/avidin and biotin-PE/PC/neutravidin coatings are referred to as PCbAv and PCbNeu coatings, respectively.
Dehydration testing
After preparation of rSBM or SBM-coated microchips, the channels were rinsed for 10 min with deionized water and dehydrated for 30 min in a vacuum chamber until all channels were visibly dry. Before use, channels were rehydrated by placing tris-sep buffer in the wells for 10 min.
μeo measurements
The electroosmotic mobility (μeo) was measured using the current-monitoring method and Tris-sep buffer as previously described.2 A field strength of 133 V/cm was used.
On-chip detection
An argon ion laser (488 nm, JDS Uniphase) provided fluorescence excitation. The excitation beam transited an excitation filter (482 ± 17.5 nm) and was reflected with a dichroic (506 nm) onto the microchannel. Emitted photons traveling through the dichroic filter were filtered with a second filter (536 ± 20 nm) and spatially filtered before detection by a photomultiplier tube (R9110, Hamamatsu, Bridgetwater, NJ). The electrical output was sent to a preamplifier before being digitized by a USB data acquisition card (DAQ/109, Measurement Computing, Norton, MA). A CCD (Sony) was used to acquire images.
Separations
Tris-sep buffer was used for all separations. To load sample into the separation channel, the pinched-injection method was used. Further details can be found in the supporting information.
Single cell cytometry
A 2 µL height difference between the cell reservoir and waste was sufficient to cause cells to flow past the separation channel. Just before reaching the separation channel, the cells were lysed with a single laser pulse from a pulsed laser (Arctic Photonics) and the fluorescent contents were electrophoresed into the separation channel. The semi-intact cell body was carried by the flow to the waste reservoir. The separation channel (tris-sep buffer) was 20 µm wide and the cell transport channel (ECB buffer) was 60 µm wide.
Results and Discussion
Resevoir adhesion using plasma oxidation
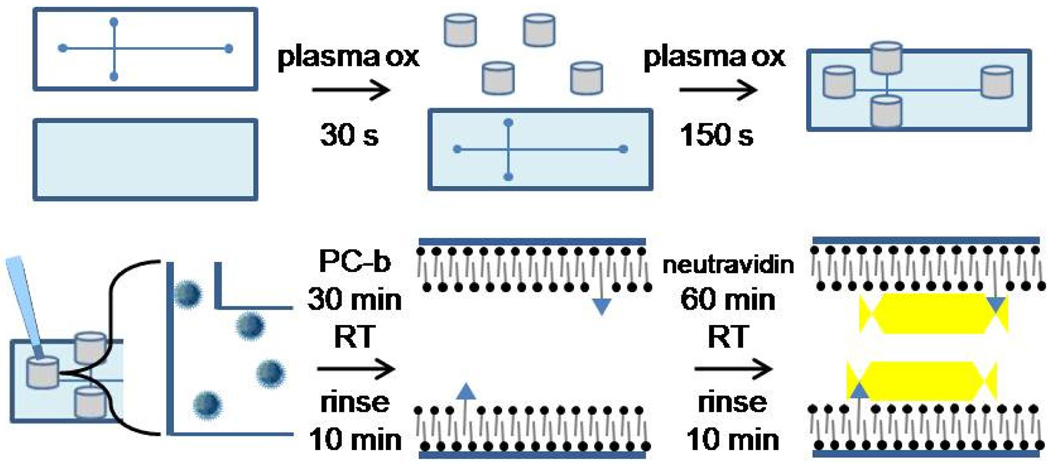
In order to fabricate PDMS chips with rSBMs, a method was needed to rapidly attach reservoirs after chip assembly. There is a short time window after plasma oxidation and assembly in which PDMS channels are hydrophilic and suitable for SBM fusion (Figure 1). Plasma oxidation can be used to permanently seal PDMS with other surfaces such as glass, silicon oxide, polyethylene, and other materials. Evidence suggests that the high-energy plasma breaks down the polymer chains and produces polar terminal groups such as COOH and OH that can condense with another silicon-containing surface to form Si-O-Si crosslinks.18 Plasma-based adhesion was tested on silicon tubing to determine whether it might bond to PDMS microchips following surface oxidation. When the tubing was cut with a razor blade or scissors, the adhesion of the tubing to the PDMS was inconsistent. In contrast, the use of a commercial tubing cutter to achieve a smooth and uniform surface resulted in high quality bonding. Figure S2A shows the silicon tubing attached to a PDMS-coated glass slide. As long as the wells were pressed together for 1 min, the wells did not leak when filled with water (30 µL). This initial press time was likely required to bring the silicon tubing material into molecular contact with the PDMS surface. Figure S2D, which was assembled in 3 min, highlights the strength and speed of the attachment method to bond highly stressed tubing, a situation in which epoxy-based adhesive would normally be pulled apart before the cure process could be completed. The plasma attachment procedure was also found to bond silastic tubing to glass.
Fig. 1.
Fabrication of PDMS/glass hybrid microchip with reservoirs and rSBM coatings. In the initial steps (upper panel) the components of the device are plasma oxidized and then assembled into a microchip with reservoirs. In the second phase (lower panel), the vesicles are loaded into the freshly oxidized device and an SBM layer forms with an occasional lipid bearing a biotin head group (triangular head groups in lower center graphic). In the final step neutravidin is loaded into the channels to covert the SBM into an rSBM coating.
The adhesion strength of this new method was quantified and compared with two other standard methods for securing reservoirs on PDMS (Figure S2 and S3). These strategies employed the standard 2-part elastomer and curing agent (Sylgard 184), whereas the other used a 1-part PDMS (RTV108) that employs an acetoxy curing system. The stress required to remove the tubing from the PDMS surface was measured to compare the performance of these PDMS-based methods with each other as well as other strategies for connecting reservoirs to PDMS microfluidic devices. The strongest adhesion was clearly seen with tubing attached using 1-part PDMS (RTV108). At 8 N/cm2 the integrity of the tubing failed but the tubing:PDMS interface remained intact. When 2-part PDMS was used to attach the tubing, a pull-off force of 2.6 N/cm2 was obtained. Plasma oxidation-based bonding between the silicon tubing and PDMS substrate supported up to 5.2 N/cm2 before failure. Tubing attachment by plasma bonding was not only much faster to implement, but was also stronger than the 2-part PDMS-based adhesion. Furthermore, the 2-part PDMS possessed a sufficiently low viscosity that it frequently flowed into and clogged the microchannels during attachment of wells. Comparing these methods to specially engineered epoxy/friction based connectors for PDMS found in the literature, the pull-out force for plasma bonding (1.5 N) was slightly weaker than the epoxy process (2 N), but twice as strong as a friction-based method (0.8 N).19 Chiou et. al. achieved 7× greater pull-out strength using tiny capillaries aligned inside 40-µm, PDMS channels formed by a wire20, but this was with an insertion depth of 1 cm, which is not suitable for thin microdevices.
μeo of PC/biotin-PE/neutravidin (PCbNeu)-coated channels
Channels of the PDMS microdevices were coated with a single-membrane bilayer of PC and biotin-PE by flowing vesicles composed of PC/biotin-PE through the channels (Figure 1). To form the PCbNeu coating, neutravidin was then introduced into the channel. The devices were then washed and μeo of the PCbNeu-coated channels was determined using the current monitoring method. PCbNeu-coated chips possessed a μeo of 2.6 ± 0.3 × 10−4 cm2 V−1S−1. For comparison microchannels coated with only PC, a zwitterion, possessed a μeo of 1.7 ± 0.4 × 10−4 cm2 V−1S−1. The greater μeo of PCbNeu-coated channels relative to that of the PC-only coating was likely due to the influence of the neutravidin coating which is negative in charge at pH 8.4 in the Tris-sep buffer. Although PC composes 95 mol % of the surface of the PCbNeu-coated channels, the PC headgroup size is much smaller than that of Neutravidin, resulting in a greatly reduced effective contribution to the overall zeta potential. For comparison, channels coated by nonspecific adsorption with bovine serum albumin (BSA), which is highly negatively charged at pH 8.4, possessed a significantly greater μeo of 5.7 ± 0.2 × 10−4 cm2 V−1S−1. BSA is a commonly used non-specific blocking coating.21
Stability of PCbNeu-coated channels
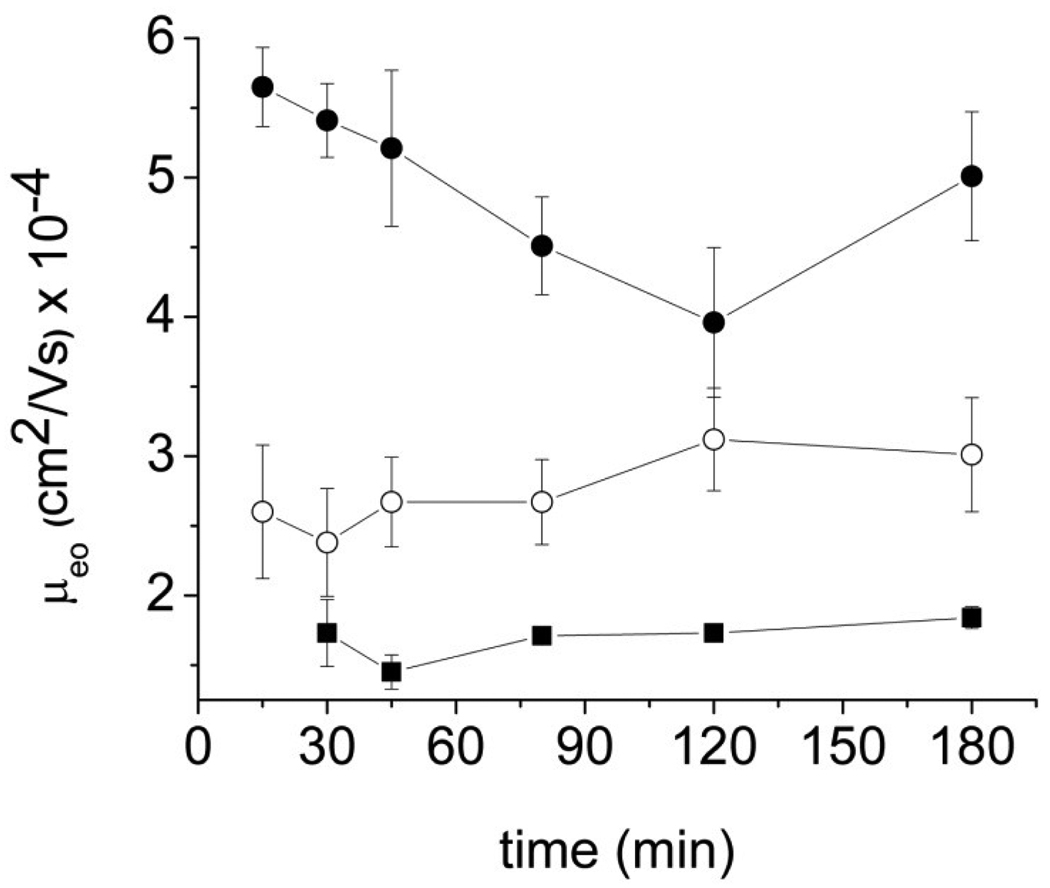
The stability of PDMS microchips coated with PCbNeu was determined by continuously applying 133V/cm across a microchannel and then measuring μeo at fixed intervals over time in continuously hydrated channels. The results were compared with the performance of microchips coated with PC only or non-specifically adsorbed BSA (Figure 2). The μeo of PCbNeu-coated chips drifted slightly from 2.6 ± 0.3 × 10−4 cm2 V−1S−1 to 3.0 ± 0.5 × 10−4 cm2 V−1S−1 (n=3) over 3 h but this change was similar to the standard deviation of the measurements. The stability of the μeo of PCbNeu-coated channels was better than that of BSA-coated channels, which ranged from 5.7 ± 0.2 × 10−4 cm2 V−1S−1 to 4.0 ± 0.1 × 10−4 cm2 V−1S−1 cm2 V−1S−1 over 3h, and close to that of pure PC-coated surfaces, which changed from 1.7 ± 0.4 × 10−4 cm2 V−1S−1 to 1.8 ± 0.4 × 10−4 cm2 V−1S−1. The stability of the μeo of the PCbNeu-coated microchips suggested that the membranes were not measurably removed or reorganized by the electric field over several hours.
Fig.2.
μeo stability in PDMS microchips. Closed squares, open circles, closed circles represent the average of the data for the PC-coated channels, PCbNeu-coated channels, and BSA-coated channels, respectively.
Separation of fluorescent dyes and peptides on PCbNeu-coated devices
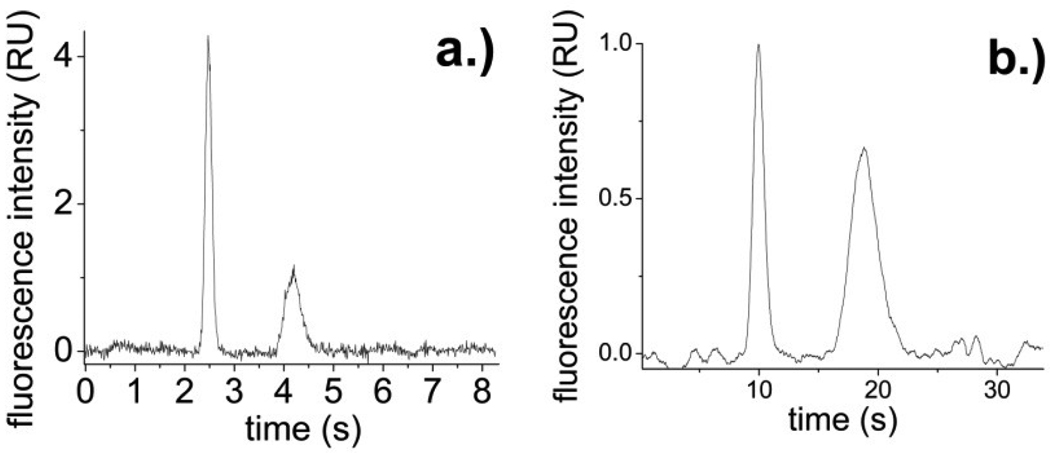
The PCbNeu-coated devices were used for separations of fluorescein and Oregon Green in order to benchmark their performance against normal SBM-coated channels and other types of surface coatings. For PCbNeu-coated channels, 722,000 ± 39,000 plates/m were obtained for Oregon Green and 504,000 ± 55,000 plates/m were obtained for fluorescein with a migration times of 2.62 ± 0.15 s and 4.37 ± 0.20 s respectively and a resolution of 4.2 (n=3) (Figure 3a). For comparison, under similar conditions PC-coated channels possessed slight lower efficiencies with 611,000 ± 39,000 plates/m for Oregon green and 499,000 ± 46,000 plates/m for fluorescein. The migration times were 3.50 ± 0.10 s (Oregon green) and 4.80 ± 0.15s (fluorescein) with a resolution of 2.4 (n=3).
Fig.3.
(a) Separation of 1 nM Oregon green (2.5 s) and 1 nM fluorescein (4.2 s) in PCbNeu-coated channels. (b) Separation of 100 nM pF-ABL (9.9 s) and 50 nM F-ABL (18.8 s) in PCbNeu-coated channels.
To determine whether Neutravidin might be replaced with avidin, channels were coated with PC and biotin-PE and then avidin was introduced into the channel to yield a PC/biotin-PE/avidin (PCbAv)-coated channel. When Oregon Green and fluorescein were loaded into and separated within this channel, 13,000 ± 1,000 plates/m were obtained for Oregon green and 15,000 ± 4,000 plates/m for fluorescein with migration time RSDs of 3.57 ± 0.14 s and 3.73 ± 0.07 s respectively. The peaks were not baseline resolved. PCbAv-coated channels had lower separation efficiencies and were less reproducible than PCbNeu-coated channels. This may be due to the heavy glycosylation of native avidin which can promote nonspecific interactions. Alternatively avidin which has a pI of 10.5 possesses a net positive charge in the electrophoretic solution (pH 8.4) and thus may have electrostatic interactions with the negatively charged dyes. In contrast Neutravidin possesses no carbohydrate and a pI of 6.3 making it negatively charged at the separation pH.
A peptide (F-ABL) which is a substrate of abl kinase was separated from its phosphorylated form (pF-ABL) in PCbNeu-coated microchips. pF-ABL and F-ABL were separated (Figure 3b) with an efficiency of 424,000 ± 59,000 plates/m and 228,000 ± 8,000 plates/m, respectively, and migration times of 10.04 ± 0.13 s and 18.90 ± 0.07 s respectively (n=3). The resolution was 3.6. These separation parameters were improved relative to that of PC-coated channels which possessed an efficiency of 365,000 ± 55,000 plates/m (pF-ABL) and 183,000 ± 34,000 plates/m (F-ABL) with a resolution of 1.7.
Stability of PCbNeu-coated channels after repeated dehydration/rehydration events
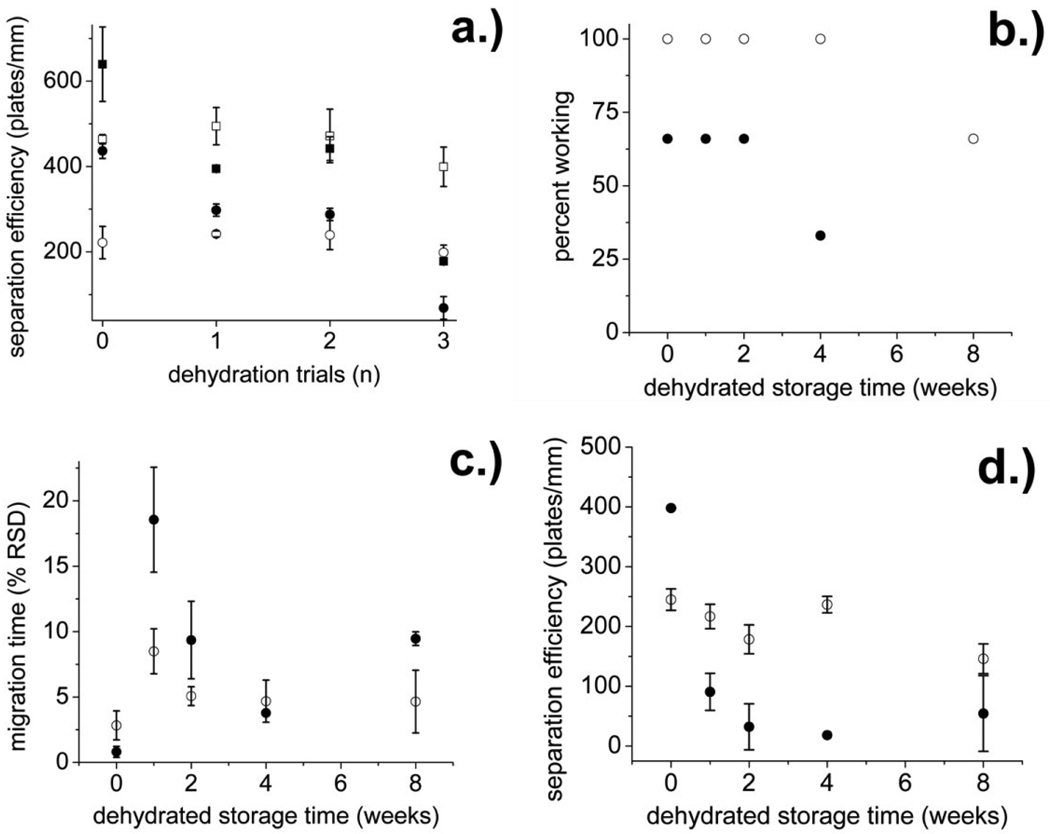
The PCbNeu- and PC-coated channels were subjected to dehydration and storage to determine their long term stability (Figure 4). In the first test, the chips were dehydrated in a vacuum for 30 min and then rehydrated. The process was repeated multiple times and the performance as indicated by the ability to electrophoretically separate Oregon green and fluorescein on the chip was evaluated after each cycle. As expected, the initial performance of PC-coated channels was excellent (639,000 ± 87,000 plates/m for Oregon green and 437,000 ± 18,000 plates/m for fluorescein). The efficiency decreased to 394,000 ± 4,000 plates/m for Oregon green and 298,000 ± 14,000 plates/m for fluorescein after a single dehydration/rehydration cycle. After three cycles the efficiency was further decreased to 178,000 ± 3,000 plates/m for Oregon green and 69,000 ± 27,000 plates/m for fluorescein (Figure 4a), most likely indicating structural rearrangement or damage to the lipid coating. The initial efficiency of the PCbNeu-coated channels prior to dehydration was 463,641 ± 11,000 plates/m for Oregon green and 221,561 ± 38,000 plates/m for fluorescein. After a single dehydration/rehydration cycle, the efficiency for Oregon green and fluorescein was 471,000 ± 63,000 plates/m for Oregon green and 239,000 ± 34,000 plates/m for fluorescein, similar to that of the original channel. After three dehydration cycles, the efficiency had decreased by only 10% to 399,000 ± 46,000 plates/m for Oregon green and 198,000 ± 17,000 plates/m for fluorescein. PCbNeu-coated channels exhibited considerably better stability over time when subjected to repeated dehydration/rehydration than the PC-coated surfaces. Thus PCbNeu-coatings were robust and could withstand adverse conditions within the microchannels.
Fig.4.
(a) Separation efficiency of Oregon green (squares) and fluorescein (circles) in PC (solid symbols) and PCbNeu (open symbols)-coated channels after the indicated number of dehydration/rehydration cycles (X axis). (b) Percentage of chips on which Oregon green and fluorescein could be separated after dehydration and storage for the indicated number of weeks. (c) The RSD of the fluorescein migration time expressed as a percentage was plotted against the storage time. (d) The separation efficiency for fluorescein versus the storage time is shown. For panels b, c, and d, the solid and open circles are PC and PCbNeu-coated channels, respectively.
Stability of PCbNeu-coated channels after prolonged dehydration and storage
Another important and useful property for a microfluidic chip is the ability to fabricate the chip, coat the channels, and then store the coated chip for future use. A major weakness of unreinforced SBM coatings is that they must be maintained in an aqueous environment at all times. In addition these coatings suffer a decrease in performance after several days of wet storage, potentially due to membrane dissolution.22 To investigate membrane integrity following storage, both PC and PCbNeu-coated channels were dehydrated and stored at 4° C for 1, 2, 4 or 8 weeks, after which time the channels were filled with an aqueous solution. Fluorescein and Oregon green were loaded into the separation channel and a separation of the dyes attempted. A chip was designated as a failure if the two dyes did not separate. Two of three PC-coated devices failed by 4 weeks of storage and all PC-coated devices (3 of 3) failed by 8 weeks (Figure 4b). In contrast at 4 weeks, a separation was achieved on all 3 PCbNeu-coated chips and only a single chip failed after 8 weeks of storage. The migration time reproducibility over time for PCbNeu-coated channels was also substantially better than that for PC-coated channels (Figure 4c). The maximum RSD for the migration time of fluorescein in the stored PC-coated channels was as high as 19% whereas the stored PCbNeu-coated chips exhibited a maximum RSD of 8%. The separation efficiency of PCbNeu-coated channels was also more stable than that of PC-coated channels over long storage times (Figure 4d). The separation efficiency of fluorescein on PC-coated chips decreased from 398,000 ± 500 plates/m to 91,000 ± 31,000 plates/m after one week in storage and to 18,000 plates/m after 4 weeks. In contrast, the fluorescein separation efficiency on PCbNeu-coated chips remained nearly constant with an initial separation efficiency of 245,000 ± 18,000 plates/m and an efficiency of 217,000 ± 20,000 plates/m and 237,000 ± 14,000 plates/m after 1 week and 4 weeks, respectively. By 8 weeks the PCbNeu-coated channels continued to maintain a respectable albeit decreased separation efficiency of 146,000 ± 25,000 plates/m. While standard PC-coated chips need to be used immediately after coating, PCbNeu-coated chips can be stored for up to 1 month without a significant decrease in separation efficiency. The greater stability of the PCbNeu coatings relative to that of standard PC coatings in a simulated “rehydrate and use” scenario resulted in improved reliability and separation performance for the microchips.
Single-cell cytometry with pristine and stored PCbNeu-coated PDMS chips
To determine whether dehydrated/stored PCbNeu-coated channels could be applied to a real-world separation challenge, PCbNeu-coated devices were rehydrated after a 2 week storage time and then used to electrophoretically separate and detect the contents of single cells. Cells loaded with fluorescein and carboxyfluorescein were introduced into the sample arm of a cross channel using hydrodynamic flow. As the cells reached the intersection of the sample and separation channels, the cells were lysed using a focused laser pulse. The contents of the lysed cell were then moved into the separation channel under an applied electric field. Fluorescein and carboxyfluorescein were then detected at a point downstream (4 mm) in the separation channel. Channels coated with PCbNeu without prior dehydration/storage were suitable for separation of carboxyfluorescein and fluorescein from individual cells (n = 30 cells) with average migration times of 3.4 and 5.2 s (with a 9.6 and 6.0% RSD) and peak widths of 0.76 ± 0.71 s and 0.65 ± 0.41 s, respectively (Figure 5). The resolution for the 2 dyes was 2.3 ± 1.5. After dehydration and storage at 4°C for two weeks, similar migration times, efficiencies, and resolutions were obtained. Carboxyfluorescein and fluorescein possessed migration times of 3.5 and 5.6 s (5 mm separation distance, 11.7 and 12.5% RSD) and peak widths of 1.15 ± 0.91s and1.34 ± 0 .92s, respectively (n = 30 cells, 2 chips yielded similar results). The resolution was 2.2 ± 2.2. Remarkably, when the different separation distances are accounted for, the average separation efficiency of stored/rehydrated PCbNeu-coated chips and pristine chips is nearly identical (4% difference). The large variation in peak width and resolution was likely due to the different cell location and velocity at the point of cell lysis by the laser beam. Nevertheless, it was remarkable that the dehydrated/stored/rehydrated PCbNeu-coated channels performed extremely well in the presence of biofouling cellular constituents such as membranes, DNA, organelles, and other cellular debris.
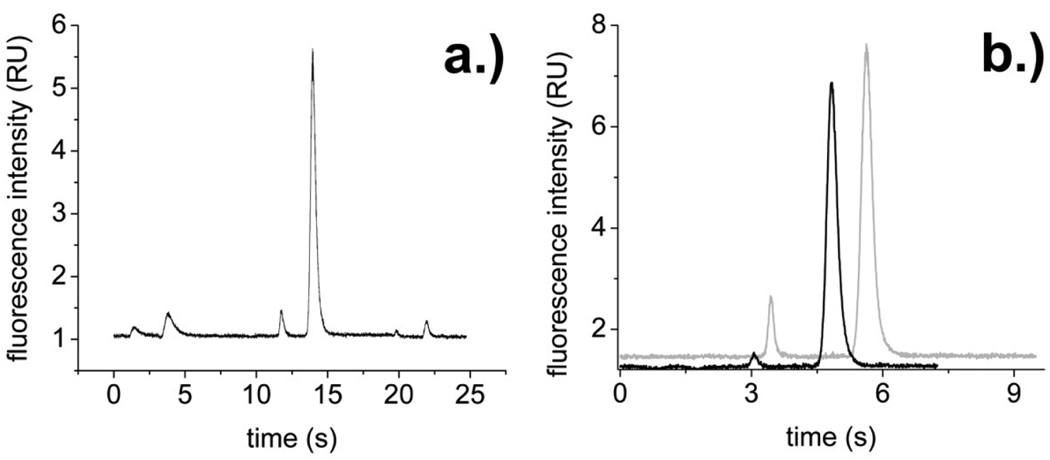
Fig.5.
(a) Continuous separation of the contents from 3 single cells (carboxyfluorescein at 1.4 s, 11.7 s, and 19.8 s; fluorescein at 3.8 s, 14.0 s, and 21.9 s). (b) Separation of carboxyfluorescein (3.1 s- black line, 3.4 s- grey line) and fluorescein (4.8 s- black line, 5.6 s- grey line) from a single cell in PCbNeu-coated channels. The channel used in the grey trace was dehydrated and stored for 2 weeks prior to rehydration and use. The channel represented by the black line was freshly coated with the lipid.
Conclusions
In this work, air-stable and storable membrane-coated PDMS devices for separations and single cell cytometry were demonstrated. An alternative strategy for fast, room-temperature reservoir attachment was developed to satisfy requirements necessary for the vesicle fusion process to be carried out in newly fabricated chips. The plasma oxidation reservoir attachment process is simple, robust, and does not require new instrumentation or synthesis. Taking advantage of this process, protein-supported SBMs were used to coat PDMS devices and tested for stability in an electric field, separation performance, and dehydration stability and found to exceed performance by conventional SBMs in all aspects of testing. Underscoring the robust nature of rSBMs was the fact that separation performance does not change after as many as 3 dehydration/rehydration cycles, or after storage times as long as 1 month. The performance for single cell cytometry, a stringent test, yielded nearly identical results (after a 1 month storage time) compared with devices that were used immediately after fabrication and coating. Due to the need to separate analytes in high-salt physiological buffer, surface coatings on this device need to perform extremely well under challenging conditions that include cell debris and very narrow separation channels. These results demonstrate that rSBMs are an excellent functional coating for PDMS devices and possess unique properties for electrophoresis and emerging live cell analysis strategies.
Supplementary Material
Acknowledgements
This work was supported by grants from the NIH (F32CA126258 to K.S.P., R01EB004436 to N.L.A.).
Footnotes
Electronic Supplementary Information (ESI) available: Methods for Separations; Schematic of pull-ofv test, Fig. S1; Images of tubing attached to a PDMS chip, Fig. S2; Adhesion strength of silicon tubing attached to PDMS, Fig. S3. See DOI: 10.1039/b000000x/
Notes and references
- 1.Cunliffe JM, Baryla NE, Lucy CA. Anal. Chem. 2002;74:776–783. doi: 10.1021/ac015627u. [DOI] [PubMed] [Google Scholar]
- 2.Phillips KS, Kottegoda S, Kang KM, Sims CE, Allbritton NL. Anal. Chem. 2008;80:9756–9762. doi: 10.1021/ac801850z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albertorio F, Chapa VA, Chen X, Diaz AJ, Cremer PS. J. Am. Chem. Soc. 2007;129:10567–10574. doi: 10.1021/ja0731266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennun SV, Faller R, Longo ML. Langmuir. 2008;24:10371–10381. doi: 10.1021/la8016694. [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Wang Y, Holtz B, Li JY, Traaseth N, Veglia G, Stottrup BJ, Elde R, Pei DQ, Guo A, Zhu XY. J. Am. Chem. Soc. 2008;130:6267–6271. doi: 10.1021/ja800049f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberts BP, Blanchard GJ. Langmuir. 2009;25:2962–2970. doi: 10.1021/la803486g. [DOI] [PubMed] [Google Scholar]
- 7.Oliver AE, Kendall EL, Howland MC, Sanii B, Shreve AP, Parikh AN. Lab Chip. 2008;8:892–897. doi: 10.1039/b800370j. [DOI] [PubMed] [Google Scholar]
- 8.Harland CW, Botyanszki Z, Rabuka D, Bertozzi CR, Parthasarathy R. Langmuir. 2009;25:5193–5198. doi: 10.1021/la804007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holden MA, Jung S-Y, Yang T, Castellana ET, Cremer PS. J. Am. Chem. Soc. 2004;126:6512–6513. doi: 10.1021/ja048504a. [DOI] [PubMed] [Google Scholar]
- 10.Dong Y, Phillips KS, Cheng Q. Lab Chip. 2006;6:675–681. doi: 10.1039/b514902a. [DOI] [PubMed] [Google Scholar]
- 11.Naruishi N, Tanaka Y, Higashi T, Wakida S-i. Journal of Chromatography A. 2006;1130:169–174. doi: 10.1016/j.chroma.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Wang A-J, Xu J-J, Chen H-Y. Journal of Chromatography A. 2006;1107:257–264. doi: 10.1016/j.chroma.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Dolnik V. Electrophoresis. 2004;25:3589–3601. doi: 10.1002/elps.200406113. [DOI] [PubMed] [Google Scholar]
- 14.Tsai J-H, Lin L. Journal of Micromechanics and Microengineering. 2001;11:577–581. [Google Scholar]
- 15.Pan T, Baldi A, Ziaie B. Journal of Microelectromechanical Systems. 2006;15:267–272. [Google Scholar]
- 16.Pattekar AV, Kothare MV. Journal of Micromechanics and Microengineering. 2003;13:337–345. [Google Scholar]
- 17.Puntambekar A, Ahn CH. Journal of Micromechanics and Microengineering. 2002;12:35–40. [Google Scholar]
- 18.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Anal. Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 19.Li SF, Chen SC. Ieee Transactions on Advanced Packaging. 2003;26:242–247. [Google Scholar]
- 20.Chiou C-H, Lee G-B. Journal of Micromechanics and Microengineering. 2004;14:1484–1490. [Google Scholar]
- 21.Knecht BG, Strasser A, Dietrich R, Martlbauer E, Niessner R, Weller MG. Anal. Chem. 2004;76:646–654. doi: 10.1021/ac035028i. [DOI] [PubMed] [Google Scholar]
- 22.Phillips KS. unpublished observation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.