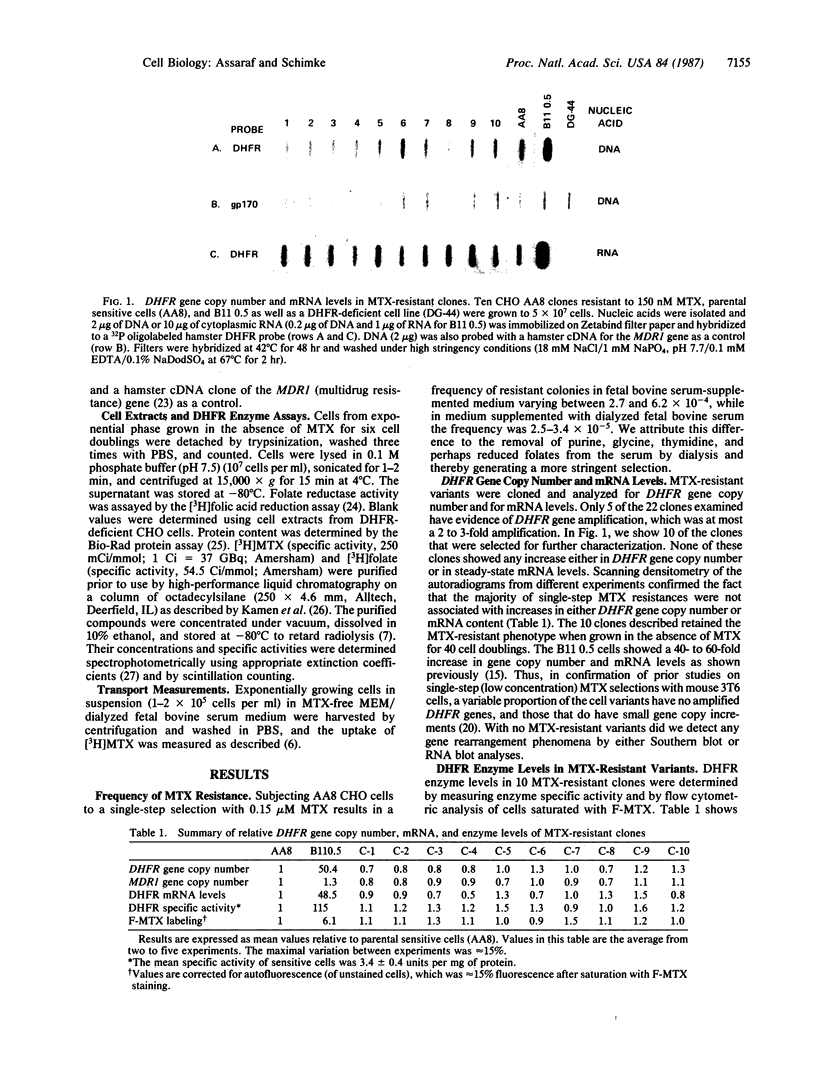
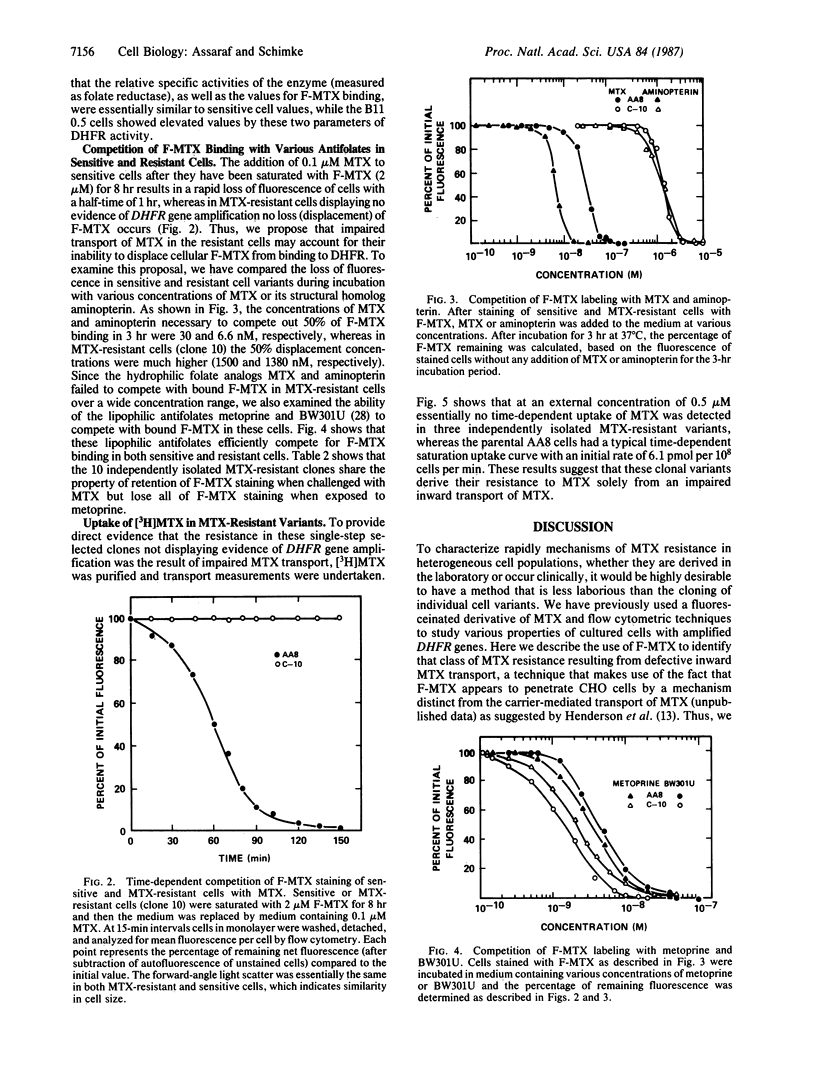
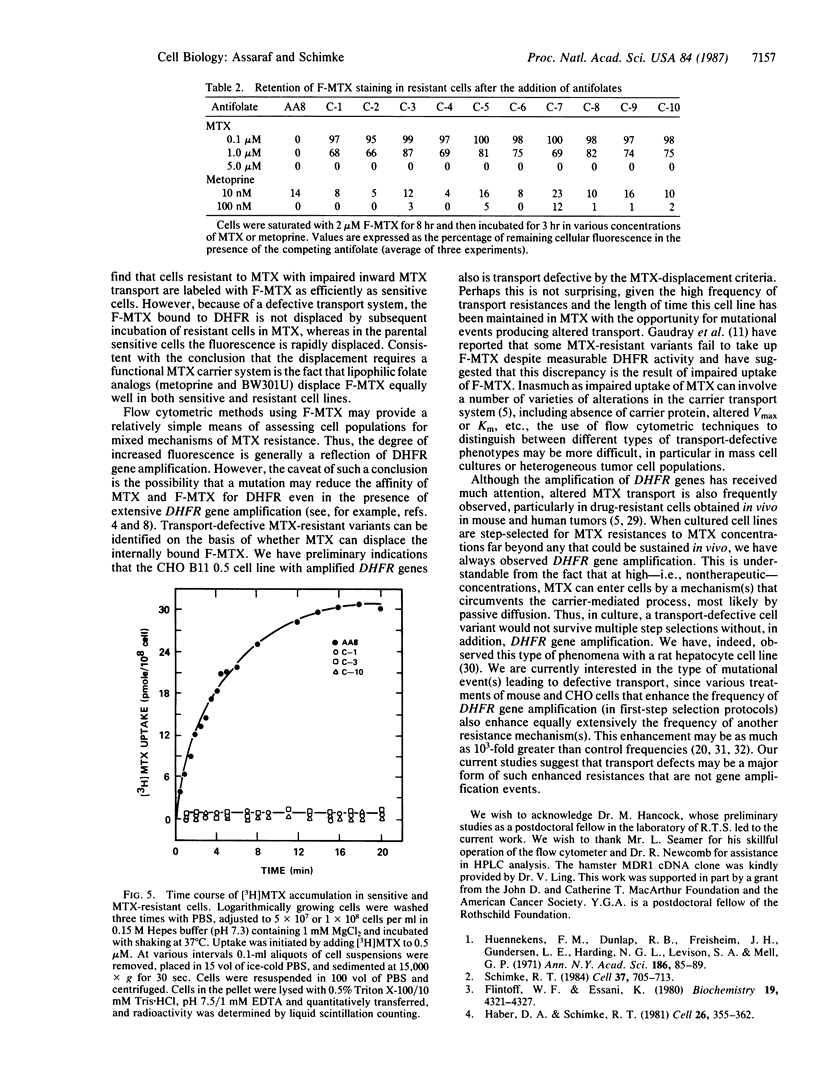
Abstract
We have studied the frequency of transport mutations in methotrexate-resistant Chinese hamster ovary cells using a rapid-flow cytometric technique. After saturating cells with fluoresceinated methotrexate, we examined the ability of hydrophilic and lipophilic antifolates to displace fluoresceinated methotrexate binding to dihydrofolate reductase. Cells with methotrexate transport deficiency are unable to take up methotrexate and thus retain the fluorescence, whereas the lipophilic antifolates displace fluoresceinated methotrexate equally well in sensitive and resistant cell lines. These resistant clones fail to take up methotrexate and occur with high frequencies upon single-step selections at methotrexate concentrations approximately equal to 7-fold the 50% killing concentration. The majority of such first-step resistant clones appear to derive their resistance solely from transport deficiency; they exhibit no overproduction of dihydrofolate reductase and no increase in either steady-state mRNA levels or gene copy number. Possible applications of the use of fluoresceinated methotrexate to the characterization of various mechanisms of methotrexate resistance in mixed cell populations are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown P. C., Tlsty T. D., Schimke R. T. Enhancement of methotrexate resistance and dihydrofolate reductase gene amplification by treatment of mouse 3T6 cells with hydroxyurea. Mol Cell Biol. 1983 Jun;3(6):1097–1107. doi: 10.1128/mcb.3.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duch D. S., Edelstein M. P., Bowers S. W., Nichol C. A. Biochemical and chemotherapeutic studies on 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine (BW 301U), a novel lipid-soluble inhibitor of dihydrofolate reductase. Cancer Res. 1982 Oct;42(10):3987–3994. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Essani K. Methotrexate-resistant Chinese hamster ovary cells contain a dihydrofolate reductase with an altered affinity for methotrexate. Biochemistry. 1980 Sep 2;19(18):4321–4327. doi: 10.1021/bi00559a027. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Nagainis C. R. Transport of methotrexate in Chinese hamster ovary cells: a mutant defective in methotrexate uptake and cell binding. Arch Biochem Biophys. 1983 Jun;223(2):433–440. doi: 10.1016/0003-9861(83)90607-0. [DOI] [PubMed] [Google Scholar]
- Fougere-Deschatrette C., Schimke R. T., Weil D., Weiss M. C. A study of chromosomal changes associated with amplified dihydrofolate reductase genes in rat hepatoma cells and their dedifferentiated variants. J Cell Biol. 1984 Aug;99(2):497–502. doi: 10.1083/jcb.99.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei E., 3rd, Rosowsky A., Wright J. E., Cucchi C. A., Lippke J. A., Ervin T. J., Jolivet J., Haseltine W. A. Development of methotrexate resistance in a human squamous cell carcinoma of the head and neck in culture. Proc Natl Acad Sci U S A. 1984 May;81(9):2873–2877. doi: 10.1073/pnas.81.9.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapski G. R., Whiteley J. M., Rader J. I., Cramer P. L., Henderson G. B., Neef V., Huennekens F. M. Synthesis of a fluorescent derivative of amethopterin,. J Med Chem. 1975 May;18(5):526–528. doi: 10.1021/jm00239a020. [DOI] [PubMed] [Google Scholar]
- Gaudray P., Trotter J., Wahl G. M. Fluorescent methotrexate labeling and flow cytometric analysis of cells containing low levels of dihydrofolate reductase. J Biol Chem. 1986 May 15;261(14):6285–6292. [PubMed] [Google Scholar]
- Goding C. R., Russell W. C. S1 sensitive sites in adenovirus DNA. Nucleic Acids Res. 1983 Jan 11;11(1):21–36. doi: 10.1093/nar/11.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber D. A., Beverley S. M., Kiely M. L., Schimke R. T. Properties of an altered dihydrofolate reductase encoded by amplified genes in cultured mouse fibroblasts. J Biol Chem. 1981 Sep 25;256(18):9501–9510. [PubMed] [Google Scholar]
- Haber D. A., Schimke R. T. Unstable amplification of an altered dihydrofolate reductase gene associated with double-minute chromosomes. Cell. 1981 Nov;26(3 Pt 1):355–362. doi: 10.1016/0092-8674(81)90204-x. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Russell A., Whiteley J. M. A fluorescent derivative of methotrexate as an intracellular marker for dihydrofolate reductase in L1210 cells. Arch Biochem Biophys. 1980 Jun;202(1):29–34. doi: 10.1016/0003-9861(80)90402-6. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Suresh M. R., Vitols K. S., Huennekens F. M. Transport of folate compounds in L1210 cells: kinetic evidence that folate influx proceeds via the high-affinity transport system for 5-methyltetrahydrofolate and methotrexate. Cancer Res. 1986 Apr;46(4 Pt 1):1639–1643. [PubMed] [Google Scholar]
- Huennekens F. M., Dunlap R. B., Freisheim J. H., Gundersen L. E., Harding N. G., Levison S. A., Mell G. P. Dihydrofolate reductases: structural and mechanistic aspects. Ann N Y Acad Sci. 1971 Nov 30;186:85–99. [PubMed] [Google Scholar]
- Kamen B. A., Cashmore A. R., Dreyer R. N., Moroson B. A., Hsieh P., Bertino J. R. Effect of [3H]methotrexate impurities on apparent transport of methotrexate by a sensitive and resistant L1210 cell line. J Biol Chem. 1980 Apr 25;255(8):3254–3257. [PubMed] [Google Scholar]
- Kaufman R. J., Schimke R. T. Amplification and loss of dihydrofolate reductase genes in a Chinese hamster ovary cell line. Mol Cell Biol. 1981 Dec;1(12):1069–1076. doi: 10.1128/mcb.1.12.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. C., Hoy C., Schimke R. T. Transient hypoxia enhances the frequency of dihydrofolate reductase gene amplification in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5978–5982. doi: 10.1073/pnas.83.16.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Rosowsky A., Wright J. E., Cucchi C. A., Boeheim K., Frei E., 3rd Transport of a fluorescent antifolate by methotrexate-sensitive and methotrexate-resistant human leukemic lymphoblasts. Biochem Pharmacol. 1986 Jan 15;35(2):356–360. doi: 10.1016/0006-2952(86)90541-1. [DOI] [PubMed] [Google Scholar]
- Rothenberg S. P. A rapid radioassay for folic acid reductase and amethopterin. Anal Biochem. 1966 Jul;16(1):176–178. doi: 10.1016/0003-2697(66)90095-9. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M., Moccio D. M., Kelleher L. E., Goutas L. J. Relative frequency and kinetic properties of transport-defective phenotypes among methotrexate-resistant L1210 clonal cell lines derived in vivo. Cancer Res. 1981 Nov;41(11 Pt 1):4447–4452. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Fong S., Brookman K. Validation of conditions for efficient detection of HPRT and APRT mutations in suspension-cultured Chinese hamster ovary cells. Mutat Res. 1980 Feb;74(1):21–36. doi: 10.1016/0165-1161(80)90188-0. [DOI] [PubMed] [Google Scholar]
- Tlsty T. D., Brown P. C., Schimke R. T. UV radiation facilitates methotrexate resistance and amplification of the dihydrofolate reductase gene in cultured 3T6 mouse cells. Mol Cell Biol. 1984 Jun;4(6):1050–1056. doi: 10.1128/mcb.4.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]



