Abstract
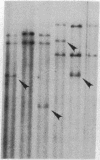
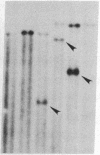
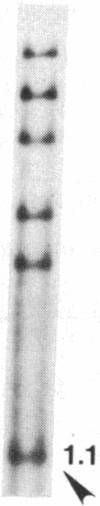
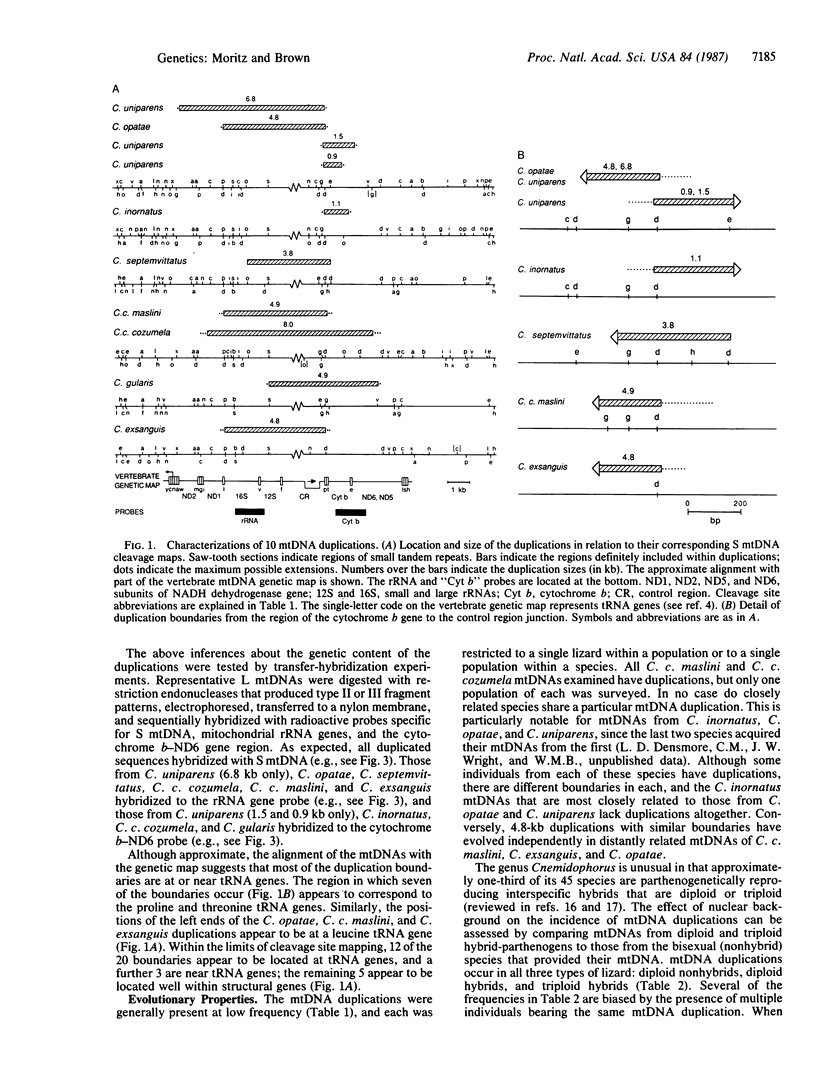
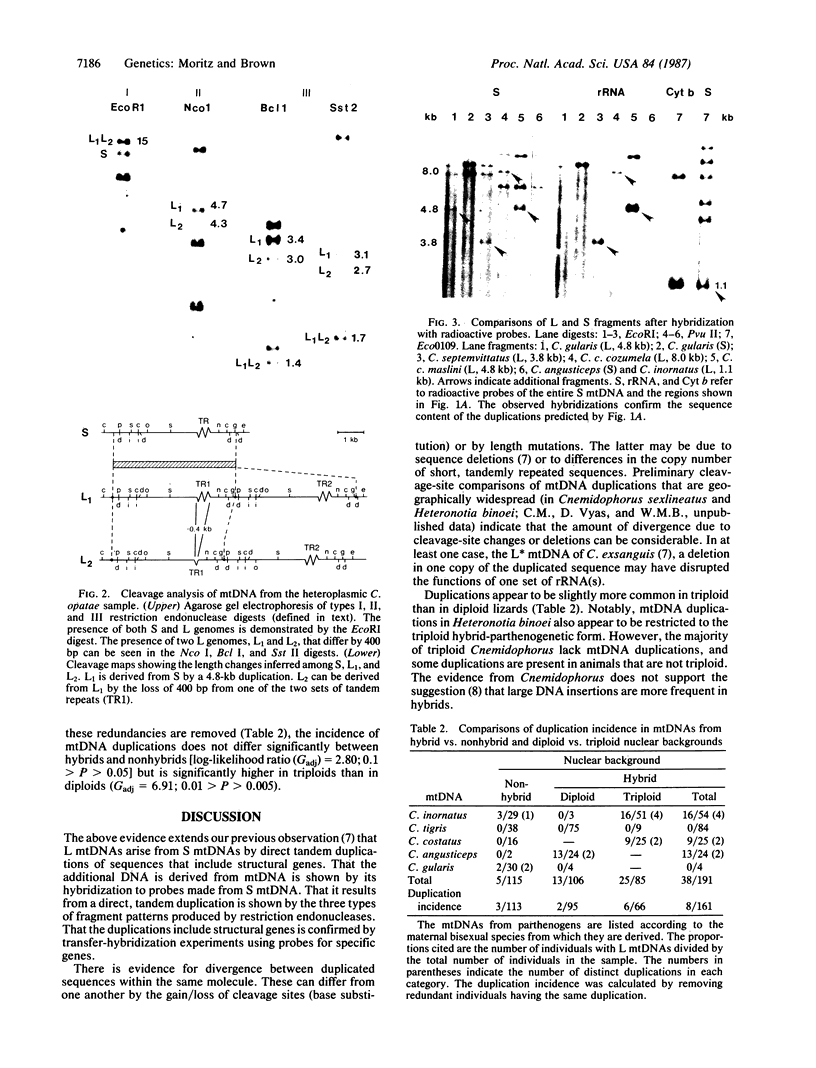
Size, location, gene content, and incidence were determined for 10 lizard mitochondrial DNA duplications. These range from 0.8 to 8.0 kilobases (kb) and account for essentially all of the observed size variation (17-25 kb). Cleavage-site mapping and transfer-hybridization experiments indicate that each duplication is tandem and direct, includes at least one protein or rRNA gene, and is adjacent to or includes the D loop-containing control region. Duplication boundaries are nonrandomly distributed, and most appear to align with tRNA genes, suggesting that these may play a role in the duplication process. Duplications are infrequent and usually restricted to particular individuals or populations. They appear to be ephemeral; in no case is the same duplication shared by mitochondrial DNAs from closely related species. Mitochondrial DNA duplications occur significantly more often in triploid than diploid lizards and at similar frequencies in hybrids and nonhybrids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Attardi G. Animal mitochondrial DNA: an extreme example of genetic economy. Int Rev Cytol. 1985;93:93–145. doi: 10.1016/s0074-7696(08)61373-x. [DOI] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., Wright J. W. Mitochondrial DNA analyses and the origin and relative age of parthenogenetic lizards (genus Cnemidophorus). Science. 1979 Mar 23;203(4386):1247–1249. doi: 10.1126/science.424751. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication of animal mitochondrial DNA. Cell. 1982 Apr;28(4):693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Densmore L. D., Wright J. W., Brown W. M. Length variation and heteroplasmy are frequent in mitochondrial DNA from parthenogenetic and bisexual lizards (genus Cnemidophorus). Genetics. 1985 Aug;110(4):689–707. doi: 10.1093/genetics/110.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Hale L. R., Singh R. S. Extensive variation and heteroplasmy in size of mitochondrial DNA among geographic populations of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8813–8817. doi: 10.1073/pnas.83.22.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson J. E., Brown W. M. A comparison of the small ribosomal RNA genes from the mitochondrial DNA of the great apes and humans: sequence, structure, evolution, and phylogenetic implications. Mol Biol Evol. 1986 Jan;3(1):1–18. doi: 10.1093/oxfordjournals.molbev.a040379. [DOI] [PubMed] [Google Scholar]
- Kessler L. G., Avise J. C. A comparative description of mitochondrial DNA differentiation in selected avian and other vertebrate genera. Mol Biol Evol. 1985 Mar;2(2):109–125. doi: 10.1093/oxfordjournals.molbev.a040339. [DOI] [PubMed] [Google Scholar]
- Moritz C., Brown W. M. Tandem duplication of D-loop and ribosomal RNA sequences in lizard mitochondrial DNA. Science. 1986 Sep 26;233(4771):1425–1427. doi: 10.1126/science.3018925. [DOI] [PubMed] [Google Scholar]
- Palmer J. D. Comparative organization of chloroplast genomes. Annu Rev Genet. 1985;19:325–354. doi: 10.1146/annurev.ge.19.120185.001545. [DOI] [PubMed] [Google Scholar]
- Powers T. O., Platzer E. G., Hyman B. C. Large mitochondrial genome and mitochondrial DNA size polymorphism in the mosquito parasite, Romanomermis culicivorax. Curr Genet. 1986;11(1):71–77. doi: 10.1007/BF00389428. [DOI] [PubMed] [Google Scholar]
- Rand D. M., Harrison R. G. Mitochondrial DNA transmission genetics in crickets. Genetics. 1986 Nov;114(3):955–970. doi: 10.1093/genetics/114.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederoff R. R. Structural variation in mitochondrial DNA. Adv Genet. 1984;22:1–108. doi: 10.1016/s0065-2660(08)60038-3. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Vawter L., Brown W. M. Nuclear and mitochondrial DNA comparisons reveal extreme rate variation in the molecular clock. Science. 1986 Oct 10;234(4773):194–196. doi: 10.1126/science.3018931. [DOI] [PubMed] [Google Scholar]
- Wallis G. P. Mitochondrial DNA insertion polymorphism and germ line heteroplasmy in the Triturus cristatus complex. Heredity (Edinb) 1987 Apr;58(Pt 2):229–238. doi: 10.1038/hdy.1987.37. [DOI] [PubMed] [Google Scholar]
- Wright J. W. Parthenogenetic lizards. Science. 1978 Sep 22;201(4361):1152–1154. doi: 10.1126/science.201.4361.1152-a. [DOI] [PubMed] [Google Scholar]